Abstract
Diabetes mellitus is the leading cause of end stage renal disease and is responsible for more than 40% of all cases in the United States. Several therapeutic interventions for the treatment of diabetic nephropathy have been developed and implemented over the past few decades with some degree of success. However, the renal protection provided by these therapeutic modalities is incomplete. More effective approaches are therefore urgently needed. Recently, several novel therapeutic strategies have been explored in treating DN patients including Islet cell transplant, Aldose reductase inhibitors, Sulodexide (GAC), Protein Kinase C (PKC) inhibitors, Connective tissue growth factor (CTGF) inhibitors, Transforming growth factor-beta (TGF-β) inhibitors and bardoxolone. The benefits and risks of these agents are still under investigation. This review aims to summarize the utility of these novel therapeutic approaches.
Keywords: Markers, Albuminuria, Diabetes, Therapy
1. Introduction
Diabetes mellitus (DM) is a leading cause of morbidity and mortality in the United States. DM is often complicated by micro- and macrovascular involvement which contribute to damage to one or more target organs. Diabetic nephropathy (DN) is a well-known microvascular complication of diabetes and is responsible for 40% – 50% of all cases of end stage renal disease (ESRD) in the U.S. adult population [1,2].
DN is defined by the presence of persistent pathologic albuminuria of greater than 300 mg/24 hrs (macroalbuminuria) accompanied by abnormally elevated plasma creatinine or diminished glomerular filtration rate (GFR) [2]. Histologically, DN manifests as diffuse or nodular mesangial expansion, tubular and glomerular basement membrane thickening, as well as interstitial fibrosis.
DN is usually preceded by microalbuminuria (urinary albumin excretion > 30 mg but <300 mg/24 hrs) which is the earliest clinical manifestation of renal involvement in diabetic patients [3]. The onsets of microalbuminuria and overt nephropathy are variable in DM type 1 (DMT1) and type 2 (DMT2). Patients with DMT1 have a more predictable natural history and may present with microalbuminuria 7 – 10 years after being diagnosed with diabetes. About 20% – 45% of these patients progress to DN over the next 10 years (almost 20 years after the diagnosis of DMT1) [4]. On the other hand, patients with DMT2, which comprise approximately 80% of all diabetics, may have overt DN at the time of diagnosis since the duration of diabetes is often not precisely known in this population. The rate of progression of DN towards ESRD is influenced by complex interactions between genetic predisposition, dietary and lifestyle factors as well as therapeutic interventions. Compared to patients with normoalbuminuria (urine albumin excretion < 30 mg/24 hrs), patients with persistent macroalbuminuria (overt DN) have an almost 10-fold higher risk of developing ESRD [5]. Overt proteinuria is also an independent predictor of cardiovascular morbidity and death in diabetic patients [6].
Current therapeutic options directed at delaying the progression of diabetic nephropathy (DN) include intensive blood glucose control, improved blood pressure control, interruption of the RAAS using angiotensin-converting enzyme (ACE) inhibitors and/or angiotensin type-1 (AT1) receptor blockers (ARB) along with dietary modification and cholesterol-lowering agents (for review please see: [7]). Despite aggressive multifactorial interventions, (DN) remains the single leading cause of ESRD in the United States. The cost of ESRD care for these patients exceeds $10 billion/year. Therefore, more effective approaches are urgently needed.
In this article, we will review several novel therapeutic strategies that have been explored recently in patients with DN which may stop or even reverse disease progression.
2. Diabetic Nephropathy Markers
Early diagnosis of DN is crucial for its effective management. Thus, the search for reliable markers for this disease has been the focus of many studies. Traditionally, GFR has been considered the gold standard in the evaluation of overt nephropathy. Several markers have been used to measure GFR including inulin, iohexol, and iothalamate. However these methods require complex measurements and are expensive, time consuming and not readily feasible in clinical practice. The clearance of endogenous creatinine is another index for GFR. However, it requires timed urine collections and tends to overestimate GFR due to tubular creatinine secretion. This has led to the development of several equations for GFR based on serum creatinine such as the Cockcroft-Gault formula (C-G) [8], the Modification of Diet in Renal Disease four-variable (MDRD-4) formula [9], and the CKD-EPI formula [10] and the measurement of serum cystatin C as an alternative to serum creatinine [11]. A study of patients with DMT2 found that serum cystatin C more accurately identified those with a GFR < 60 ml/min/1.73 m2 than did serum creatinine, MDRD-4 and C-G formulas [12]. Therefore, cystatin C may be regarded as a superior measure of GFR, especially at lower GFR levels, than serum creatinine. Unfortunately, cystatin C measurements are more expensive than creatinine and may yield falsely low GFR in certain circumstances such as inflammation, steroid therapy and hyperthyroidism.
Urine albumin excretion has received considerable attention and has achieved widespread clinical use as a marker of early DN and as a target for intervention. Increased urinary albumin excretion is the hallmark of DN and estimating urinary albumin excretion is now considered the most reliable marker for assessing disease progression and determining efficacy of treatment in diabetic patients. Nonetheless, urine microalbumin measurements are subject to several limitations relating to specimen collection, indexing to urine creatinine or volume, intrasubject variability and influences of medications, diet and activity which confound its interpretation [11–15]. Thus, a prospective longitudinal study of 232 patients with DM found that the positive predictive value of microalbuminuria as a marker of risk for DN was 43% and the negative predictive value was 77% [13]. Therefore, microalbuminuria may not serve as a strong predictor of DN [14]. On the other hand, macroalbuminuria (overt proteinuria) develops at advanced stage of DN when attempts to prevent progression to ESRD can be very challenging. The discovery of better markers for early detection of DN is an area of active investigation.
3. Candidate Markers in Future
Recently, certain cytokines, such as connective tissue growth factor (CTGF), transforming growth factor-β (TGF-β), and tumor necrosis factor-α (TNF-α) have emerged as potential markers of progression of DN (Table 1). For instance, the number of CTGF messenger RNA positive cells in the kidney biopsy was closely related to the renal biopsy fibrosis score and urinary CTGF levels in 65 subjects, three of whom had diabetes [15]. Additional studies have suggested that the urinary excretion of CTGF is related to both albuminuria and GFR in DMT1 [16]. Jaffa and colleagues measured the circulating and urinary levels of CTGF in 1050 subjects with DMT1 from the DCCT/Epidemiology of Diabetes Interventions and Complications (EDIC) study [17]. They showed that significantly higher levels of plasma CTGF are apparent in advanced kidney disease as measured by increased urinary albumin excretion rate (AER), concluding that plasma CTGF is a risk marker of diabetic renal and vascular disease. More recently, urinary TGF-β excretion was shown to be attenuated by ACE inhibition in DMT2 patients with nephropathy [18].
Table 1.
Diabetic nephropathy markers.
| Current markers | Candidate markers in future |
|---|---|
| 1. Creatinine, Cystatin C (estimated GFR). | 1. Urinary podocytes |
| 2. Microalbuminuria | 2. NGAL |
| 3. Macroalbuminuria or Proteinuria | 3. KIM-1 |
| 4. Smad 1 | |
| 5. CTGF | |
| 6. TGF-β | |
| 7. TNF-α |
NGAL = Neutrophil Gelatinase-Associated Lipocalin; KIM-1 = Kidney Injury Molecule 1; CTGF = Connective tissue growth factor; TGF-β = Transforming growth factor beta; TNF-α = Tumor necrosis factor alpha.
In animal models of DMT1, the renal TNF-β level (renal interstitial fluid and urinary TNF-α) showed an early rise after the induction of diabetes [19], which preceded the rise in urinary albumin excretion by about 2 weeks suggesting a possible contribution of TNF-α in the complicated pathogenic process resulting in microalbuminuria in diabetes. Further studies are necessary to assess value of urinary CTGF, TGF-β and TNF-α as markers of DN progression.
Podocyte loss, effacement, and alterations of the podocyte cytoskeleton and structural proteins play a pivotal role in the pathogenesis of DN. Podocytes have been shown in the urine of diabetic patients with microalbuminuria (53%) and with macroalbuminuria (80%) using immunofluorescence microscopy [20]. The number of podocytes in the urine of patients with macroalbuminuria was significantly greater than in patients with microalbuminuria (p < 0.01). Preliminary studies in animal models of diabetes show that flow cytometry is a feasible and less expensive method for assessing urinary podocytes (Awad AS, unpublished data). Whether the measurement of urinary podocytes may serve as a surrogate marker not only for the progression of DN, but also for the efficacy of potential therapies, is not clear at this point. Additional research is needed to explore this possibility.
More recently, Mima et al. demonstrated the critical role of Smad1 in the development of mesangial matrix expansion in the early phase of DN in Streptozotocin-induced diabetic rats [21]. Under diabetic conditions, Smad1 regulates the genetic expression of type IV collagen (Col4) which is a key component involved in mesangial expansion. They also showed a direct correlation between urinary Smad1 levels and the severity of mesangial expansion [22].
Additional promising biomarkers include neutrophil gelatinase-associated lipocalin (NGAL) and kidney injury molecule 1 (KIM-1). NGAL is a small, 25-kD protein that belongs to the lipocalin protein family and is produced in epithelial cells and neutrophils. NGAL is an established novel biomarker for early diagnosis of acute kidney injury (AKI) [23–27]. It has also been linked as an independent biomarker for predicting chronic kidney disease progression [28]. In a cohort of 56 patients with DMT2, serum and urinary NGAL levels were evaluated in 3 groups of varying degrees of proteinuria: normoalbuminuria, microalbuminuria and overt DN [28]. The results revealed that all groups had increased NGAL levels as compared to controls and both serum and urinary NGAL levels correlated with the severity of renal disease reaching highest levels in patients with overt DN. The presence of elevated NGAL levels even in normoalbuminuric patients, who had no signs of glomerular damage, raised the possibility that NGAL may be a useful, noninvasive tool for early detection of incipient DN.
Urinary KIM-1 has also been proposed to be a novel biomarker of AKI in humans [29,30]. KIM-1 is a transmembrane protein exclusively located in the proximal tubules of the kidney and is markedly upregulated in ischemic kidney damage. Nielsen et al. [31] studied both NGAL and KIM-1 in DMT1 patients with different levels of albuminuria (normo-, micro- and macroalbuminuria) compared to non-diabetic control subjects. They also evaluated the effect of ACE inhibition (lisinopril) on urinary NGAL excretion in patients with DN. The results of the study showed both urinary NGAL and KIM-1 to be elevated in all groups of diabetic patients compared to non-diabetics, reflecting possible utility of both NGAL and KIM-1 as independent biomarkers of early diabetic kidney disease. They also found a reduction, albeit not statistically significant, of urinary NGAL with ACE inhibition. However, these biomarkers did not provide additional prognostic information to that of known traditional markers in predicting the decline of kidney function in diabetic patients who have already developed overt nephropathy [32].
Until more studies are available, periodic measurements of microalbuminuria and serum creatinine (for estimated GFR) still remain the standard of care for screening of DN in the diabetic population.
4. Potential Future Therapeutic Agents for Diabetic Nephropathy
Currently available measures to control DN are mostly preventative. Recently, several emerging as well as potential therapies for future have been proposed for treating DN based on both animal and human studies (Table 2). Emerging therapeutic agents include thiazolidinediones/PPAR-gamma agonists, angiotensin converting enzyme-2 (ACE-2), endothelin receptor blockers, advanced glycation endproduct (AGE) inhibitors, and selective vitamin D activation which have been suggested to have a protective role in DN by causing a reduction or even reversal of proteinuria (for review please see: [7]).
Table 2.
Therapeutic modalities in diabetic nephropathy.
| Current therapy | Emerging therapy | Potential therapy for future |
|---|---|---|
| 1. Intensive glycemic control. | 1. Thiazolidinediones/PPAR-gamma agonists. | 1. Islet cell transplant. |
| - Pharmacologic measures. | 2. ACE-2. | 2. Aldose reductase inhibitors. |
| - Pancreas transplant. | 3. Endothelin receptor blockers. | 3. Sulodexide (GAG). |
| 2. Blood pressure control. | 4. AGE inhibitors. | 4. Protein kinase C (PKC) inhibitors. |
| - Drugs affecting RAAS: | 5. Vitamin D activation. | 5. Connective tissue growth factor (CTGF) inhibitors. |
| . ACE inhibitor | 6. Transforming growth factor beta (TGF-B) inhibitors. | |
| . ARB | 7. Bardoxolone | |
| . Direct renin inhibitor | ||
| . Aldosterone antagonist | ||
| - Drugs not affecting RAAS: | ||
| . NDHP CCB | ||
| . B-blocker | ||
| . Diuretics | ||
| 3. Lipid lowering agents. | ||
| 4. Lifestyle modification. |
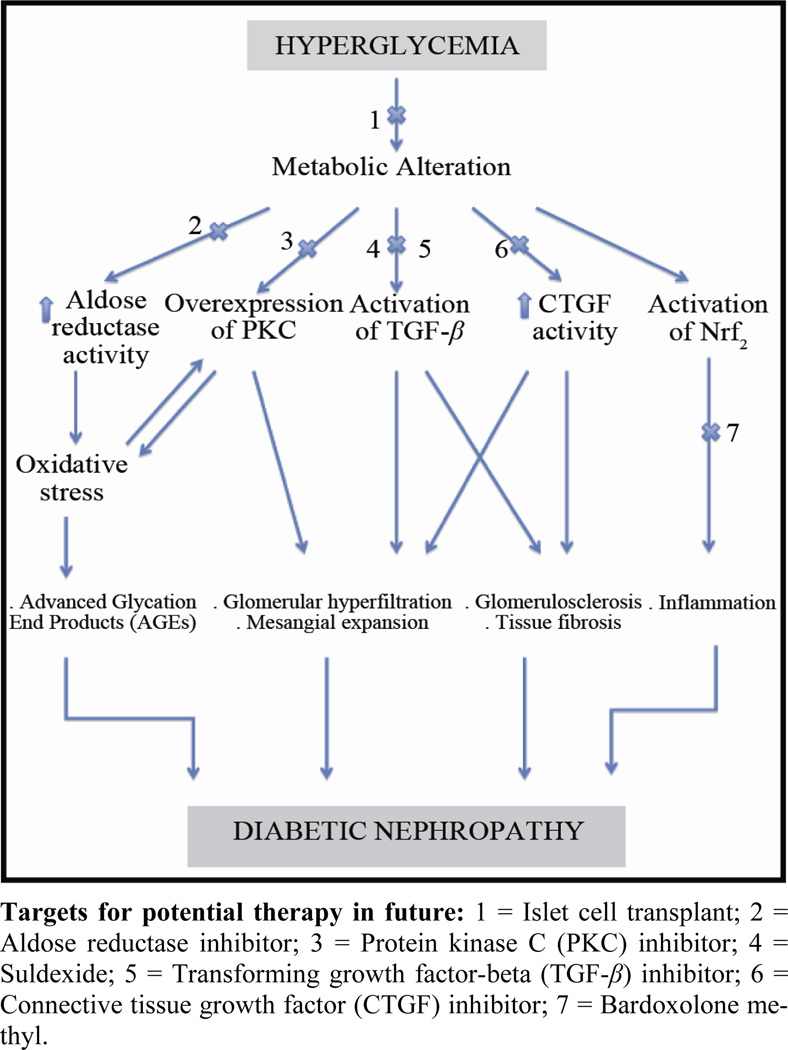
In this article, we will review some of the potential future therapeutic agents for treating DN (Figure 1) as follows.
Figure 1.
Pathogenesis of Diabetic Nephropathy (DN) and Potential Future Therapeutic Measures for Treatment and/or Reversal of DN.
4.1. Islet Cell Transplant
Several studies have shown that islet transplantation is associated with improved diabetic control with a possibility of protection against diabetic complications. Warnok et al. [33] performed a prospective crossover, cohort study of 42 patients with DM for more than 5 years and with established diabetic complications such as retinopathy and mild nephropathy. All patients were initially enrolled in group I and treated with intensive medical therapy. Thirty one patients from group I subsequently received islet cell transplants and crossed over to group II. After almost 3 years of follow up, group II patients showed better glycemic control (HBA1c 7.5% (I) vs. 6.6% (II); p < 0.01) and less progression on retinopathy. However, both groups showed similar declines in kidney function suggesting no additional benefit of islet cell transplantation in preserving GFR (eGFR = −0.45 ml/min (I) vs. −0.12 ml/min (II); p = 0.1). While islet cells are typically obtained from a deceased organ donor, another technique involving transplant of autologous islets has been developed. The basic technique requires total pancreatectomy,fragmentation of the pancreas followed by collagenase digestion and then differential centrifugation. The isolated islets are then re-implanted in the patient’s liver via the portal vein [34]. Webb et al. [34] studied 46 patients who received auto islet transplantation. After 10 years of follow up, the median serum creatinine increased very little from 0.8 mg/dl to 0.87 mg/dl, suggesting a role for auto islet cell transplantation in possible protection against diabetic complications.
4.2. Aldose Reductase Inhibitors
Aldose reductase catalyzes the first and rate-limiting step of the polyol pathway of glucose metabolism [35]. Activation of the polyol pathway is implicated in diabetes induced renal dysfunction via de novo synthesis of diacylglycerol (DAG), activation of protein kinase C (PKC) with increased production of TGF-β, extracellular matrix proteins and prostaglandins. Increased aldose reductase activity also results in depletion of NADPH, a decrease in cellular levels of reduced glutathione, and increased oxidative stress. The complex interaction between hyperglycemia-induced oxidative stress from aldose reductase activation, increased formation of advanced glycation endproducts (AGEs) and activation of vascular PKC isoforms ultimately result in microvascular diabetic complications. Increased aldose reductase expression has been shown in DMT2 patients [36]. A number of studies have shown a decrease in urinary albumin excretion in animals treated with aldose reductase inhibitors [37–39]. For instance, the aldose reductase inhibitor, sorbinil, was found to reduce albuminuria and glomerular basement membrane thickening in STZ diabetic rats treated for five months [38]. These actions were attributed to a reduction in the renal cortical activity of glucosyl-galactosyl-hydroxylysyl-glucohydrolase, an enzyme involved in the catabolism of collagen disaccharide units [39].
Small clinical trials have assessed the efficacy of aldose reductase inhibitors in the treatment of DN in both DMT1 [40] and DMT2 [41]. Both studies showed reduced urinary albumin excretion rate after aldose reductase inhibitor treatment for 6 months [40] or 5 years [41]. In contrast to these results, McAuliffe et al. reported that aldose reductase inhibitors had no effect on proteinuria in 16 diabetic subjects treated for 12 months [42]. Drugs which block aldose reductase activity include spirohydantoins (sorbinil), carboxylic acid derivatives (tolrestat, epalrestat, ponalrestat) and flavonoids. Sorbinil and tolrestat have been withdrawn from the worldwide market because of severe toxicity (hepatotoxic). Taken together, these studies of aldose reductase inhibitors have not shown convincing evidence of benefit in the treatment of DN.
4.3. Sulodexide (GAG)
Sulodexide is an oral formulation of a highly purified mixture of glycosaminoglycans. It is composed of 80% fast-moving heparin sulfate and 20% dermatan sulfate and is the most extensively studied glycosaminoglycan for diabetic patients. It bears strong chemical similarity to heparin but does not have anticoagulation properties when given orally [43]. Sulodexide has emerged as a potential treatment of DN as multiple studies have demonstrated reductions in urinary albumin excretion with glycosaminoglycan therapy [44–47].
The precise physiology of the sulodexide-mediated renoprotection in DN is not clear, but several mechanisms have been proposed. Sulodexide has been shown to block heparinase-1 activity [48,49], an enzyme that is upregulated in hyperglycemia and can degrade heparin sulfate molecules of the glomerular basement membrane. As sulodexide is a mixture of glycosaminoglycans, it may help in restoring the glycoproteins present in the GBM and mesangium. Another mechanism involves restoring the anionic heparin sulfate charge on the GBM. Finally, sulodexide may suppress high-glucose induced overexpression of TGF-β1 that is responsible for enhanced expression of mesangial matrix and collagens [50]. In a study of the db/db mouse model of diabetes, sulodexide was shown to reduce proteinuria significantly in early stage kidney disease but not late kidney disease (12 weeks and after) [51].
The efficacy of sulodexide in diabetes was also evaluated in the DiNAS study [52]. DiNAS was a randomized, double blind and placebo controlled trial involving 223 patients with DMT1 or DMT2 and microalbuminuria or macroalbuminuria. Patients were randomized to receive sulodexide (50 to 200 mg daily) or placebo for 4 months. After 4 months of therapy, albuminuria decreased by as much as 74% compared with the placebo group. Four months after drug discontinuation, albuminuria remained 69% lower in those randomized to 200 mg of sulodexide compared with the placebo group. This sustained response suggests that some anatomical or structural changes had occurred with sulodexide treatment. Sulodexide was well tolerated in that study. Another study showed a significant reduction in albuminuria with long term use of oral sulodexide at a moderate dose in patients with DN [53]. In this study, thirty patients (both DMT1 and DMT2) treated with 50 mg per day of oral sulodexide for 12 months were compared with thirty matched diabetic patients in the control group. The degree of albuminuria was greatly reduced in patients treated with sulodexide at the end of 12 months but was increased in the control group (−260% and +29% respectively; p = 0.0001).
Another recent study included 149 patients with DMT2 and microalbuminuria [54] who were randomized to receive 200 or 400 mg of sulodexide versus placebo. The primary endpoint at 6 months was a 50% reduction in albuminuria or return to normoalbuminuria. This was achieved in 33.3% of the sulodexide 200 mg group and 18.4% of the sulodexide 400 mg group as compared to 15.4% of the placebo group (p = 0.075 and 0.781 respectively) [54]. Based on the experience gained from these smaller studies, two large multicenter double-blinded, randomized placebo controlled trials were designed to establish the renoprotective potential of sulodexide. The results, unfortunately, were disappointing. The first study was the Sulodexide Microalbuminuria (SUN-micro) Trial, which examined the efficacy of sulodexide given over 26 weeks in 1000 patients with DMT2, hypertension and microalbuminuria [55]. The second study was the Sulodexide Overt Nephropathy (SUN-macro) Trial which aimed to examine the efficacy of sulodexide in 2240 patients with DMT2, hypertension and proteinuria ≥ 900 mg/24 h [55]. Both SUN-micro and SUN-macro trials used Sulodexide 200 mg daily vs. placebo in patients being treated with maximum approved or tolerated dose of ACE inhibitor or ARB in both arms. The primary outcome of the SUN-micro Trial was the conversion to normoalbuminuria and at least a 25% decrease in the urinary albumin creatinine ratio (UACR) or at least a 50% reduction in UACR. The primary outcome of the SUN-macro Trial was time to a composite end point of doubling of serum creatinine or ESRD. However, the SUN-micro trial failed to show a reduction of albuminuria in DN. With the failure of the SUN-micro trial, the SUN-macro trial was cancelled.
4.4. Protein Kinase C (PKC) Inhibitors
Activation of PKC is one of the key metabolic pathways involved in the pathogenesis of the DN. PKC is a family of at least 12 serine-threonine protein kinases that play an important role in intracellular signal transduction [56]. Hyperglycemia-induced oxidative stress has been strongly implicated in microvascular complications from diabetes. High ambient blood glucose levels increase diacylglycerol levels, advanced glycation end products, and enhance mitochondrial synthesis of reactive oxygen species, thereby activating protein kinase C (PKC), particularly in organs that are susceptible to developing diabetic micro-and macro-vascular complications [57]. Activated PKC causes kidney damage through a number of mechanisms including NADPH oxidase-dependent generation of oxidants, signaling TGF-β to induce extracellular matrix production and increased secretion of vasodilatory prostanoids which contribute to glomerular hyperfiltration [58,59]. Ruboxistaurin mesylate (RTX) (previously known as LY333531) is a bisindolylmaleimide with a high degree of specificity for inhibiting PKC-β1 and –β2 isoforms [60] which has been studied in animal models of DM [61–63].
Ruboxistaurin was shown to have several positive impacts on the pathogenesis of DN. It was able to normalize glomerular hyperfiltration, reduce extracellular matrix protein production and TGF-β1, reduce mesangial expansion, glomerulosclerosis and tubulointerstitial fibrosis and decrease albuminuria.
A randomized, double blind, placebo-controlled, multicenter, pilot study was conducted to evaluate the effect of LY333531 in type 2 DN patients [64]. In this study, 123 patients with DN and macroalbuminuria were randomized to either 32 mg daily of RTX or placebo for 1 year [64]. Patients in both arms were continued on ACEIs or ARBs during the trial. The primary endpoint was a reduction in ACR. After one year, active treatment was associated with a reduction in albuminuria and stabilization of GFR whereas the placebo group experienced no change in albuminuria and worsening of GFR. The reduction in albuminuria appeared as early as 1 month following treatment initiation. Although the study showed beneficial effects of LY333531 in DN (reduction in albuminuria and prevention in loss of eGFR), this study had some limitations. It was underpowered to detect any significant differences in albumin-creatinine ratio and eGFR. Another limitation of this study was its short duration of follow-up that limited conclusions about safety of RTX. Unfortunately, the PKC diabetic retinopathy study 2 (PKC-DRS 2) seemed to show an increased frequency of the adverse event of “diabetic nephropathy” in ruboxistaurin-treated patients compared with placebo-treated patients [65]. Furthermore, Tuttle et al. analyzed results from studies investigating the effects of ruboxistaurin on renal outcomes and found that the rate of kidney outcomes was similar in ruboxistaurin-treated patients and individuals receiving placebo [66].
4.5. Connective Tissue Growth Factor (CTGF) Inhibitors
CTGF is a recently identified potent profibrotic peptide that has been shown to play a role in the pathogenesis of kidney diseases, micro- and macrovascular complications of diabetes [67,68]. Several agents regulate CTGF expression such as TGF-β, high glucose and fibroblast growth factor [69].
CTGF stimulates cell adhesion and migration, production and deposition of extracellular matrix (ECM) proteins, and angiogenesis [70,71]. Zhou et al. showed that AGE-induced CTGF expression plays a critical role in renal ECM accumulation leading to DN [72]. CTGF has been implicated in promoting tissue fibrosis in DN by activating several intracellular signaling molecules in human mesangial cells (HMC) including receptor tyrosine kinases (TrkA) and induction of transcription factor TGF-B-inducible early gene [73]. Multiple in vitro and animal studies have demonstrated that inhibition of CTGF prevents the production of key proteins that compose scar [74] and prevents development of renal fibrosis [75,76]. Several animal and human studies have been undertaken to evaluate the role of CTGF inhibition using a monoclonal antibody that targets CTGF (FG-3019). Flyvbjerg and colleagues investigated the effects of FG-3019 in obese mice with DMT2 [77]. FG-3019 reduced urinary albumin excretion, GBM thickening and normalized hyperfiltration in these mice. A similar study in rats showed that FG-3019 reduced diabetic proteinuria [78].
Likewise, encouraging results were noted in human studies examining FG-3019 as a therapeutic agent in patients with DN. Adler and colleagues studied 24 micro-albuminuric subjects (21% with DMT1 and 79% with DMT2) who received 3 or 10 mg/kg FG-3019 (total 4 doses 2 weeks apart) with one year follow up. The results showed that FG-3019 was associated with a significant reduction of urinary albumin/creatinine ratio (mean pretreatment value of 48 mg/g to a mean post-treatment value of 20 mg/g, p = 0.027) without evidence for a dose-response relationship [79]. Similarly, Schwartz and colleagues showed that FG-3019 reduced microalbuminuria in patients with diabetes [80]. These preliminary results suggest that CTGF does play a role in the pathogenesis of DN, a role that needs further clarification. Inhibition of CTGF is promising as a therapeutic target for patients with DN.
4.6. Transforming Growth Factor-Beta (TGF-β) Inhibitors
TGF-β1 is a powerful cytokine that plays several roles in the kidney; including cell proliferation, migration, differentiation, immunomodulation and ECM turnover regulation [81]. The role of TGF-β in diabetic nephropathy has been examined in both animal and human studies. Langham et al. [18] extracted RNA from 12 human renal biopsies taken from participants in the Diabiopsies study, a randomized controlled 2-year trial that reported a reduction in proteinuria and cortical matrix expansion in DMT2 patients treated with perindopril (an ACE inhibitor) vs. placebo. The study showed a substantial diminution in TGF-β mRNA gene expression (mean 83% reduction, p < 0.05) in patients who had reduced proteinuria, reflecting a potential role of TGF-β inhibition in the treatment of DN. Therapeutic strategies were developed to block the production/activity of the renal TGF-β1 system to limit DN. Among these strategies are indirect approaches to decrease the TGF-β effects using renin-angiotensin inhibition, tight glycemic control, statin therapy and/or tight blood pressure control. Other direct approaches decrease TGF-β effect using neutralizing anti-TGF-β antibodies and antisense or using novel antifibrotic agents such as Pirfenidone [82].
Several animal studies have been performed to evaluate the therapeutic role of TGF-β blockers in DN. Sharma and colleagues administered TGF-β neutralizing antibodies to diabetic rats and showed that TGF-β antibodies prevented glomerular enlargement and suppressed the expression of genes encoding ECM components [83]. Ziyadeh and colleagues further showed that administration of anti-TGF-β antibody could attenuate progressive diabetic kidney disease in diabetic mice by preventing pathological changes of glomerulosclerosis [84]. Pirfenidone (PFD; 5-methyl-1-phenyl-2-(1H)-pyridone), a novel antifibrotic agent, is a low molecular weight synthetic molecule that inhibits TGF-β production and exerts antifibrotic properties in cell culture and various animal models of fibrosis [85,86]. Recently RamachandraRao and colleagues evaluated the therapeutic efficacy of PFD in db/db diabetic mice [87]. DN developed in the db/db mice as evidenced by albuminuria and mesangial matrix expansion by 12 to 16 wk of age. PFD was then given to the db/db mice from week 17 to week 21. Four weeks of PFD treatment led to a significant reduction in the degree of mesangial matrix expansion. They concluded that PFD can promote resolution of mesangial matrix when administered after the onset of nephropathy. They also showed that PFD did not worsen renal blood flow, lower BP, affect glycemic parameters, or cause hyperkalemia [87].
Several clinical studies have confirmed that TGF-β is increased in the kidneys of diabetic patients. Glomerular expression of TGF-β is also increased in early [85,88] and late stages [86,89] of DMT1 and DMT2 and correlates with the degree of glycemic control in these patients [88]. These data were the grounds for a recent clinical trial to evaluate the role of TGF-β inhibitors on the course of DN. A double blind, placebo-controlled study with 77 subjects of DN were randomized to treatment with placebo, low dose (1200 mg daily) or high dose (2400 mg daily) PFD for one year [90]. Treatment with low dose, but not high dose of PFD resulted in an improvement in GFR with no change in albuminuria. The major side effects were gastrointestinal symptoms and fatigue. The results of the study suggested that PFD could be a potential promising therapeutic agent in patients with DN. Additional studies in larger numbers of patients, ideally with histologic assessment of the kidneys, appear to be warranted to support this benefit.
4.7. Anti-Inflammatory Agents—Bardoxolone
Recently, an orally available synthetic triterpenoid, Bardoxolone methyl, has shown promising results in DN. Bardoxolone methyl exerts potent anti-oxidant and anti-inflammatory activity via induction of the Nrf2 transcription factor. A Phase 2 trial of bardoloxone methyl treatment for 8 weeks in 20 patients with moderate-severe CKD and DMT2 demonstrated improved renal function as evidenced by increased eGFR paralleled by a significant reduction in serum creatinine and BUN [91]. A subsequent trial examined the effect of bardoloxone methyl (25 – 150 mg/d) administered for 52 weeks to 227 patients with moderate to severe CKD and DMT2 [92]. Bardoloxone methyl produced a significant increase in GFR of 8 – 11 ml/min/1.73 m2. The improvement in GFR was evident by 8 – 12 weeks of treatment and persisted for the entire 52 week treatment period. Likewise, bardoloxone treatment reduced the proportion of patients who experienced a 25% fall in GFR from 13% in the placebo group to only 2% in treatment group. Although hard outcomes, such as dialysis dependency and death, were not evaluated, these results are very encouraging and justify further study of bardoxolone methyl and related compounds.
5. Summary and Conclusions
There is clear evidence that optimal glycemic control [93–99] and blood pressure control [100–109] are of paramount importance in preventing progression of DN. The renoprotective benefits of agents that block reninangiotensin aldosterone system (RAAS) in preventing progression of DN is well-established [110–121]. Non-dihydropyrdine CCBs (diltiazem, verapamil), that do not affect RAAS, have also been shown to reduce proteinuria and slow progression of kidney disease in diabetics [122–127]. Similarly, lipid lowering agents (such as statins) have been shown to slow the rate of progression of DN [128], but the data supporting this are scant. Even so, it is important to understand that currently available strategies are geared towards limiting or slowing the rate of progression of DN to ESRD. These modalities do not actually stop the progression of DN. In this modern era of medical advancement, we are in dire need of novel strategies that can halt or even reverse the disease progression.
While pancreas transplantation [129] is an effective approach in preventing DN in patients with DMT1, islet cell transplantation appears to be a reasonable alternative to improve glycemic control but has questionable benefit in terms of preserving GFR in patients with established DN. Aldose-reductase is a rate-limiting enzyme in glucose metabolism pathway, and its activation leads to increased oxidative stress, formation of AGE products and activation of protein kinase C, ultimately resulting in micro-vascular complications such as DN. Although aldose-reductase inhibitors have been shown to reduce albuminuria and prevent glomerular basement membrane thickening in animal studies, their efficacy in humans has been inconclusive. Sulodexide, an oral formulation of a purified mixture of glycosaminoglycans reduced proteinuria by uncertain mechanisms. It produced sustained reductions in albuminuria in both animal and human studies even after discontinuation of the drug, suggesting the possibility for anatomical or structural changes with long term protection against DN. Unfortunately, larger multi-center studies (SUN-micro and SUN-macro trials) failed to confirm these benefits. Protein kinase C inhibitors (Ruboxistaurin) have shown promising results with respect to improving glomerular hyperfiltration, reducing mesangial expansion and reduction of albuminuria in diabetic animal models as well as humans. They have been associated with a reduction in albuminuria and prevention of loss of GFR in a few randomized pilot studies, although large scale prospective studies have yet to confirm their beneficial effects on renal outcomes. CTGF is a potent profibrotic peptide that has been implicated in extracellular matrix deposition and promotion of tissue fibrosis. Therefore, CTGF inhibition is a promising therapeutic target in patients with DN. FG-3019, a CTGF-inhibitor, has been shown to reduce glomerular basement membrane thickening and normalize hyperfiltration diabetic mice and reduce albuminuria in humans. Similarly, pirfenidone is a TGF-β inhibitor that is recognized as a novel anti-fibrotic agent. In humans, at a low treatment dose, it was shown to improve GFR without improvement in albuminuria. Similarly, the use of bardoxalone is encouraging and justifies its further study.
In summary, these data indicate that there are several promising therapeutic targets that could potentially be utilized to treat patients with established DN. While currently available measures to control DN are largely preventative, there is hope that therapies capable of stopping, or even reversing progression of DN will soon become available. The current cost of ESRD care exceeds $10 billion per year, much of which is devoted to care of DN. In addition, overt proteinuria in DN is a known independent risk factor for cardiovascular events, including death. Therefore, the potential ability to halt or reverse DN with these candidate markers in future may offer significant benefits in terms of minimizing cardiovascular morbidity and mortality and also reducing tremendous health-care spending. However, additional human studies are warranted to prove the effectiveness of these agents in treating DN.
Acknowledgements
This work was supported by NIH Grant DK077444.
Footnotes
Conflict of interest: None.
REFERENCES
- 1.United States Renal Data System (USRDS) Annual Data Report. Bethesda: 2005. The National Institutes of Diabetes and Digestive and Kidney Diseases. [Google Scholar]
- 2.Eknoyan G, Hostetter T, Bakris GL, Hebert L, Levey AS, Parving HH, Steffes MW, Toto R. Proteinuria and Other Markers of Chronic Kidney Disease: A Position Statement of the National Kidney Foundation (NKF) and the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) Journal of Kidney Diseases. 2003;Vol. 42(No. 4):617–622. doi: 10.1016/s0272-6386(03)00826-6. [DOI] [PubMed] [Google Scholar]
- 3.Mogensen CE. Microalbuminuria as a predictor of clinical diabetic nephropathy. Kidney International. 1987;Vol. 31:673–689. doi: 10.1038/ki.1987.50. [DOI] [PubMed] [Google Scholar]
- 4.Krolewski AS, Warram JH, Rand LI, Kahn CR. Epidemiologic Approach to the Etiology of Type I Diabetes Mellitus and Its Complications. The New England Journal of Medicine. 1987;Vol. 317(No. 22):1390–1398. doi: 10.1056/NEJM198711263172206. [DOI] [PubMed] [Google Scholar]
- 5.Berhane AM, Weil EJ, Knowler WC, Nelson RG, Hanson RL. Albuminuria and Estimated Glomerular Filtration Rate as Predictors of Diabetic End-Stage Renal Disease and Death. Clinical Journal of the American Society of Nephrology. 2011;Vol. 6(No. 10):2444–2451. doi: 10.2215/CJN.00580111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gerstein HC, Mann JF, Yi Q, Zinman B, Dinneen SF, Hoogwerf B, Halle JP, Young J, Rashkow A, Joyce C, Nawaz S, Yusuf S. Albuminuria and Risk of Cardiovascular Events, Death, and Heart Failure in Diabetic and Nondiabetic Individuals. The Journal of the American Medical Association. 2001;Vol. 286(No. 4):421–426. doi: 10.1001/jama.286.4.421. [DOI] [PubMed] [Google Scholar]
- 7.Abdel-Rahman EM, Saadulla L, Reeves WB, Awad AS. Therapeutic Modalities in Diabetic Nephropathy: Standard and Emerging Approaches. Journal of General Internal Medicine. 2011;Vol. 27(No. 4):458–468. doi: 10.1007/s11606-011-1912-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cockcroft DW, Gault MH. Prediction of Creatinine Clearance from Serum Creatinine. Nephron. 1976;Vol. 16(No. 1):31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 9.Levey AS, Coresh J, Balk E, Kausz AT, Levin A, Steffes MW, Hogg RJ, Perrone RD, Lau J, Eknoyan G. National Kidney Foundation Practice Guidelines for Chronic Kidney Disease: Evaluation, Classification, and Stratification. Annals of Internal Medicine. 2003;Vol. 139(No. 2):137–147. doi: 10.7326/0003-4819-139-2-200307150-00013. [DOI] [PubMed] [Google Scholar]
- 10.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J. A New Equation to Estimate Glomerular Filtration Rate. Annals of Internal Medicine. 2009;Vol. 150(No. 9):604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Perkins BA, Nelson RG, Ostrander BE, Blouch KL, Krolewski AS, Myers BD, Warram JH. Detection of Renal Function Decline in Patients with Diabetes and Normal or Elevated GFR by Serial Measurements of Serum Cystatin C Concentration: Results of a 4-Year Follow-up Study. Journal of the American Society of Nephrology. 2005;Vol. 16(No. 5):1404–1412. doi: 10.1681/ASN.2004100854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Macisaac RJ, Tsalamandris C, Thomas MC, Premaratne E, Panagiotopoulos S, Smith TJ, Poon A, Jenkins MA, Ratnaike SI, Power DA, Jerums G. The Accuracy of Cystatin C and Commonly Used Creatinine-Based Methods for Detecting Moderate and Mild Chronic Kidney Disease in Diabetes. Diabetic Medicine. 2007;Vol. 24(No. 4):443–448. doi: 10.1111/j.1464-5491.2007.02112.x. [DOI] [PubMed] [Google Scholar]
- 13.Tabaei BP, Al-Kassab AS, Ilag LL, Zawacki CM, Herman WH. Does Microalbuminuria Predict Diabetic Nephropathy? Diabetes Care. 2001;Vol. 24(No. 9):1560–1566. doi: 10.2337/diacare.24.9.1560. [DOI] [PubMed] [Google Scholar]
- 14.Perkins BA, Ficociello LH, Ostrander BE, Silva KH, Weinberg J, Warram JH, Krolewski AS. Microalbuminuria and the Risk for Early Progressive Renal Function Decline in Type 1 Diabetes. Journal of the American Society of Nephrology. 2007;Vol. 18(No. 4):1353–1361. doi: 10.1681/ASN.2006080872. [DOI] [PubMed] [Google Scholar]
- 15.Ito Y, Aten J, Bende RJ, Oemar BS, Rabelink TJ, Weening JJ, Goldschmeding R. Expression of Connective Tissue Growth Factor in Human Renal Fibrosis. Kidney International. 1998;Vol. 53:853–861. doi: 10.1111/j.1523-1755.1998.00820.x. [DOI] [PubMed] [Google Scholar]
- 16.Nguyen TQ, Tarnow L, Andersen S, Hovind P, Parving HH, Goldschmeding R, van Nieuwenhoven FA. Urinary Connective Tissue Growth Factor Excretion Correlates with Clinical Markers of Renal Disease in a Large Population of Type 1 Diabetic Patients with Diabetic Nephropathy. Diabetes Care. 2006;Vol. 29(No. 1):83–88. doi: 10.2337/diacare.29.1.83. [DOI] [PubMed] [Google Scholar]
- 17.Jaffa AA, Usinger WR, McHenry MB, Jaffa MA, Lipstiz SR, Lackland D, Lopes-Virella M, Luttrell LM, Wilson PW. Connective Tissue Growth Factor and Susceptibility to Renal and Vascular Disease Risk in Type 1 Diabetes. The Journal of Clinical Endocrinology & Metabolism. 2008;Vol. 93(No. 5):1893–1900. doi: 10.1210/jc.2007-2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Langham RG, Kelly DJ, Gow RM, Zhang Y, Cordonnier DJ, Pinel N, Zaoui P, Gilbert RE. Transforming Growth Factor-β in Human Diabetic Nephropathy: Effects of ACE Inhibition. Diabetes Care. 2006;Vol. 29(No. 12):2670–2675. doi: 10.2337/dc06-0911. [DOI] [PubMed] [Google Scholar]
- 19.Kalantarinia K, Awad AS, Siragy HM. Urinary and Renal Interstitial Concentrations of TNF-α Increase Prior to the Rise in Albuminuria in Diabetic Rats. Kidney International. 2003;Vol. 64:1208–1213. doi: 10.1046/j.1523-1755.2003.00237.x. [DOI] [PubMed] [Google Scholar]
- 20.Nakamura T, Ushiyama C, Suzuki S, Hara M, Shimada I. Ebihara N, Koide H. Urinary Excretion of Podocytes in Patients with Diabetic Nephropathy. Nephrology Dialysis Transplantation. 2000;Vol. 15(No. 9):1379–1383. doi: 10.1093/ndt/15.9.1379. [DOI] [PubMed] [Google Scholar]
- 21.Matsubara T, Abe H, Arai H, Nagai K, Mima A, Kanamori H, Sumi E, Takahashi T, Matsuura M, Iehara N, Fukatsu A, Kita T, Doi T. Expression of Smad1 is Directly Associated with Mesangial Matrix Expansion in Rat Diabetic Nephropathy. Laboratory Investigation. 2006;Vol. 86:357–368. doi: 10.1038/labinvest.3700400. [DOI] [PubMed] [Google Scholar]
- 22.Mima A, Arai H, Matsubara T, Abe H, Nagai K, Tamura Y, Torikoshi K, Araki M, Kanamori H, Takahashi T, Tominaga T, Matsuura M, Iehara N, Fukatsu A, Kita T, Doi T. Urinary Smad1 Is a Novel Marker to Predict Later Onset of Mesangial Matrix Expansion in Diabetic Nephropathy. Diabetes. 2008;Vol. 57(No. 6):1712–1722. doi: 10.2337/db07-1726. [DOI] [PubMed] [Google Scholar]
- 23.Mishra J, Dent C, Tarabishi R, Mitsnefes MM, Ma Q, Kelly C, Ruff SM, Zahedi K, Shao M, Bean J, Mori K, Barasch J, Devarajan P. Neutrophil Gelatinase-Associated Lipocalin (NGAL) as a Biomarker for Acute Renal Injury after Cardiac Surgery. The Lancet. 2005;Vol. 365(No. 9466):1231–1238. doi: 10.1016/S0140-6736(05)74811-X. [DOI] [PubMed] [Google Scholar]
- 24.Parikh CR, Mishra J, Thiessen-Philbrook H, Dursun B, Ma Q, Kelly C, Dent C, Devarajan P, Edelstein CL. Urinary IL-18 Is an Early Predictive Biomarker of Acute Kidney Injury after Cardiac Surgery. Kidney International. 2006;Vol. 70:199–203. doi: 10.1038/sj.ki.5001527. [DOI] [PubMed] [Google Scholar]
- 25.Bennett M, Dent CL, Ma Q, Dastrala S, Grenier F, Workman R, Syed H, Ali S, Barasch J, Devarajan P. Urine NGAL Predicts Severity of Acute Kidney Injury after Cardiac Surgery: A Prospective Study. Clinical Journal of the American Society of Nephrology. 2008;Vol. 3(No. 3):665–673. doi: 10.2215/CJN.04010907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ling W, Zhaohui N, Ben H, Leyi G, Jianping L, Huili D, Jiaqi Q. Urinary IL-18 and NGAL as Early Predictive Biomarkers in Contrast-Induced Nephropathy after Coronary Angiography. Nephron Clinical Practice. 2008;Vol. 108(No. 3):176–181. doi: 10.1159/000117814. [DOI] [PubMed] [Google Scholar]
- 27.Nickolas TL, O’Rourke MJ, Yang J, Sise ME, Canetta PA, Barasch N, Buchen C, Khan F, Mori K, Giglio J, Devarajan P, Barasch J. Sensitivity and Specificity of a Single Emergency Department Measurement of Urinary Neutrophil Gelatinase-Associated Lipocalin for Diagnosing Acute Kidney Injury. Annals of Internal Medicine. 2008;Vol. 148(No. 11):810–819. doi: 10.7326/0003-4819-148-11-200806030-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bolignano D, Lacquaniti A, Coppolino G, Donato V, Campo S, Fazio MR, Nicocia G, Buemi M. Neutrophil Gelatinase-Associated Lipocalin (NGAL) and Progression of Chronic Kidney Disease. Clinical Journal of the American Society of Nephrology. 2009;Vol. 4(No. 2):337–344. doi: 10.2215/CJN.03530708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Han WK, Bailly V, Abichandani R, Thadhani R, Bonventre JV. Kidney Injury Molecule-1 (KIM-1): A Novel Biomarker for Human Renal Proximal Tubule Injury. Kidney International. 2002;Vol. 62:237–244. doi: 10.1046/j.1523-1755.2002.00433.x. [DOI] [PubMed] [Google Scholar]
- 30.van Timmeren MM, van den Heuvel MC, Bailly V, Bakker SJ, van Goor H, Stegeman CA. Tubular Kidney Injury Molecule-1 (KIM-1) in Human Renal Disease. The Journal of Pathology. 2007;Vol. 212(No. 2):209–217. doi: 10.1002/path.2175. [DOI] [PubMed] [Google Scholar]
- 31.Nielsen SE, Schjoedt KJ, Astrup AS, Tarnow L, Lajer M, Hansen PR, Parving HH, Rossing P. Neutrophil Gelatinase-Associated Lipocalin (NGAL) and Kidney Injury Molecule 1 (KIM1) in Patients with Diabetic Nephropathy: A Cross-Sectional Study and the Effects of Lisinopril. Diabetic Medicine. 2010;Vol. 27(No. 10):1144–1150. doi: 10.1111/j.1464-5491.2010.03083.x. [DOI] [PubMed] [Google Scholar]
- 32.Nielsen SE, Andersen S, Zdunek D, Hess G, Parving HH, Rossing P. Tubular Markers Do Not Predict the Decline in Glomerular Filtration Rate in Type 1 Diabetic Patients with Overt Nephropathy. Kidney International. 2011;Vol. 79(No. 10):1113–1118. doi: 10.1038/ki.2010.554. [DOI] [PubMed] [Google Scholar]
- 33.Warnock GL, Thompson DM, Meloche RM, Shapiro RJ, Ao Z, Keown P, Johnson JD, Verchere CB, Partovi N, Begg IS, Fung M, Kozak SE, Tong SO, Alghofaili KM, Harris C. A Multi-Year Analysis of Islet Transplantation Compared with Intensive Medical Therapy on Progression of Complications in Type 1 Diabetes. Transplantation. 2008;Vol. 86(No. 12):1762–1766. doi: 10.1097/TP.0b013e318190b052. [DOI] [PubMed] [Google Scholar]
- 34.Webb MA, Illouz SC, Pollard CA, Gregory R, Mayberry JF, Tordoff SG, Bone M, Cordle CJ, Berry DP, Nicholson ML, Musto PP, Dennison AR. Islet Auto Transplantation Following Total Pancreatectomy: A Long-Term Assessment of Graft Function. Pancreas. 2008;Vol. 37(No. 3):282–287. doi: 10.1097/mpa.0b013e31816fd7b6. [DOI] [PubMed] [Google Scholar]
- 35.Vander Jagt DL, Robinson B, Taylor KK, Hunsaker LA. Aldose Reductase from Human Skeletal and Heart Muscle. Interconvertible Forms Related by Thiol-Disulfide Exchange. The Journal of Biological Chemistry. 1990;Vol. 265(No. 34):20982–20987. [PubMed] [Google Scholar]
- 36.Kasajima H, Yamagishi S, Sugai S, Yagihashi N, Yagihashi S. Enhanced in Situ Expression of Aldose Reductase in Peripheral Nerve and Renal Glomeruli in Diabetic Patients. Virchows Archiv. 2001;Vol. 439(No. 1):46–54. doi: 10.1007/s004280100444. [DOI] [PubMed] [Google Scholar]
- 37.Tilton RG, Chang K, Pugliese G, Eades DM, Province MA, Sherman WR, Kilo C, Williamson JR. Prevention of Hemodynamic and Vascular Albumin Filtration Changes in Diabetic Rats by Aldose Reductase Inhibitors. Diabetes. 1989;Vol. 38(No. 10):1258–1270. doi: 10.2337/diab.38.10.1258. [DOI] [PubMed] [Google Scholar]
- 38.Robison WG, Jr, Tillis TN, Laver N, Kinoshita JH. Diabetes-Related Histopathologies of the Rat Retina Prevented with an Aldose Reductase Inhibitor. Experimental Eye Research. 1990;Vol. 50(No. 4):355–366. doi: 10.1016/0014-4835(90)90136-i. [DOI] [PubMed] [Google Scholar]
- 39.Kassab JP, Guillot R, Andre J, Claperon N, Bellon G, Feldmann G, Peyroux J, Sternberg M. Renal and Microvascular Effects of an Aldose Reductase Inhibitor in Experimental Diabetes. Biochemical, Functional and Ultrastructural Studies. Biochemical Pharmacology. 1994;Vol. 48(No. 5):1003–1008. doi: 10.1016/0006-2952(94)90371-9. [DOI] [PubMed] [Google Scholar]
- 40.Passariello N, Sepe J, Marrazzo G, de Cicco A, Peluso A, Pisano MC, Sgambato S, Tesauro P, D’Onofrio F. Effect of Aldose Reductase Inhibitor (Tolrestat) on Urinary Albumin Excretion Rate and Glomerular Filtration Rate in Iddm Subjects with Nephropathy. Diabetes Care. 1993;Vol. 16(No. 5):789–795. doi: 10.2337/diacare.16.5.789. [DOI] [PubMed] [Google Scholar]
- 41.Iso K, Tada H, Kuboki K, Inokuchi T. Long-Term Effect of Epalrestat, an Aldose Reductase Inhibitor, on the Development of Incipient Diabetic Nephropathy in Type 2 Diabetic Patients. Journal of Diabetes and Its Complications. 2001;Vol. 15(No. 5):241–244. doi: 10.1016/s1056-8727(01)00160-x. [DOI] [PubMed] [Google Scholar]
- 42.McAuliffe AV, Brooks BA, Fisher EJ, Molyneaux LM, Yue DK. Administration of Ascorbic Acid and an Aldose Reductase Inhibitor (Tolrestat) in Diabetes: Effect on Urinary Albumin Excretion. Nephron. 1998;Vol. 80(No. 3):277–284. doi: 10.1159/000045187. [DOI] [PubMed] [Google Scholar]
- 43.Andriuoli G, Mastacchi R, Barbanti M. Antithrombotic Activity of a Glycosaminoglycan (Sulodexide) in Rats. Thrombosis Research. 1984;Vol. 34(No. 1):81–86. doi: 10.1016/0049-3848(84)90108-7. [DOI] [PubMed] [Google Scholar]
- 44.Solini A CA, Barzon I, Crepaldi G. Therapy with Glycosaminoglycans Lowers Albumin Excretion Rate in Non-Insulin Dependent Diabetic Patients with Microalbuminuria. Diabetes, Nutrition & Metabolism. 1994;Vol. 7:304–307. [Google Scholar]
- 45.Skrha J, Perusicova J, Pontuch P, Oksa A. Glycosaminoglycan Sulodexide Decreases Albuminuria in Diabetic Patients. Diabetes Research and Clinical Practice. 1997;Vol. 38(No. 1):25–31. doi: 10.1016/s0168-8227(97)00076-4. [DOI] [PubMed] [Google Scholar]
- 46.Velussi M, et al. Glycosaminoglycans Oral Therapy Reduces Microalbuminuria, Blood Fibrinogen Levels and Limb Arteriopathy Clinical Signs in Patients with Non-Insulin Dependent Diabetes Mellitus. Diabetes, Nutrition & Metabolism. 1996;Vol. 9:53–58. [Google Scholar]
- 47.Solini A, Vergnani L, Ricci F, Crepaldi G. Glycosaminoglycans Delay the Progression of Nephropathy in NIDDM. Diabetes Care. 1997;Vol. 20(No. 5):819–823. doi: 10.2337/diacare.20.5.819. [DOI] [PubMed] [Google Scholar]
- 48.Xu X, et al. Mechanism of Action of Sulodexide-Mediated Control of Diabetic Proteinuria: Inhibition of Heparanase-1 Activity. Journal of the American Society of Nephrology. 2005;Vol. 16:673. [Google Scholar]
- 49.Maxhimer JB, Somenek M, Rao G, Pesce CE, Baldwin D, Gattuso P, Schwartz MM, Lewis EJ, Prinz RA, Xu X. Heparanase-1 Gene Expression and Regulation by High Glucose in Renal Epithelial Cells: A Potential Role in the Pathogenesis of Proteinuria in Diabetic Patients. Diabetes. 2005;Vol. 54(No. 7):2172–2178. doi: 10.2337/diabetes.54.7.2172. [DOI] [PubMed] [Google Scholar]
- 50.Gambaro G, Cavazzana AO, Luzi P, Piccoli A, Borsatti A, Crepaldi G, Marchi E, Venturini AP, Baggio B. Glycosaminoglycans Prevent Morphological Renal Alterations and Albuminuria in Diabetic Rats. Kidney International. 1992;Vol. 42:285–291. doi: 10.1038/ki.1992.288. [DOI] [PubMed] [Google Scholar]
- 51.Rossini M, Naito T, Yang H, Freeman M, Donnert E, Ma LJ, Dunn SR, Sharma K, Fogo AB. Sulodexide Ameliorates Early But Not Late Kidney Disease in Models of Radiation Nephropathy and Diabetic Nephropathy. Nephrology Dialysis Transplantation. 2010;Vol. 25(No. 6):1803–1810. doi: 10.1093/ndt/gfp724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gambaro G, Kinalska I, Oksa A, Pontuch P, Hertlova M, Olsovsky J, Manitius J, Fedele D, Czekalski S, Perusicova J, Skrha J, Taton J, Grzeszczak W, Crepaldi G. Oral Sulodexide Reduces Albuminuria in Microalbuminuric and Macroalbuminuric Type 1 and Type 2 Diabetic Patients: The Di.N.A.S. Randomized Trial. Journal of the American Society of Nephrology. 2002;Vol. 13(No. 6):1615–1625. doi: 10.1097/01.asn.0000014254.87188.e5. [DOI] [PubMed] [Google Scholar]
- 53.Achour A, Kacem M, Dibej K, Skhiri H, Bouraoui S, El May M. One Year Course of Oral Sulodexide in the Management of Diabetic Nephropathy. Journal of Nephrology. 2005;Vol. 18(No. 5):568–574. [PubMed] [Google Scholar]
- 54.Heerspink HL, Greene T, Lewis JB, Raz I, Rohde RD, Hunsicker LG, Schwartz SL, Aronoff S, Katz MA, Eisner GM, Mersey JH, Wiegmann TB. Effects of Sulodexide in Patients with Type 2 Diabetes and Persistent Albuminuria. Nephrology Dialysis Transplantation. 2008;Vol. 23(No. 6):1946–1954. doi: 10.1093/ndt/gfm893. [DOI] [PubMed] [Google Scholar]
- 55.Lambers Heerspink HJ, et al. Rationale for and Study Design of the Sulodexide Trials in Type 2 Diabetic, Hypertensive Patients with Microalbuminuria or Overt Nephropathy. Diabetic Medicine. 2007;Vol. 24(No. 11):1290–1295. doi: 10.1111/j.1464-5491.2007.02249.x. [DOI] [PubMed] [Google Scholar]
- 56.Goekjian PG, Jirousek MR. Protein Kinase C in the Treatment of Disease: Signal Transduction Pathways, Inhibitors and Agents in Development. Current Medicinal Chemistry. 1999;Vol. 6(No. 9):877–903. [PubMed] [Google Scholar]
- 57.Tuttle KR. Protein Kinase C-β Inhibition for Diabetic Kidney Disease. Diabetes Research and Clinical Practice. 2008;Vol. 82(Suppl. 1):70–74. doi: 10.1016/j.diabres.2008.09.041. [DOI] [PubMed] [Google Scholar]
- 58.Yamagishi S, Fukami K, Ueda S, Okuda S. Molecular Mechanisms of Diabetic Nephropathy and Its Therapeutic Intervention. Current Drug Targets. 2007;Vol. 8(No. 8):952–959. doi: 10.2174/138945007781386884. [DOI] [PubMed] [Google Scholar]
- 59.Kunisaki M, Bursell SE, Umeda F, Nawata H, King GL. Normalization of Diacylglycerol-Protein Kinase C Activation by Vitamin E in Aorta of Diabetic Rats and Cultured Rat Smooth Muscle Cells Exposed to Elevated Glucose Levels. Diabetes. 1994;Vol. 43(No. 11):1372–1377. doi: 10.2337/diab.43.11.1372. [DOI] [PubMed] [Google Scholar]
- 60.Jirousek MR, Gillig JR, Gonzalez CM, Heath WF, McDonald JH, Neel DA, Rito CJ, Singh U, Stramm LE, Melikian-Badalian A, Baevsky M, Ballas LM, Hall SE, Winneroski LL, Faul MM. (S)13-[(Dimethylamino)methyl]-10,11,14,15-tetrahydro-4,9:16, 21-dimetheno-1H, 13H-dibenzo[e,k]pyrrolo[3,4-h][1,4,13] oxadiazacyclohexadecene-1,3(2H)-dione (LY333531) and Related Analogues: Isozyme Selective Inhibitors of Protein Kinase Cβ. Journal of Medicinal Chemistry. 1996;Vol. 39(No. 14):2664–2671. doi: 10.1021/jm950588y. [DOI] [PubMed] [Google Scholar]
- 61.Ishii H, Jirousek MR, Koya D, Takagi C, Xia P, Clermont A, Bursell SE, Kern TS, Ballas LM, Heath WF, Stramm LE, Feener EP, King GL. Amelioration of Vascular Dysfunctions in Diabetic Rats by an Oral PKC β Inhibitor. Science. 1996;Vol. 272(No. 5262):728–731. doi: 10.1126/science.272.5262.728. [DOI] [PubMed] [Google Scholar]
- 62.Koya D, Haneda M, Nakagawa H, Isshiki K, Sato H, Maeda S, Sugimoto T, Yasuda H, Kashiwagi A, Ways DK, King GL, Kikkawa R. Amelioration of Accelerated Diabetic Mesangial Expansion by Treatment with a PKC β Inhibitor in Diabetic db/db Mice, a Rodent Model for Type 2 Diabetes. The FASEB Journal. 2000;Vol. 14(No. 3):439–447. doi: 10.1096/fasebj.14.3.439. [DOI] [PubMed] [Google Scholar]
- 63.Kelly DJ, Zhang Y, Hepper C, Gow RM, Jaworski K, Kemp BE, Wilkinson-Berka JL, Gilbert RE. Protein Kinase Cβ Inhibition Attenuates the Progression of Experimental Diabetic Nephropathy in the Presence of Continued Hypertension. Diabetes. 2003;Vol. 52(No. 2):512–518. doi: 10.2337/diabetes.52.2.512. [DOI] [PubMed] [Google Scholar]
- 64.Tuttle KR, Bakris GL, Toto RD, McGill JB, Hu K, Anderson PW. The Effect of Ruboxistaurin on Nephropathy in Type 2 Diabetes. Diabetes Care. 2005;Vol. 28(No. 11):2686–2690. doi: 10.2337/diacare.28.11.2686. [DOI] [PubMed] [Google Scholar]
- 65.Aiello LP, Davis MD, Girach A, Kles KA, Milton RC, Sheetz MJ, Vignati L, Zhi XE. Effect of Ruboxistaurin on Visual Loss in Patients with Diabetic Retinopathy. Ophthalmology. 2006;Vol. 113(No. 12):2221–2230. doi: 10.1016/j.ophtha.2006.07.032. [DOI] [PubMed] [Google Scholar]
- 66.Tuttle KR, McGill JB, Haney DJ, Lin TE, Anderson PW. Kidney Outcomes in Long-Term Studies of Ruboxistaurin for Diabetic Eye Disease. Clinical Journal of the American Society of Nephrology. 2007;Vol. 2(No. 4):631–636. doi: 10.2215/CJN.00840207. [DOI] [PubMed] [Google Scholar]
- 67.van Nieuwenhoven FA, Jensen LJ, Flyvbjerg A, Goldschmeding R. Imbalance of Growth Factor Signalling in Diabetic Kidney Disease: Is Connective Tissue Growth Factor (CTGF, CCN2) the Perfect Intervention Point? Nephrology Dialysis Transplantation. 2005;Vol. 20(No. 1):6–10. doi: 10.1093/ndt/gfh570. [DOI] [PubMed] [Google Scholar]
- 68.Wahab NA, Yevdokimova N, Weston BS, Roberts T, Li XJ, Brinkman H, Mason RM. Role of Connective Tissue Growth Factor in the Pathogenesis of Diabetic Nephropathy. Biochemical Journal. 2001;Vol. 359, Pt. 1:77–87. doi: 10.1042/0264-6021:3590077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lau LF, Lam SC. The CCN Family of Angiogenic Regulators: The Integrin Connection. Experimental Cell Research. 1999;Vol. 248(No. 1):44–57. doi: 10.1006/excr.1999.4456. [DOI] [PubMed] [Google Scholar]
- 70.Riser BL, Denichilo M, Cortes P, Baker C, Grondin JM, Yee J, Narins RG. Regulation of Connective Tissue Growth Factor Activity in Cultured Rat Mesangial Cells and Its Expression in Experimental Diabetic Glomerulosclerosis. Journal of the American Society of Nephrology. 2000;Vol. 11(No. 1):25–38. doi: 10.1681/ASN.V11125. [DOI] [PubMed] [Google Scholar]
- 71.Twigg SM, Joly AH, Chen MM, Tsubaki J, Kim HS, Hwa V, Oh Y, Rosenfeld RG. Connective tissue Growth Factor/IGF-Binding Protein-Related Protein-2 Is a Mediator in the Induction of Fibronectin by Advanced Glycosylation End-Products in Human Dermal Fibroblasts. Endocrinology. 2002;Vol. 143(No. 4):1260–1269. doi: 10.1210/endo.143.4.8741. [DOI] [PubMed] [Google Scholar]
- 72.Zhou G, Li C, Cai L. Advanced Glycation End-Products Induce Connective Tissue Growth Factor-Mediated Renal Fibrosis Predominantly through Transforming Growth Factor β-Independent Pathway. American Journal of Pathology. 2004;Vol. 165(No. 6):2033–2043. doi: 10.1016/s0002-9440(10)63254-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wahab NA, Weston BS, Mason RM. Connective Tissue Growth Factor CCN2 Interacts with and Activates the Tyrosine Kinase Receptor TrkA. Journal of the American Society of Nephrology. 2005;Vol. 16(No. 2):340–351. doi: 10.1681/ASN.2003100905. [DOI] [PubMed] [Google Scholar]
- 74.Roestenberg P, van Nieuwenhoven FA, Joles JA, Trischberger C, Martens PP, Oliver N, Aten J, Hoppener JW, Goldschmeding R. Temporal Expression Profile and Distribution Pattern Indicate a Role of Connective Tissue Growth Factor (CTGF/CCN-2) in Diabetic Nephropathy in Mice. American Journal of Physiology. 2006;Vol. 290(No. 6):1344–1354. doi: 10.1152/ajprenal.00174.2005. [DOI] [PubMed] [Google Scholar]
- 75.Yokoi H, Mukoyama M, Nagae T, Mori K, Suganami T, Sawai K, Yoshioka T, Koshikawa M, Nishida T, Takigawa M, Sugawara A, Nakao K. Reduction in Connective Tissue Growth Factor by Antisense Treatment Ameliorates Renal Tubulointerstitial Fibrosis. Journal of the American Society of Nephrology. 2004;Vol. 15(No. 6):1430–1440. doi: 10.1097/01.asn.0000130565.69170.85. [DOI] [PubMed] [Google Scholar]
- 76.Okada H, Kikuta T, Inoue T, Kanno Y, Ban S, Sugaya T, Takigawa M, Suzuki H. Dexamethasone Induces Connective Tissue Growth Factor Expression in Renal Tubular Epithelial Cells in a Mouse Strain-Specific Manner. American Journal of Pathology. 2006;Vol. 168(No. 3):737–747. doi: 10.2353/ajpath.2006.050656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Flyvbjerg A, et al. Long-Term Renal Effects of a Neutralizing Connective Tissue Growth Factor (Ctgf)-Antibody in Obese Type 2 Diabetic Mice. Journal of the American Society of Nephrology. 2004;Vol. 15:261. [Google Scholar]
- 78.Wang Q, et al. Amelioration of Diabetic Nephropathy (DN) Incused by Renal Ischemia-Reperfusion (IR) in Rats with Diabetes Mellitus (DM) by Treatment with FG-3019, a Monoclonal Antibody Against Connective Tissue Growth Factor (CTGF) Journal of the American Society of Nephrology. 2004:731. [Google Scholar]
- 79.Adler SG, Schwartz S, Williams ME, Arauz-Pacheco C, Bolton WK, Lee T, Li D, Neff TB, Urquilla PR, Sewell KL. Phase 1 Study of Anti-CTGF Monoclonal Antibody in Patients with Diabetes and Microalbuminuria. Clinical Journal of the American Society of Nephrology. 2010;Vol. 5(No. 8):1420–1428. doi: 10.2215/CJN.09321209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Schwartz S. Phase 1 Study of FG-3019, an Anti-CTGF Monoclonal Antibody, in Type 1/2 Diabetes Mellitus with Microalbuminuria. Diabetes. 2007;Vol. 56:151. [Google Scholar]
- 81.Qi W, Chen X, Poronnik P, Pollock CA. Transforming Growth Factor-β/Connective Tissue Growth Factor Axis in the Kidney. The International Journal of Biochemistry & Cell Biology. 2008;Vol. 40(No. 1):9–13. doi: 10.1016/j.biocel.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 82.McGowan TA, Zhu Y, Sharma K. Transforming Growth Factor-β: A Clinical Target for the Treatment of Diabetic Nephropathy. Current Diabetes Reports. 2004;Vol. 4(No. 6):447–454. doi: 10.1007/s11892-004-0055-z. [DOI] [PubMed] [Google Scholar]
- 83.Sharma K, Jin Y, Guo J, Ziyadeh FN. Neutralization of TGF-β by Anti-TGF-β Antibody Attenuates Kidney Hypertrophy and the Enhanced Extracellular Matrix Gene Expression in STZ-Induced Diabetic Mice. Diabetes. 1996;Vol. 45:522–530. doi: 10.2337/diab.45.4.522. [DOI] [PubMed] [Google Scholar]
- 84.Ziyadeh FN, Hoffman BB, Han DC, Iglesias-de la Cruz MC, Hong SW, Isono M, Chen S, McGowan TA, Sharma K. Long-Term Prevention of Renal Insufficiency, Excess Matrix Gene Expression and Glomerular Mesangial Matrix Expansion by Treatment with Monoclonal Antitransforming Growth Factor-β Antibody in db/db Diabetic Mice. Proceedings of the National Academy of Sciences. 2000;Vol. 97(No. 14):8015–8020. doi: 10.1073/pnas.120055097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sharma K, Ziyadeh FN, Alzahabi B, McGowan TA, Kapoor S, Kurnik BR, Kurnik PB, Weisberg LS. Increased Renal Production of Transforming Growth Factor-β1 in Patients with Type II Diabetes. Diabetes. 1997;Vol. 46(No. 5):854–859. doi: 10.2337/diab.46.5.854. [DOI] [PubMed] [Google Scholar]
- 86.Yamamoto T, Nakamura T, Noble NA, Ruoslahti E, Border WA. Expression of Transforming Growth Factor β Is Elevated in Human and Experimental Diabetic Nephropathy. Proceedings of the National Academy of Sciences. 1993;Vol. 90(No. 5):1814–1818. doi: 10.1073/pnas.90.5.1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.RamachandraRao SP, Zhu Y, Ravasi T, Mc-Gowan TA, Toh I, Dunn SR, Okada S, Shaw MA, Sharma K. Pirfenidone Is Renoprotective in Diabetic Kidney Disease. Journal of the American Society of Nephrology. 2009;Vol. 20(No. 8):1765–1775. doi: 10.1681/ASN.2008090931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Iwano M, Kubo A, Nishino T, Sato H, Nishioka H, Akai Y, Kurioka H, Fujii Y, Kanauchi M, Shiiki H, Dohi K. Quantification of Glomerular TGF-β1 mRNA in Patients with Diabetes Mellitus. Kidney International. 1996;Vol. 49:1120–1126. doi: 10.1038/ki.1996.162. [DOI] [PubMed] [Google Scholar]
- 89.Yamamoto T, Noble NA, Cohen AH, Nast CC, Hishida A, Gold LI, Border WA. Expression of Transforming Growth Factor-β Isoforms in Human Glomerular Diseases. Kidney International. 1996;Vol. 49:461–469. doi: 10.1038/ki.1996.65. [DOI] [PubMed] [Google Scholar]
- 90.Sharma K, Ix JH, Mathew AV, Cho M, Pflueger A, Dunn SR, Francos B, Sharma S, Falkner B, McGowan TA, Donohue M, Ramachandrarao S, Xu R, Fervenza FC, Kopp JB. Pirfenidone for Diabetic Nephropathy. Journal of the American Society of Nephrology. 2011;Vol. 22(No. 6):1144–1151. doi: 10.1681/ASN.2010101049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Pergola PE, Krauth M, Huff JW, Ferguson DA, Ruiz S, Meyer CJ, Warnock DG. Effect of Bardoxolone Methyl on Kidney Function in Patients with T2D and Stage 3b-4 CKD. American Journal of Nephrology. 2011;Vol. 33(No. 5):469–476. doi: 10.1159/000327599. [DOI] [PubMed] [Google Scholar]
- 92.Pergola PE, Raskin P, Toto RD, Meyer CJ, Huff JW, Grossman EB, Krauth M, Ruiz S, Audhya P, Christ-Schmidt H, Wittes J, Warnock DG. Bardoxolone Methyl and Kidney Function in CKD with Type 2 Diabetes. The New England Journal of Medicine. 2011;Vol. 365:327–336. doi: 10.1056/NEJMoa1105351. [DOI] [PubMed] [Google Scholar]
- 93.Retinopathy and Nephropathy in Patients with Type 1 Diabetes Four Years after a Trial of Intensive Therapy. The Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group. The New England Journal of Medicine. 2000;Vol. 342:381–389. doi: 10.1056/NEJM200002103420603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sustained Effect of Intensive Treatment of Type 1 Diabetes Mellitus on Development and Progression of Diabetic Nephropathy: The Epidemiology of Diabetes Interventions and Complications (EDIC) Study. The Journal of the American Medical Association. 2003;Vol. 290(No. 16):2159–2167. doi: 10.1001/jama.290.16.2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Nathan DM, Cleary PA, Backlund JY, Genuth SM, Lachin JM, Orchard TJ, Raskin P, Zinman B. Intensive Diabetes Treatment and Cardiovascular Disease in Patients with Type 1 Diabetes. The New England Journal of Medicine. 2005;Vol. 353:2643–2653. doi: 10.1056/NEJMoa052187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Stratton IM, Adler AI, Neil HA, Matthews DR, Manley SE, Cull CA, Hadden D, Turner RC, Holman RR. Association of Glycaemia with Macrovascular and Microvascular Complications of Type 2 Diabetes (UKPDS 35): Prospective Observational Study. British Medical Journal. 2000;Vol. 321:405–412. doi: 10.1136/bmj.321.7258.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-Year Follow-up of Intensive Glucose Control in Type 2 Diabetes. The New England Journal of Medicine. 2008;Vol. 359:1577–1589. doi: 10.1056/NEJMoa0806470. [DOI] [PubMed] [Google Scholar]
- 98.UK Prospective Diabetes Study (UKPDS) Group. Intensive Blood-Glucose Control with Sulphonylureas or Insulin Compared with Conventional Treatment and Risk of Complications in Patients with Type 2 Diabetes (UKPDS 33) The Lancet. 1998;Vol. 352(No. 9131):837–853. [PubMed] [Google Scholar]
- 99.Patel A, MacMahon S, Chalmers J, Neal B, Billot L, Woodward M, Marre M, Cooper M, Glasziou P, Grobbee D, Hamet P, Harrap S, Heller S, Liu L, Mancia G, Mogensen CE, Pan C, Poulter N, Rodgers A, Williams B, Bompoint S, de Galan BE, Joshi R, Travert F. Intensive Blood Glucose Control and Vascular Outcomes in Patients with Type 2 Diabetes. The New England Journal of Medicine. 2008;Vol. 358:2560–2572. doi: 10.1056/NEJMoa0802987. [DOI] [PubMed] [Google Scholar]
- 100.Mogensen CE. Long-Term Antihypertensive Treatment Inhibiting Progression of Diabetic Nephropathy. British Medical Journal. 1982;Vol. 285:685–688. doi: 10.1136/bmj.285.6343.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Schrier RW, Estacio RO, Esler A, Mehler P. Effects of Aggressive Blood Pressure Control in Normotensive Type 2 Diabetic Patients on Albuminuria, Retinopathy and Strokes. Kidney International. 2002;Vol. 61:1086–1097. doi: 10.1046/j.1523-1755.2002.00213.x. [DOI] [PubMed] [Google Scholar]
- 102.UK Prospective Diabetes Study Group. Tight Blood Pressure Control and Risk of Macrovascular and Microvascular Complications in Type 2 Diabetes: UKPDS 38. British Medical Journal. 1998;Vol. 317:703–713. [PMC free article] [PubMed] [Google Scholar]
- 103.UK Prospective Diabetes Study Group. Cost Effectiveness Analysis of Improved Blood Pressure Control in Hypertensive Patients with Type 2 Diabetes: UKPDS 40. British Medical Journal. 1998;Vol. 317:720–726. [PMC free article] [PubMed] [Google Scholar]
- 104.Patel A, MacMahon S, Chalmers J, Neal B, Woodward M, Billot L, Harrap S, Poulter N, Marre M, Cooper M, Glasziou P, Grobbee DE, Hamet P, Heller S, Liu LS, Mancia G, Mogensen CE, Pan CY, Rodgers A, Williams B. Effects of a Fixed Combination of Perindopril and Indapamide on Macrovascular and Microvascular Outcomes in Patients with Type 2 Diabetes Mellitus (the ADVANCE trial): A Randomised Controlled Trial. The Lancet. 2007;Vol. 370(No. 9590):829–840. doi: 10.1016/S0140-6736(07)61303-8. [DOI] [PubMed] [Google Scholar]
- 105.de Galan BE, Perkovic V, Ninomiya T, Pillai A, Patel A, Cass A, Neal B, Poulter N, Harrap S, Mogensen CE, Cooper M, Marre M, Williams B, Hamet P, Mancia G, Woodward M, Glasziou P, Grobbee DE, MacMahon S, Chalmers J. Lowering Blood Pressure Reduces Renal Events in Type 2 Diabetes. Journal of the American Society of Nephrology. 2009;Vol. 20(No. 4):883–892. doi: 10.1681/ASN.2008070667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jones DW, Materson BJ, Oparil S, Wright JT, Roccella EJ. Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation and Treatment of High Blood Pressure. Hypertension. 2003;Vol. 42:1206–1252. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 107.American Diabetes Association. Standards of Medical Care in Diabetes. Diabetes Care. 2005;Vol. 28(Suppl. 1):4–36. [Google Scholar]
- 108.Kidney Disease Outcomes Quality Initiative (K/DOQI) K/DOQI Clinical Practice Guidelines on Hypertension and Antihypertensive Agents in Chronic Kidney Disease. American Journal of Kidney Disease. 2004;Vol. 43(No. 5):1–290. [PubMed] [Google Scholar]
- 109.The ACCORD Study Group. Effects of Intensive Blood-Pressure Control in Type 2 Diabetes Mellitus. The New England Journal of Medicine. 2010;Vol. 362:1575–1585. doi: 10.1056/NEJMoa1001286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.The EUCLID Study Group. Randomised Placebo-Controlled Trial of Lisinopril in Normotensive Patients with Insulin-Dependent Diabetes and Normoalbuminuria or Microalbuminuria. Lancet. 1997;Vol. 349(No. 9068):1787–1792. [PubMed] [Google Scholar]
- 111.Ravid M, Brosh D, Levi Z, Bar-Dayan Y, Ravid D, Rachmani R. Use of enalapril to attenuate decline in renal function in normotensive, normoalbuminuric patients with type 2 diabetes mellitus. Annals of Internal Medicine. 1998;Vol. 128(No. 12):982–988. doi: 10.7326/0003-4819-128-12_part_1-199806150-00004. [DOI] [PubMed] [Google Scholar]
- 112.Estacio RO, Jeffers BW, Gifford N, Schrier RW. Effect of Blood Pressure Control on Diabetic Microvascular Complications in Patients with Hypertension and Type 2 Diabetes. Diabetes Care. 2000;Vol. 23(Suppl. 2):54–64. [PubMed] [Google Scholar]
- 113.Ravid M, Savin H, Jutrin I, Bental T, Katz B, Lishner M. Long-Term Stabilization of Angiotensin-Converting Enzyme Inhibition on Plasma Creatinine and on Proteinuria in Normotensive Type II Diabetic Patients. Annals of Internal Medicine. 1993;Vol. 118:577–581. doi: 10.7326/0003-4819-118-8-199304150-00001. [DOI] [PubMed] [Google Scholar]
- 114.Andersen S, Tarnow L, Rossing P, Hansen BV, Parving HH. Renoprotective Effects of Angiotensin II Receptor Blockade in Type 1 Diabetic Patients with Diabetic Nephropathy. Kidney International. 2000;Vol. 57:601–606. doi: 10.1046/j.1523-1755.2000.00880.x. [DOI] [PubMed] [Google Scholar]
- 115.Brenner BM, Cooper ME, de Zeeuw D, Keane WF, Mitch WE, Parving HH, Remuzzi G, Snapinn SM, Zhang Z, Shahinfar S. Effects of Losartan on Renal and Cardiovascular Outcomes in Patients with Type 2 Diabetes and Nephropathy. The New England Journal of Medicine. 2001;Vol. 345:861–869. doi: 10.1056/NEJMoa011161. [DOI] [PubMed] [Google Scholar]
- 116.Lewis EJ, Hunsicker LG, Clarke WR, Berl T, Pohl MA, Lewis JB, Ritz E, Atkins RC, Rohde R, Raz I. Renoprotective Effect of the Angiotensin-Receptor Antagonist Irbesartan in Patients with Nephropathy Due to Type 2 Diabetes. The New England Journal of Medicine. 2001;Vol. 345:851–860. doi: 10.1056/NEJMoa011303. [DOI] [PubMed] [Google Scholar]
- 117.Parving HH, Lehnert H, Brochner-Mortensen J, Gomis R, Andersen S, Arner P. The Effect of Irbesartan on the Development of Diabetic Nephropathy in Patients with Type 2 Diabetes. The New England Journal of Medicine. 2001;Vol. 345:870–878. doi: 10.1056/NEJMoa011489. [DOI] [PubMed] [Google Scholar]
- 118.Uresin Y, Taylor AA, Kilo C, Tschope D, Santonastaso M, Ibram G, Fang H, Satlin A. Efficacy and Safety of the Direct Renin Inhibitor Aliskiren and Ramipril Alone or in Combination in Patients with Diabetes and Hypertension. Journal of the Renin-Angiotensin-Aldosterone System. 2007;Vol. 8(No. 4):190–198. doi: 10.3317/jraas.2007.028. [DOI] [PubMed] [Google Scholar]
- 119.Parving HH, Persson F, Lewis JB, Lewis EJ, Hollenberg NK. Aliskiren Combined with Losartan in Type 2 Diabetes and Nephropathy. The New England Journal of Medicine. 2008;Vol. 358:2433–2446. doi: 10.1056/NEJMoa0708379. [DOI] [PubMed] [Google Scholar]
- 120.Dunn MJ. Prostaglandins, Angiotension II, and Proteinuria. Nephron. 1990;Vol. 55(Suppl. 1):30–37. doi: 10.1159/000186032. [DOI] [PubMed] [Google Scholar]
- 121.Melchior WR, Bindlish V, Jaber LA. Angiotensin-Converting Enzyme Inhibitors in Diabetic Nephropathy. The Annals of Pharmacotherapy. 1993;Vol. 27:344–350. doi: 10.1177/106002809302700318. [DOI] [PubMed] [Google Scholar]
- 122.Bakris GL, Weir MR, Secic M, Campbell B, Weis-McNulty A. Differential Effects of Calcium Antagonist Subclasses on Markers of Nephropathy Progression. Kidney International. 2004;Vol. 65:1991–2002. doi: 10.1111/j.1523-1755.2004.00620.x. [DOI] [PubMed] [Google Scholar]
- 123.Remuzzi G, Ruggenenti P, Benigni A. Understanding the Nature of Renal Disease Progression. Kidney International. 1997;Vol. 51:2–15. doi: 10.1038/ki.1997.2. [DOI] [PubMed] [Google Scholar]
- 124.Kloke HJ, Branten AJ, Huysmans FT, Wetzels JF. Antihypertensive Treatment of Patients with Proteinuric Renal Diseases: Risks or Benefits of Calcium Channel Blockers? Kidney International. 1998;Vol. 53:1559–1573. doi: 10.1046/j.1523-1755.1998.00912.x. [DOI] [PubMed] [Google Scholar]
- 125.Gansevoort RT, Sluiter WJ, Hemmelder MH, de Zeeuw D, de Jong PE. Antiproteinuric Effect of Blood-Pressure-Lowering Agents: A Meta-Analysis of Comparative Trials. Nephrology Dialysis Transplantation. 1995;Vol. 10:1963–1974. [PubMed] [Google Scholar]
- 126.Bakris GL. Effects of Diltiazem or Lisinopril on Massive Proteinuria Associated with Diabetes Mellitus. Annals of Internal Medicine. 1990;Vol. 112(No. 9):707–708. doi: 10.7326/0003-4819-112-9-707. [DOI] [PubMed] [Google Scholar]
- 127.Bakris GL, Copley JB, Vicknair N, Sadler R, Leurgans S. Calcium Channel Blockers Versus Other Antihypertensive Therapies on Progression of NIDDM Associated Nephropathy. Kidney International. 1996;Vol. 50:1641–1650. doi: 10.1038/ki.1996.480. [DOI] [PubMed] [Google Scholar]
- 128.Tonolo G, Velussi M, Brocco E, Abaterusso C, Carraro A, Morgia G, Satta A, Faedda R, Abhyankar A, Luthman H, Nosadini R. Simvastatin Maintains Steady Patterns of GFR and Improves AER and Expression of Slit Diaphragm Proteins in Type II Diabetes. Kidney International. 2006;Vol. 70:177–186. doi: 10.1038/sj.ki.5001515. [DOI] [PubMed] [Google Scholar]
- 129.Bilous RW, Mauer SM, Sutherland DE, Najarian JS, Goetz FC, Steffes MW. The Effects of Pancreas Transplantation on the Glomerular Structure of Renal Allografts in Patients with Insulin-Dependent Diabetes. The New England Journal of Medicine. 1989;Vol. 321(No. 2):80–85. doi: 10.1056/NEJM198907133210204. [DOI] [PubMed] [Google Scholar]