Abstract
The conjunctival and cerebral vasculatures share similar embryological origins, with similar structural and physiological characteristics. Tracking the conjunctival microvasculature may provide useful information for predicting the onset, progression and prognosis of both systemic and central nervous system (CNS) vascular diseases. The bulbar conjunctival vasculature was imaged using a retinal function imager (RFI, Optical Imaging Ltd, Rehovot, Israel). Hemoglobin in red blood cells was used as an intrinsic motion-contrast agent in the generation of detailed noninvasive capillary-perfusion maps (nCPMs) and the calculation of the blood flow velocity. Five healthy subjects were imaged under normal conditions and again under the stress condition of wearing a contact lens. The retina was also imaged in one eye of one subject for comparison. The nCPMs showed the conjunctival microvasculature in exquisite detail, which appeared as clear as the retinal nCPMs. The blood flow velocities in the temporal conjunctival microvasculature were 0.86 ± 0.08 (mean ± SD, mm/s) for the bare eye and 0.99 ± 0.11 mm/s with contact lens wear. It is feasible to use RFI for imaging the conjunctival vasculature.
Keywords: retina, conjunctiva, microvasculature, quantitative analysis, segmentation, image processing, blood flow
Introduction
The vasculatures in the eye and cerebral cortex have the same main blood supply, which is the internal carotid artery (ICA). The bulbar conjunctival vasculature is regarded as the terminal vascular bed of the human ICA (Duke-Elder S, 1961). The bulbar conjunctiva vessels can be accessed directly and non-invasively, and studies of the conjunctival microvasculature have provided sensitive indicators of both systemic and CNS vascular diseases (Cheung et al., 2001a;Cheung et al., 2001b;Cheung et al., 2002a;Ohtani, 1996;Schaser et al., 2003). Retinal vascular function, particularly blood flow velocity and capillary perfusion, has been assessed without contrast using the retinal function imager (RFI,) (Nelson et al., 2011;Nelson et al., 2005). However, capillary perfusion and blood flow velocity in a wide field have not been generated previously for the conjunctiva. The purpose was to ascertain the feasibility of utilizing a commercially available RFI instrument to image the conjunctival microvasculature and compare findings to RFI image characteristics of the retina. In addition, the feasibility of imaging the conjunctival microvasculature through the contact lens was also demonstrated.
Materials and Methods
The study was approved by the Institutional Review Board of the University of Miami. All participants provided written informed consent. To test the feasibility of using RFI to measure the conjunctival vessels, five healthy subjects (3 males and 2 females, aged 36.4 ± 8.4 years) were recruited. The temporal conjunctiva of one randomly selected eye in each subject was imaged by RFI. In addition, the retina was imaged in 1 eye for comparison. The device has been described in details elsewhere (Nelson et al., 2011;Nelson et al., 2005;Beutelspacher et al., 2011;Landa et al., 2012;Landa and Rosen, 2010). Briefly, the RFI is a fundus camera-based device with an attachment of a specific camera (a 60-Hz, 1024 × 1024-pixel digital camera) that captures reflectance changes as a function of time under stroboscopic illumination (wavelengths between 530 and 590 nm). Eight consecutive flashes with an interflash interval of less than 20 ms were used to take 8 images (Nelson et al., 2011). Hemoglobin in red blood cells acted as an intrinsic motion-contrast agent in the generation of detailed noninvasive capillary-perfusion maps (nCPMs), and the blood flow velocity was calculated using the proprietary software of the device (Nelson et al., 2011;Izhaky et al., 2009). After image acquisition, imaging algorithm registered a set of 8 images. After that, ratio images were created by dividing each image by the average of the set to obtain the motion signal of the hemoglobin in red blood cells along the path, resulting in the visualization of the microvasculature (nCPM). Blood flow velocity map was also calculated based on the motion signal.
Conjunctival vessels are in the transparent or semi-transparent conjunctival tissue, which provides the possibility being imaged by RFI. In the present study, the system was adapted for imaging the conjunctiva by focusing on the ocular surface when the subject was fixed at approximately 45 degrees to the nasal side. The temporal conjunctiva of the test eye (Figure 2) was imaged under normal conditions, and the imaging was repeated after wearing a contact lens (CL: PureVision, BC 8.3 mm, −3.00D) for 5 minutes. The blood flow velocities were measured at approximately twenty vessel segments. The measurement was performed in a dedicated room with a controlled temperature (between 22°C and 24°C) and humidity to avoid the possible impact of the external environment on conjunctival microcirculation (Duench et al., 2007). The subjects were asked to relax for 15 minutes before imaging. At another visit, the fovea of one subject was imaged, and a series of eight images were acquired. Multiple image sessions were performed to calculate the nCPMs. Blood flow velocity was calculated in the second or third branches of both major retinal arteries and veins (Figure 1). It took about 10 minutes to complete imaging one eye for each visit.
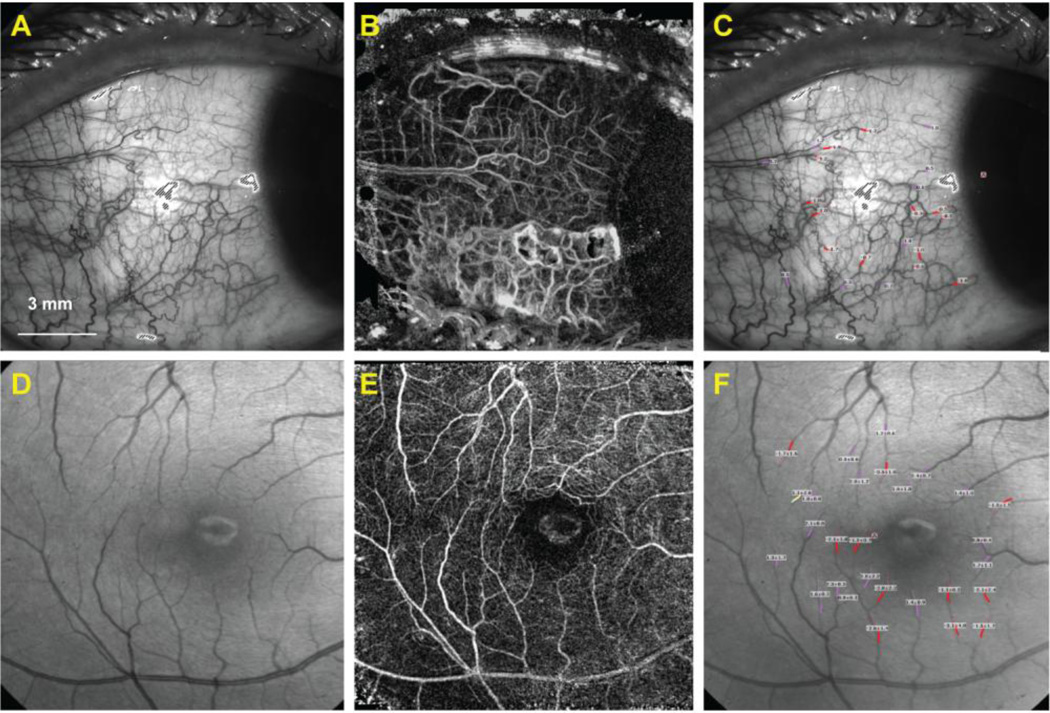
Figure 2. Human conjunctival microvasculature underneath a contact lens.
The bulbar conjunctival region (A) of the same healthy subject shown in Figure 1 was imaged with a soft contact lens in situ, and measurements of the bulbar conjunctival capillary perfusion (B) and blood flow velocity (unit: mm/s) (C) were obtained. Note, bulbar conjunctival capillary perfusion is clearly shown, (B) and the lens edge is visible (A-C). Note that negative values (red) indicate blood flow moving away from the heart and that the vessels are arteries. Positive values (pink) represent the veins.
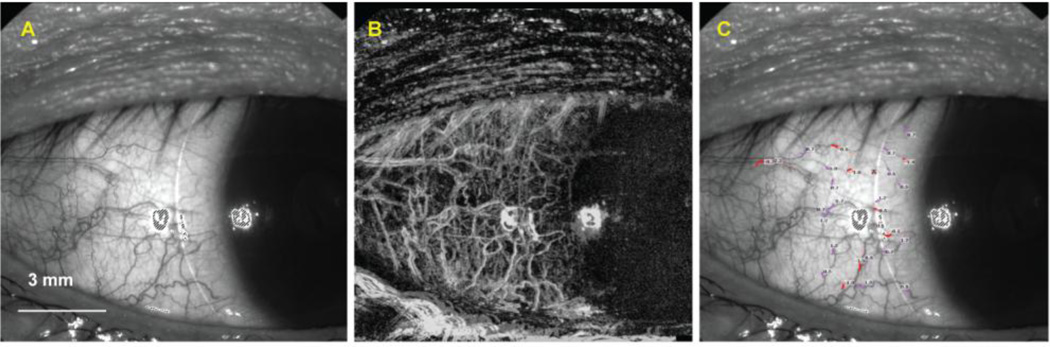
Figure 1. Human retinal and conjunctival microvascular assessed with a retinal function imager (RFI).
The bulbar conjunctiva (A) of a healthy subject was imaged, and measurements of the conjunctival capillary perfusion (B) and blood flow velocity (unit: mm/s) (C) were obtained. Similarly, the fovea of another healthy subject (D) was imaged, and measurements of the capillary perfusion (E) and blood flow velocity expressed in mean ± SD (unit: mm/s) (F) were obtained. The avascular zone was evident in the fovea. Note that negative values (red) indicate blood flow moving away from the heart and that the vessels are arteries. Positive values (pink) represent the veins.
Results
The nCPMs were successfully acquired, and the capillaries were shown in exquisite detail on the temporal bulbar conjunctiva with and without the CL in situ (Figures 1 and 2), which appeared to be as clear as the nCPMs in the retina. Using the flow movie and velocity measurements, the conjunctival arteries and veins were clearly visualized and identified (Figure 3). An avascular zone was evident in the retina (Figure 1). The blood flow velocities in the temporal conjunctival microvessels without and with the CL were 0.86 ± 0.08 (mean ± SD, mm/s) and 0.99 ± 0.11 mm/s, respectively (p = 0.09). The blood flow velocities of the retinal artery and vein were 3.70 mm/s and 2.73 mm/s, respectively.
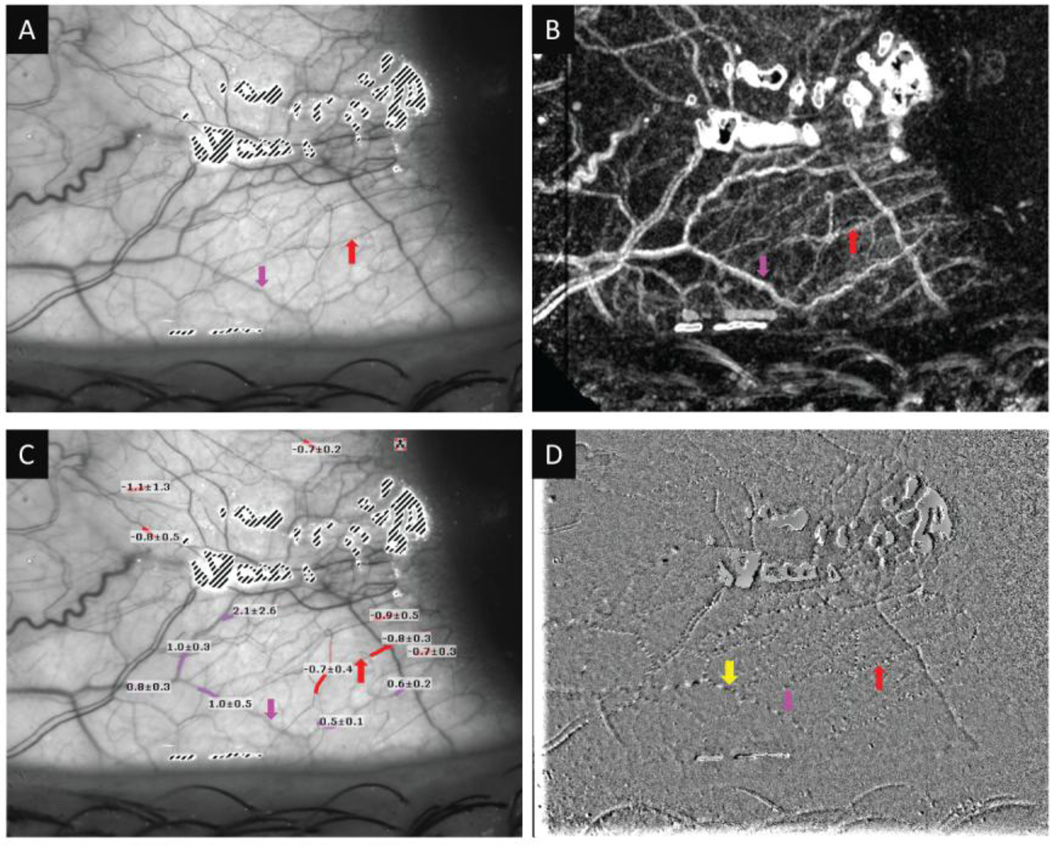
Figure 3. The microvasculature of the human conjunctiva.
A: An RFI image showing vessels on the temporal region of the conjunctiva. B: nCPM of the same region. C: Quantification of the conjunctival blood-flow velocity expressed in mean ± SD (unit: mm/s). The images show several arteries (red) and veins (pink) that were confirmed by viewing the flow movie from the same region. Note that negative values (red) indicate blood flow moving away from the heart and that the vessels are arteries. Positive values (pink) represent the veins. D: A single frame of a flow movie showing erythrocyte clusters (black) and gaps (white) moving in the small vessels marked as yellow arrow. Red arrow: artery; Pink arrow: Vein.
Discussion
Tracking both conjunctival and retinal microvasculature may provide useful information in predicting the onset, progression and prognosis of both systemic and CNS vascular diseases (Cheung et al., 2001a;Cheung et al., 2001b;Cheung et al., 2002a;Ohtani, 1996;Schaser et al., 2003). RFI was designed to directly and non-invasively measure the retinal vessel blood-flow velocity and to perform capillary perfusion mapping (Izhaky et al., 2009), which can be used to measure the vasoreactivity of vessels with a smaller diameter. In the present study, we demonstrated that RFI could be used to study the conjunctival microvasculature and its flow velocity, which may provide more information on the early stage of cerebral microvascular changes. To the best of our knowledge, this pilot study is the first to demonstrate that optical imaging using RFI is feasible for obtaining high-resolution nCPMs and providing the blood flow velocity of the bulbar conjunctiva. The image quality appeared as good as that obtained in the retina. The blood flow velocity of the conjunctiva was lower than the retina, indicating the terminal vascular beds of the conjunctival microvasculature (Duke-Elder S, 1961). This may also be an indication of the different physiologic needs because the conjunctiva is a protective membrane that lubricates and protects the ocular surface. In contrast, the retina is a complex sensory organ with a high metabolic demand for oxygen to sense light and process vision.
The feasibility of the study of both the bulbar conjunctival and retinal microvasculature using the same device provides the opportunity for non-invasive functional and structural studies of central nervous system and systemic vascular diseases. Bulbar conjunctival blood flow is sensitive to the change of the supplying vessels and correlates with cerebral blood flow during aortic arch surgery (Ohtani, 1996). It has also been used for intraoperative monitoring during carotid artery surgery, with microcirculation attenuation when the ICA was clamped and restoration immediately after ICA reperfusion (Schaser et al., 2003). Cheung et al reported conjunctival microangiopathy in type I diabetic patients that reversed following simultaneous pancreas-kidney transplantation (Cheung et al., 1999;Cheung et al., 1997;Cheung et al., 1994) and sickle cell-related conjunctival microangiopathy that improved following poloxamer 188 treatments (Cheung et al., 2004). Diabetic conjunctival microangiopathy was identified earlier than diabetic retinopathy (To et al., 2011).
RFI with its image-processing software within the device has the capability to directly and noninvasively image high-resolution and wide-field nCPMs, as demonstrated in previous studies (Nelson et al., 2005;Nelson et al., 2011). We demonstrated that the bulbar conjunctival nCPM can also be imaged. The results are well within the range of previously reported results using other methods (Shahidi et al., 2010;Cheung et al., 2002b) RFI may be a unique tool for quantitatively analyzing microvasculature because both capillary perfusion and blood velocity can be imaged in the retina and conjunctiva. Although large-scale studies will be needed to justify its routine clinic use, the robust image analysis and ease of use may be beneficial for both clinicians and patients, compared to other methods. Duench and associates modified a Heidelberg Retinal Flowmeter (HRF; Heidelberg Engineering, Heidelberg, Germany) to measure bulbar conjunctival blood flow (Duench et al., 2007), but only arbitrary results were provided (Duench et al., 2007). Orthogonal polarized spectral imaging was performed to measure conjunctival capillary perfusion in a single vessel (Schaser et al., 2003). The image field was very small (Schaser et al., 2003). Cheung and associates used a computer-assisted video camera to analyze conjunctival microcirculation with in-house developed software (Cheung et al., 1999). Up to 15 minutes of recording was typically required, and the field was approximately 8 mm2 (Cheung et al., 1999). High-speed video microcinematography (MSN) has also been used to measure pre-capillary arteriole blood flow velocity, including the minimum of the end diastolic values and the maximum of all of the peak systolic values and their average (Koutsiaris et al., 2010;Koutsiaris et al., 2007). A manual approach to the registration of sequential images may be time consuming (Koutsiaris et al., 2010).
The eye is sensitive to the outside environment, and wearing a contact lens causes the eye to adapt. We demonstrated the feasibility of imaging conjunctival vessels through the contact lens and measuring the conjunctival response to ocular stress by placing a contact lens on the eye. Conjunctival hyperemia is a normal response to initial lens wear, indicating that the microcirculation may be altered (Fonn et al., 2002). Cheung and associates demonstrated microvascular abnormalities in the conjunctival vessel after long-term lens wear (Cheung et al., 2012). Conjunctival tissue compression and distortion at the limbal region due to contact lens wear have been documented (Shen et al., 2011). Although significant differences in velocity were not found in the small sample size, the trend of increased velocity may indicate microcirculatory compromise underneath the contact lens. The compromise may be due to localized pressure underneath the lens (Shen et al., 2011). Future studies with a larger sample size and a variety of lenses for an extended wearing period may further validate the biomarkers for ocular integrity during lens wear and shed light on contact-lens-induced corneal neovascularization and conjunctival hyperemia.
In conclusion, this pilot study demonstrated that optical imaging using RFI is feasible for making high-resolution nCPMs and determining the blood flow velocity on the conjunctiva and the microvascular changes in response to soft contact lens wear. Further validity studies of RFI as well as clinical studies using RFI for imaging both the conjunctival and retinal vasculature in normal controls and various types of vascular diseases, such as stroke, may lead to the development of sensitive markers of ocular, cerebral and systemic vascular diseases.
Supplementary Material
Acknowledgments
Grant/financial support: Supported in part by the research grants NIH R01EY020607, NIH R01EY020607S, NIH Center Grant P30 EY014801 and the grant from Research to Prevent Blindness (RPB).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Commercial relationship: None
Financial Disclosures: All authors of the manuscript report no disclosures.
References
- Beutelspacher SC, Serbecic N, Barash H, Burgansky-Eliash Z, Grinvald A, Jonas JB. Central serous chorioretinopathy shows reduced retinal flow circulation in retinal function imaging (RFI) Acta Ophthalmol. 2011;89:e479–e482. doi: 10.1111/j.1755-3768.2011.02136.x. [DOI] [PubMed] [Google Scholar]
- Cheung AT, Chan MS, Ramanujam S, Rangaswami A, Curl K, Franklin P, Wun T. Effects of poloxamer 188 treatment on sickle cell vaso-occlusive crisis: computer-assisted intravital microscopy study. J. Investig. Med. 2004;52:402–406. doi: 10.1136/jim-52-06-35. [DOI] [PubMed] [Google Scholar]
- Cheung AT, Chen PC, Larkin EC, Duong PL, Ramanujam S, Tablin F, Wun T. Microvascular abnormalities in sickle cell disease: a computer-assisted intravital microscopy study. Blood. 2002a;99:3999–4005. doi: 10.1182/blood.v99.11.3999. [DOI] [PubMed] [Google Scholar]
- Cheung AT, Chen PC, Leshchinsky TV, Wiltse SL, Basadonna GP, Katznelson S, Perez RV. Improvement in conjunctival microangiopathy after simultaneous pancreas-kidney transplants. Transplant. Proc. 1997;29:660–661. doi: 10.1016/s0041-1345(96)00387-9. [DOI] [PubMed] [Google Scholar]
- Cheung AT, Harmatz P, Wun T, Chen PC, Larkin EC, Adams RJ, Vichinsky EP. Correlation of abnormal intracranial vessel velocity, measured by transcranial Doppler ultrasonography, with abnormal conjunctival vessel velocity, measured by computer-assisted intravital microscopy, in sickle cell disease. Blood. 2001a;97:3401–3404. doi: 10.1182/blood.v97.11.3401. [DOI] [PubMed] [Google Scholar]
- Cheung AT, Hu BS, Wong SA, Chow J, Chan MS, To WJ, Li J, Ramanujam S, Chen PC. Microvascular abnormalities in the bulbar conjunctiva of contact lens users. Clin. Hemorheol. Microcirc. 2012;51:77–86. doi: 10.3233/CH-2011-1513. [DOI] [PubMed] [Google Scholar]
- Cheung AT, Perez RV, Basadonna GP, Cox KL, Bry WI. Microangiopathy reversal in successful simultaneous pancreas-kidney transplantation. Transplant. Proc. 1994;26:493–495. [PubMed] [Google Scholar]
- Cheung AT, Perez RV, Chen PC. Improvements in diabetic microangiopathy after successful simultaneous pancreas-kidney transplantation: a computer-assisted intravital microscopy study on the conjunctival microcirculation. Transplantation. 1999;68:927–932. doi: 10.1097/00007890-199910150-00005. [DOI] [PubMed] [Google Scholar]
- Cheung AT, Price AR, Duong PL, Ramanujam S, Gut J, Larkin EC, Chen PC, Wilson DM. Microvascular abnormalities in pediatric diabetic patients. Microvasc. Res. 2002b;63:252–258. doi: 10.1006/mvre.2001.2386. [DOI] [PubMed] [Google Scholar]
- Cheung AT, Ramanujam S, Greer DA, Kumagai LF, Aoki TT. Microvascular abnormalities in the bulbar conjunctiva of patients with type 2 diabetes mellitus. Endocr. Pract. 2001b;7:358–363. doi: 10.4158/EP.7.5.358. [DOI] [PubMed] [Google Scholar]
- Duench S, Simpson T, Jones LW, Flanagan JG, Fonn D. Assessment of variation in bulbar conjunctival redness, temperature blood flow. Optom. Vis. Sci. 2007;84:511–516. doi: 10.1097/OPX.0b013e318073c304. [DOI] [PubMed] [Google Scholar]
- Duke-Elder S. System of Ophthalmology. CV Mosby: St. Louis; 1961. [Google Scholar]
- Fonn D, MacDonald KE, Richter D, Pritchard N. The ocular response to extended wear of a high Dk silicone hydrogel contact lens. Clin. Exp. Optom. 2002;85:176–182. doi: 10.1111/j.1444-0938.2002.tb03032.x. [DOI] [PubMed] [Google Scholar]
- Izhaky D, Nelson DA, Burgansky-Eliash Z, Grinvald A. Functional imaging using the retinal function imager: direct imaging of blood velocity, achieving fluorescein angiography-like images without any contrast agent, qualitative oximetry functional metabolic signals. Jpn. J. Ophthalmol. 2009;53:345–351. doi: 10.1007/s10384-009-0689-0. [DOI] [PubMed] [Google Scholar]
- Koutsiaris AG, Tachmitzi SV, Batis N, Kotoula MG, Karabatsas CH, Tsironi E, Chatzoulis DZ. Volume flow and wall shear stress quantification in the human conjunctival capillaries and post-capillary venules in vivo. Biorheology. 2007;44:375–386. [PubMed] [Google Scholar]
- Koutsiaris AG, Tachmitzi SV, Papavasileiou P, Batis N, Kotoula MG, Giannoukas AD, Tsironi E. Blood velocity pulse quantification in the human conjunctival pre-capillary arterioles. Microvasc. Res. 2010;80:202–208. doi: 10.1016/j.mvr.2010.05.001. [DOI] [PubMed] [Google Scholar]
- Landa G, Jangi AA, Garcia PM, Rosen RB. Initial report of quantification of retinal blood flow velocity in normal human subjects using the Retinal Functional Imager (RFI) Int. Ophthalmol. 2012;32:211–215. doi: 10.1007/s10792-012-9547-z. [DOI] [PubMed] [Google Scholar]
- Landa G, Rosen RB. A new vascular pattern for idiopathic juxtafoveal telangiectasia revealed by the retinal function imager. Ophthalmic Surg. Lasers Imaging. 2010;41:413–417. doi: 10.3928/15428877-20100325-04. [DOI] [PubMed] [Google Scholar]
- Nelson DA, Burgansky-Eliash Z, Barash H, Loewenstein A, Barak A, Bartov E, Rock T, Grinvald A. High-resolution wide-field imaging of perfused capillaries without the use of contrast agent. Clin. Ophthalmol. 2011;5:1095–1106. doi: 10.2147/OPTH.S20103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson DA, Krupsky S, Pollack A, Aloni E, Belkin M, Vanzetta I, Rosner M, Grinvald A. Special report: Noninvasive multi-parameter functional optical imaging of the eye. Ophthalmic Surg. Lasers Imaging. 2005;36:57–66. [PubMed] [Google Scholar]
- Ohtani N. Laser Doppler flowmetry of the bulbar conjunctiva as a monitor of the cerebral blood flow. Nihon Kyobu Geka Gakkai Zasshi. 1996;44:1721–1728. [PubMed] [Google Scholar]
- Schaser KD, Settmacher U, Puhl G, Zhang L, Mittlmeier T, Stover JF, Vollmar B, Menger MD, Neuhaus P, Haas NP. Noninvasive analysis of conjunctival microcirculation during carotid artery surgery reveals microvascular evidence of collateral compensation and stenosis-dependent adaptation. J. Vasc. Surg. 2003;37:789–797. doi: 10.1067/mva.2003.139. [DOI] [PubMed] [Google Scholar]
- Shahidi M, Wanek J, Gaynes B, Wu T. Quantitative assessment of conjunctival microvascular circulation of the human eye. Microvasc. Res. 2010;79:109–113. doi: 10.1016/j.mvr.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen M, Cui L, Riley C, Wang MR, Wang J. Characterization of soft contact lens edge fitting using ultra-high resolution and ultra-long scan depth optical coherence tomography. Invest Ophthalmol. Vis. Sci. 2011;52:4091–4097. doi: 10.1167/iovs.10-6507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- To WJ, Telander DG, Lloyd ME, Chen PC, Cheung AT. Correlation of conjunctival microangiopathy with retinopathy in type-2 diabetes mellitus (T2DM) patients. Clin. Hemorheol. Microcirc. 2011;47:131–141. doi: 10.3233/CH-2010-1374. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.