Abstract
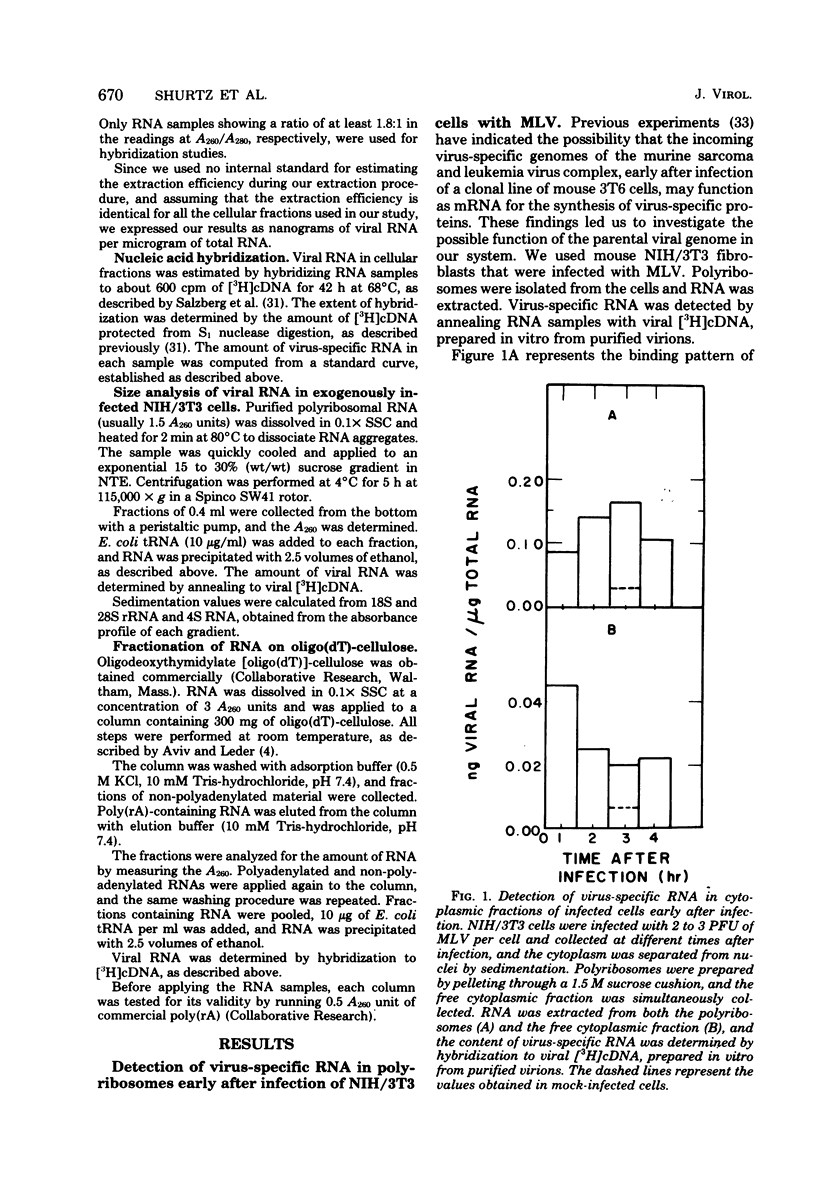
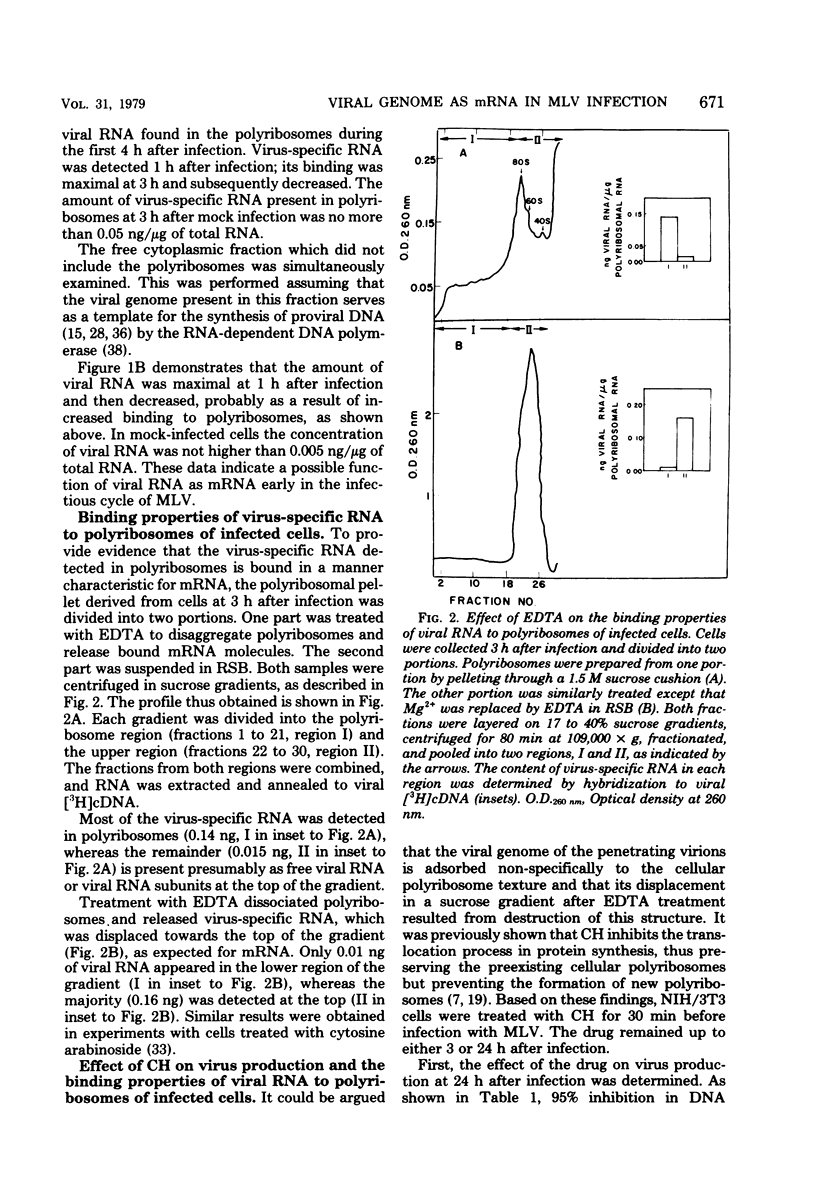
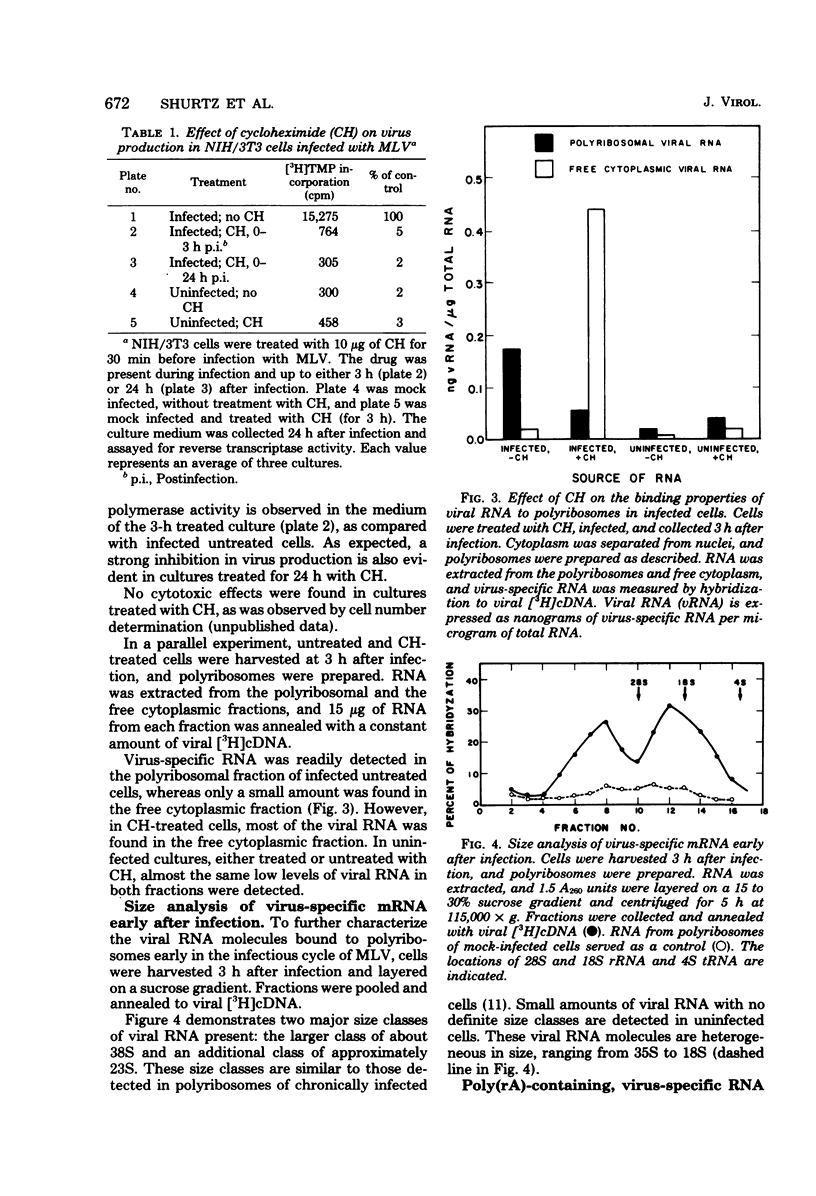
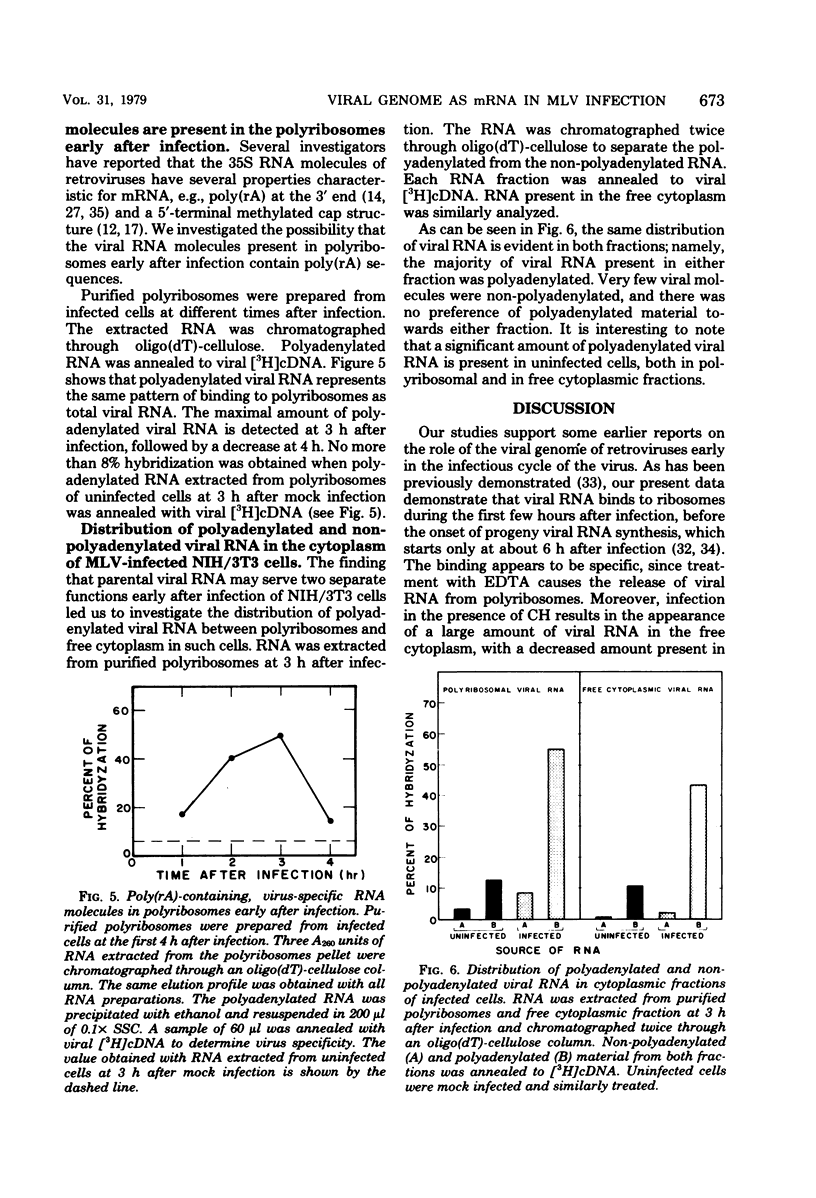
When NIH/3T3 mouse fibroblasts were infected with the Moloney strain of murine leukemia virus, part of the viral genome RNA molecules were detected in polyribosomes of the infected cells early in the infectious cycle. The binding appears to be specific, since we could demonstrate the release of viral RNA from polyribosomes with EDTA. Moreover, when infection occurred in the presence of cycloheximide, most viral RNA molecules were detected in the free cytoplasm. Size analysis on polyribosomal viral RNA molecules indicated that two size class molecules, 38S and 23S, are present in polyribosomes at 3 h after infection. Analysis of the polyriboadenylate [poly(rA)] content of viral RNA extracted from infected polyribosomes demonstrated that such molecules bind with greatest abundance at 3 h after infection, as has been detected with total viral RNA. No molecules lacking poly(rA) stretches could be detected in polyribosomes. Furthermore, when a similar analysis was performed on unbound molecules present in the free cytoplasm, identical results were obtained. We conclude that no selection towards poly(rA)-containing viral molecules is evident on binding to polyribosomes. These findings suggest that the incoming viral genome of the Moloney strain of murine leukemia virus may serve as a messenger for the synthesis of one or more virus-specific proteins early after infection of mouse fibroblasts.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aaronson S. A., Dunn C. Y. High-frequency C-type virus induction by inhibitors of protein synthesis. Science. 1974 Feb 1;183(4123):422–424. doi: 10.1126/science.183.4123.422. [DOI] [PubMed] [Google Scholar]
- Aboud M., Shoor R., Salzberg S. Effect of interferon on exogenous murine leukemia virus infection. Virology. 1978 Jan;84(1):134–141. doi: 10.1016/0042-6822(78)90225-8. [DOI] [PubMed] [Google Scholar]
- Aboud M., Weiss O., Salzberg S. Rapid quantitation of interferon with chronically oncornavirus-producing cells. Infect Immun. 1976 Jun;13(6):1626–1632. doi: 10.1128/iai.13.6.1626-1632.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beemon K., Hunter T. In vitro translation yields a possible Rous sarcoma virus src gene product. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3302–3306. doi: 10.1073/pnas.74.8.3302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COLOMBO B., FELICETTI L., BAGLIONI C. INHIBITION OF PROTEIN SYNTHESIS BY CYCLOHEXIMIDE IN RABBIT RETICULOCYTES. Biochem Biophys Res Commun. 1965 Feb 3;18:389–395. doi: 10.1016/0006-291x(65)90719-9. [DOI] [PubMed] [Google Scholar]
- Clayman C. H., Mosharrafa E., Faras A. J. In vitro synthesis of infectious transforming DNA by the avian sarcoma virus reverse transcriptase. J Virol. 1979 Jan;29(1):242–249. doi: 10.1128/jvi.29.1.242-249.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimock K., Stoltzfus C. M. Sequence specificity of internal methylation in B77 avian sarcoma virus RNA subunits. Biochemistry. 1977 Feb 8;16(3):471–478. doi: 10.1021/bi00622a021. [DOI] [PubMed] [Google Scholar]
- Fan H., Baltimore D. RNA metabolism of murine leukemia virus: detection of virus-specific RNA sequences in infected and uninfected cells and identification of virus-specific messenger RNA. J Mol Biol. 1973 Oct 15;80(1):93–117. doi: 10.1016/0022-2836(73)90235-0. [DOI] [PubMed] [Google Scholar]
- Fan H. Expression of RNA tumor viruses at translation and transcription lebels. Curr Top Microbiol Immunol. 1978;79:1–41. doi: 10.1007/978-3-642-66853-1_1. [DOI] [PubMed] [Google Scholar]
- Fan H., Verma I. M. Size analysis and relationship of murine leukemia virus-specific mRNA's: evidence for transposition of sequences during synthesis and processing of subgenomic mRNA. J Virol. 1978 May;26(2):468–478. doi: 10.1128/jvi.26.2.468-478.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuichi Y., Shatkin A. J., Stavnezer E., Bishop J. M. Blocked, methylated 5'-terminal sequence in avian sarcoma virus RNA. Nature. 1975 Oct 16;257(5527):618–620. doi: 10.1038/257618a0. [DOI] [PubMed] [Google Scholar]
- Gallis B. M., Eisenman R. N., Diggelmann H. Synthesis of the precursor to avian RNA tumor virus internal structural proteins early after infection. Virology. 1976 Oct 15;74(2):302–313. doi: 10.1016/0042-6822(76)90337-8. [DOI] [PubMed] [Google Scholar]
- Green M., Cartas M. The genome of RNA tumor viruses contains polyadenylic acid sequences. Proc Natl Acad Sci U S A. 1972 Apr;69(4):791–794. doi: 10.1073/pnas.69.4.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatanaka M., Kakefuda T., Gilden R. V., Callan E. A. Cytoplasmic DNA synthesis induced by RNA tumor viruses. Proc Natl Acad Sci U S A. 1971 Aug;68(8):1844–1847. doi: 10.1073/pnas.68.8.1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph D. R., Ihle J. N. Comparison of sequence homology of poly(A) and non-poly(A) containing 34S RNA of AKR murine leukemia virus. Biochem Biophys Res Commun. 1977 Jan 24;74(2):499–505. doi: 10.1016/0006-291x(77)90332-1. [DOI] [PubMed] [Google Scholar]
- Keith J., Fraenkel-Conrat H. Identification of the 5' end of Rous sarcoma virus RNA. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3347–3350. doi: 10.1073/pnas.72.9.3347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King A. M., Wells R. D. All intact subunit RNAs from Rous sarcoma virus contain poly (A). J Biol Chem. 1976 Jan 10;251(1):150–152. [PubMed] [Google Scholar]
- Lin S. Y., Mosteller R. D., Hardesty B. The mechanism of sodium fluoride and cycloheximide inhibition of hemoglobin biosynthesis in the cell-free reticulocyte system. J Mol Biol. 1966 Oct 28;21(1):51–69. doi: 10.1016/0022-2836(66)90079-9. [DOI] [PubMed] [Google Scholar]
- Mueller-Lantzsch N., Fan H. Monospecific immunoprecipitation of murine leukemia virus polyribosomes: identification of p30 protein-specific messenger RNA. Cell. 1976 Dec;9(4 Pt 1):579–588. doi: 10.1016/0092-8674(76)90040-4. [DOI] [PubMed] [Google Scholar]
- Panet A., Gorecki M., Bratosin S., Aloni Y. Electron microscopic evidence for splicing of Moloney murine leukemia virus RNAs. Nucleic Acids Res. 1978 Sep;5(9):3219–3230. doi: 10.1093/nar/5.9.3219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons J. T., Lewis P., Dierks P. Purification of virus-specific RNA from chicken cells infected with avian sarcoma virus: identification of genome-length and subgenome-leghth viral RNAs. J Virol. 1978 Jul;27(1):227–238. doi: 10.1128/jvi.27.1.227-238.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawson T., Martin G. S., Smith A. E. Cell-free translation of virion RNA from nondefective and transformation-defective Rous sarcoma viruses. J Virol. 1976 Sep;19(3):950–967. doi: 10.1128/jvi.19.3.950-967.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purchio A. F., Erikson E., Erikson R. L. Translation of 35S and of subgenomic regions of avian sarcoma virus RNA. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4661–4665. doi: 10.1073/pnas.74.10.4661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rho H. M., Green M. The homopolyadenylate and adjacent nucleotides at the 3'-terminus of 30-40s RNA subunits in the genome of murine sarcoma-leukemia virus. Proc Natl Acad Sci U S A. 1974 Jun;71(6):2386–2390. doi: 10.1073/pnas.71.6.2386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roa R. C., Bose S. K. Inhibition by ethidium bromide of the establishment of infection by murine sarcoma virus. J Gen Virol. 1974 Nov;25(2):197–205. doi: 10.1099/0022-1317-25-2-197. [DOI] [PubMed] [Google Scholar]
- Roa R. C., Bose S. K. Requirement for cellular protein synthesis in reversal of ethidium-bormide-induced inhibition of cell transformation by murine sarcoma virus. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4337–4340. doi: 10.1073/pnas.72.11.4337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robin M. S., Salzberg S., Green M. Cytoplasmic synthesis of viral DNA early during infection and cell transformation by the murine sarcoma-leukemia virus. Intervirology. 1974;4(5):268–278. doi: 10.1159/000149859. [DOI] [PubMed] [Google Scholar]
- Rothenberg E., Smotkin D., Baltimore D., Weinberg R. A. In vitro synthesis of infectious DNA of murine leukaemia virus. Nature. 1977 Sep 8;269(5624):122–126. doi: 10.1038/269122a0. [DOI] [PubMed] [Google Scholar]
- Salden M. H., Bloemendal H. Translation of avian myeloblastosis virus RNA in a reticulocyte cell-free system. Biochem Biophys Res Commun. 1976 Jan 12;68(1):249–255. doi: 10.1016/0006-291x(76)90036-x. [DOI] [PubMed] [Google Scholar]
- Salzberg S., Levi Z., Aboud M., Goldberger A. Isolation and characterization of DNA-DNA and DNA-RNA. Biochemistry. 1977 Jan 11;16(1):25–29. doi: 10.1021/bi00620a004. [DOI] [PubMed] [Google Scholar]
- Salzberg S., Robin M. S., Green M. A possible requirement for protein synthesis early in the infectious cycle of the murine sarcoma-leukemia virus. Virology. 1977 Jan;76(1):341–351. doi: 10.1016/0042-6822(77)90307-5. [DOI] [PubMed] [Google Scholar]
- Salzberg S., Robin M. S., Green M. Appearance of virus-specific RNA, virus particles, and cell surface changes in cells rapidly transformed by the murine sarcoma virus. Virology. 1973 May;53(1):186–195. doi: 10.1016/0042-6822(73)90477-7. [DOI] [PubMed] [Google Scholar]
- Schincariol A. L., Joklik W. K. Early synthesis of virus-specific RNA and DNA in cells rapidly transformed with Rous sarcoma virus. Virology. 1973 Dec;56(2):532–548. doi: 10.1016/0042-6822(73)90056-1. [DOI] [PubMed] [Google Scholar]
- Stephenson M. L., Scott J. F., Zamecnik P. C. Evidence that the polyadenylic acid segment of "35S" RNA of avian myeloblastosis virus is located at the 3'-OH terminus. Biochem Biophys Res Commun. 1973 Nov 1;55(1):8–16. doi: 10.1016/s0006-291x(73)80052-x. [DOI] [PubMed] [Google Scholar]
- Varmus H. E., Guntaka R. V., Fan W. J., Heasley S., Bishop J. M. Synthesis of viral DNA in the cytoplasm of duck embryo fibroblasts and in enucleated cells after infection by avian sarcoma virus. Proc Natl Acad Sci U S A. 1974 Oct;71(10):3874–3878. doi: 10.1073/pnas.71.10.3874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varmus H. E., Heasley S., Kung H. J., Oppermann H., Smith V. C., Bishop J. M., Shank P. R. Kinetics of synthesis, structure and purification of avian sarcoma virus-specific DNA made in the cytoplasm of acutely infected cells. J Mol Biol. 1978 Mar 25;120(1):55–82. doi: 10.1016/0022-2836(78)90295-4. [DOI] [PubMed] [Google Scholar]
- Verma I. M. The reverse transcriptase. Biochim Biophys Acta. 1977 Mar 21;473(1):1–38. doi: 10.1016/0304-419x(77)90005-1. [DOI] [PubMed] [Google Scholar]
- Vogt P. K., Hu S. S. The genetic structure of RNA tumor viruses. Annu Rev Genet. 1977;11:203–238. doi: 10.1146/annurev.ge.11.120177.001223. [DOI] [PubMed] [Google Scholar]
- Weiss S. R., Varmus H. E., Bishop J. M. The size and genetic composition of virus-specific RNAs in the cytoplasm of cells producing avian sarcoma-leukosis viruses. Cell. 1977 Dec;12(4):983–992. doi: 10.1016/0092-8674(77)90163-5. [DOI] [PubMed] [Google Scholar]


