Abstract
Extremely premature infants are often exposed to supra-physiologic concentrations of oxygen, and frequently have hypoxemic episodes. These preterm infants are at high risk (~40%) for neurodevelopmental impairment (NDI) even in the absence of obvious intracranial pathology such as intraventricular hemorrhage or periventricular leukomalacia. The etiology for NDI has not been determined, and there are no animal models to simulate neurodevelopmental outcomes of prematurity. Our objectives were to develop and characterize a mouse model to determine long-term effects of chronic hypoxia or hyperoxia exposure on neurodevelopment. Newborn C57BL/6 mice were exposed to hypoxia (12% O2) or hyperoxia (85% O2) from postnatal day 1 to 14 and then returned to air. At 12–14 weeks of age, neurobehavioral assessment (Water Maze test, Novel Object Recognition test, Open Field test, Elevated Plus Maze, and Rotarod test) was performed, followed by MRI and brain histology. Neurobehavioral testing revealed that hyperoxia-exposed mice did poorly on the water maze and novel object recognition tests compared to air-exposed mice. MRI demonstrated smaller hippocampi in hyperoxia- and hypoxia-exposed mice with a greater reduction in hyperoxia-exposed mice, including a smaller cerebellum in hyperoxia-exposed mice. Brain histology showed reduced CA1 and CA3 and increased dentate gyral width in hippocampus. In conclusion, neonatal hyperoxia in mice leads to abnormal neurobehavior, primarily deficits in spatial and recognition memory, associated with smaller hippocampal sizes, similar to findings in ex-preterm infants. This animal model may be useful to determine mechanisms underlying developmental programming of NDI in preterm infants, and for evaluation of therapeutic strategies.
Keywords: Disease models, animal, Infant, premature, Developmental programming, Hippocampus, Cerebellum, Oxidative stress
Introduction
Extremely low birth weight infants are at high risk (~40%) for neurodevelopmental impairment (NDI) even in the absence of known intracranial complications of prematurity such as intraventricular hemorrhage or periventricular leukomalacia (Broitman et al., 2007; Laptook et al., 2005; Neubauer et al., 2008). The etiology for NDI in the absence of such obvious intracranial pathology is not clear, and there are no animal models to simulate neurodevelopmental outcomes of prematurity. Preterm infants are often exposed to supraphysiological levels of oxygen supplementation, sometimes alternating with periods of hypoxemia, that are known to affect normal lung and retinal development (Auten et al., 2009; Dorfman et al., 2008; Londhe et al., 2011). Impairment of lung development (bronchopulmonary dysplasia) or abnormal retinal development (retinopathy of prematurity) in extremely preterm infants are associated with an increased risk of NDI (Anderson and Doyle, 2006; Hintz et al., 2005a; Schmidt et al., 2003). It is important to determine if oxygen supplementation also alters developmental programming of brain development, leading to neurodevelopmental impairment and alterations in neurobehavior. Our objective in this project was to test the hypothesis that exposure of newborn mice to chronic hypoxia or hyperoxia during the first two postnatal weeks would lead to permanent impairment of brain development and function, when assessed in adult life.
Materials and methods
All protocols were approved by the Institutional Animal Care and Use Committee (IACUC) of UAB, and were consistent with the PHS policy on Humane Care and Use of Laboratory Animals (Office of Laboratory Animal Welfare, Aug 2002) and the Guide for the Care and Use of Laboratory Animals (National Research Council, National Academy Press, 1996). All experiments, unless otherwise specified, were done with a minimum of six mice of either gender from at least two litters for each experimental condition.
Animal model
C57BL/6 mice of either sex were exposed continuously (except for 10 minutes three days a week to change cages) to either normobaric 12% O2 (hypoxia), 85% O2 (hyperoxia), or air (normoxia) from soon after birth (second postnatal day or P2) until 14 days of age (P14) as described previously (Ambalavanan et al., 2005; Ambalavanan et al., 2008a; Ambalavanan et al., 2008b; James et al., 2010; Nicola et al., 2009). Pups that were exposed to hyperoxia were cross-fostered with dams that were alternated every 48h from normoxia to hyperoxia. After the 14th postnatal day (P14), mice were returned to air, and maintained on standard rodent diet and light/dark cycling in microisolator cages until assessment at 12–14 weeks of age. A total of 54 mice were studied in this project, in two sets (air: n=13 in first set, 11 in second set; hypoxia: 6 in set 1, 7 in set 2; hyperoxia: n=9 in set 1, 8 in set 2). All animals were ear tagged and were identified only by a three digit number on the ear tag during analysis, in order to maintain blinding of the observer to initial study group assignment.
Measurements
Neurobehavioral assessment was done at 12 weeks of age, followed by MRI and histology of the brain at 14 weeks of age. Mice were weighed weekly from P14 until 14 weeks of age.
Brain Development and Injury Assessment
Neurobehavioral Studies
As described previously by Van Groen (Handattu et al., 2009; Perry et al., 2010) and others (Rogers et al., 1999; Rogers et al., 2001), a modified comprehensive phenotyping protocol (SHIRPA) was used at 12 weeks of age. Mice were assessed for locomotor activity (Open field test), balance and coordination (Rotarod test) anxiety (Elevated Plus Maze), spatial learning and memory (Novel Object Recognition test, Morris Water Maze Task), and vision (Cliff test). All the behavioral tests described below were analyzed and measured using a camera-driven tracking system (Ethovision 3.1 by Noldus, The Netherlands).
Novel Object Recognition Test (Clarke et al., 2008)
The mouse was placed with two small objects in a square arena with black plexiglass sides (20 cm high) and observed for 10 min. After 24 hours, the animal was tested again in the arena with one new object and one familiar object and observed for 4 min. Memory (recognition) of the animal was assessed by analyzing the amount of time spent by the animal with the new and the old object.
Morris Water Maze Navigation Task (Brandeis et al., 1989; D’Hooge and De Deyn, 2001)
The mouse was gently placed in a blue plastic pool filled with water, in which a platform was submerged 0.5 cm below the water surface. Mice were trained on four trials (for a maximum of 60 seconds per trial) a day, and for a total of twenty trials over five days to learn and find the platform. Spatial learning and memory of the animal were measured by analyzing the swimming speed, latency to find the platform, path length, and percentage of trials in which the platform was found by each mouse.
Elevated Plus Maze (Anxiety) (Carobrez and Bertoglio, 2005; Pellow et al., 1985)
The mouse was placed in a maze consisting of four arms (31 × 5 cm) that were raised 40 cm above the table with 15 cm high opaque sides on two arms and observed for 4 minutes. For analysis the maze divided into the three regions, open, closed, and the center area where the arms meet. Anxiety of the animal was assessed by analyzing the amount of time spent by the animal in the open versus the closed, “safe” arms.
Open Field Test (Locomotor Activity) (Walsh and Cummins, 1976)
Mice were placed in a 42 by 42 cm square clear plexiglass arena and the distance travelled by the animal along the walls, sides and center of the arena were recorded over a period of 4 minutes.
Rotarod Test (Coordination) (Jones and Roberts, 1968)
Fine motor coordination, balance, and resistance to fatigue were quantified by measuring the amount of time that an animal could remain standing on a rotating, accelerating drum (by 2 rpms every 30s) (San Diego Instruments, USA) for a maximum period of 5 minutes.
The Cliff Test (Vision) (Lione et al., 1999)
Mice were placed in a square arena with a clear plexiglass bottom (20 cm on the table [safe area] and 20 cm off the table [over the edge area]) and observed for 10 minutes. Vision of the animal was measured by analyzing the amount of time spent by animal in the “safe” area versus the “over-the-edge” area.
Structural Analysis of the Brain
Magnetic Resonance Image of the Brain
Using isoflurane, mice were anaesthetized and T2 -weighted non-contrast MRI (slice thickness 1 mm) was done. Hippocampal area and other brain areas were defined in accordance with The Mouse Brain in Sterotaxic Coordinates (George Paxinos and Keith Franklin; Academic Press; 2nd edition, 2001; ISBN-10: 012547637X). Using ImageJ software (http://rsbweb.nih.gov/ij/index.html) the area occupied by the hippocampi and the whole brain at approximately 2.1 mm post-bregma and the cerebellum at approximately 6 mm post-bregma were measured, by a single observer masked to study group (MR). Total brain area, total hippocampal area, right and left hippocampal areas, and cerebellar area were then calculated.
Histochemical Analysis of the Brain Sections
After the MRI, mice were sacrificed and their brains were removed carefully and stored in formalin overnight. The brain was then transferred to 30% sucrose with antifreeze solution and stored at −20°C for sections. As described previously (Kadish and Van Groen, 2002), six coronal 30μm sections of the brain were stained using cresyl violet (Nissl staining).
Photomicrographs of CA1, CA3, and dentate gyrus regions of the hippocampi were taken at 100x magnification. Boundaries of hippocampus and dentate gyrus were defined in accordance with Mouse Brain Atlas (http://www.mbl.org/mbl_main/atlas.html). Widths of granular cell layers of hippocampi (CA1 and CA3) and dentate gyrus were measured using MetaMorph v.6.2 (Universal Imaging Corporation, Downingtown, PA) software interfaced with a Nikon Eclipse TE2000 microscope equipped with a QiCam Fast Cooled high resolution CCD camera. In addition, brain sections stained for white matter (CNPase), synapse density (synaptophysin) and astrocytes (GFAP) were analyzed qualitatively.
Statistical Analysis
Results are expressed as means ±SE. The data were analyzed by one-way ANOVA to test for effects of oxygen concentration (air vs. hypoxia vs. hyperoxia) on outcomes. Multiple comparisons testing (Student-Newman-Keuls) was performed if statistical significance (p < 0.05) was noted by ANOVA.
Results
Neurobehavioral Analysis
Neonatal Hyperoxia Exposure Decreased Adult Spatial Learning and Memory in Adult Mice
Air- and hypoxia-exposed mice were able to find the submerged platform in the Morris water maze task in a comparable reduced amount of time with successive trials. However, neonatal hyperoxia markedly impaired the ability of mice to find the platform (Figure 1). Neonatal hyperoxia also decreased performance in the novel object recognition, with less time spent on exploring the new objects compared to air-exposed mice (Figure 2A).
Figure 1. Neonatal hyperoxia exposure decreased adult spatial learning and memory.
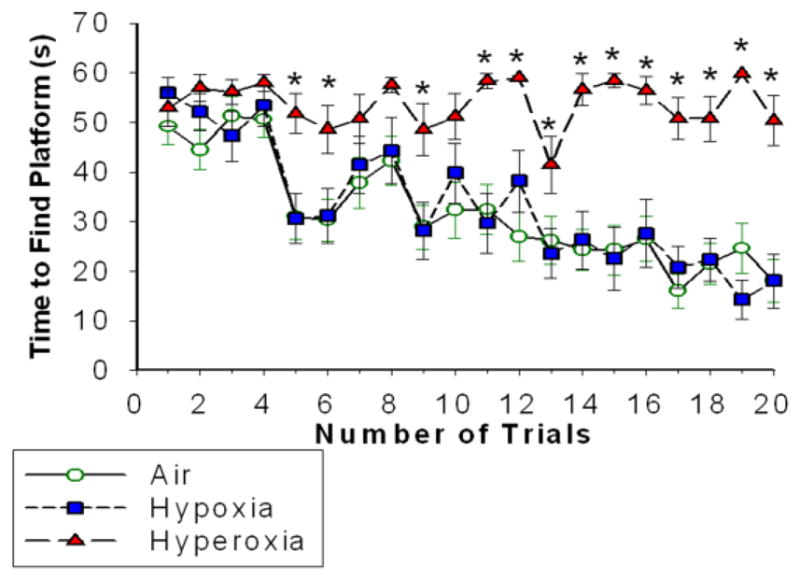
Time (seconds) taken by the adult mice exposed to Air (line with open green circles), Hypoxia (dashed line with blue squares) and Hyperoxia (dashed line with red triangles) to find the platform in water maze navigation task. Mice exposed to air or hypoxia in the neonatal period performed comparably, but neonatal exposure to hyperoxia impaired the ability of mice to find the submerged platform. means ± SEM; n= 22 in air, 13 in hypoxia, and 16 in hyperoxia.*p<0.05 vs. air-exposed mice RM ANOVA).
Figure 2. Neonatal hyperoxia exposure decreased memory, increased exploratory behavior and decreased anxiety in adult mice.
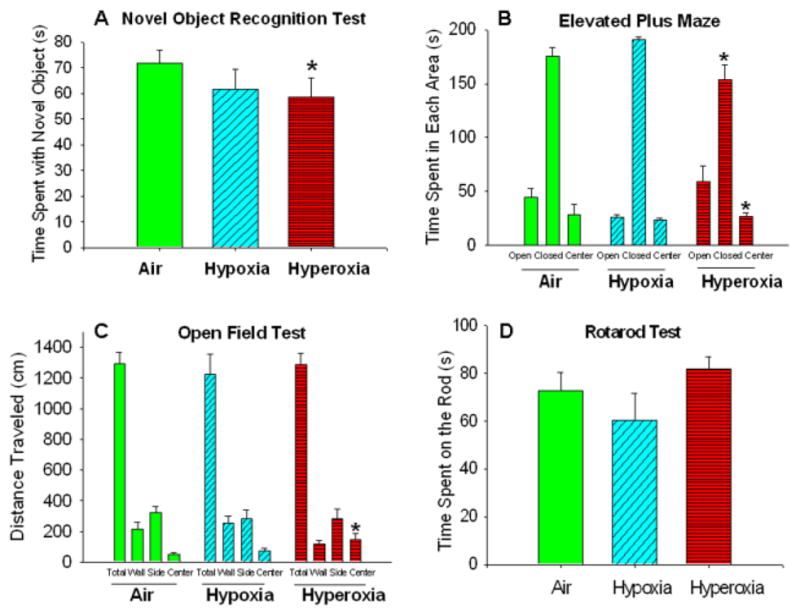
(A) Novel Object Recognition test: Hyperoxia-exposed mice spent less time with novel objects as compared to air-exposed mice. (B) Elevated Plus Maze: Hyperoxia-exposed mice spent more time in the open center and less time in the closed area. (C) Open Field Test: Hyperoxia-exposed mice traveled a greater distance in the center area (D) Rotarod test: No statistical differences were noted in time spent on the rod. Air-exposed: solid green bars, hypoxia-exposed mice: cyan angled stripes, hyperoxia-exposed: red bars with horizontal stripes; means± SEM; n= 10 in air, 7 in hypoxia, and 7 in hyperoxia.*p<0.05 vs. air-exposed mice.
Neonatal Hyperoxia Exposure Increased Exploratory Behaviour and Decreased Anxiety in Adult Mice
In the elevated plus maze test, hyperoxia-exposed (but not hypoxia-exposed) mice spent less time in the closed arm and engaged in riskier behavior with more time exploring in the open and center areas, compared to air-exposed mice (Figure 2B).
In the open field test, adult mice exposed to neonatal hypoxia or hyperoxia were comparable to air-exposed mice in the total distance travelled, but hyperoxia-exposed mice spent more time in the center area of the open field compared to other groups (Figure 2C). In addition, neonatal exposure to hypoxia or hyperoxia did not affect coordination of adult mice on the Rotorod test (Figure 1D) or vision on the Cliff test (data not shown).
Growth Assessment
Effects of Neonatal Hypoxia or Hyperoxia Exposure on Body Weight
Initially, both hypoxia- and hyperoxia-exposed mice had poor weight gain compared with air-exposed mice (weight at 4 weeks of age: air: 10.8 ±0.5 g; hypoxia: 9.6 ±0.6 g, p=0.13; hyperoxia: 8.6±0.4 g, p<0.01 for air vs. other groups) but all three groups were comparable at 8 weeks of age (air: 19.4±0.4 g; hypoxia: 18.4±0.5g; hyperoxia: 18.6 ±0.6 g; p NS). At 14 weeks of age, hyperoxia-exposed mice had a trend toward increased weight (air: 22.5±0.6 g; hypoxia: 22.9±0.7 g, p= 0.65; hyperoxia: 24.3±0.9 g, p< 0.10 vs. air).
Brain Structural Analysis
Magnetic Resonance Imaging
Neonatal Hypoxia and Hyperoxia Exposure Decreased Hippocampal and Cerebellar Size in Adult Mice
Measurements of total brain cross-sectional area (at Bregma -2.1mm) were similar in all three groups of mice (air: n=8, hypoxia: n=7, hyperoxia: n=8) (Figure 3D). Total (right+left) hippocampal area (at Bregma-2.1mm) in both hypoxia- and hyperoxia-exposed mice trended lower (p = 0.06) as compared to air-exposed mice. Analysis of right and left hippocampal areas indicated that the reduction in the hippocampal area was primarily in the right hippocampus (Figure 3E). Cerebellar area (at Bregma-6mm) (Figure F) was reduced in hyperoxia-exposed mice.
Figure 3. Neonatal hypoxia and hyperoxia exposure decreased hippocampal and cerebellar size in adult mice.
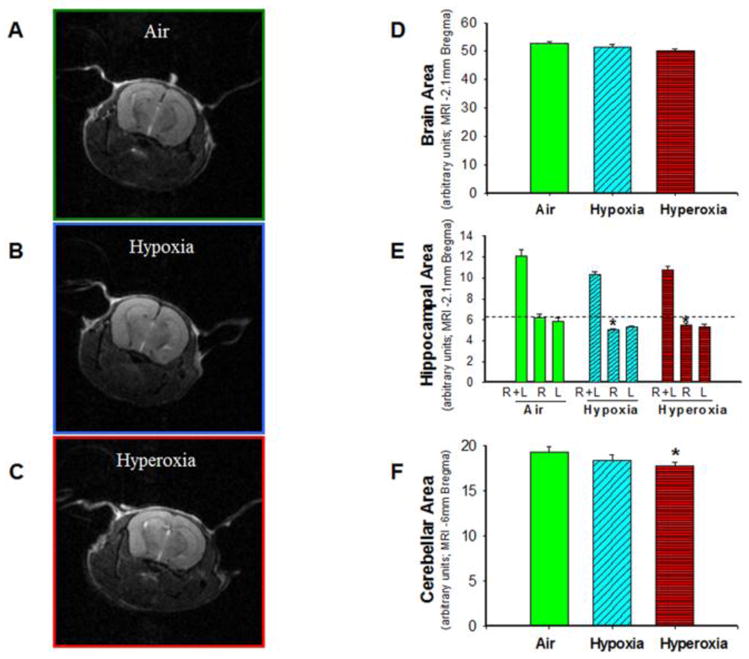
T2-weighted non-contrast MR images of brain from air-exposed (A), hypoxia-exposed (B), and hyperoxia-exposed (C) mice were used to measure brain, hippocampal, and cerebellar areas by tracing the periphery of the specific regions. (D) Cross-sectional brain area in adult mice at approximately −2.1 mm bregma did not differ by neonatal exposure to air, hypoxia or hyperoxia. (E) Cross-sectional area of the right hippocampus at −2.1 mm bregma was lower in hypoxia- and hyperoxia-exposed mice, as compared to air-exposed mice. (F) Cross-sectional cerebellar area at −6 mm bregma was reduced in neonatal hyperoxia-exposed mice as compared to air-exposed mice. Air-exposed: solid green bars, hypoxia-exposed mice: cyan angled stripes, hyperoxia-exposed: red bars with horizontal stripes; means± SEM; n= 11 in air, 7 in hypoxia, and 6 in hyperoxia.*p<0.05 vs. air-exposed mice.
Histology
Neonatal Hyperoxia Exposure Decreased CA1 and CA3 Width and Increased Dentate Gyral Width of Hippocampus in Adult Mice
Histological analysis of Nissl-stained brain sections showed that neonatal hyperoxia resulted in reduced width of CA1 (Figure 4D) and CA3 (Figure 4E) regions of the hippocampus, while hypoxia exposure did not significantly alter CA1 or CA3 thickness compared to air-exposed mice. In addition, hyperoxia-exposed mice had increased granular cell layer width in the dentate gyrus (Figure 4F). We also confirmed the MRI findings that hyperoxia-exposed mice had reduced total hippocampal area (data not shown). Brain sections stained for white matter (CNPase), astrocytes (GFAP) and synapse density (synaptophysin) were qualitatively similar among the three groups (data not shown).
Figure 4. Neonatal hyperoxia exposure decreased CA1 and CA3 width but increased dentate gyral width of hippocampus in adult mice.
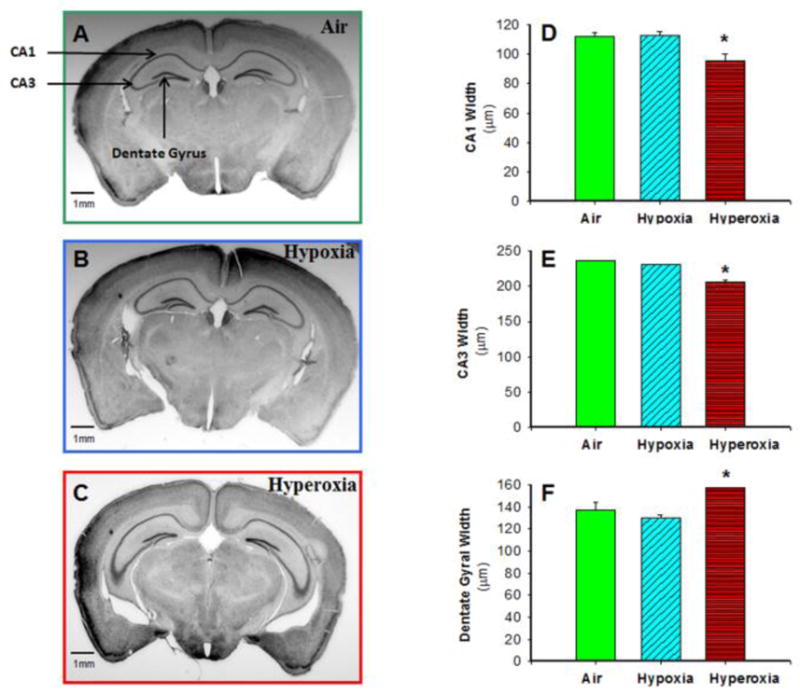
Photomicrographs of Nissl-stained adult mouse brain sections from air-exposed (A), hypoxia-exposed (B), and hyperoxia-exposed (C) mice were used to measure CA1, CA3, and dentate gyral width by image analysis. CA1 width (D) and CA3 width (E) in adult mice at approximately −2.1 mm bregma were lower, but dentate gyral width (F) was increased in adult mice exposed to neonatal hyperoxia, as compared to mice exposed to air or hypoxia. Air-exposed: solid green bars, hypoxia-exposed mice: cyan angled stripes, hyperoxia-exposed: red bars with horizontal stripes; means± SEM; n= 11 in air, 7 in hypoxia, and 6 in hyperoxia.*p<0.05 vs. air-exposed mice. Calibration bar = 1 mm.
Gender effects
There were no statistically significant differences in the gender ratio among the groups of adult mice which had been exposed to air, hyperoxia, or hypoxia in the neonatal period (data not shown). We did not find any statistically significant gender effects in this study in neurobehavioral analysis or brain structural analysis, although it is possible that use of a larger sample size may demonstrate some differences. In the growth assessment, male mice were larger than female mice (10–20%) as expected, but no interaction with neonatal exposure to air, hyperoxia, or hypoxia were noted.
Discussion
The present study is the first to determine the effect of neonatal exposure to chronic hypoxia or hyperoxia on adult neurobehavior and brain structure in a mouse model. The major finding of our study was that exposure of newborn mice to chronic hyperoxia led to deficits in spatial and recognition memory associated with smaller hippocampi in adult mice. This finding is important and highly clinically relevant as human preterm infants, despite a relatively uncomplicated neonatal intensive care course, frequently have poor executive and memory function in adolescence (Aarnoudse-Moens et al., 2009; Luu et al., 2011), associated with reductions in hippocampal volume (Gimenez et al., 2004). The hippocampal volume in preterm infants at term equivalent age is lower than in term infants (Peterson et al., 2000), and the magnitude of such reduction correlates with later working memory deficits (Beauchamp et al., 2008). The etiology for such neurodevelopmental impairment without obvious intracranial pathology had not been previously determined (Broitman et al., 2007; Hintz et al., 2005b). Our study suggests that exposure to supraphysiologic concentrations of oxygen during a vulnerable period of brain development may preferentially affect hippocampal development and lead to deficits in memory. The fetus in the third trimester of pregnancy is normally exposed to relatively low oxygen tensions in utero, which is conducive for normal organ development. Very preterm infants who are born early and therefore miss out on varying durations of in utero development in the third trimester are often exposed postnatally to high concentrations of oxygen for prolonged periods of time due to lung immaturity, and even preterm infants not on supplemental oxygen have higher oxygen saturations (normal target range in most neonatal intensive care units is 88–95%) than in utero levels (oxygen saturation in utero is 70–75%).
Strengths of our study include the evaluation of adult mice, rather than newborn or juvenile mice during or immediately after the exposure to hypoxia or hyperoxia. We also evaluated both brain structure and function and identified structure–function correlation, in that we noted both a reduction in hippocampal size and an impairment of abilities known to be associated with hippocampal function.
However, our study has some limitations. Studies in mouse models may not closely resemble the human due to interspecies differences, especially as mice do not have the marked cerebral cortical development and resulting enhanced cognitive abilities found in humans. Also, chronic hypoxia or hyperoxia, while good reproducible models in mice, may not exactly simulate environmental effects in a human preterm infant due to differences in the timing of the insult, severity of the hyperoxia and hypoxia, and co-existing pathology (e.g. preterm infants often have respiratory distress syndrome that reduce arterial oxygen tension during hyperoxia exposure). We did not find any gender effects in this study, although it is possible that use of a larger sample size may demonstrate some differences. We noticed a trend towards increased weight gain in hyperoxia-exposed mice, we are initiating experiments to evaluate feeding behavior, growth parameters, energy balance, sleep-wake cycle, and body composition in these mice to determine alterations in such metabolic pathways linked to hypothalamic networks but that is beyond the scope of this report.
The hippocampus is generally considered critical for spatial learning, and to a lesser extent for novel object recognition (Squire, 1992). Poor performance on the Morris water maze task is an indicator of impaired spatial memory (an inability to remember the location of the submerged platform) and is often associated with hippocampal injury (Jarrard, 1993). We observed poor performance on the water maze and novel object recognition in the hyperoxia-exposed mice, but not in the hypoxia-exposed mice, although both hyperoxia- and hypoxia-exposed mice had reductions in hippocampal size. It is possible that a threshold effect exists, for hyperoxia-exposed mice had a greater reduction in hippocampal size as compared to hypoxia-exposed mice.
In addition to the impairments in spatial memory and novel object recognition, we also noted that hyperoxia-exposed mice spent less time in the closed area of the elevated plus maze. Mice generally prefer dark and enclosed areas over bright and exposed areas in order to avoid predators, and a decrease in the amount of time in the closed area usually indicates a reduction in fear and increased exploratory behavior (Lister, 1987). Preterm infants are at 3–4 fold higher risk (compared to term infants) of psychiatric disorders in later life, mainly attention deficit-hyperactivity (ADHD), autism spectrum disorder (ASD), and emotional disorders (Johnson and Marlow, 2011). Mouse models of ADHD are characterized by decreases in time spent in the closed area (Avale et al., 2004), suggesting that our mouse model of neonatal hyperoxia exposure may also simulate ADHD resulting from preterm birth.
Deficits in the Rotarod test, a test of coordination and cerebellar function, were not noted even though cerebellar area was reduced in hyperoxia exposed mice. It is possible that the reduction in cerebellar size did not reach the threshold required to cause deficits in coordination. Reductions in cerebellar size are known to be associated with neurodevelopmental impairment in preterm infants (Lind et al., 2011).
The mechanism underlying the impairment in spatial learning and object recognition in our model system is probably the reduction in hippocampal size, and the pathophysiological process associated with the reduction in hippocampal size with alterations in environmental oxygen during brain development is probably oxidative stress. Elevations of arterial PaO2 may directly impact vulnerable areas of the brain via oxidative stress, or hyperoxia-induced lung injury may indirectly lead to brain injury through increases in circulating cytokines. Other investigators have shown that hyperoxia exposure in neonatal rats increases apoptosis and reduces neuronal number in the hippocampus as well as other brain areas (Yis et al., 2008) and that such vulnerability to oxygen neurotoxicity is temporally confined to the first 2 postnatal weeks (Felderhoff-Mueser et al., 2004). Hyperoxia exposure caused oxidative stress, decreased neurotrophin expression, and inactivation of survival signaling proteins Ras, extracellular signal-regulated kinase (ERK 1/2), and protein kinase B (Akt) (Felderhoff-Mueser et al., 2004). Transgenic mice overexpressing constitutively activated Ras and phosphorylated kinases ERK1/2 in the brain were protected against oxygen-induced apoptosis, indicating that this pathway plays a mechanistic role in hyperoxia-induced neurotoxicity (Felderhoff-Mueser et al., 2004).
It is interesting that we noted that the reduction in hippocampal size was mainly in the right hippocampus. Hippocampi are asymmetric in human children and adults (the right hippocampus is larger) and volumetric MRI has shown that even preterm infants have rightward asymmetry indicating that this difference develops in utero (Thompson et al., 2009). Compared to children born at term, preterm children have smaller left hippocampi (16%, p =0.001) and right hippocampi (12%, p=0.007) (Peterson et al., 2000). Even in C57BL/6 mice, cerebral and hippocampal asymmetry has been described using high resolution MRI. Unlike humans, the left hippocampus (specifically the dentate gyrus) is larger than the right in mice (Spring et al., 2010). There is some evidence from split-brain mice that the usage of the right hippocampus improves the accuracy of spatial memory(Shinohara et al., 2012). Further study of this phenomenon is necessary.
Our data indicate that exposure to hyperoxia during critical developmental periods may cause permanent memory deficits associated with structural abnormalities (smaller hippocampal size) later in life. Over the past few decades, there has been increasing awareness of early life exposures that result in “fetal reprogramming” with cardiovascular and metabolic disease in adult humans (the “Barker Hypothesis”) (Hales and Barker, 1992; Tamashiro and Moran, 2010). Similarly, there is also increasing recognition of the effect of early life exposures that may lead to non-optimal neurological outcomes. Prenatal and early life events have been associated with schizophrenia and affective disorders (Bale et al., 2010). There is evidence that autism may at least in part be a disorder of fetal programming (Szatmari, 2011), and that attention-deficit hyperactivity disorder may result from exposure to maternal stress while in utero (Talge et al., 2007) or childhood stress (Teicher et al., 2002). The programming may occur at any point during development, with the induced epigenetic changes occurring following either in utero exposures and alterations in the maternal environment, or following ex-utero exposure in early childhood, either in responses to changes in maternal care (Bagot et al., 2012; Caldji et al., 2011) or in response to physical changes in the environment (such as hyperoxia in our study).
Conclusions
In conclusion, neonatal exposure to chronic hyperoxia leads to impaired spatial learning and memory deficits associated with reduced hippocampal size in adult mice. Neonatal exposure to chronic hypoxia also reduces hippocampal size, and may lead to more subtle neurological effects. These mouse models may be useful to investigate the mechanisms underlying abnormal neurodevelopment in preterm infants who are often exposed to hyperoxia or have hypoxemic episodes.
Highlights.
Adult mice exposed to hyperoxia in the newborn period had impaired spatial and recognition memory.
Adult hyperoxia- and hypoxia-exposed mice had smaller hippocampi by MRI and histology
Adult mice exposed to hyperoxia in the newborn period had reduced cerebellar area but performance on the Rotorod test was not affected.
Neonatal hyperoxia exposure increased exploratory behavior and decreased anxiety in adult mice.
Acknowledgments
This work was partially funded by P30 NS47466, and IKARIA, R01 HL092906, a Research Facilities Improvement Program Grant C06 RR 15490, and the Division of Neonataology, Department of Pediatrics, University of Alabama at Birmingham, Alabama USA
Footnotes
Disclosure: None
Conflict of interest: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aarnoudse-Moens CS, et al. Meta-analysis of neurobehavioral outcomes in very preterm and/or very low birth weight children. Pediatrics. 2009;124:717–28. doi: 10.1542/peds.2008-2816. [DOI] [PubMed] [Google Scholar]
- Ambalavanan N, et al. Endothelin-A receptor blockade prevents and partially reverses neonatal hypoxic pulmonary vascular remodeling. Pediatr Res. 2005;57:631–6. doi: 10.1203/01.PDR.0000159512.55862.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambalavanan N, et al. Transforming growth factor-beta signaling mediates hypoxia-induced pulmonary arterial remodeling and inhibition of alveolar development in newborn mouse lung. Am J Physiol Lung Cell Mol Physiol. 2008a;295:L86–95. doi: 10.1152/ajplung.00534.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambalavanan N, et al. Role of matrix metalloproteinase-2 in newborn mouse lungs under hypoxic conditions. Pediatr Res. 2008b;63:26–32. doi: 10.1203/PDR.0b013e31815b690d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson PJ, Doyle LW. Neurodevelopmental outcome of bronchopulmonary dysplasia. Semin Perinatol. 2006;30:227–32. doi: 10.1053/j.semperi.2006.05.010. [DOI] [PubMed] [Google Scholar]
- Auten RL, et al. Hyperoxia impairs postnatal alveolar epithelial development via NADPH oxidase in newborn mice. Am J Physiol Lung Cell Mol Physiol. 2009;297:L134–42. doi: 10.1152/ajplung.00112.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avale ME, et al. The dopamine D4 receptor is essential for hyperactivity and impaired behavioral inhibition in a mouse model of attention deficit/hyperactivity disorder. Mol Psychiatry. 2004;9:718–26. doi: 10.1038/sj.mp.4001474. [DOI] [PubMed] [Google Scholar]
- Bagot RC, et al. Maternal Care Influences Hippocampal N-Methyl-D-Aspartate Receptor Function and Dynamic Regulation by Corticosterone in Adulthood. Biol Psychiatry. 2012 doi: 10.1016/j.biopsych.2012.03.016. [DOI] [PubMed] [Google Scholar]
- Bale TL, et al. Early life programming and neurodevelopmental disorders. Biol Psychiatry. 2010;68:314–9. doi: 10.1016/j.biopsych.2010.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchamp MH, et al. Preterm infant hippocampal volumes correlate with later working memory deficits. Brain. 2008;131:2986–94. doi: 10.1093/brain/awn227. [DOI] [PubMed] [Google Scholar]
- Brandeis R, et al. The use of the Morris Water Maze in the study of memory and learning. Int J Neurosci. 1989;48:29–69. doi: 10.3109/00207458909002151. [DOI] [PubMed] [Google Scholar]
- Broitman E, et al. Clinical data predict neurodevelopmental outcome better than head ultrasound in extremely low birth weight infants. J Pediatr. 2007;151:500–5. 505, e1–2. doi: 10.1016/j.jpeds.2007.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldji C, et al. Environmental regulation of the neural epigenome. FEBS Lett. 2011;585:2049–58. doi: 10.1016/j.febslet.2011.03.032. [DOI] [PubMed] [Google Scholar]
- Carobrez AP, Bertoglio LJ. Ethological and temporal analyses of anxiety-like behavior: the elevated plus-maze model 20 years on. Neurosci Biobehav Rev. 2005;29:1193–205. doi: 10.1016/j.neubiorev.2005.04.017. [DOI] [PubMed] [Google Scholar]
- Clarke JR, et al. Posttraining activation of CB1 cannabinoid receptors in the CA1 region of the dorsal hippocampus impairs object recognition long-term memory. Neurobiol Learn Mem. 2008;90:374–81. doi: 10.1016/j.nlm.2008.04.009. [DOI] [PubMed] [Google Scholar]
- D’Hooge R, De Deyn PP. Applications of the Morris water maze in the study of learning and memory. Brain Res Brain Res Rev. 2001;36:60–90. doi: 10.1016/s0165-0173(01)00067-4. [DOI] [PubMed] [Google Scholar]
- Dorfman A, et al. Early manifestations of postnatal hyperoxia on the retinal structure and function of the neonatal rat. Invest Ophthalmol Vis Sci. 2008;49:458–66. doi: 10.1167/iovs.07-0916. [DOI] [PubMed] [Google Scholar]
- Felderhoff-Mueser U, et al. Oxygen causes cell death in the developing brain. Neurobiol Dis. 2004;17:273–82. doi: 10.1016/j.nbd.2004.07.019. [DOI] [PubMed] [Google Scholar]
- Gimenez M, et al. Hippocampal gray matter reduction associates with memory deficits in adolescents with history of prematurity. Neuroimage. 2004;23:869–77. doi: 10.1016/j.neuroimage.2004.07.029. [DOI] [PubMed] [Google Scholar]
- Hales CN, Barker DJ. Type 2 (non-insulin-dependent) diabetes mellitus: the thrifty phenotype hypothesis. Diabetologia. 1992;35:595–601. doi: 10.1007/BF00400248. [DOI] [PubMed] [Google Scholar]
- Handattu SP, et al. Oral apolipoprotein A-I mimetic peptide improves cognitive function and reduces amyloid burden in a mouse model of Alzheimer’s disease. Neurobiol Dis. 2009;34:525–34. doi: 10.1016/j.nbd.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hintz SR, et al. Neurodevelopmental and growth outcomes of extremely low birth weight infants after necrotizing enterocolitis. Pediatrics. 2005a;115:696–703. doi: 10.1542/peds.2004-0569. [DOI] [PubMed] [Google Scholar]
- Hintz SR, et al. Changes in neurodevelopmental outcomes at 18 to 22 months’ corrected age among infants of less than 25 weeks’ gestational age born in 1993–1999. Pediatrics. 2005b;115:1645–51. doi: 10.1542/peds.2004-2215. [DOI] [PubMed] [Google Scholar]
- James ML, et al. Vitamin A and retinoic acid act synergistically to increase lung retinyl esters during normoxia and reduce hyperoxic lung injury in newborn mice. Pediatr Res. 2010;67:591–7. doi: 10.1203/PDR.0b013e3181dbac3d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarrard LE. On the role of the hippocampus in learning and memory in the rat. Behavioral and neural biology. 1993;60:9–26. doi: 10.1016/0163-1047(93)90664-4. [DOI] [PubMed] [Google Scholar]
- Johnson S, Marlow N. Preterm birth and childhood psychiatric disorders. Pediatr Res. 2011;69:11R–8R. doi: 10.1203/PDR.0b013e318212faa0. [DOI] [PubMed] [Google Scholar]
- Jones BJ, Roberts DJ. The quantiative measurement of motor inco-ordination in naive mice using an acelerating rotarod. J Pharm Pharmacol. 1968;20:302–4. doi: 10.1111/j.2042-7158.1968.tb09743.x. [DOI] [PubMed] [Google Scholar]
- Kadish I, Van Groen T. Low levels of estrogen significantly diminish axonal sprouting after entorhinal cortex lesions in the mouse. J Neurosci. 2002;22:4095–102. doi: 10.1523/JNEUROSCI.22-10-04095.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laptook AR, et al. Adverse neurodevelopmental outcomes among extremely low birth weight infants with a normal head ultrasound: prevalence and antecedents. Pediatrics. 2005;115:673–80. doi: 10.1542/peds.2004-0667. [DOI] [PubMed] [Google Scholar]
- Lind A, et al. Associations between regional brain volumes at term-equivalent age and development at 2 years of age in preterm children. Pediatr Radiol. 2011;41:953–61. doi: 10.1007/s00247-011-2071-x. [DOI] [PubMed] [Google Scholar]
- Lione LA, et al. Selective discrimination learning impairments in mice expressing the human Huntington’s disease mutation. J Neurosci. 1999;19:10428–37. doi: 10.1523/JNEUROSCI.19-23-10428.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lister RG. The use of a plus-maze to measure anxiety in the mouse. Psychopharmacology (Berl) 1987;92:180–5. doi: 10.1007/BF00177912. [DOI] [PubMed] [Google Scholar]
- Londhe VA, et al. Hyperoxia impairs alveolar formation and induces senescence through decreased histone deacetylase activity and up-regulation of p21 in neonatal mouse lung. Pediatr Res. 2011;69:371–7. doi: 10.1203/PDR.0b013e318211c917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luu TM, et al. Executive and memory function in adolescents born very preterm. Pediatrics. 2011;127:e639–46. doi: 10.1542/peds.2010-1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neubauer AP, et al. Outcome of extremely low birth weight survivors at school age: the influence of perinatal parameters on neurodevelopment. Eur J Pediatr. 2008;167:87–95. doi: 10.1007/s00431-007-0435-x. [DOI] [PubMed] [Google Scholar]
- Nicola T, et al. Loss of Thy-1 inhibits alveolar development in the newborn mouse lung. Am J Physiol Lung Cell Mol Physiol. 2009;296:L738–50. doi: 10.1152/ajplung.90603.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellow S, et al. Validation of open:closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J Neurosci Methods. 1985;14:149–67. doi: 10.1016/0165-0270(85)90031-7. [DOI] [PubMed] [Google Scholar]
- Perry GM, et al. Mitochondrial calcium uptake capacity as a therapeutic target in the R6/2 mouse model of Huntington’s disease. Hum Mol Genet. 2010;19:3354–71. doi: 10.1093/hmg/ddq247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson BS, et al. Regional brain volume abnormalities and long-term cognitive outcome in preterm infants. JAMA. 2000;284:1939–47. doi: 10.1001/jama.284.15.1939. [DOI] [PubMed] [Google Scholar]
- Rogers DC, et al. Use of SHIRPA and discriminant analysis to characterise marked differences in the behavioural phenotype of six inbred mouse strains. Behav Brain Res. 1999;105:207–17. doi: 10.1016/s0166-4328(99)00072-8. [DOI] [PubMed] [Google Scholar]
- Rogers DC, et al. SHIRPA, a protocol for behavioral assessment: validation for longitudinal study of neurological dysfunction in mice. Neurosci Lett. 2001;306:89–92. doi: 10.1016/s0304-3940(01)01885-7. [DOI] [PubMed] [Google Scholar]
- Schmidt B, et al. Impact of bronchopulmonary dysplasia, brain injury, and severe retinopathy on the outcome of extremely low-birth-weight infants at 18 months: results from the trial of indomethacin prophylaxis in preterms. JAMA. 2003;289:1124–9. doi: 10.1001/jama.289.9.1124. [DOI] [PubMed] [Google Scholar]
- Shinohara Y, et al. Right-hemispheric dominance of spatial memory in split-brain mice. Hippocampus. 2012;22:117–21. doi: 10.1002/hipo.20886. [DOI] [PubMed] [Google Scholar]
- Spring S, et al. Cerebral asymmetries in 12-week-old C57Bl/6J mice measured by magnetic resonance imaging. Neuroimage. 2010;50:409–15. doi: 10.1016/j.neuroimage.2009.12.043. [DOI] [PubMed] [Google Scholar]
- Squire LR. Memory and the hippocampus: a synthesis from findings with rats, monkeys, and humans. Psychol Rev. 1992;99:195–231. doi: 10.1037/0033-295x.99.2.195. [DOI] [PubMed] [Google Scholar]
- Szatmari P. Is autism, at least in part, a disorder of fetal programming? Arch Gen Psychiatry. 2011;68:1091–2. doi: 10.1001/archgenpsychiatry.2011.99. [DOI] [PubMed] [Google Scholar]
- Talge NM, et al. Antenatal maternal stress and long-term effects on child neurodevelopment: how and why? J Child Psychol Psychiatry. 2007;48:245–61. doi: 10.1111/j.1469-7610.2006.01714.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamashiro KL, Moran TH. Perinatal environment and its influences on metabolic programming of offspring. Physiol Behav. 2010;100:560–6. doi: 10.1016/j.physbeh.2010.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teicher MH, et al. Developmental neurobiology of childhood stress and trauma. Psychiatr Clin North Am. 2002;25:397–426. vii–viii. doi: 10.1016/s0193-953x(01)00003-x. [DOI] [PubMed] [Google Scholar]
- Thompson DK, et al. MR-determined hippocampal asymmetry in full-term and preterm neonates. Hippocampus. 2009;19:118–23. doi: 10.1002/hipo.20492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh RN, Cummins RA. The Open-Field Test: a critical review. Psychol Bull. 1976;83:482–504. [PubMed] [Google Scholar]
- Yis U, et al. Hyperoxic exposure leads to cell death in the developing brain. Brain Dev. 2008;30:556–62. doi: 10.1016/j.braindev.2008.01.010. [DOI] [PubMed] [Google Scholar]


