Abstract
Calcium/calmodulin-dependent protein kinase II (CaMKII) activity is necessary for the long-lasting expression of locomotor sensitization and enhanced drug-taking observed in rats previously exposed to psychostimulants. Exposure to these drugs also transiently increases αCaMKII levels in the nucleus accumbens (NAcc), an effect that, when mimicked by transient viral-mediated overexpression of αCaMKII in NAcc shell neurons, leads to long-lasting enhancement in locomotor responding to amphetamine and NAcc α-amino-3-hydroxyl-5-methyl-4-isoxazole-propionate (AMPA). The present experiments characterized the dopamine (DA) dependence of the functional AMPA receptor upregulation observed long after transient overexpression of αCaMKII. Rats infected with herpes simplex virus-αCaMKII in the NAcc shell showed a transient increase in αCaMKII levels that peaked at 4 days post-infection and returned to baseline 8 days later. When challenged with AMPA (0.8 nmol/side) in the NAcc shell at 20 days post-infection, these rats showed enhanced locomotion compared with controls. This sensitized locomotor response was blocked when AMPA was coinfused with either the DA type-1 receptor antagonist SCH23390 (0.8 nmol/side) or the protein kinase A inhibitor Rp-cAMPS (80 nmol/side). Neither SCH23390 nor Rp-cAMPS produced locomotor effects when infused by itself into the NAcc shell. Furthermore, these antagonists did not block the acute non-sensitized locomotor response to AMPA observed in control rats. These findings show that transient viral-mediated overexpression of αCaMKII in neurons of the NAcc shell leads to long-lasting functional upregulation of AMPA receptors that is DA type-1 receptor and protein kinase A dependent. Thus, transient increases in levels of αCaMKII in the NAcc shell produce long-lasting changes in the way that DA and glutamate interact in this site to generate locomotor behavior.
Keywords: dopamine type-1 receptor, herpes simplex virus, locomotion, protein kinase A, rat, sensitization
Introduction
Repeatedly exposing rats to psychostimulants enhances their drug-seeking behavior, a manifestation of sensitization that is argued to model the transition from casual drug use to drug craving and abuse (Robinson & Berridge, 1993; Vezina, 2004). This sensitization is long-lasting, such that re-exposure to the drug weeks to months later in the rat produces enhanced locomotor responding and nucleus accumbens (NAcc) dopamine (DA) overflow as well as increased work output aimed at obtaining the drug (Kalivas & Stewart, 1991; Paulson & Robinson, 1995; Vezina et al., 2002). Calcium/calmodulin-dependent protein kinase II (CaMKII) is known to contribute importantly to the expression of these forms of sensitization. Microinjecting the CaMKII inhibitor KN-93 into the NAcc shell of rats previously exposed to amphetamine or cocaine prevents the expression of sensitized locomotion (Pierce et al., 1998), NAcc DA overflow (Pierce & Kalivas, 1997) and drug self-administration (Loweth et al., 2008). Similarly, overexpressing an inactive mutant form of αCaMKII (K42M) in the NAcc shell prevents the expression of amphetamine locomotor sensitization (Vezina et al., 2009). Conversely, increasing αCaMKII levels in neurons of the NAcc shell, but not the NAcc core, using a transient protein overexpression system that mimics the transient increase in αCaMKII observed following exposure to amphetamine or cocaine (Boudreau et al., 2009; Loweth et al., 2010), enhances amphetamine-induced locomotion and self-administration (Loweth et al., 2010). In this latter report, sensitized-like behaviors were expressed both when αCaMKII levels were increased and long after they had returned to baseline, indicating that αCaMKII contributes to the expression of psychostimulant sensitization directly and via post-phosphorylation cascades that support long-lasting neuroadaptations in the NAcc shell. One of the long-lasting neuroadaptations observed was functional upregulation of glutamate α-amino-3-hydroxyl-5-methyl-4-isoxazole-propionate (AMPA) receptors; locomotor responding to NAcc shell AMPA was enhanced as were phosphorylation levels of serine residue 831 of AMPA glutamate receptor subunit (GluR)1 (ser831) (Loweth et al., 2010), a CaMKII site associated with increased channel conductance (Barria et al., 1997; Song & Huganir, 2002).
A number of recent reports indicate that activation of DA type-1 (D1) receptors can influence psychostimulant-induced behaviors by initiating a protein kinase A (PKA)-calcium-CaMKII pathway that regulates AMPA receptor signaling in the NAcc (Anderson et al., 2008; Chao et al., 2002a,b; Mangiavacchi & Wolf, 2004; Sun et al., 2008). In this pathway, activation of D1 receptors activates PKA, leading to phosphorylation by PKA of L-type calcium channels, an increase in inward calcium conductance and activation of CaMKII (Anderson et al., 2008; Hernandez-Lopez et al., 1997; Surmeier et al., 1995). As post-synaptic interactions between DA and glutamate in the NAcc are critical for the expression of psychostimulant-induced sensitization (Vanderschuren & Kalivas, 2000; Vezina & Suto, 2003; Wolf, 1998), it is likely that this pathway contributes to the long-lasting functional upregulation of AMPA receptors observed in the NAcc of sensitized rats (Pierce et al., 1996; Suto et al., 2004). This possibility was assessed in the present experiments by determining the D1 receptor and PKA dependence of the long-lasting AMPA receptor upregulation produced in the NAcc following transient viral-mediated overexpression of αCaMKII in NAcc neurons.
Materials and methods
Animals
Sprague-Dawley rats (Harlan Sprague-Dawley, Madison, WI, USA) weighing 275–300 g on arrival were housed individually in a reverse cycle room (12/12-h light/dark cycle) with freely available food and water. Testing occurred in the dark period of the cycle. All experiments were conducted in accordance with the Guide for the Care and Use of Laboratory Animals as promulgated by the National Institutes of Health. All surgical procedures were conducted according to an approved Institutional Animal Care and Use Committee protocol.
Surgical procedures
Starting at 3–5 days after arrival, rats were anesthetized with ketamine (100 mg/kg, i.p.) and xylazine (6 mg/kg, i.p.) and mounted on a stereotaxic apparatus with the incisor bar positioned at 5.0 mm above the interaural line. They were surgically implanted with chronic bilateral indwelling guide cannulas (22 gauge; Plastics One, Roanoke, VA, USA) aimed at the NAcc shell (anterior/posterior, +3.4; lateral, ± 0.8; dorsal/ventral, −7.5 mm from bregma and skull) (Pellegrino et al., 1979). Guide cannulas were angled at 10° to the vertical, positioned 4 mm above the final injection site and anchored in place with a dental cement cap secured by six screws drilled into the skull. Finally, 28 gauge obturators were inserted into the guide cannulas flush with their tips. After surgery, the rats were returned to their cages to recover for 7–10 days.
Viral infection
Replication-deficient viral vectors encoding rat wild-type or constitutively active (T286D) αCaMKII were constructed and packaged as previously described (Neve et al., 1997). In the constitutively active CaMKII isoform, the threonine 286 residue on the autoinhibitory domain was replaced with aspartate, thereby enabling calcium-independent kinase activity (Lisman et al., 2002). The complementary DNA was inserted into the herpes simplex virus (HSV) amplicon [replication-deficient herpes simplex virus amplicon (HSV-PrpUC)], packaged with the replication-deficient IE2 deletion mutant 5d1.2 helper virus derived from the KOS strain and resuspended in 10% sucrose. The average titer of the resulting viral stocks was 4.0 × 107 infectious units/mL. Transgene expression was regulated by HSV IE4/5. Additional HSV control vectors were engineered to express either a fusion αCaMKII-green fluorescent protein construct or the reporter gene LacZ.
Following recovery from stereotaxic surgery, all rats were moved to a biosafety level 2 facility for bilateral microinjections into the NAcc shell of either HSV-αCaMKII or control infusions. Rats randomly assigned to the groups infected with HSV-αCaMKII were infused with vectors leading to overexpression of either wild-type or T286D αCaMKII. Because similar effects were produced by both αCaMKII constructs in the present as well as previous experiments (Loweth et al., 2010), data obtained with each were combined. The remaining rats were assigned to the control groups and infused with 10% sucrose or vectors leading to expression of the LacZ product β-galactosidase. Again, these control infusions were used interchangeably as both were without detectable effects.
Microinjections were performed in freely moving rats using 10 µL syringes (Hamilton, Reno, NV, USA) connected to injection cannulas (28 gauge) via polyethylene PE20 tubing. Injectors were inserted to a depth 4 mm below the guide cannula tips and 2 µL of the solution was infused into each side over a 10 min period. Following a diffusion time of 5 min, injectors were removed and obturators replaced. Rats were returned to the colony room 1 day after the microinjections. This procedure led to infection of neurons localized specifically to the NAcc shell. As illustrated in Fig. 1B, infusion of the control vector HSV-LacZ revealed infection patterns localized to this subnucleus. The present experiments focused on the NAcc shell because previous studies using pharmacological inhibitors (Loweth et al., 2008; Pierce & Kalivas, 1997; Pierce et al., 1998) and viral-mediated gene transfer (Loweth et al., 2010; Vezina et al., 2009) support a role for αCaMKII in the expression of sensitization selectively in this site. All procedures were conducted according to an approved Institutional Biosafety Committee protocol.
Fig. 1.
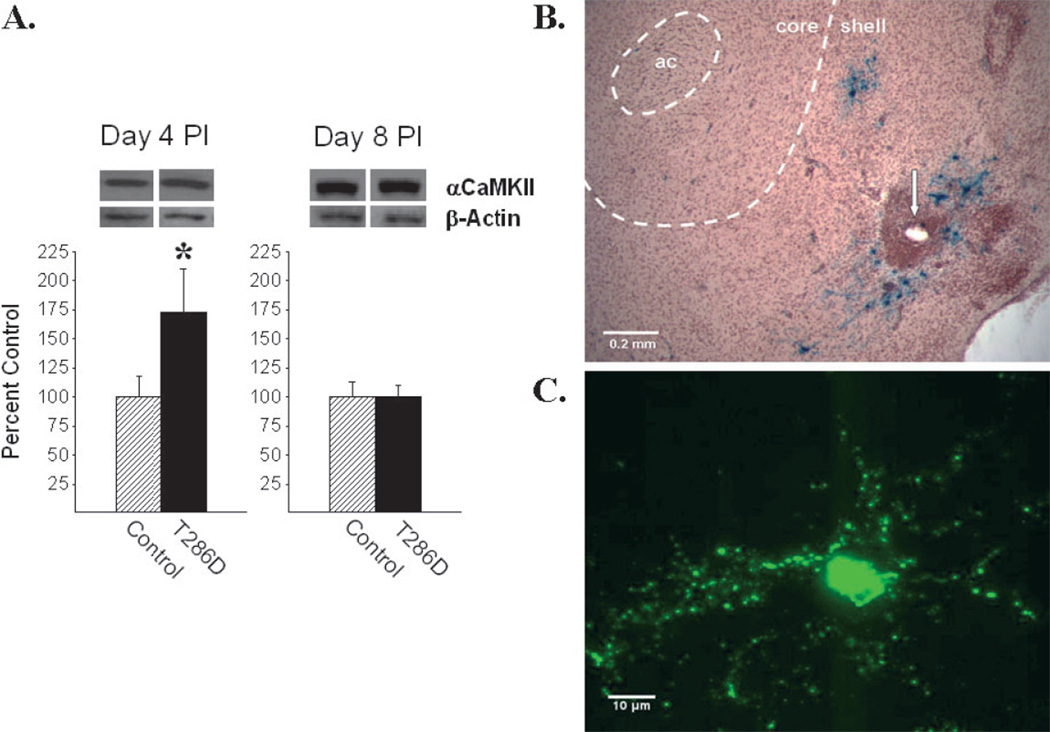
Viral-mediated gene transfer in the NAcc causes a transient increase in levels of the transgene. (A) Western blots of αCaMKII and β-actin (internal loading control) at 4 and 8 days after microinjection of HSV-T286D αCaMKII in the NAcc shell. αCaMKII was significantly increased at 4 days, but not 8 days, post-infection (PI). Protein levels are expressed as group mean (+SEM) % change from controls (HSV-LacZ or 10% sucrose) (n = 3–4/group; *P < 0.05, HSV-T286D αCaMKII vs. control). (B) Photomicrograph of a section of the NAcc (+1.2 mm from bregma) obtained at 4 days after infection with HSV-LacZ displaying β-galactosidase-positive neurons in close proximity to the injection cannula tip in the NAcc shell (arrow). Dashed lines delineating the NAcc core and shell subregions are superimposed from Paxinos & Watson (1997). ac, anterior commissure. (C) A green fluorescent protein (GFP)-positive neuron in the NAcc shell photographed at 4 days following infection with HSV-αCaMKII-GFP.
Immunoblotting
Rats were decapitated and brains were rapidly removed and flash-frozen on dry ice at either 4 or 8 days post-infection. Sections (1 mm thick) were obtained with a brain matrix and tissue punches were taken bilaterally around the injection cannula tips. Punches were 2 mm in diameter in order to obtain sufficient protein, keeping in mind that, following infusion of the viral vectors into the NAcc shell, neuronal infection was localized to this subnucleus (Fig. 1B). Punches were subsequently frozen on dry ice and processed as previously described (Carlezon & Neve, 2003). Briefly, tissue was homogenized in radioimmunoprecipitation assay buffer containing protease and phosphatase inhibitor cocktails (#1 and #2; Sigma-Aldrich Inc., St Louis, MO, USA) and protein levels were measured by the Bradford method; 7.5 µg of protein in homogenate containing 1% sodium dodecyl sulfate was loaded per lane. Following transfer, membranes were incubated in blocking solution (5% milk in Tris-buffered saline containing 0.1% Tween) sequentially containing no antibody, a rabbit-derived primary antibody for αCaMKII (1 : 1000; Millipore, Billerica, MA, USA) and a horseradish peroxidase-conjugated anti-rabbit immunoglobulin G (1 : 100 000; Jackson Labs, West Grove, PA, USA). Membranes were then stripped and probed with a mouse-derived antibody for β-actin as a loading control (1 : 2000; Sigma-Aldrich Inc.), incubated in an horseradish peroxidase-conjugated anti-mouse immunoglobulin G (1 : 100 000; Jackson Labs) and developed. Bands were visualized using the enhanced chemiluminescence detection system (ECL Advanced; GE Healthcare, Waukesha, WI, USA).
Immunohistochemistry
In separate rats, the non-fluorescent control vector HSV-LacZ, encoding β-galactosidase, was used to visualize the pattern of infection around injection cannula tips. Four days following infection in the NAcc shell, rats were anesthetized with sodium pentobarbital (65 mg/kg, i.p., Sigma-Aldrich Inc.) and perfused via intracardiac infusion with saline and 4% paraformaldehyde. Brains were then harvested and tissue was processed as previously described (Carlezon & Neve, 2003). 5-Bromo-4-chloro-3-indolyl β-D-galactopyranoside solution (0.2 mg/mL) was used to detect β-galactosidase expression. An HSV vector encoding the fusion αCaMKII-green fluorescent protein construct was also used to visualize αCaMKII overexpression. Brains were again harvested at 4 days following infection, flash-frozen in chilled isopentane and stored at −80°C. To detect green fluorescent protein fluorescence, 20 µm sections were prepared, mounted in ProGold antifade mountant (Molecular Probes, Eugene, OR, USA) and analyzed using a confocal fluorescence microscope with an argon-krypton laser and appropriate performance filters.
Behavioral testing
Assessments of the locomotor response to NAcc shell AMPA were made at 20 days post-infection, a time when NAcc αCaMKII protein levels had returned to baseline and infected rats maintained enhanced locomotor responding to amphetamine or AMPA (Loweth et al., 2010). In these experiments, HSV-αCaMKII-infected and control rats separate from those used for immunoblotting and immunohistochemistry were first placed in photocell chambers and left to habituate for 1 h. Rats were then administered their respective bilateral microinjections into the NAcc shell and returned to the photocell chambers for an additional 2 h of locomotor testing. Rats in separate groups were administered saline (0.5 µL/side) or the AMPA receptor agonist (±) AMPA hydrobromide (0.8 nmol/0.5 µL/side; Sigma-Aldrich Inc.) infused either alone or with the D1 receptor antagonist SCH23390 HCl (0.8 nmol/0.5 µL/side; Tocris Bioscience, Ellisville, MO, USA) or the PKA inhibitor Rp-cAMPS (80 nmol/1 µL/side; Axxora LLC, San Diego, CA, USA). Thus, rats in 12 separate groups were tested: two infection conditions (HSV-αCaMKII-infected or control) × two AMPA challenge conditions (AMPA or saline) × three inhibition conditions (none, SCH23390 or Rp-cAMPS). All drugs were dissolved in 0.9% sterile saline. Doses refer to the weight of the salt and were selected based on preliminary findings and previously published results (Self et al., 1998; Suto et al., 2004; Vezina, 1996).
Microinjections were again made in the freely moving rat using the same guide cannulas used to deliver the viral vectors to the NAcc shell. Injection cannulas (28 gauge) connected via PE20 tubing to 1 µL syringes (Hamilton) were inserted to a depth 4 mm below the guide cannula tips. Microinjections were made over 30 s (AMPA, SCH23390 and saline; 0.5 µL/side) or 1 min (Rp-cAMPS; 1 µL/side) followed by a diffusion time of 1 min before the injectors were removed and the obturators replaced.
Rats were randomly assigned to the locomotor chambers and assignment to a specific chamber was counterbalanced between αCaMKII and control group rats. Of the 12 locomotor chambers, 8–10 were used at any one time with half assigned to rats in the αCaMKII group and half to rats in the control group.
Locomotor chambers
A bank of 12 locomotor chambers was used to estimate locomotor activity. Each chamber (22 × 43 × 33 cm) was constructed of opaque plastic (rear and two side walls), a Plexiglas front-hinged door and a tubular stainless steel ceiling and floor. Interruptions of two photocell beams, positioned 2.5 cm above the floor and spaced evenly along the longitudinal axis of each box, estimated locomotion. The resulting locomotor counts were recorded by a computer located in an adjacent room using locally developed software.
Histology
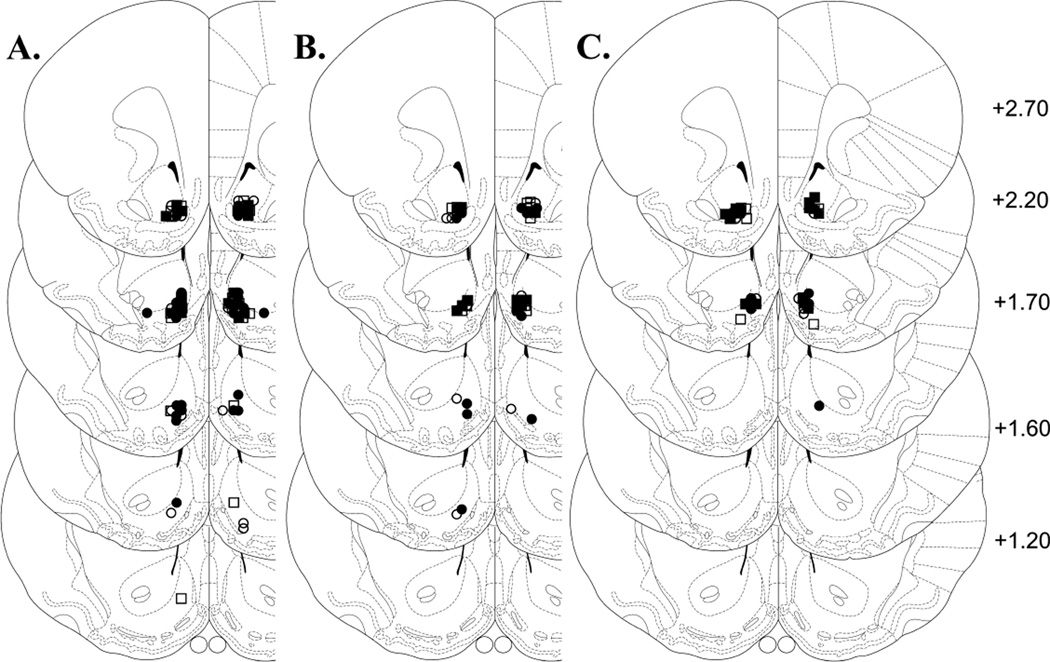
Following locomotor testing, rats were anesthetized with sodium pentobarbital (65 mg/kg, i.p.; Sigma-Aldrich Inc.) and perfused via intracardiac infusion of saline and 10% formalin. The brains were then removed and post-fixed in 10% formalin for at least 24 h. For verification of cannula tip placements in the NAcc shell, coronal sections (40 µm) were mounted onto gelatin-coated slides and stained with cresyl violet. Only rats with both injection cannula tips located in the NAcc shell were used in the statistical analyses (Fig. 3). The following rats were dropped for failing to meet this criterion: αCaMKII AMPA, 3; control AMPA, 5; αCaMKII saline, 3; control saline, 1; αCaMKII AMPA+SCH23390, 7; control AMPA+SCH23390, 3; αCaMKII saline+SCH23390, 2; control saline+SCH23390, 0; αCaMKII AMPA+Rp-cAMPS, 1; control AMPA+Rp-cAMPS, 2; αCaMKII saline+Rp-cAMPS, 1 and control saline+Rp-cAMPS, 1.
Fig. 3.
Location of microinjection cannula tips in the NAcc shell for all rats included in the data analyses. Line drawings of coronal sections (adapted from Paxinos and Watson, 1997) show the location of the microinjection cannula tips in the NAcc shell for rats tested with no inhibitor (A), with SCH23390 (B) or with Rp-cAMPS (C). Numbers to the right indicate mm from bregma. Symbols denote group affiliation: filled circles, αCaMKII-AMPA; open circles, control-AMPA; filled squares, αCaMKII-saline; open ssquares, control-saline.
Data analysis
Immunoblot data were analyzed with t-tests for independent samples. Pre- and post-challenge locomotor counts (1 and 2 h totals) were analyzed with two-way between-group anovas with infection (αCaMKII infected or control) and AMPA challenge (AMPA or saline) as the between-group factors. These analyses were conducted for each of the three inhibition conditions (none, SCH23390 or Rp-cAMPS). Post-hoc comparisons were made using the Scheffé test.
Results
Immunoblotting
As previously reported with the HSV vector system (Neve et al., 1997; Carlezon et al., 1997), microinjection into the NAcc shell of either the wild-type or the T286D construct of HSV-αCaMKII produced a transient increase in transgene expression in this site. Figure 1A illustrates the magnitude and temporal pattern of αCaMKII overexpression observed following infection in the NAcc shell with HSV-T286D αCaMKII. Western blot analyses revealed that αCaMKII levels were significantly increased (approximately 70%) at 4 days (t4 = 2.63, P < 0.05) but no longer at 8 days post-infection (t6 = 0.06, n.s.). Both the magnitude and time-course of overexpression were identical to that observed following infection in this site with HSV-wild-type αCaMKII (Loweth et al., 2010).
In this experiment, a mouse-derived antibody for β-actin was used as a loading control. A significant difference in total β-actin between HSV-T286D αCaMKII and control rats was not observed either at day 4 or day 8 post-infection. It is possible that the overexpression of αCaMKII produced a change in the ratio of filamentous actin to globular actin without altering total actin levels, as reported to occur following repeated psychostimulant administration (Toda et al., 2006). However, given the importance of the β but not the α isoform of CaMKII in regulating cytoskeletal dynamics including interactions with filamentous actin in dendritic spines, it is likely that filamentous actin and globular actin levels remained unchanged following over-expression of αCaMKII alone (Shen et al., 1998; Fink et al., 2003).
Immunohistochemistry
Immunohistological examination of brain sections at 4 days following infection with HSV-LacZ in the NAcc shell revealed localized overexpression of β-galactosidase around the injection cannula tip in this subnucleus (Fig. 1B). As previously reported (Loweth et al., 2010), there was no evidence for spread of the virus to the NAcc core or other adjacent brain regions. As shown in Fig. 1C, CaMKII-green fluorescent protein-expressing cells exhibited large dendritic processes consistent with the morphology of medium spiny neurons in the NAcc and the neuron-preferring characteristics of HSV (Carlezon et al., 2000).
Locomotor activity
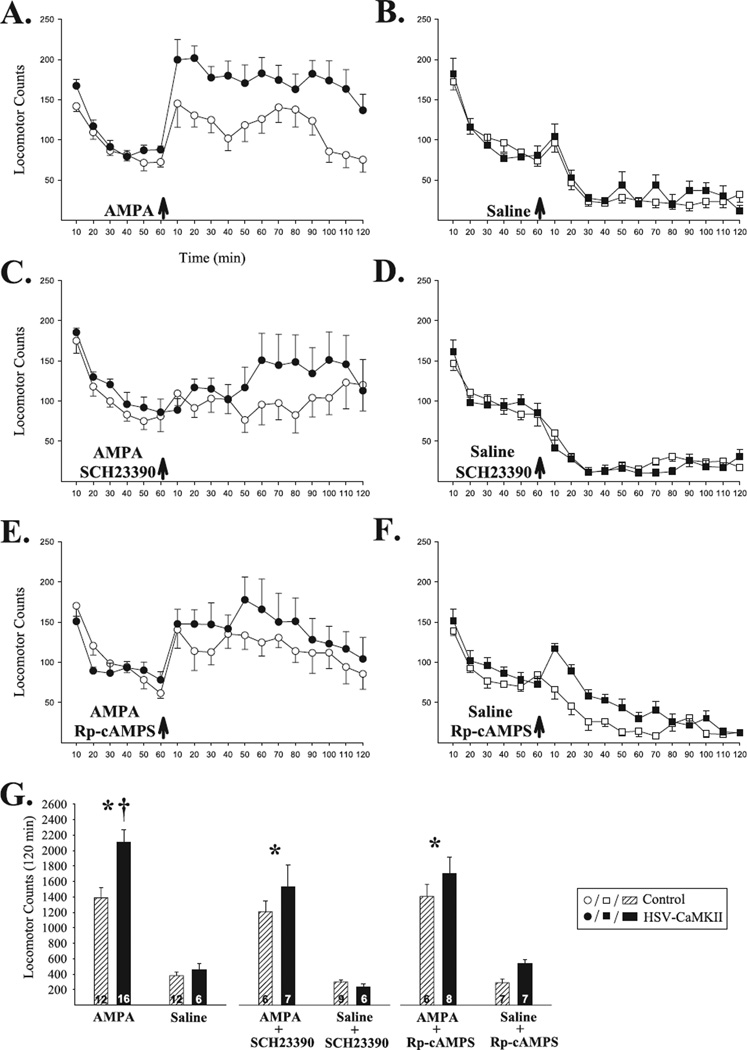
Consistent with previous reports (Loweth et al. 2010), transient overexpression of αCaMKII in the NAcc shell led to a long-lasting enhancement in locomotor responding to AMPA in this site. In a test conducted at 20 days post-infection, NAcc shell AMPA increased locomotion in both αCaMKII and control groups relative to NAcc shell saline but this response was significantly greater in αCaMKII group rats (Fig. 2A, B and G). The anova conducted on the 2 h total locomotor counts obtained post-challenge revealed significant effects of infection (F1,42 = 7.19, P < 0.05) and AMPA (F1,42 = 82.08, P < 0.001) and a significant infection × AMPA interaction (F1,42 = 4.81, P < 0.05). Post-hoc Scheffé comparisons confirmed that, compared with controls, HSV-αCaMKII-infected rats showed enhanced locomotor responding to AMPA (P < 0.01).
Fig. 2.
Enhanced locomotor responding to NAcc shell AMPA at 20 days post-infection requires D1 receptor and PKA activation. Data in (A–F) are shown as group mean (±SEM) locomotor counts obtained before and after the challenge injection (arrows). (A and B) The locomotor response to NAcc shell AMPA was significantly enhanced in αCaMKII compared with control group rats. Little locomotor activity was observed in either group following NAcc shell saline. Coadministration of either SCH23390 (C and D) or Rp-cAMPS (E and F) with AMPA into the NAcc shell blocked the enhanced locomotor response in αCaMKII compared with control group rats but spared the locomotor effects of AMPA in both groups compared with NAcc shell saline. Neither SCH23390 nor Rp-cAMPS produced effects that differed significantly from saline when administered alone. (G) Summary of post-challenge injection results illustrated as group mean (+SEM) 2 h total locomotor counts. *P < 0.001, AMPA vs. saline; †P < 0.01, αCaMKII vs. control; revealed by post-hoc Scheffé comparisons following anova.
To investigate the contribution of D1 receptor and PKA activation to the enhanced NAcc shell AMPA response observed in αCaMKII group rats, the effects of the D1 receptor antagonist SCH23390 and the PKA inhibitor Rp-cAMPS were assessed. Following infusion of AMPA+SCH23390 into the NAcc shell, increased locomotion relative to NAcc shell saline was still produced in both αCaMKII and control group rats but the enhanced locomotor response normally observed in αCaMKII group rats was blocked (Fig. 2C, D and G). The anova conducted on these data only showed a significant effect of AMPA (F1,24 = 48.66, P < 0.001). Infusing SCH23390 with saline into the NAcc shell produced no effects that were statistically different from those produced by saline. Similar results were obtained with Rp-cAMPS; the locomotor response to NAcc shell AMPA was spared but the enhanced locomotor response normally observed in HSV-αCaMKII-infected rats was blocked (Fig. 2E–G). Again, the anova only showed a significant effect of AMPA (F1,24 = 62.65, P < 0.001) and Rp-cAMPS by itself produced no effects that differed significantly from those produced by saline. Finally, no significant differences between groups were detected for locomotion in the habituation period immediately preceding the challenge injection.
Discussion
In the present experiments, transient viral-mediated overexpression of αCaMKII in neurons of the NAcc shell that mimics the transient increase in αCaMKII observed in this site following exposure to amphetamine or cocaine led to long-lasting enhancement of the locomotor response to NAcc shell AMPA. This enhancement was observed long after levels of αCaMKII in the NAcc shell had returned to baseline and required activation of D1 receptors and PKA. Because post-synaptic interactions between DA and glutamate in the NAcc are critical for the expression of sensitization by psychostimulant drugs, the functional upregulation of AMPA receptors observed may contribute to the long-lasting maintenance of behavioral sensitization by these drugs. Thus, transient increases in αCaMKII levels in the NAcc shell produce long-lasting changes in the way that DA and glutamate interact in this site to generate locomotor behavior.
Four different CaMKII subunits are known to exist to form the dodecameric holoenzyme. Of these, the principal CaMKII subunits found in brain are the α and β isoforms (Bennett et al., 1983; Miller & Kennedy, 1986). Work in cultured hippocampal neurons suggests that the α : β expression ratio shifts toward α during periods of increased activity and toward β during decreased activity, an effect reflecting greater sensitivity to changes in calmodulin, and thus calcium, of the α subunit (Thiagarajan et al., 2002). In addition, αCaMKII-transfected cells show increased miniature excitatory post-synaptic current amplitude and slowed miniature excitatory post-synaptic current decay compared with control and βCaMKII-transfected cells (Thiagarajan et al., 2002). These results suggest that the enhanced responding to amphetamine or AMPA in HSV-αCaMKII-infected rats may be a consequence of the long-lasting increase in the excitability of NAcc medium spiny neurons (Loweth et al., 2010). Repeated cocaine administration enhances αCaMKII but not βCaMKII gene expression and lentiviral knockdown of αCaMKII, but not βCaMKII, in the NAcc shell decreases motivation to self-administer cocaine (Wang et al., 2010). Similarly, overexpression of an inactive mutant form of αCaMKII (K42M) in neurons of the NAcc shell blocks sensitized locomotor responding to amphetamine (Vezina et al., 2009). These results further demonstrate the importance of the α isoform of CaMKII in the expression of sensitization phenotypes. In contrast, evidence suggests that the β isoform of CaMKII regulates changes in dendritic morphology and synapse formation (Shen et al., 1998; Fink et al., 2003). Thus, overexpressing αCaMKII but not βCaMKII in the NAcc shell may have enhanced locomotor responding to AMPA (present findings) and amphetamine (Loweth et al., 2010) without altering dendritic spine morphology. Sensitization has widely been associated with increases in dendritic morphological traits (Robinson & Kolb, 2004). Recent findings, however, indicate that amphetamine locomotor sensitization can be expressed in the absence of increased dendritic length, branching or spine density (Singer et al., 2009).
Although the relative contributions of its α and β isoforms remain to be determined, CaMKII is known to regulate AMPA receptor function in a number of ways (Lisman et al., 2002), most notably by phosphorylating its GluR1 subunit at the ser831 to enhance channel conductance (Oh & Derkach, 2005; Song & Huganir, 2002) and by promoting GluR1 insertion into the synapse (Hayashi et al., 2000). Less is known about the post-phosphorylation cascades initiated by CaMKII in NAcc neurons that lead to long-lasting functional upregulation of AMPA receptors. A number of pathways exist that may contribute, including decreases in protein phosphatase activity (Hu et al., 2005), phosphorylation by protein kinase C of GluR1 (ser831), as this kinase is also known to contribute to psychostimulant sensitization (Gnegy, 2000; Pierce et al., 1998), as well as long-lasting CaMKII-initiated changes leading to enlargement of spines on NAcc medium spiny neurons and increases in AMPA receptor-mediated transmission (Matsuzaki et al., 2004). Nonetheless, whatever the pathways involved, the present findings suggest that the neuroadaptations produced by αCaMKII in NAcc neurons that underlie the long-lasting functional upregulation of AMPA receptors observed require D1 receptor and PKA activation. Other findings showing that inhibiting CaMKII in the NAcc shell blocks the expression of long-lasting sensitization (Pierce et al., 1998; Pierce & Kalivas, 1997; Loweth et al., 2008) suggest that activation of CaMKII may also be required.
Enhanced AMPA receptor-mediated glutamatergic transmission is known to play an important role in the expression of psychostimulant sensitization (Vanderschuren & Kalivas, 2000; Vezina & Suto, 2003; Wolf, 1998). AMPA receptor antagonists block its expression (Bell et al., 2000; Karler et al., 1991; Mead & Stephens, 1998; Pierce et al., 1996; Tzschentke & Schmidt, 1997; cf, Karler et al., 1994; Li et al., 1997) and NAcc AMPA produces enhanced responding in psychostimulant-exposed rats (Pierce et al., 1996; Suto et al., 2004). These rats also show increased cell surface expression of GluR1 and GluR2 AMPA receptor subunits (cocaine exposed; Boudreau & Wolf, 2005; Boudreau et al., 2007, 2009) and increased phosphorylated GluR1 (ser831) levels in the NAcc (amphetamine exposed; Loweth et al., 2010). The latter effect was also observed following transient viral-mediated overexpression of αCaMKII (Loweth et al., 2010). Considering that phosphorylation of GluR1 (ser831) increases channel conductance in GluR2-lacking AMPA receptors (Oh & Derkach, 2005), these receptors contribute to synaptic transmission in the NAcc (Boudreau et al., 2007; Campioni et al., 2009) and their contribution is increased following exposure to cocaine (Conrad et al., 2008), it is conceivable that increased phosphorylated GluR1 (ser831) contributed to the enhanced NAcc shell AMPA-stimulated locomotion observed in HSV-αCaMKII-infected rats in the present experiments. Given the ability of repeated cocaine (Boudreau & Wolf, 2005; Boudreau et al., 2007, 2009) but not repeated amphetamine (Nelson et al., 2009) to increase cell surface expression of AMPA receptor subunits, it remains possible that this enhanced NAcc AMPA-induced locomotion resulted from a long-lasting increase in AMPA receptor surface expression. This possibility remains to be determined.
A number of studies indicate that CaMKII can be recruited in NAcc neurons by a pathway initiated by activation of D1 receptors. The ensuing activation of PKA and phosphorylation of L-type calcium channels lead to a rise in inward calcium flow and activation of CaMKII (Anderson et al., 2008; Hernandez-Lopez et al., 1997; Surmeier et al., 1995). Activation of this pathway and phosphorylation by CaMKII of GluR1 (ser831) in NAcc shell neurons is required for reinstatement of cocaine seeking (Anderson et al., 2008). Further, increased surface expression of GluR1 in primary NAcc neuron cultures requires D1 receptor and PKA activation (Chao et al., 2002a; Mangiavacchi & Wolf, 2004; Sun et al., 2008). The present results, showing that functional upregulation of AMPA receptors requires D1 receptor and PKA activation, are consistent with these findings.
Expression of sensitization by psychostimulants is also associated with long-lasting enhancement of DA overflow in the NAcc (Vezina, 2004). It is conceivable that the resulting increases in extracellular DA can activate the above PKA-calcium-CaMKII pathway, possibly in a manner involving cooperative D1 and D2 receptor signaling (Hopf et al., 2003), to enhance AMPA-mediated glutamatergic signaling in NAcc neurons. Interestingly, activation of CaMKII is also required for sensitized DA release from DA terminals (Kantor et al., 1999). Thus, CaMKII may act pre- and post-synaptically in the NAcc to generate a sensitizing feed-forward loop that promotes sensitized behavioral output in psychostimulant-exposed rats (Loweth & Vezina, 2010). Because sensitized locomotor responding to NAcc shell AMPA was produced in the present experiments by transiently overexpressing αCaMKII in NAcc neurons, the nature of the contribution of presynaptic DA to the present findings remains unclear. Glutamate is known to act at ionotropic glutamate receptors in the NAcc to increase extracellular levels of DA in this site and this probably provided the necessary D1 receptor stimulus to activate the above pathway in the present experiments (Howland et al., 2002). Indeed, NAcc AMPA-evoked DA overflow is enhanced in the NAcc of amphetamine-sensitized rats (Steinmiller et al., 2003). It is possible that transiently overexpressing αCaMKII in NAcc neurons led to a similar enhancement in the ability of AMPA to increase DA overflow in this site via descending projections to the ventral tegmental area, the site of the DA cell bodies projecting to the NAcc (Loweth & Vezina, 2010). This possibility remains to be determined. In a manner analogous to the inability of CaMKII inhibition to prevent acute amphetamine-induced DA release in non-sensitized controls (Kantor et al., 1999), the present findings show that D1 receptor and PKA activation are not required for acute non-sensitized NAcc shell AMPA-induced locomotion in control rats. Rather, D1 receptor-activated signaling leading to activation of CaMKII may be necessary for the expression of sensitized locomotor responding in rats previously exposed to a psychostimulant or subjected to transient overexpression of αCaMKII in the NAcc.
Acknowledgements
This study was supported by grants from the Peter F. McManus Charitable Trust (P.V.) as well as grants R01-DA-09397 (P.V.), T32-DA-07255 (B.F.S.) and F31-DA-022834 (J.A.L.) from the National Institutes of Health. The authors are grateful to Drs K. U. Bayer and S. A. Josselyn for the T286D CaMKII construct.
Abbreviations
- AMPA
α-amino-3-hydroxyl-5-methyl-4-isoxazole-propionate
- CaMKII
calcium/calmodulin-dependent protein kinase II
- D1
dopamine type-1
- DA
dopamine
- GluR
α-amino-3-hydroxyl-5-methyl-4-isoxazole-propionate glutamate receptor subunit
- HSV
herpes simplex virus
- NAcc
nucleus accumbens
- PKA
protein kinase A
- ser831
serine residue 831 of α-amino-3-hydroxyl-5-methyl-4-isoxazole-propionate glutamate receptor subunit 1
References
- Anderson SM, Famous KR, Sadri-Vakili G, Kumaresan V, Schmidt HD, Bass CE, Terwilliger EF, Cha JJ, Pierce RC. CaMKII: a biochemical bridge linking accumbens dopamine and glutamate systems in cocaine seeking. Nat. Neurosci. 2008;11:344–353. doi: 10.1038/nn2054. [DOI] [PubMed] [Google Scholar]
- Barria A, Derkach V, Soderling T. Identification of the Ca2+/calmodulin-dependent protein kinase II regulatory phosphorylation site in the alpha-amino-3-hydroxyl-5-methyl-4-isoxazole-propinate-typeglutamate receptor. J. Biol. Chem. 1997;272:32727–32730. doi: 10.1074/jbc.272.52.32727. [DOI] [PubMed] [Google Scholar]
- Bell K, Duffy P, Kalivas PW. Context-specific enhancement of glutamate transmission by cocaine. Neuropsychopharmacology. 2000;23:335–344. doi: 10.1016/S0893-133X(00)00100-7. [DOI] [PubMed] [Google Scholar]
- Bennett MK, Erondu NE, Kennedy MB. Purification and characterization of a calmodulin-dependent protein kinase that is highly concentrated in brain. J. Biol. Chem. 1983;258:12735–12744. [PubMed] [Google Scholar]
- Boudreau AC, Wolf ME. Behavioral sensitization to cocaine is associated with increased AMPA receptor surface expression in the nucleus accumbens. J. Neurosci. 2005;25:9144–9151. doi: 10.1523/JNEUROSCI.2252-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudreau AC, Reimers JM, Milovanovic M, Wolf ME. Cell surface AMPA receptors in the rat nucleus accumbens increase during cocaine withdrawal but internalize after cocaine challenge in association with altered activation of mitogen-activated protein kinases. J. Neurosci. 2007;27:10621–10635. doi: 10.1523/JNEUROSCI.2163-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudreau AC, Ferrario CR, Glucksman MJ, Wolf ME. Signaling pathway adaptations and novel protein kinase A substrates related to behavioral sensitization to cocaine. J. Neurochem. 2009;110:363–377. doi: 10.1111/j.1471-4159.2009.06140.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campioni MR, Xu M, McGehee DS. Stress-induced changes in nucleus accumbens glutamate synaptic plasticity. J. Neurophysiol. 2009;101:3192–3198. doi: 10.1152/jn.91111.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlezon WA, Jr, Neve RL. Viral-mediated gene transfer to study the behavioral correlates of CREB function in the nucleus accumbens of rats. Methods Mol. Med. 2003;79:331–350. doi: 10.1385/1-59259-358-5:331. [DOI] [PubMed] [Google Scholar]
- Carlezon WA, Jr, Boundy VA, Haile CN, Lane SB, Kalb RG, Neve RL, Nestler EJ. Sensitization to morphine induced by viral-mediated gene transfer. Science. 1997;277:812–814. doi: 10.1126/science.277.5327.812. [DOI] [PubMed] [Google Scholar]
- Carlezon WA, Nestler EJ, Neve RL. Herpes simplex virus-mediated gene transfer as a tool for neuropsychiatric research. Crit. Rev. Neurobiol. 2000;14:47–67. doi: 10.1080/08913810008443546. [DOI] [PubMed] [Google Scholar]
- Chao SZ, Ariano MA, Peterson DA, Wolf ME. D1 dopamine receptor stimulation increases GluR1 surface expression in nucleus accumbens neurons. J. Neurochem. 2002a;83:704–712. doi: 10.1046/j.1471-4159.2002.01164.x. [DOI] [PubMed] [Google Scholar]
- Chao SZ, Lu W, Lee HK, Huganir RL, Wolf ME. D(1) dopamine receptor stimulation increases GluR1 phosphorylation in postnatal nucleus accumbens cultures. J. Neurochem. 2002b;81:984–992. doi: 10.1046/j.1471-4159.2002.00877.x. [DOI] [PubMed] [Google Scholar]
- Conrad KL, Tseng KY, Uejima JL, Reimers JM, Heng L, Shaham Y, Marinelli M, Wolf ME. Formation of accumbens GluR2-lacking AMPA receptors mediates incubation of cocaine craving. Nature. 2008;454:118–121. doi: 10.1038/nature06995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink CC, Bayer KU, Myers JW, Ferrell JE, Jr, Schulman H, Meyer T. Selective regulation of neurite extension and synapse formation by the beta but not the alpha isoform of CaMKII. Neuron. 2003;39:283–297. doi: 10.1016/s0896-6273(03)00428-8. [DOI] [PubMed] [Google Scholar]
- Gnegy ME. Ca2+/calmodulin signaling in NMDA-induced synaptic plasticity. Crit. Rev. Neurobiol. 2000;14:91–129. [PubMed] [Google Scholar]
- Hayashi Y, Shi SH, Esteban JA, Piccini A, Poncer JC, Malinow R. Driving AMPA receptors into synapses by LTP and CaMKII: requirement for GluR1 and PDZ domain interaction. Science. 2000;287:2262–2267. doi: 10.1126/science.287.5461.2262. [DOI] [PubMed] [Google Scholar]
- Hernandez-Lopez S, Bargas J, Surmeier DJ, Reyes A, Galarraga E. D1 receptor activation enhances evoked discharge in neostriatal medium spiny neurons by modulating an L-type Ca2+ conductance. J. Neurosci. 1997;17:3334–3342. doi: 10.1523/JNEUROSCI.17-09-03334.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopf FW, Cascini MG, Gordon AS, Diamond I, Bonci A. Cooperative activation of dopamine D1 and D2 receptors increases spike firing of nucleus accumbens neurons via G-protein βγ subunits. J. Neurosci. 2003;23:5079–5087. doi: 10.1523/JNEUROSCI.23-12-05079.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howland JG, Taepavarapruk P, Phillips AG. Glutamate receptor-dependent modulation of dopamine efflux in the nucleus accumbens by basolateral, but not central, nucleus of the amygdala in rats. J. Neurosci. 2002;22:1137–1145. doi: 10.1523/JNEUROSCI.22-03-01137.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu XT, Ford K, White FJ. Repeated cocaine administration decreases calcineurin (PP2B) but enhances DARPP-32 modulation of sodium currents in rat nucleus accumbers neurons. Neuropsychopharmacology. 2005;30:916–926. doi: 10.1038/sj.npp.1300654. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Stewart J. Dopamine transmission in the initiation and expression of drug- and stress-induced sensitization of motor activity. Brain Res. Brain Res. Rev. 1991;16:223–244. doi: 10.1016/0165-0173(91)90007-u. [DOI] [PubMed] [Google Scholar]
- Kantor L, Hewlett GJK, Gnegy ME. Enhanced amphetamine-and K+-mediated dopamine release in rat striatum after repeated amphetamine: differential requirements for Ca2+- and calmodulin-dependent phosphorylation and synaptic vesicles. J. Neurosci. 1999;19:3801–3808. doi: 10.1523/JNEUROSCI.19-10-03801.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karler R, Calder LD, Turkanis SA. DNQX blockade of amphetamine behavioral sensitization. Brain Res. 1991;552:295–300. doi: 10.1016/0006-8993(91)90095-d. [DOI] [PubMed] [Google Scholar]
- Karler R, Calder LD, Bedingfield JB. Cocaine behavioral sensitization and the excitatory amino acids. Psychopharmacology. 1994;115:305–310. doi: 10.1007/BF02245070. [DOI] [PubMed] [Google Scholar]
- Li Y, Vartanian AJ, White FJ, Xue CJ, Wolf ME. Effects of the AMPA receptor antagonist NBQX on the development and expression of behavioral sensitization to cocaine and amphetamine. Psychopharmacology. 1997;134:266–276. doi: 10.1007/s002130050449. [DOI] [PubMed] [Google Scholar]
- Lisman J, Schulman H, Cline H. The molecular basis of CaMKII function in synaptic and behavioural memory. Nat. Rev. Neurosci. 2002;3:175–190. doi: 10.1038/nrn753. [DOI] [PubMed] [Google Scholar]
- Loweth JA, Vezina P. Sensitization. Calcium-calmodulin-dependent protein kinase II and the expression of stimulant sensitization. In: Olmstead MC, Walz W, editors. Animal Models of Drug Addiction. Totawa, N.J.: The Humana Press Inc.; 2010. in press. [Google Scholar]
- Loweth JA, Baker LK, Guptaa T, Guillory AM, Vezina P. Inhibition of CaMKII in the nucleus accumbens shell decreases enhanced amphetamine intake in sensitized rats. Neurosci. Lett. 2008;444:157–160. doi: 10.1016/j.neulet.2008.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loweth JA, Singer BF, Baker LK, Wilke G, Inamine H, Bubula N, Alexander JK, Carlezon WA, Jr, Neve RL, Vezina P. Transient overexpression of αCaMKII in the nucleus accumbens shell enhances behavioral responding to amphetamine. J. Neurosci. 2010;30:939–949. doi: 10.1523/JNEUROSCI.4383-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangiavacchi S, Wolf ME. D1 dopamine receptor stimulation increases the rate of AMPA receptor insertion onto the surface of cultured nucleus accumbens neurons through a pathway dependent on protein kinase A. J. Neurochem. 2004;88:1261–1271. doi: 10.1046/j.1471-4159.2003.02248.x. [DOI] [PubMed] [Google Scholar]
- Matsuzaki M, Honkura N, Ellis-Davies GCR, Kasai H. Structural basis of long-term potentiation in single dendritic spines. Nature. 2004;429:761–766. doi: 10.1038/nature02617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mead AN, Stephens DN. AMPA-receptors are involved in the expression of amphetamine-induced behavioural sensitisation, but not in the expression of amphetamine-induced conditioned activity in mice. Neuropharmacology. 1998;37:1131–1138. doi: 10.1016/s0028-3908(98)00101-4. [DOI] [PubMed] [Google Scholar]
- Miller SG, Kennedy MB. Regulation of brain type II Ca2+/calmodulin-dependent protein kinase by autophosphorylation: a Ca2+-triggered molecular switch. Cell. 1986;44:861–870. doi: 10.1016/0092-8674(86)90008-5. [DOI] [PubMed] [Google Scholar]
- Nelson CL, Milovanovic M, Wetter JB, Ford KA, Wolf ME. Behavioral sensitization to amphetamine is not accompanied by changes in glutamate receptor surface expression in the rat nucleus accumbens. J. Neurosci. 2009;109:35–51. doi: 10.1111/j.1471-4159.2009.05911.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neve RL, Howe JR, Hong S, Kalb RG. Introduction of the glutamate receptor subunit 1 into motor neurons in vitro and in vivo using a recombinant herpes simplex virus. Neuroscience. 1997;79:435–447. doi: 10.1016/s0306-4522(96)00645-8. [DOI] [PubMed] [Google Scholar]
- Oh MC, Derkach VA. Dominant role of the GluR2 subunit in regulation of AMPA receptors by CAMKII. Nat. Neurosci. 2005;8:853–854. doi: 10.1038/nn1476. [DOI] [PubMed] [Google Scholar]
- Paulson PE, Robinson TE. Amphetamine-induced time-dependent sensitization of dopamine neurotransmission in the dorsal and ventral striatum: a microdialysis study in behaving rats. Synapse. 1995;19:56–65. doi: 10.1002/syn.890190108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Compact 3rd Edn. San Diego: Academic Press; 1997. [Google Scholar]
- Pellegrino LJ, Pellegrino AS, Cushman AJ. A Stereotaxic Atlas of the Rat Brain. New York: Plenum Press; 1979. [Google Scholar]
- Pierce RC, Kalivas PW. Repeated cocaine modifies the mechanism by which amphetamine releases dopamine. J. Neurosci. 1997;17:3254–3261. doi: 10.1523/JNEUROSCI.17-09-03254.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce RC, Bell K, Duffy P, Kalivas PW. Repeated cocaine augments excitatory amino acid transmission in the nucleus accumbens only in rats having developed behavioral sensitization. J. Neurosci. 1996;16:1550–1560. doi: 10.1523/JNEUROSCI.16-04-01550.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce RC, Quick EA, Reeder DC, Morgan ZR, Kalivas PW. Calcium-mediated second messengers modulate the expression of behavioral sensitization to cocaine. J. Pharmacol. Exp. Ther. 1998;286:1171–1176. [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res. Brain Res. Rev. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Kolb B. Structural plasticity associated with exposure to drugs of abuse. Neuropharmacology. 2004;47:33–46. doi: 10.1016/j.neuropharm.2004.06.025. [DOI] [PubMed] [Google Scholar]
- Self DW, Genova LM, Hope BT, Barnhart WJ, Spencer JL, Nestler EJ. Involvement of cAMP-dependent protein kinase in the nucleus accumbens in cocaine self-administration and relapse of cocaine-seeking behavior. J. Neurosci. 1998;18:1848–1859. doi: 10.1523/JNEUROSCI.18-05-01848.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen K, Teruel MN, Subramanian K, Meyer T. CaMKIIbeta functions as an F-actin targeting module that localizes CaMKIIalpha/beta heterooligomers to dendritic spines. Neuron. 1998;21:593–606. doi: 10.1016/s0896-6273(00)80569-3. [DOI] [PubMed] [Google Scholar]
- Singer BF, Tanabe LM, Gorny G, Jake-Matthews C, Li Y, Kolb B, Vezina P. Amphetamine-induced changes in dendritic morphology in rat forebrain correspond to associative drug conditioning rather than nonassociative drug sensitization. Biol. Psychiatry. 2009;65:835–840. doi: 10.1016/j.biopsych.2008.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song I, Huganir RL. Regulation of AMPA receptors during synaptic plasticity. Trends Neurosci. 2002;25:578–588. doi: 10.1016/s0166-2236(02)02270-1. [DOI] [PubMed] [Google Scholar]
- Steinmiller CL, Kennedy RH, Baker LK, Suto N, Vezina P. Evidence that previous exposure to amphetamine can sensitize AMPA-evoked dopamine overflow in the nucleus accumbens. Soc. for Neurosci. Abst. 2003;29:113.8. [Google Scholar]
- Sun X, Milovanovic M, Zhao Y, Wolf ME. Acute and chronic dopamine receptor stimulation modulates AMPA receptor trafficking in nucleus accumbens neurons cocultured with prefrontal cortex neurons. J. Neurosci. 2008;28:4216–4230. doi: 10.1523/JNEUROSCI.0258-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surmeier DJ, Bargas J, Hemmings HC, Jr, Nairn AC, Greengard P. Modulation of calcium currents by a D1 dopaminergic protein kinase/phosphatase cascade in rat neostriatal neurons. Neuron. 1995;14:385–397. doi: 10.1016/0896-6273(95)90294-5. [DOI] [PubMed] [Google Scholar]
- Suto N, Tanabe LM, Austin JD, Creekmore E, Pham CT, Vezina P. Previous exposure to psychostimulants enhances the reinstatement of cocaine seeking by nucleus accumbens AMPA. Neuropsychopharmacology. 2004;29:2149–2159. doi: 10.1038/sj.npp.1300533. [DOI] [PubMed] [Google Scholar]
- Thiagarajan TC, Piedras-Renteria ES, Tsien RW. Alpha- and betaCaMKII. Inverse regulation by neuronal activity and opposing effects on synaptic strength. Neuron. 2002;36:1103–1114. doi: 10.1016/s0896-6273(02)01049-8. [DOI] [PubMed] [Google Scholar]
- Toda S, Shen HW, Peters J, Cagle S, Kalivas PW. Cocaine increases actin cycling: effects in the reinstatement model of drug seeking. J. Neurosci. 2006;26:1579–1587. doi: 10.1523/JNEUROSCI.4132-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzschentke TM, Schmidt WJ. Interactions of MK-801 and GYKI 52466 with morphine and amphetamine in place preference conditioning and behavioural sensitization. Behav. Brain Res. 1997;84:99–107. doi: 10.1016/s0166-4328(97)83329-3. [DOI] [PubMed] [Google Scholar]
- Vanderschuren LJMJ, Kalivas PW. Alterations in dopaminergic and glutamatergic transmission in the induction and expression of behavioral sensitization: a critical review of preclinical studies. Psychopharmacology. 2000;151:99–120. doi: 10.1007/s002130000493. [DOI] [PubMed] [Google Scholar]
- Vezina P. D1 dopamine receptor activation is necessary for the induction of sensitization by amphetamine in the ventral tegmental area. J. Neurosci. 1996;16:2411–2420. doi: 10.1523/JNEUROSCI.16-07-02411.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vezina P. Sensitization of midbrain dopamine neuron reactivity and the self-administration of psychomotor stimulant drugs. Neurosci. Biobehav. Rev. 2004;27:827–839. doi: 10.1016/j.neubiorev.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Vezina P, Suto N. Glutamate and the self-administration of psychomotor stimulant drugs. In: Herman BH, editor. Glutamate and Addiction. Totowa, NJ: Humana Press; 2003. pp. 183–220. [Google Scholar]
- Vezina P, Lorrain DS, Arnold GM, Austin JD, Suto N. Sensitization of midbrain dopamine neuron reactivity promotes the pursuit of amphetamine. J. Neurosci. 2002;22:4654–4662. doi: 10.1523/JNEUROSCI.22-11-04654.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vezina P, Wilke G, Jeyifous O, Bayer KU, Neve RL, Loweth JA. Viral-mediated overexpression of an inactive mutant form of αCaMKII in the NAcc shell inhibits the expression of sensitized locomotor responding to amphetamine. Soc. for Neurosci. Abst. 2009;35:66.22. [Google Scholar]
- Wang L, Lv Z, Hu Z, Sheng J, Hui B, Sun J, Ma L. Chronic cocaine-induced H3 acetylation and transcriptional activation of CaMKIIalpha in the nucleus accumbens is critical for motivation for drug reinforcement. Neuropsychopharmacology. 2010;35:913–928. doi: 10.1038/npp.2009.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf ME. The role of excitatory amino acids in behavioral sensitization to psychomotor stimulant drugs. Prog. Neurobiol. 1998;54:679–720. doi: 10.1016/s0301-0082(97)00090-7. [DOI] [PubMed] [Google Scholar]