Abstract
AIM
Brain systems supporting higher cognitive and motor control develop in a parallel manner, dependent on functional integrity and maturation of related regions, suggesting neighbouring neural circuitry. Concurrent examination of motor and cognitive control can provide a window into neurological development. However, identification of performance-based measures that do not correlate with IQ has been a challenge.
METHOD
Timed motor performance from the Physical and Neurological Examination of Subtle Signs and IQ were analysed in 136 children aged 6 to 16 (mean age 10y 2.6mo, SD 2y 6.4mo; 98 female, 38male) attending an outpatient neuropsychology clinic and 136 right-handed comparison individuals aged 6 to 16 (mean age 10y 3.1mo, SD 2y 6.1mo; 98 female, 38male). Timed activities – three repetitive movements (toe tapping, hand patting, finger tapping) and three sequenced movements (heel–toe tap, hand pronate/supinate, finger sequencing) each performed on the right and left – were included in exploratory factor analyses.
RESULTS
Among comparison individuals, factor analysis yielded two factors – repetitive and sequenced movements – with the sequenced factor significantly predictive of Verbal IQ (VIQ) (ΔR2=0.018, p=0.019), but not the repetitive factor (ΔR2=0.004, p=0.39). Factor analysis within the clinical group yielded two similar factors (repetitive and sequenced), both significantly predictive of VIQ, (ΔR2=0.028, p=0.015; ΔR2=0.046, p=0.002 respectively).
INTERPRETATION
Among typical children, repetitive timed tasks may be independent of IQ; however, sequenced tasks share more variance, implying shared neural substrates. Among neurologically vulnerable populations, however, both sequenced and repetitive movements covary with IQ, suggesting that repetitive speed is more indicative of underlying neurological integrity.
The overlap between higher intellectual skills and measures of specific neuropsychological functions has been the topic of considerable debate. Given the frequency with which IQ tests are used in neuropsychological examinations, their use as ‘reference points’ for interpretation of deficient function in other neurobehavioural domains has been called into question for both adults and children.1–3 Basic motor speed, however, may represent a neuropsychological function that is dissociable from other, more complex, cognitive functions. Research investigating the relationship between motor speed and higher cognitive functions has produced mixed results.4–7 The failure to find a clear dissociation may be because brain systems necessary for higher cognitive and motor control develop in a parallel manner and depend on functional integrity and maturation of related regions, suggesting neighbouring neural circuitry.8,9 For example, the neocerebellum (important in motor function) and the dorsolateral prefrontal cortex (considered critical for complex cognition) have similar developmental trajectories.4
Children with neurodevelopmental disorders commonly present with motor dysfunction.4,10 For example, children with autism have been shown to have motor disturbances,11 with as many as 55% of children displaying motor impairments in one study.12 Additionally, in children with attention-deficit–hyperactivity disorder (ADHD), Davis et al.13 found that those with more pronounced sensory–motor deficits also do poorly on academic achievement measures. In children with other neurodevelopmental disorders, however, the degree of motor impairment does not necessarily correspond to the level of cognitive–intellectual impairment (e.g. cerebral palsy, spina bifida14,15). These inconsistencies may be related to the variability in methods of measuring both motor speed and IQ.16 It may also be that the motor impairment is associated with brain regions and/or connections remote from cognitive processing regions (higher cognitive functions, e.g. midbrain, brainstem, spinal cord). Yet another possibility may be that the inconsistencies are due to the specificity of input and output of the cerebellum. For example, within the cerebellum, some nuclei both project to and receive information from the dorsolateral prefrontal cortex (which is associated with ADHD deficits), whereas others do not.4 Therefore, it is possible that motor and cognitive dysfunction may be dissociable in some individuals.
There are multiple methods of assessing motor speed in children, including pegboards, finger tapping, writing and copying speed, and structured timed motor examinations – each measuring a somewhat different aspect of motor function. However, the timed motor examination may be considered more ‘pure’ because it eliminates the visual–motor integration demands present in tasks involving writing, drawing, copying, or placing pegs in pegboard holes (i.e. even when tapping on a key or button, one must hit with accurate aim). The timed motor portion of the Physical and Neurological Examination of Subtle Signs (PANESS)17 is particularly useful because it allows for differential assessment of repetitive and sequenced movements. This distinction is important, as a dissociation has been found between brain regions involved in ordinal and temporal control of movements – the latter primarily involving the presupplementary motor regions, inferior frontal gyri, precentral sulci, and superior temporal gyri. This regional specialization may be important for the flexibility required in these voluntary timed motor tasks.18 Furthermore, sequenced movements (e.g. toe–heel sequences, hand pronation– supination, finger apposition) have been shown to be influenced by the ipsilateral as well as contralateral motor cortex in studies using transcranial magnetic stimulation,19,20 whereas repetitive movements may represent a more basic neurobehavioural function – potentially dissociable from other higher-order skills. Additionally, the left motor cortex has been shown to be more involved than the right in both simple and complex motor sequenced tasks, regardless of handedness. 8 By examining age-related changes in right (vs left) hand superiority using six timed tasks from the PANESS (three repetitive, three sequenced) with typically developing right-handed children, Roeder et al.21 reported age–sex interactions in right-side superiority (i.e. right–left gap) on two of the three sequenced motor tasks. This more rapid ‘equalization’ of left and right speed for females on the sequenced tasks parallels the earlier patterns of cerebral and corpus callosum maturation in females. Thus, improvement in the speed of sequenced movements may be more closely linked with brain development than speed improvement in repetitive movements, which may be more separable from higher cognitive function. This ‘separability’ of sequenced and repetitive movements (and the relationship of each with higher cognitive skills) has not yet been examined in paediatric populations.
Although a variety of studies have emphasized the significant overlap between IQ and executive function,16,22,23 others have also acknowledged the association between IQ and non-executive skills, including memory, language, visuospatial skills, and academic achievement.24–26 Given the multidimensional nature of the IQ score, it is not surprising that other performance-based tests of cognitive function share considerable variance with IQ.27 In a series of papers examining ‘the myths of neuropsychology’, Dodrill22,25 argued that the strong association between IQ and neuropsychological function may not exist for all individuals – especially those with above average IQ. Similarly, in a study of executive functions in children with ADHD, Mahone et al.23 found IQ to be a powerful moderator variable influencing the impact of ADHD on children’s cognitive performance, suggesting that those with average IQs are less able than children with above average IQs to meet the added demands of the disorder. In the same manner, Jepsen et al.28 reported that inattention, as measured on the Test of Variables of Attention, accounted for as much as 19% of the variation in Full-scale IQ (FSIQ) in children with ADHD when not taking medication. More recently, McGee et al.3 reported that children and adolescents with high IQ scores had significantly lower performance on the Delis–Kaplan Executive Function System, whereas those with low IQ scores had significantly higher Delis–Kaplan Executive Function System performance, suggesting that the relationship between IQ and executive control may be less robust at both ends of the bell curve. Among children with learning disorders (i.e. reading, spelling, arithmetic), IQ and academic skills are thought to be affected by the same developmental disadvantages, including both genetic and environmental influences.1,29 Therefore, although other methods of assessment (e.g. rating scales) have shown separability from performance-based IQ tests,30 it has been a challenge for neuropsychologists to identify performance-based measures that do not share substantial variance with IQ in clinical populations.
The present study had two primary goals. First, the factor structure of a timed motor examination, including both sequenced and repetitive timed movements, was examined in samples of typically developing children and clinically referred children to clarify nature of these movements. We hypothesized that a similar factor structure would be observed in both comparison individuals and clinically referred participants, and that repetitive movements would load on different factors than sequenced movements. Second, the study examined the relationship between factors comprising motor speed and performance-based measures of IQ. We hypothesized that latent variables emphasizing sequenced movements would share more variance with higher intellectual function (e.g. IQ) than variables emphasizing repetitive motor speed in both participant and comparison groups.
METHOD
Participants
Two samples of participants were included in the analyses. Participants in the typically developing group of children were drawn from the National Institutes of Health (NIH) Magnetic Resonance Imaging (MRI) Study of Normal Brain Development. 31,32 Detailed methods for selection of these participants have been published.32 The sample was collected from six sites across the USA and screened for neurological, psychiatric, and learning disorders. The participants in the clinical sample were drawn from a large outpatient neuropsychology clinic at a regional developmental disabilities–rehabilitation hospital. Informed consent to be included in a clinical database was signed by each participant (or by parent/guardian) at the time of assessment. The clinical sample included children with both acquired conditions (e.g. stroke, traumatic brain injury, leukaemia, brain tumour) and congenital disorders (e.g. hydrocephalus, mild cerebral palsy, autism spectrum disorders, ADHD, learning disabilities). Sample characteristics for both groups are reported in Tables I and II. There were a total of 136 right-handed participants in each group (272 total), ranging in age from 6 to 16 years. Both samples comprised 98 females and 38 males. Data from both samples were anonymized in accordance with requirements of the Johns Hopkins Medicine Institutional Review Boards.
Table I.
Study sample characteristics
| Variable | Comparison individuals (n=136) | Clinical (n=136) |
|---|---|---|
| Sex (M:F) | 38:98 | 38:98 |
| Mean age (SD) | 10y 2.6mo (2y 6.4mo) | 10y 3.1mo (2y 6.1mo) |
| Mean VIQ/VCI (SD) | 111.68 (12.27) | 97.90 (17.48) |
VCI, Verbal Comprehension Index from Wechsler Intelligence Scale for Children 3rd or 4th edition; VIQ, Verbal IQ from Wechsler Abbreviated Scale of Intelligence.
Table II.
Primary referral diagnoses for clinical group
| Primary diagnosis (n=136) | No. of participants |
|---|---|
| Acutemyelogenous leukaemia | 1 |
| Agenesis of the corpus callosum | 1 |
| Arachnoid cyst | 1 |
| Cerebral palsy (mild) | 1 |
| Craniosynostosis | 1 |
| Seizure disorder | 1 |
| HIV (congenital) | 1 |
| Prenatal drug/alcohol exposure | 1 |
| Encephalitis | 1 |
| Pervasive developmental disorder | 1 |
| Mental retardation* – unspecified | 2 |
| Lead poisoning | 3 |
| Pretermbirth | 3 |
| Stroke | 4 |
| Traumatic brain injury | 5 |
| Congenital hydrocephalus | 6 |
| Genetic disorder | 6 |
| Learning disabilities | 16 |
| Obstructive sleep apnoea | 18 |
| Tourette syndrome | 19 |
| Attention-deficit–hyperactivity disorder or disruptive behavioural disorder | 21 |
| Acute lymphoblastic leukaemia | 23 |
Conditions listed are the primary referral diagnoses. Many children in the sample had more than one condition.
UK usage: learning disability.
Study procedures
Participants were included in the clinical group if they had been administered the PANESS and an IQ test as part of their routine outpatient assessment. PANESS administration for participants in the clinical group was completed by a trained neuropsychologist (EMM). Participants in the comparison group were acquired from the online database from the NIH MRI Study of Normal Brain Development.32 Participants were included in the comparison group if they had been administered the timed motor portion of the PANESS and IQ test. Participants in the comparison group were chosen such that they individually matched each participant in the clinical group on the basis of handedness, age, and sex.
Measures
PANESS
The timed motor examination used in this study is part of the revised PANESS,17 with detailed scoring procedures outlined by Gidley Larson et al.33 The PANESS has been found to have adequate test–retest reliability,34 interrater reliability, and internal consistency.35 For the timed variables in particular, Vitiello et al.35 reported an interrater reliability range of 0.70 to 0.92 (preferred hand) and test–retest reliability ranging from 0.60 to 0.87 (preferred hand). The timed activities examined in this study include three repetitive movements and three sequenced movements, each performed on the right and left while seated, producing 12 total movements. The repetitive movements are simple flexion movements that are repeated as quickly as possible, including toe tapping (e.g. heel on floor lifting only toes), hand patting (e.g. wrist on thigh lifting hand), and finger tapping (e.g. hand open, tapping index finger to thumb). Sequenced movements include alternating patterns of more complex movements performed as quickly as possible, including heel–toe tap (e.g. rock from heel to toe), hand pronate– supinate, and finger sequence (i.e. open hand, touch every finger to thumb, always in the following order: index, middle, ring, pinkie, index, etc.). The examiner verbally explains each movement (‘Now, rock one foot back and forth, heel–toe, heel–toe, as fast as you can’) while also demonstrating it for the participant, and records the time in seconds it takes to complete 20 movements after the child demonstrates a steady pace. For the sequenced movements, 20 complete movements are considered to be 10 pairs of movements; for example, heel-toe is counted as two movements, not one.
Wechsler Intelligence Scale for Children, Third Edition (WISC-III) and Fourth Edition (WISC-IV)
The WISC-IV provides four index scores: Verbal Comprehension Index (VCI), Perceptual Reasoning Index, Working Memory Index, Processing Speed Index, and a FSIQ. When supplemental tests are administered, the WISC-III also produces four index scores: VCI, Perceptual Organization Index, Freedom from Distractibility Index, and Processing Speed Index. Because of the potential overlap between motor and/or processing speed and variables included in the FSIQ, the Verbal Comprehension Index was used as the representative IQ measure for the clinical group. Within the clinical group, 90 participants were administered the WISC-III and 46 were administered the WISC-IV. The WISC-III VCI includes four subtests (Information, Vocabulary, Similarities, and Comprehension), whereas the WISC-IV VCI includes only three of the four subtests (Vocabulary, Similarities, and Comprehension). None of the subtests from either version of the VCI is timed or requires a manual motor response.
Wechsler Abbreviated Scale of Intelligence (WASI)
As part of the NIH MRI Study of Normal Brain Development, children in the comparison group were administered the four-subtest version of the WASI as an abbreviated scale of intelligence. The WASI has normative data for ages 6 to 89, with stratified normative data for the entire age range of the present sample (6–16y). Correlation between WASI and the FSIQ of the WISC-III ranges from the 1970s to the 1990s, and from age 6 to 16 years. For the present study, the WASI Verbal IQ(VIQ) was used as the IQ measure in the comparison group, to eliminate the confound of motor and/or processing speed in tests comprising the Performance IQ. The WASI VIQ includes two subtests (Vocabulary and Similarities) – neither of which is timed.
Data analysis
Exploratory factor analyses were conducted separately for the comparison and clinical groups using principal component analysis extraction with promax rotation. The resulting factors were used to examine the relationship between motor function and IQ. Given the wide age range of the sample (6–16y) and considering that females perform faster than age-matched males on timed movements,36,37 age and sex were used as covariates in hierarchical linear regression analyses when examining the relationship between factor scores and IQ. Throughout the analyses, a significance level (two-tailed) of p<0.05 was used.
RESULTS
Demographic information
Demographic characteristics are summarized in Table I. All participants were right-handed. The comparison and clinical samples were, by design, highly similar in age (comparison individuals: mean age 10y 2.6mo, SD 2y 6.4mo; clinical participants: mean 10y 3.1mo, SD 2y 6.1mo). There were no differences between males and females in age in either the comparison (F1,135=1.64; p=0.20) or the clinical groups (F1,135=1.68; p=0.19). The comparison group had a mean VIQ score of 111.68 (SD 12.27; range 73–147), whereas the clinical group had a mean VCI score of 97.90 (SD 17.48; range 50– 146). There were no differences between males and females in WASI VIQ (F1,135=2.98, p=0.09) in the comparison group or in WISC III/IV VCI (F1,135=2.88; p=0.09) in the clinical group. However, as females had a trend towards higher IQ in both groups, sex (along with age) was used as a covariate in subsequent regression analyses.
Exploratory factor analysis
The factor structures for each group are listed in Tables III and IV. Among comparison individuals, the factor analysis yielded two factors that accounted for 73.6% of the variance – factor 1 comprised sequenced movements (60.6%) and factor 2 comprised repetitive movements (13.0%). All the 12 timed movements strongly loaded on their respective components, with the left foot tap variable being the only exception; factor loading was 0.75 on the repetitive factor and 0.72 on the sequenced factor. The factor analysis within the clinical group produced two nearly identical factors, accounting for 63% of the variance (sequenced=53.8%, repetitive=9.2%). In the clinical group, the left hand pronate–supinate variable loaded nearly evenly on the repetitive (0.72) and sequenced (0.70) factors. Similarly, the right foot tap variable was evenly distributed between the repetitive (0.56) and sequenced (0.56) factors.
Table III.
Factor loadings from exploratory factor analysis in typically developing children
| Variable | Component
|
|
|---|---|---|
| 1 | 2 | |
| Right | ||
| Foot tap | 0.693 | 0.755 |
| Foot heel–toe tap | 0.898 | 0.501 |
| Hand pat | 0.472 | 0.890 |
| Hand pronate–supinate | 0.812 | 0.643 |
| Finger tap | 0.523 | 0.891 |
| Appose finger succession | 0.813 | 0.514 |
| Left | ||
| Foot tap | 0.722 | 0.754 |
| Foot heel–toe tap | 0.849 | 0.448 |
| Hand pat | 0.542 | 0.894 |
| Hand pronate–supinate | 0.834 | 0.619 |
| Finger tap | 0.562 | 0.852 |
| Appose finger succession | 0.843 | 0.506 |
Component 1 is sequenced and component 2 is repetitive. Bold values indicate which component each factor loads on.
Table IV.
Factor loadings from exploratory factor analysis in a mixed clinical sample
| Variable | Component
|
|
|---|---|---|
| 1 | 2 | |
| Right | ||
| Foot tap | 0.545 | 0.547 |
| Foot heel–toe tap | 0.860 | 0.557 |
| Hand pat | 0.533 | 0.782 |
| Hand pronate–supinate | 0.756 | 0.486 |
| Finger tap | 0.648 | 0.722 |
| Appose finger succession | 0.850 | 0.534 |
| Left | ||
| Foot tap | 0.534 | 0.773 |
| Foot heel–toe tap | 0.817 | 0.553 |
| Hand pat | 0.494 | 0.883 |
| Hand pronate–supinate | 0.695 | 0.715 |
| Finger tap | 0.525 | 0.802 |
| Appose finger succession | 0.824 | 0.569 |
Component 1 is sequenced and component 2 is repetitive. Bold values indicate which component each factor loads on.
Relationship between IQ and latent motor speed factors
Comparison group
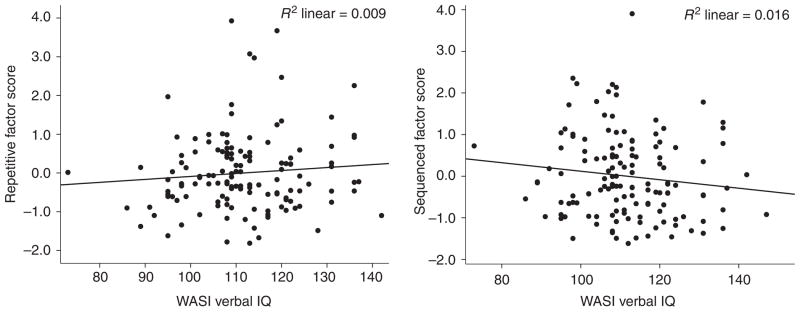
Hierarchical linear regression analyses were used to determine the association between VIQ and each of the two motor speed factors identified via factor analysis. Age was entered on the first step, followed by sex on the second step, and then IQ on the third step, thus controlling for age and sex when considering the association between IQ and motor speed. Assumptions of linear regression were examined. Residuals for each of the predictor variables were consistent across values, showing homoscedasticity. Error terms were normally distributed and were without significant autocorrelation (the Durbin-Watson statistic for n=136 with three independent variables was 1.9 for the sequenced factor and 2.2 for the repetitive factor). The results of regression analyses are shown in Table V. As expected, age was a significant predictor of motor speed for both factors, and sex added a significant proportion of unique variance in predicting the sequenced factor, after controlling for age (females faster than males). After controlling for age and sex, VIQ added a small, but significant proportion of unique variance to the prediction of the sequenced motor speed factor in comparison individuals (ΔR2=0.018; ΔF1,132=5.61; p=0.019). For the repetitive factor, VIQ was not a significant predictor of motor speed, after controlling for age and sex (ΔR2=0.004, ΔF1,132=0.72; p=0.39).
Table V.
Hierarchical regression analysis predicting motor speed from IQ
| Motor speed factor | Predictor | β | ΔR2 | p value |
|---|---|---|---|---|
| Comparison group | ||||
| Sequenced | Age | −0.728 | 0.529 | <0.001 |
| Sex | 0.136 | 0.018 | 0.022 | |
| VIQ | −0.137 | 0.018 | 0.019 | |
| Repetitive | Age | −0.552 | 0.304 | <0.001 |
| Sex | −0.083 | 0.007 | 0.251 | |
| VIQ | 0.062 | 0.004 | 0.399 | |
| Clinical group | ||||
| Sequenced | Age | −0.599 | 0.359 | <0.001 |
| Sex | −0.071 | 0.005 | 0.312 | |
| VCI | −0.219 | 0.046 | 0.002 | |
| Repetitive | Age | −0.590 | 0.348 | <0.001 |
| Sex | −0.108 | 0.011 | 0.126 | |
| VCI | −0.172 | 0.028 | 0.015 | |
VCI, Verbal Comprehension Index from the Wechsler Intelligence
Scale for Children 3rd or 4th edition; VIQ, Verbal IQ from the Wechsler Abbreviated Scale of Intelligence.
Clinical group
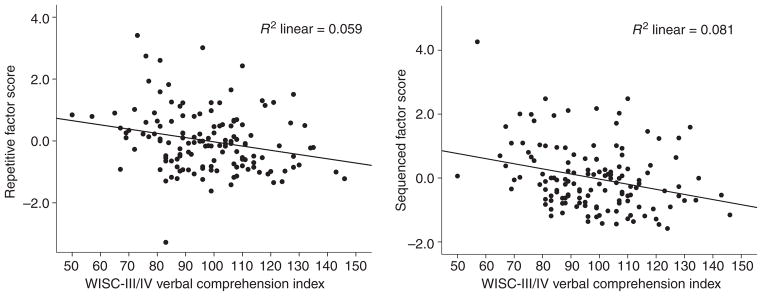
Similar hierarchical linear regression analyses were used to determine the association between WISC-III/IV VCI and each of the two motor speed factors identified via factor analysis in the mixed clinical group. Residuals for each of the predictor variables were consistent across values in both regression analyses, showing homoscedasticity. Error terms were normally distributed for both analyses, and were without significant autocorrelation (the Durbin–Watson statistic for n=136 with three independent variables was 2.1 for the sequenced factor and 2.0 for the repetitive factor). Age was a significant predictor of motor speed for both factors, but sex did not contribute unique variance in predicting either factor, after controlling for age. After controlling for age and sex, VCI added a significant proportion of unique variance to the prediction of both the sequenced motor speed factor (ΔR2=0.046; ΔF1,132=10.37; p=0.002), and the repetitive motor speed factor (ΔR2=0.028; ΔF1,132=6.13; p=0.015).
DISCUSSION
Among separate large samples of typically developing and clinically referred children, the PANESS timed motor examination yielded two robust factors – one emphasizing sequenced or patterned movements and one emphasizing repetitive (less controlled) movements. Although a wide range of disorders were represented in the clinically referred sample, the exploratory factor analysis yielded similar findings in both samples (clinical and typically developing), suggesting different under-lying brain mechanisms in the two types of movements. Thus, the current findings are consistent with the results of published functional magnetic resonance imaging (fMRI) studies highlighting the separability of repetitive and sequenced movements. For example, using fMRI, Chan et al.38 reported different patterns of activation for basic (i.e. hand patting; with activation in contralateral sensorimotor, thalamus, superior temporal–inferior frontal, and ipsilateral cerebellum) and more complex motor tasks in healthy adults, such that when performing the most complex sequenced motor task (i.e. fist– edge–palm), increased activation was observed in distributed brain regions, including bilateral sensorimotor, supplementary motor area (SMA), and left parietal and right cerebellum, with increased signal change (left sensorimotor, left thalamus, right cerebellum) associated with increased complexity of movements.
The relationship between higher cognitive function (as represented by IQ) and basic motor speed is different between clinic-referred individuals and healthy comparison individuals. Repetitive movements, such as basic response time have long been thought to be tightly coupled with IQ. However, among carefully screened comparison individuals, speed of repetitive movements appears separable and distinct from verbal intelligence (note that a similar finding was present when using FSIQ) (Figure 1), whereas, among clinic-referred individuals, even these basic repetitive movements were associated with IQ, albeit with modest effect size (Figure 2). This relationship remained significant after controlling for age and sex, and when using a measure of IQ (i.e. the VCI) that minimizes motor speed demands by not requiring timed responses. Sequenced movements, however, share a significant proportion of common variance with IQ in both samples, again suggesting that the more complex movements may be associated with neurological integrity in general, and probably involve a more widely distributed neural substrate,18 but emphasizing left hemisphere representation,8,38 perhaps as a key example of limb kinetic praxis.39
Figure 1.
Relationships between factor scores and Verbal IQ: comparison individuals. WASI, Wechsler Abbreviated Scale of Intelligence.
Figure 2.
Relationships between factor scores and Verbal Comprehension Index: mixed clinical sample. WISC-III/IV, Wechsler Intelligence Scale for Children, 3rd and 4th editions.
Parallel motor and cognitive anomalies represent a characteristic feature of many neurodevelopmental disorders. For example, there is consistent evidence suggesting that the dopaminergic system is involved with the pathophysiology of ADHD,40 with at least half of the children with ADHD manifesting motor coordination difficulties.10 Similarly, children with autism spectrum disorders frequently have difficulties in performing novel movements and skilled movements, suggesting that frontostriatal–cerebellum anomalies affecting motor control in autism41 may also control social and communication skills in this group.42 Sequenced motor tasks have gained clinical significance in their ability to distinguish healthy comparison individuals from those with schizophrenia.38 The relationship between sequenced movements and cognitive development may be a common feature among clinical and typical populations, whereas repetitive movements (such as response times in general) are separable from higher cognitive skills among comparison individuals, suggesting that reduced repetitive speed is a marker for anomalous brain development (i.e. as a fever is to many infections, so sustained speed is to the ‘well-wired’ brain). This observation of importance of repetitive movements in a clinical sample provides another symptom for clinicians to consider during diagnosis and treatment plans.
Strengths of the present study include assessment of two large, separately recruited, diverse samples matched on age, handedness, and sex distribution. Additionally, the IQ range in both the clinical and comparison groups was very wide, and thus did not limit the range of scores used in regression analyses. At the same time, there are limitations of our methods, which should be considered when interpreting the findings. First, slightly different IQ tests were used for clinical and comparison groups. The impact of this difference was minimized, however, because the subtests of the WISC VCI and WASI VIQ are highly similar, and are not timed. It is noteworthy that identical findings were obtained when using the FSIQ from both tests, although (as expected) the associations were stronger between motor speed and FSIQ than between motor speed and VCI/VIQ. Another possible limitation to interpretation is the disproportionate number of females in both samples. As females are known to develop motor skills earlier than males,37 the findings may be influenced by the greater proportion of females; however, as sex was used as a covariate in the hierarchical regression analyses, and because the two samples were age matched, these effects were minimized. Future research should continue to investigate the neural substrates underlying repetitive versus sequenced movements in children, using structural and functional neuroimaging, with emphasis on how brain regions supporting these skills develop over time and how they are affected in neurodevelopmental disorders.
What this paper adds.
This study identified a common two-factor structure (repetitive and sequenced movements), in both clinical and comparison samples, of the timed motor portion of the Revised Physical and Neurological Examination of Subtle Signs.
Sequenced movements were associated with IQ in both samples, whereas repetitive movements were associated with IQ only in the clinical sample.
Sequenced movements were predictive of VIQ in both populations, however, repetitive movements only predicted VIQ in the clinical sample, suggesting an association of even simple motor movements with neurological integrity.
Acknowledgments
Portions of this manuscript were presented as a poster at the 37th annual meeting of the International Neuropsychological Society, 13th February 2009, Atlanta, Georgia. Supported by HD-24061 (Intellectual and Developmental Disabilities Research Center), the Johns Hopkins University School of Medicine Institute for Clinical and Translational Research, an NIH/NCRR CTSA Program, UL1-RR025005, and the NIHMRI Study of Normal Brain Development.
LIST OF ABBREVIATIONS
- PANESS
Physical and Neurological Examination of Subtle Signs
- VCI
Verbal Comprehension Index
- ADHD
Attention-deficit-hyperactivity disorder
- VIQ
Verbal IQ
- FSIQ
Full Scale IQ
References
- 1.Dennis M, Francis DJ, Cirino PT, Schachar R, Barnes MA, Fletcher JM. Why IQ is not a covariate in cognitive studies of neurodevelopmental disorders. J Int Neuropsychol Soc. 2009;15:331–43. doi: 10.1017/S1355617709090481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lezak MD. IQ: R.IP. J Clin Exp Neuropsychol. 2008;10:351–61. doi: 10.1080/01688638808400871. [DOI] [PubMed] [Google Scholar]
- 3.McGee CL, Delis DC, Holdnack JA. Cognitive discrepancies in children at the ends of the bell curve: a note of caution for clinical interpretation. Clin Neuropsychol. 2009;23:1160–72. doi: 10.1080/13854040902794995. [DOI] [PubMed] [Google Scholar]
- 4.Diamond A. Close interrelation of motor development and cognitive development and of the cerebellum and prefrontal cortex. Child Dev. 2000;71:44–56. doi: 10.1111/1467-8624.00117. [DOI] [PubMed] [Google Scholar]
- 5.Piek JP, Dyck MJ, Nieman A, et al. The relationship between motor coordination, executive functioning and attention in school aged children. Arch Clin Neuropsychol. 2004;19:1063–76. doi: 10.1016/j.acn.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 6.Roebers CM, Kauer M. Motor and cognitive control in a normative sample of 7-year-olds. Dev Sci. 2009;12:175–81. doi: 10.1111/j.1467-7687.2008.00755.x. [DOI] [PubMed] [Google Scholar]
- 7.Wassenberg R, Feron FJ, Kessels AG, et al. Relation between cognitive and motor performance in 5- to 6-year-old children: results from a large-scale cross-sectional study. Child Dev. 2005;76:1092–103. doi: 10.1111/j.1467-8624.2005.00899.x. [DOI] [PubMed] [Google Scholar]
- 8.Haaland KY. Left hemisphere dominance for movement. Clin Neuropsychol. 2006;20:609–22. doi: 10.1080/13854040590967577. [DOI] [PubMed] [Google Scholar]
- 9.Mahone EM, Powell SK, Loftis CW, Goldberg MC, Denckla MB, Mostofsky SH. Motor persistence and inhibition in autism and ADHD. J Int Neuropsychol Soc. 2006;12:622–31. doi: 10.1017/S1355617706060814. [DOI] [PubMed] [Google Scholar]
- 10.Pitcher TM, Piek JP, Hay DA. Fine and gross motor ability in boys with attention-deficit–hyperactivity disorder. Dev Med Child Neurol. 2003;45:525–35. doi: 10.1017/s0012162203000975. [DOI] [PubMed] [Google Scholar]
- 11.Hughes C. Brief report: planning problems in autism at the level of motor control. J Autism Dev Disord. 1996;26:99–107. doi: 10.1007/BF02276237. [DOI] [PubMed] [Google Scholar]
- 12.Page J, Boucher J. Motor impairments in children with autistic disorder. Child Lang Teach Ther. 1998;14:233–59. [Google Scholar]
- 13.Davis AS, Pass LA, Finch WH, Dean RS, Woodcock RW. The canonical relationship between sensory-motor functioning and cognitive processing in children with attention-deficit/hyperactivity disorder. Arch Clin Neuropsychol. 2009;24:273–86. doi: 10.1093/arclin/acp032. [DOI] [PubMed] [Google Scholar]
- 14.Whitaker TM, Palmer FB. The developmental history. In: Accardo PJ, editor. Capute & Accardo’s Neurodevelopmental disabilities in infancy and childhood. 3. Baltimore: Paul H. Brookes Publishing Co; 2008. pp. 297–310. [Google Scholar]
- 15.Blondis TA. Neurodevelopmental motor disorders: cerebral palsy and neuromuscular diseases. In: Dewey D, Tupper DE, editors. Developmental motor disorders: a neuropsychological perspective. New York: The Guildford Press; 2004. pp. 113–36. [Google Scholar]
- 16.Yeates KO, Donders J. The WISC-IV and neuropsychological assessment. In: Prifitera A, Saklofske DH, Weiss LG, editors. WISC-IV: Clinical use and interpretation: scientist-practitioner perspectives. Burlington, MA: Elsevier Academic Press; 2005. pp. 415–34. [Google Scholar]
- 17.Denckla MB. Revised neurological examination for subtle signs. Psychopharmacol Bull. 1985;21:773–9. [PubMed] [Google Scholar]
- 18.Ullen F, Bengtsson SL, Ehrsson HH, Forssberg H. Neural control of rhythmic sequences. Ann N Y Acad Sci. 2005;1060:368–76. doi: 10.1196/annals.1360.031. [DOI] [PubMed] [Google Scholar]
- 19.Avanzino L, Bove M, Trompetto C, Tacchino A, Ogliastro C, Abbruzzese G. 1-Hz repetitive TMS over ipsilateral motor cortex influences the performance of sequential finger movements of different complexity. Eur J Neurosci. 2008;27:1285–91. doi: 10.1111/j.1460-9568.2008.06086.x. [DOI] [PubMed] [Google Scholar]
- 20.Lepage M, Beaudoin G, Boulet C, et al. Frontal cortex and the programming of repetitive tapping movements in man: lesion effects and functional neuroimaging. Brain Res Cogn Brain Res. 1999;8:17–25. doi: 10.1016/s0926-6410(98)00055-x. [DOI] [PubMed] [Google Scholar]
- 21.Roeder MB, Mahone EM, Gidley Larson J, et al. Left–right differences on timed motor examination in children. Child Neuropsychol. 2008;14:249–62. doi: 10.1080/09297040701370016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dodrill CB. Myths of neuropsychology. Clin Neuropsychol. 1997;11:1–17. doi: 10.1076/1385-4046(199911)13:04;1-Y;FT562. [DOI] [PubMed] [Google Scholar]
- 23.Mahone EM, Hagelthorn KM, Cutting LE, et al. Effects of IQ on executive function measures in children with ADHD. Child Neuropsychol. 2002;8:52–65. doi: 10.1076/chin.8.1.52.8719. [DOI] [PubMed] [Google Scholar]
- 24.Arffa S. The relationship of intelligence to executive function and non-executive function measures in a sample of average, above average, and gifted youth. Arch Clin Neuropsychol. 2007;22:969–78. doi: 10.1016/j.acn.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 25.Dodrill CB. Myths of neuropsychology: further considerations. Clin Neuropsychol. 1999;13:562–72. doi: 10.1076/1385-4046(199911)13:04;1-Y;FT562. [DOI] [PubMed] [Google Scholar]
- 26.Friedman NP, Miyake A, Corley RP, Young SE, Defries JC, Hewitt JK. Not all executive functions are related to intelligence. Psychol Sci. 2006;17:172–9. doi: 10.1111/j.1467-9280.2006.01681.x. [DOI] [PubMed] [Google Scholar]
- 27.Jung RE, Yeo RA, Chiulli SJ, Sibbitt WL, Jr, Brooks WM. Myths of neuropsychology: intelligence, neurometabolism, and cognitive ability. Clin Neuropsychol. 2000;14:535–45. doi: 10.1076/clin.14.4.535.7198. [DOI] [PubMed] [Google Scholar]
- 28.Jepsen JR, Fagerlund B, Mortensen EL. Do attention deficits influence IQ assessment in children and adolescents with ADHD? J Atten Disord. 2009;12:551–62. doi: 10.1177/1087054708322996. [DOI] [PubMed] [Google Scholar]
- 29.Hart B, Risley TR. Meaningful differences in the everyday experience of young American children. Baltimore, MD: Brookes; 1995. [Google Scholar]
- 30.Mahone EM, Cirino PT, Cutting LE, et al. Validity of the behavior rating inventory of executive function in children with ADHD and/or Tourette syndrome. Arch Clin Neuropsychol. 2002;17:643–62. [PubMed] [Google Scholar]
- 31.Evans AC. The NIH MRI study of normal brain development. Neuroimage. 2006;30:184–202. doi: 10.1016/j.neuroimage.2005.09.068. [DOI] [PubMed] [Google Scholar]
- 32.Waber DP, De Moor C, Forbes PW, et al. The NIH MRI study of normal brain development: performance of a population based sample of healthy children aged 6 to 18 years on a neuropsychological battery. J Int Neuropsychol Soc. 2007;13:729–46. doi: 10.1017/S1355617707070841. [DOI] [PubMed] [Google Scholar]
- 33.Gidley Larson JC, Mostofsky SH, Goldberg MC, Cutting LE, Denckla MB, Mahone EM. Effects of gender and age on motor exam in typically developing children. Dev Neuropsychol. 2007;32:543–62. doi: 10.1080/87565640701361013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Holden EW, Tarnowski KJ, Prinz RJ. Reliability of neurological soft signs in children: reevaluation of the PANESS. J Abnorm Child Psychol. 1982;10:163–72. doi: 10.1007/BF00915938. [DOI] [PubMed] [Google Scholar]
- 35.Vitiello B, Ricciuti AJ, Stoff DM, Behar D, Denckla MB. Reliability of subtle (soft) neurological signs in children. J Am Acad Child Adolesc Psychiatry. 1989;28:749–53. doi: 10.1097/00004583-198909000-00017. [DOI] [PubMed] [Google Scholar]
- 36.Denckla MB. Development of speed in repetitive and successive finger-movements in normal children. Dev Med Child Neurol. 1973;15:635–45. doi: 10.1111/j.1469-8749.1973.tb05174.x. [DOI] [PubMed] [Google Scholar]
- 37.Gidley Larson JC, Bastian AJ, Donchin O, Shadmehr R, Mostofsky SH. Acquisition of internal models of motor tasks in children with autism. Brain. 2008;131:2894–903. doi: 10.1093/brain/awn226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chan RC, Rao H, Chen EE, Ye B, Zhang C. The neural basis of motor sequencing: an fMRI study of healthy subjects. Neurosci Lett. 2006;398:189–94. doi: 10.1016/j.neulet.2006.01.014. [DOI] [PubMed] [Google Scholar]
- 39.Heilman KM, Meador KJ, Loring DW. Hemispheric asymmetries of limb-kinetic apraxia: a loss of deftness. Neurology. 2000;55:523–6. doi: 10.1212/wnl.55.4.523. [DOI] [PubMed] [Google Scholar]
- 40.Mehler-Wex C, Riederer P, Gerlach M. Dopaminergic dysbalance in distinct basal ganglia neurocircuits: implications for the pathophysiology of Parkinson’s disease, schizophrenia and attention deficit hyperactivity disorder. Neurotox Res. 2006;10:167–79. doi: 10.1007/BF03033354. [DOI] [PubMed] [Google Scholar]
- 41.Stefanatos GA, Joe WQ, Aguirre GK, Detre JA, Wetmore G. Activation of human auditory cortex during speech perception: effects of monaural, binaural, and dichotic presentation. Neuropsychologia. 2008;46:301–15. doi: 10.1016/j.neuropsychologia.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 42.Dziuk MA, Gidley Larson JC. Dyspraxia in autism: association with motor, social, and communicative deficits. Dev Med Child Neurol. 2007;49:734–9. doi: 10.1111/j.1469-8749.2007.00734.x. [DOI] [PubMed] [Google Scholar]