Abstract
The mutant β1,4-galactosyltransferase (β4Gal-T1), β4Gal-T1-Y289L, in contrast to wild-type β4Gal-T1, can transfer GalNAc from the sugar donor UDP-GalNAc to the acceptor, GlcNAc, with efficiency as good as that of galactose from UDP-Gal. Furthermore, the mutant can also transfer a modified sugar, C2 keto galactose, from its UDP derivative to O-GlcNAc modification on proteins that provided a functional handle for developing a highly sensitive chemoenzymatic method for detecting O-GlcNAc post-translational modification on proteins. We report herein that the modified sugar, C2 keto galactose, can be transferred to free GlcNAc residues on N-linked glycoproteins, such as ovalbumin or asialo-agalacto IgG1. The transfer is strictly dependent on the presence of both the mutant enzyme and the ketone derivative of the galactose. Moreover, the PNGase F treatment of the glycoproteins, which cleaves the N-linked oligosaccharide chain, shows that the modified sugar has been transferred to the N-glycan chains of the glycoproteins and not to the protein portion. The application of the mutant galactosyltransferase, β4Gal-T1-Y289L, to produce glycoconjugates carrying sugar moieties with reactive groups, is demonstrated. We envision a broad potential for this technology such as the possibilities to link cargo molecules to glycoproteins, such as monoclonal antibodies, via glycan chains, thereby assisting in the glycotargeting of drugs to the site of action or used as biological probes.
INTRODUCTION
In recent years, it has become evident that a number of biological processes, such as cell adhesion (1, 2), cell–cell recognition (3), formation of glycosynapses (4, 5), immune defense (6), targeting, and signaling, appear to be modulated by the glycan moieties of glycoproteins and glycolipids, where they serve as ligands in the specific protein–carbohydrate (7), lipid–carbohydrate, and carbohydrate–carbohydrate interactions (5, 8). They also serve as recognition targets for their complementary binding proteins, the lectins (7). Because of the molecular diversity of glycans, and being specific recognition ligands in various biological processes, they are also used to design drugs that target various diseases (9).
Glycans can be classified as linear or branched sugars. The linear sugars are the glycosaminoglycans comprising polymers of sulfated disaccharide repeat units that are O-linked to a core protein, forming a proteoglycan aggregate (10). The branched glycans are found as N-linked and O-linked sugars on glycoproteins or on glycolipids (11). These carbohydrate moieties of the linear and branched glycans are synthesized by a super family of enzymes, the glycosyltransferases, which transfer a sugar moiety from a sugar donor to an acceptor molecule.
X-ray crystal structures of the catalytic domain of many glycosyltransferases have been determined in recent years. These studies show that the specificity of the sugar donor is determined by a few residues in the sugar–nucleotide binding pocket of glycosyltransferases, which are conserved among family members from different species (12). This structural information has made it possible to reengineer the existing glycosyltransferases. For example, the β1,4-galactosyltranferase family in vertebrates (β4Gal-T1 to T7) is responsible for the transfer of galactose from the donor UDP-galactose (UDP-Gal) to various glycans in a β1–4 linkage (13, 14). The residue Tyr289 (or Phe) in the catalytic pocket of β4Gal-T1, determines the sugar donor substrate specificity of the enzyme toward UDP-Gal (12, 15). Mutation of Tyr289 to Leu or Ile enlarges the binding pocket such that the mutant enzyme, β4Gal-T1-Y289L, has β1,4-N-acetylgalactosaminyltransferase (β4GalNAc-T) activity, which is as efficient as its β1,4-galactosyltransferase (β4Gal-T) activity (15, 16). Similarly, in blood group A GalNAc-transferase, the residues Leu266 and Gly268, and in blood group B Gal-transferase, the residues Met266 and Ala268 determine the specificities toward their respective sugar donors, UDP-GalNAc and UDP-Gal (17). Mutation of Met266 to Leu266 in blood group B Gal-transferase changes the enzyme sugar donor specificity toward UDP–GalNAc. Furthermore, in β1,3-glucuronyltransferase-1 (GlcA-T1), His 308 (His 311 in GlcAT-P) (18, 19) determines the enzyme specificity toward the sugar donor UDP–GlcUA. Mutation of His308 to Arg308 changes the specificity of the sugar donor to UDP–Glc, UDP–Man, or UDP–GlcNAc.
The O-GlcNAc modification on proteins is the dynamic posttranslational modification in which the β-N-acetylglucosamine is covalently attached to serine or threonine residues in proteins. Several methods have been reported for the identification of O-GlcNAc modification on proteins. One of the detection methods involves the enzymatic labeling by β4Gal-T1 of O-GlcNAc using UDP-3H galactose (20). However, this method is time-consuming and expensive. The engineered β4Gal-T1-Y289L was shown to transfer from a UDP derivative, a galactose moiety that has a ketone substitution at the C2 position of the galactose ring (21). The introduced ketone was then used as a functional handle that allowed the development of a highly sensitive and rapid chemoenzymatic method to detect O-GlcNAc modification on proteins (21).
Here, we show that the C2 keto galactose moiety can also be transferred from its UDP derivative to only free GlcNAc residues on the N-linked glycan chains of ovalbumin or on asialo-agalacto IgG1 molecules. That the modified galactose is transferred only to the GlcNAc residues on the N-glycan chains was confirmed by the PNGase F treatment of the glycoprotein that cleaves the N-linked glycan chains. As previously described (21), the transfer was followed by coupling the C2 keto galactose moiety on the glycan chain to an aminooxy biotin which was then detected by a sensitive chemiluminescence assay. Using this strategy, one can couple two glycoproteins that have modified glycan chains, with each having reactive functional groups with heterobifunctional linkers (22). The advantage of this methodology is that the modification occurs in a site-directed manner, only where the carbohydrate is attached to the glycoprotein. This specificity permits the use of site-directed immunotherapy without affecting the antigen binding affinity of the immunoglobulin. The advantages and potential use of this approach in developing a drug delivery system or a biological probe are discussed.
EXPERIMENTAL PROCEDURES
Preparation and Purification of Wild-Type β4Gal-T1 and Mutant β4Gal-T1-Y289L
The β4Gal-T1 that has a Cys342 mutated to Thr342 was used as wild-type β4Gal-T1. This mutation does not affect the enzymatic properties of β4Gal-T1, and it makes the protein stable at room temperature (15). This wild-type β4Gal-T1 and the mutant β4Gal-T1-Y289L, derived from the wild type, were constructed in the pET23a vector (Novagen, Inc.) as described previously (15, 23, 24). The E. coli strain BL21(DE3)pLysS, containing these plasmids, was used for the expression of these proteins (Novagen, Inc). The recombinant proteins were expressed in the inclusion body fraction and purified according to the published method (25). From a liter of induced bacterial culture, the yield of purified inclusion bodies is generally 80–100 mg. However, the presence of PEG-400 and l-arginine during folding of the inclusion bodies increases the yields of the native protein, resulting in an overall folding efficiency of ~60% (25).
Supply of Reagents
The oligosaccharide acceptors, N-acetylglucosamine (GlcNAc), chitobiose (GlcNAcβ1,4-GlcNAc), and chitotriose (GlcNAcβ1,4-GlcNAcβ1,4-GlcNAc) were obtained from Sigma Chemicals, U.S.A.; chitotetrose (GlcNAcβ1,4-GlcNAcβ1,4-GlcNAcβ1,4-GlcNAc) and the pentasaccharide GlcNAcβ1,2-Mana1,6-(GlcNAcβ1,2-Mana1,3)-Man were purchased from Dextra-labs, U.K. The heptasaccharide tetrapeptide, Arg-[GlcNAcβ1,2-Manα1,6-(GlcNAcβ1,2-Mana1,3)-Manβ1,4-GlcNAcβ1,4-GlcNAcβ]-Asn-Glu-Gly, which carries both 1,2–1,6-arm and 1,2–1,3-arm linked to core mannose, was purchased from Calbiochem, U.S.A. The UDP derivative of C2 keto galactose was synthesized as described earlier (21). The N′-aminooxymethylcarbonylhydrazino-d-biotin (ARP) was purchased from Dojindo Laboratories. Peptide N-glycosidase F (PNGase F) was obtained from New England Labs. Albumin from chicken egg white grade VI was purchased from Sigma, and IgG1 was provided by Drs. Mei-Yun Zhang and Yang Feng from Dr. Dimiter S. Dimitrov’s laboratory at the Nanobiology program, CCR, NCI.
GalNAc-T Enzyme Assays
The protein concentrations were measured using the Bio-Rad Protein Assay kit on the basis of the method of Bradford and further verified by SDS-PAGE electrophoresis. An enzymatic assay procedure for β4Gal-T1 has been reported previously (26). The enzyme activities of the β4Gal-T1-Y289L mutant were measured using UDP–GalNAc as the sugar nucleotide donor, and at various concentrations of the acceptors, GlcNAc, chitobiose, chitotriose, chitotetrose, the mono, di-, tri-, tetrasaccharides, respectively, and the N-linked branched oligosaccharide structures. For the specific activity measurements, a 100 µL incubation mixture containing 10 mM MnCl2, 25 mM Tris–HCl, pH 8.0, 500 µM of UDP–GalNAc, 20 ng of the mutant β4Gal-T1-Y289L, and 0.5 µCi 3H-UDP–GalNAc was used for the GalNAc-T reaction. The reaction was terminated by adding 200 µL of cold water, and the mixture was passed through a 0.5 mL bed volume column of AG 1-X8 cation resin (Bio-Rad) to remove any unreacted 3H-UDP–GalNAc. The column was washed successively with 300, 400, and 500 µL of water, and the column flowthrough was diluted to 20 mL with Biosafe scintillation fluid; radioactivity was measured with a Beckman counter LS-3801. A reaction without the acceptor sugar was used as a control.
Transfer of C2 Keto Galactose from its UDP Derivative to Free GlcNAc Residues on Glycoacceptors Using the Mutant of β4Gal-T1-Y289L
Albumin from chicken egg white grade VI (40 µg) and recombinant IgG1 (2–5 µg) were incubated with 1 mM UDP-C2 keto galactose and 500 ng of the mutant β4Gal-T1-Y289L or 488 ng of wild-type β4Gal-T1 in a 10 µL final incubation mixture containing 10 mM MnCl2 and 25 mM Tris–HCl (pH 8.0). Reactions were incubated at 30 °C for 3 h.
Biotinylation of Ovalbumin and IgG1
The ketone-labeled proteins were subsequently diluted to 30 µL in a mixture containing 50 mM NaOAc (pH 3.9) and N-aminooxymethylcarbonylhydrazino-d-biotin (ARP). The biotinylation reactions were incubated with gentle shaking for 12–16 h at 25 °C. The reactions were stopped by boiling in Tris-glycine-SDS sample buffer containing β-mercaptoethanol and analyzed as described (21).
Western Blotting of Ovalbumin and Asialo-Agalacto-IgG1
Proteins were resolved in 14% Tris–glycine gel (Invitrogen) and then transferred by electrophoresis to nitrocellulose (0.45 µm pore size) for 2 h at 25 V, and biotinylated protein bands identified as described previously (21). Briefly, the nitrocellulose blots were blocked for 1 h at room temperature with shaking in a solution containing 5% dry milk in 0.02% Tween-20. Blots were rinsed thoroughly with water and probed with streptavidin conjugated with horseradish peroxidase (HRP) (Amersham Biosciences) (1:4000) in 1× PBS pH 7.4, 0.02% Tween-20 containing 3% BSA for 1 h at 25 °C. After probing with the conjugated streptavidin, the nitrocellulose membranes were washed 4× for 10 min in 5% dry milk containing 0.02% Tween-20. The streptavidin–HRP signal was visualized by chemiluminescence after 2 min exposure to ECL detection reagents (Amersham) and exposure to Kodak Biomax XAR film
Peptide: N-Glycosidase F Treatment
Ovalbumin and asialo-agalacto-IgG1 samples, approximately 3 µg each, were boiled for 10 min in 1% SDS and 1% β-mercaptoethanol. Samples were then incubated in the presence or absence of PNGase F (2500 units) for 16 h at 37 °C in a buffer containing 5 mM sodium phosphate and 1% NP-40. Samples were then boiled in SDS-PAGE loading buffer and then resolved by SDS-PAGE.
MALDI Mass Spectrometric Analyses of Oligosaccharides
An Applied Biosystems Voyager-DE Pro time-of-flight mass spectrometer was utilized for analyses. The accelerating voltage was 20 kV, guide wire 0.05%, and grid voltage 94%. The instrument was operated in linear mode under positive ion conditions. A nitrogen laser was used at 337 nm with 150 laser shots averaged per spectrum. 2,5-Dihydroxybenzoic acid (Aldrich Chemicals, St. Louis, MO) was used as the matrix for all experiments. The matrix concentration was 20 mg/mL in water. Sample preparation was a modified “dried droplet” procedure, whereby 0.3 µL of sample was spotted onto the MALDI target, followed by 0.3 µL of matrix. The mixture was then allowed to air-dry prior to analysis. Calibration was performed using instrument default settings, and data analysis was carried out using Data Explorer software residing on the Voyager mass spectrometer
MALDI Mass Spectrometric Analyses of the N-Glycans of Ovalbumin
Albumin from chicken egg white (grade VI), 1 mg in 100 µL deionized water, was washed in a microcon YM-10 centrifugal filter device with 1.5 mL of deionized water to remove free oligosaccharide chains. The washed sample was concentrated and incubated at 30 °C for 18 h in a 100 µL final incubation mixture containing 0.5 mM UDP–GalNAc, 2 µg of the mutant β4Gal-T1-Y289L, 10 mM MnCl2, and 25 mM Tris–HCl (pH 8.0). The samples were then digested for 18 h at 37 °C with 2500 units of PNGase F (NEB) in 50 mM sodium phosphate buffer. The digested samples were applied onto a Microcon YM-10 filter device, and the filtrate containing the released N-glycans was analyzed by mass spectrometry as described above
RESULTS
Sugar Donor Specificity of β4Gal-T1
Our laboratory previously showed that the sugar donor specificity of β4Gal-T1 toward UDP–Gal is determined by a single amino acid, Tyr, at position 289 (15). When Tyr289 is mutated to Leu, the sugar donor specificity of β4Gal-T1 is broadened in a way that, in contrast to the wild-type enzyme which lacks GalNAc-T activity, the mutant β4Gal-T1-Y289L exhibits both β4Gal-T and β4GalNAc-T activities (15). The Tyr289 mutant can also transfer from the UDP derivatives the galactose moiety that has, at the C2 position, substitutions other than the 2-N-acetyl group (–NH–CO–CH3 in GalNAc) (21). Here, we first investigated the transfer of GalNAc to N-glycans of glycoproteins by the mutant enzyme, β4Gal-T1-Y289L
Transfer Preferences of β4Gal-T1-Y289L to oligosaccharide acceptors
In a previous study from our laboratory, we had shown by double substrate kinetics that the true Km for both chitobiose and chitotriose was nearly the same (27), suggesting that the presence of an additional GlcNAc residue in chitotriose, not present in chitobiose, did not affect the ground-state binding of these acceptor substrates to the enzyme. Moreover, the apparent Km values for chitobiose and chitotriose were similar to the true Km values obtained by double substrate kinetics. In this study, we tested the amount of acceptor converted to product for various linear and branched structures (Table 1) In the linear structures, 25 nmol of the acceptor GlcNAc was available in the reaction, and in the branched structures with two terminal GlcNAc residues, 50 nmol of the acceptor GlcNAc was available in the reaction mixture. The linear acceptors, mono-, di-, tri- and tetrasaccharides, all showed 97% to 100% conversion of the acceptor to product (Table 1). Both chitobiose and chitotriose showed 99% conversion. In the pentasaccharide, GlcNAcβ1,2-Manα1,6-(GlcNAcβ1,2-Manα1,3)-Man, and the heptasaccharide tetrapeptide, Arg-[GlcNAcβ1,2-Manα1,6-(GlcNAcβ1,2-Mana1,3)-Manβ1,4-GlcNAcβ1,4-GlcNAcβ]-Asn-Glu-Gly (which carries GlcNAc on both the 1,2–1,6-arm and the 1,2–1,3-arm linked to core mannose), GalNAc is only transferred to 50% of the available GlcNAc residues on these acceptor substrates (Table 1).
Table 1.
Transfer of GalNAc from UDP-GalNAc by β4Gal-T1-Y289L to Oligosaccharide Acceptorsa
| nmoles of available | ||||
|---|---|---|---|---|
| sugar acceptor | substrate | GlcNAc residues |
nmoles of GalNAc transferred |
% acceptor converted to product |
| GlcNAc | 24.90 | 24.90 | 24.09 ± 0.07 | 96.74 |
| β-benzyl-GlcNAc | 25.00 | 25.00 | 24.50 ± 0.22 | 98.00 |
| chitobiose | 25.00 | 25.00 | 24.78 ± 0.21 | 99.12 |
| chitotriose | 25.00 | 25.00 | 24.90 ± 0.05 | 99.60 |
| chitotetraose | 25.00 | 25.00 | 24.88 ± 0.02 | 99.52 |
| pentasaccharideb | 25.00 | 50.00 | 24.50 ± 0.5 | 49.00c |
| heptasaccharideb | 25.00 | 50.00 | 24.68 ± 0.04 | 49.36c |
| ovalbumin | 24.00 | variabled | 9.00 ± 14 | 37.50e |
Galactosyltransferase assays with GalNAc were performed as described in the Materials and Methods section. The mutant β4Gal-T1-Y289L was assayed at a final concentration of 24 µg/mL. Assays were done at 30 °C with overnight incubations using 1.5 mM of UDP-GalNAc and 1 mM of acceptors.
The pentasaccharide substrate,GlcNAcβ1,2-Manα1,6(GlcNAcβ1,2-Manα1,3)Man, and the heptasaccharide tetrapeptide substrate, Arg-[GlcNAcβ1,2-Manα1,6-(GlcNAcβ1,2-Manα1,3)-Manβ1,4-GlcNAcβ1,4-GlcNAcβ]-Asn-Glu-Gly, are branched structures which have 2 mol of GlcNAc per mole of substrate available for the transfer reaction.
GalNAc is transferred to only 50% of the available GlcNAc residues in the branched substrate structures.
Ovalbumin also has a branched N-linked glycan. The amount of GlcNAc available for transfer is variable.
Percentage of the acceptor converted to product has been calculated on the basis of the amount of ovalbumin used in the assay.
Transfer of GalNAc Residue to N-Linked Glycoproteins
Over 20 years ago, it was shown β4Gal-T1 could transfer galactose from UDP–Gal to ovalbumin in the absence of α-lactalbumin (28). Only recently, the permissive mutant β4Gal-T1-Y289L was shown to transfer GalNAc from UDP–GalNAc to GlcNAc residues (15). Galactosyltransferase and N-acetylgalactosaminyltransferase assays with the β4Gal-T1-Y289L mutant, using ovalbumin as the acceptor, were performed by a radiochemical assay as described previously (15, 25). The kinetics of transfer of GalNAc to ovalbumin was analyzed over a period of 20 h as described in the Materials and Methods section (Figure 1). Due to the heterogeneity observed with commercial ovalbumin preparations (29), the amount of available acceptor substrate is variable, and we show that, after overnight incubation, only 37% of acceptor is converted into product (Table 1).
Figure 1.
(A) Enzyme kinetics activity of the mutant enzyme, β4Gal-T1-Y289L, with UDP–GalNAc as a donor substrate and ovalbumin as a glycoprotein acceptor substrate. GalNAc-T activity was measured using 500 µM UDP–GalNAc and 0.5 mg of ovalbumin in 100 µL incubation mix containing Mn2+ and Tris pH 8.0 (Material and Methods section). The mutant enzyme was used at a final concentration of 40 µg/mL, and activity was measured at the indicated times. For MS analysis, the reaction was carried out for a period of 20 h at 30 °C. (B) The insert shows the results of the kinetic analysis between zero and 200 min of incubation time.
MALDI Mass Spectra Determination of Glycans before and after Treatment with UDP–GalNAc and UDP-C2 Keto-Modified Galactose
The transfer of GalNAc and C2 keto-modified galactose from their respective UDP derivatives was followed by MALDI mass profiling (Figure 2). In the MALDI profile, in the range between m/z 700 and 1500, a unique peak at m/z 853.4 corresponds to the chitotetrose, prior to the transfer of the donor sugar (Figure 2A). Transfers of GalNAc (Figure 2B) and C2 keto-modified galactose (Figure 2C) are observed as peaks at m/z 1056.4 and 1055.5, respectively. The MALDI profile in a range between m/z 1099.4 and 3501.0 (Figure 2D) shows a major peak at m/z 1773.4 corresponding to the heptasaccharide tetrapeptide, Arg-[GlcNAcβ1,2-Manα1,6-(GlcNAcβ1,2-Manα1,3)-Manβ1,4-GlcNAcβ1,4-GlcNAcβ]-Asn-Glu-Gly. The addition of GalNAc (Figure 2E) and C2-keto galactose (Figure 2F) to either antenna of the heptasaccharide is observed as peaks at m/z 1976.7 and 1975.4, respectively. Under the conditions used in this experiment (Materials and Methods section), the transfer of GalNAc to the GlcNAc residue on one of the antenna of the biantennary structure was observed.
Figure 2.
MALDI mass spectra of glycans after the transfer of GalNAc to the sugar acceptors chitotetrose GlcNAcβ1,4-GlcNAcβ1,4-GlcNAcβ1,4-GlcNAcβ (A,B,C) and a heptasaccharide tetrapeptide Arg-[GlcNAcβ1,2-Manα1,6-(GlcNAcβ1,2-Manα1,3)-Manβ1,4-GlcNAcβ1,4-GlcNAcβ]-Asn-Glu-Gly (D,E,F). Major peaks are annotated with the carbohydrate structure shown in the symbols for monosaccharides, according to the nomenclature adopted by the consortium for functional glycomics, http://www.functionalglycomics.org/static/consortium/. GlcNAc (blue squares), mannose (green spheres), GalNac (yellow squares), and C2 keto galactose (yellow sphere). The symbols were drawn using the GlycoWorkbench program found in the eurocarb database http://www.eurocarbdb.org/. The transferred C2 keto galactose moiety in panels C and F is shown. Panel A shows a peak at 853.4 m/z corresponding to the linear glycan structure chitotetrose. Panel B shows a shift in the molecular mass from the starting ion at 853.4 m/z to a peak of 1056.4 m/z after addition of a GalNAc moiety (Material and Methods section). Panel C shows chitotetrose with the transferred C2 keto galactose moiety to the terminal GlcNAc residue. Panel D shows a peak at 1773.4 m/z corresponding to the starting branched heptasaccharide peptide structure, Arg-[GlcNAcβ1,2-Manα1,6-(GlcNAcβ1,2-Manα1,3)-Manβ1,4-GlcNAcβ1,4-GlcNAcβ]-Asn-Glu-Gly. Panel E shows a peak at 1976.7 m/z that corresponds to a heptasaccharide peptide having one added GalNAc moiety. Panel F shows the peak at 1975.4 m/z that corresponds to a heptasaccharide to which one C2-keto galactose moiety has been transferred to one of the available terminal GlcNAc moieties.
MALDI Mass Spectra Determination of N-Linked Carbohydrates from Ovalbumin
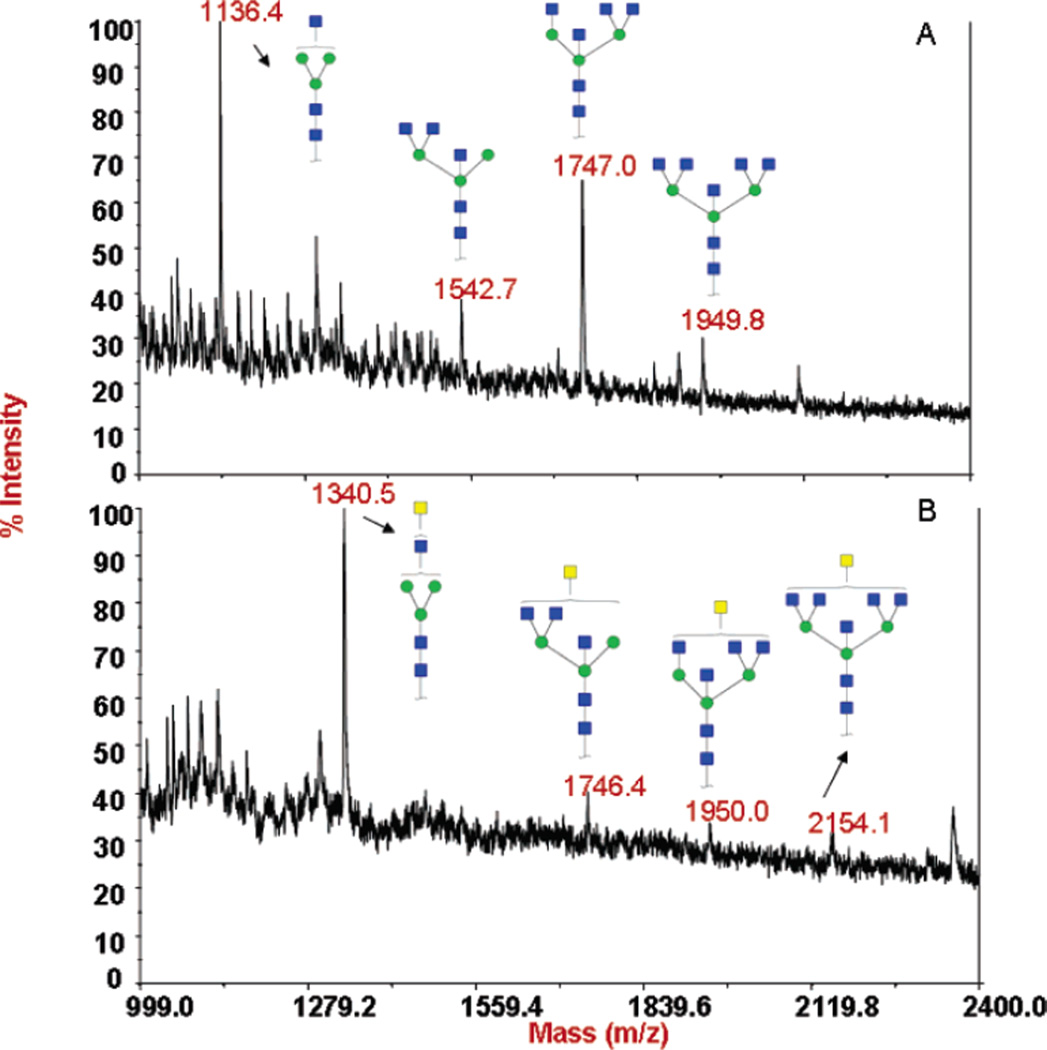
To determine the nature of the N-glycans attached to ovalbumin, glycans were released with PNGase F, purified on amicon filters, and analyzed by MALDI. In the MALDI profile, obtained from the released N-glycan chains of ovalbumin, peaks in the range between m/z 999.0 and 2400.0 were observed. Four major peaks were found at m/z 1136.4, 1542.7, 1747.0, and 1949.8 (Figure 3A). These are mainly bi- to penta-antennary structures which lack the terminal sialic acid and galactose present on N-glycans of mammalian proteins. Upon transfer of GalNAc to one antenna of the penta-antennary structures, the peaks observed at m/z 1340.5, 1746.4, 1950.0, and 2154.1 correspond to structures with one additional monosaccharide unit, the GalNAc residue that has been transferred from UDP–GalNAc by the mutant enzyme β4Gal-T1-Y289L (Figure 3B).
Figure 3.
MALDI mass spectra of the glycans released from chicken ovalbumin. After PNGase F treatment of chicken ovalbumin, the released oligosaccharides were passed through the microcon YM-10 columns and analyzed by MALDI mass spectrometry. Only the peaks of interest are annotated, showing their molecular mass and possible structures. Carbohydrate structures are shown in symbol form, as described in the Figure 2 legend. (A) Glycans released from the commercial sample (Sigma) of chicken ovalbumin before GalNAc transfer. Ions at 1136.4, 1542.7, 1747.0, and 1949.8 m/z are assigned to ovalbumin structures reported (29). (B) Glycan structures released from chicken ovalbumin after the transfer of GalNAc from UDP–GalNAc with the mutant enzyme β4Gal-T1-Y289L. Ions at 1340.5, 1746.4, 1950.0, and 2154.1 m/z are assigned to structures in the ovalbumin preparation onto which GalNAc moieties have been transferred.
Detection of GlcNAc Residue on Glycosylated Proteins by β4Gal-T1-Y289L
Figure 4 shows the schematics of the transfer of C2 keto galactose to the N-glycan moiety of a glycoprotein. The ketone moiety at the C2 position of galactose is a flexible chemical handle, which under mild conditions can be coupled to aminooxy-containing ligands (Figure 4B). When a biotinylated derivative of the aminooxy-containing ligand (N-(aminooxyacetyl)-N′-(d-biotinoyl) hydrazine) was used as a coupling agent, the transfer and coupling reaction could be followed by detecting the biotinylated product by chemiluminescence with streptavidin conjugated to horseradish peroxidase (HRP) (Figure 4C).
Figure 4.
Detection of GlcNAc glycosylated proteins by β4Gal-T1-Y289L. Strategy for the detection of a terminal GlcNAc residue in the N-linked glycoprotein by the mutant β4Gal-T1-Y289L. (A) In contrast to the wild-type β4Gal-T1, the mutant enzyme β4Gal-T1-Y289L transfers to the GlcNAc residues of the N-linked glycoprotein a Gal moiety, from the UDP derivative, that has modifications located at the C2 position of galactose, such as the N-acetyl moiety (GalNAc) (15) or a ketone moiety (21). (B) The C2 ketone moiety of the substituting group in galactose can then be coupled with aminooxy derivatives under mild conditions (21). (C) When aminooxy biotin (N-(aminooxyacetyl)-N′-(d-biotinoyl) hydrazine) is used as the coupling agent, the product can be labeled by streptavidin conjugated to horseradish peroxidase (HRP) and detected by a sensitive chemiluminescence method.
Transfer of C2 Keto Galactose from its UDP Derivative to GlcNAc Residues on the Glycan Chains of Ovalbumin and Asialo-Agalacto-IgG1 by the Mutant Enzyme β4Gal-T1-Y289L and Coupling of Aminooxy biotin to the Modified Sugar
The mutant enzyme, β4Gal-T1-Y289L, can transfer the C2 keto galactose from its UDP derivative to the GlcNAc residue on the N-glycan chain of ovalbumin (Figure 5A) or to an asialo-agalacto-IgG1 molecule (Figure 6A). The transfer of the modified sugar by the mutant enzyme is carried out at 30 °C for 3 h. Reactions were also performed in the absence of UDP donor (Figure 5B). The transfer has been followed by coupling to the ketone group at the C2 position of galactose, the biotinylated aminooxy ligand, followed by resolving proteins by SDS-PAGE analysis, transfer of proteins to nitrocellulose filters by Western blotting, and probing by streptavidin–HRP techniques. The transfer is strictly dependent on the presence of the donor and the mutant enzyme, as observed by the strong chemiluminescence of the transferred material (Figure 5B). The wild-type enzyme cannot utilize the C2 ketone derivative of the galactose (Figure 5B).
Figure 5.
Transfer of the C2 keto derivative of galactose to free GlcNAc residues on the N-glycan chains of ovalbumin by the mutant enzyme β4Gal-T1-Y289L and the chemoenzymatic detection of the transferred C2 keto derivative. (A) N-Glycan chain, attached to ovalbumin, has been shown using the symbols for monosaccharides as described in Figure 2. The C2 keto galactose (yellow spheres) is transferred to the GlcNAc residues (blue squares) at the nonreducing end of the glycan chain, by the mutant enzyme β4Gal-T1-Y289L. The mannose residues in ovalbumin are shown as the green spheres. An arrow shows the cleavage site for PNGase F. (B) Detection of the transferred C2 keto galactose is accomplished by linking with the aminooxy biotin, followed by the chemiluminescence technique. After the transfer of the sugar moiety by the transferase enzyme and subsequent linking to the aminooxy biotin, the mixtures were resolved by SDS-PAGE, transferred by Western blot to nitrocellulose membranes, and probed with streptavidin–HRP. In the absence of the sugar donor substrate (−), little signal was detected using either the wild-type enzyme β4Gal-T1 or the mutant enzyme β4Gal-T1-Y289L. The chemiluminescence was detected only in the samples that contained UDP-C2 keto galactose, mutant enzyme (+), and at least 5 ng of ovalbumin. The wild-type enzyme, β4Gal-T1, having Tyr at position 289, cannot transfer the modified C2 keto galactose. (C) PNGase F treatment of the biotinylated ovalbumin. Ovalbumin samples, after the transfer of the modified C2 keto galactose, were treated with PNGase F (Materials and Methods section), which removes the N-glycan chains from the protein. In contrast to untreated samples (−), in the PNGase F-treated samples (+) no chemiluminescence was detected, showing that the transfer of the modified C2 keto galactose is selective for the glycan portion of ovalbumin.
Figure 6.
Coupling to IgG via N-glycan chains. Selective labeling of the asialo-IgG1 molecule confirmed by PNGase F treatment. (A) Schematic diagram of an IgG molecule with the N-glycan structures attached at the Fc region. (B) The C2 keto galactose moiety was transferred to the carbohydrate in the Fc region of IgG1, using the mutant enzyme β4Gal-T1-Y289L. Mixtures were either treated without PNGase F (−) or with PNGase F (+) containing NP-40, prior to the coupling to aminooxy biotin. The mixtures were resolved by SDS-PAGE, transferred by Western blot to nitrocellulose membranes, and probed with streptavidin–HRP. No chemiluminescence was detected in PNGase F treated sample (+), showing that the transfer of the modified C2 keto galactose moiety is selective for the glycan portion of IgG1 and glycosylation has occurred in the heavy chain of IgG1 at the free GlcNAc residues (10, 25, and 50 ng).
Peptide: N-Glycosidase F (PNGase F) Treatment Removes N-Glycan Chains with Modified Sugar on Glycoproteins
The biotinylated aminooxy ligand is linked only to the N-glycan chain of ovalbumin or IgG1, as shown by PNGase F treatment, which removes N-glycan chains from the protein. No chemiluminescence was detected at ovalbumin or IgG1 protein bands in the Western blot analysis, suggesting that the transferred C2 keto-modified galactose was linked only to the released carbohydrate moiety (Figures 5C and 6B).
DISCUSSION
This study has shown that it is possible to modify free GlcNAc residues on two N-linked glycoproteins, ovalbumin and immunoglobulin, by enzymatic methods. These modifications have only been possible due to the availability of the structural information of glycosyltransferases which helped to design mutant enzymes that can transfer unnatural sugar donors (15, 21, 30). Here, we show that the transfer is strictly dependent on the presence of both the mutant enzyme β4Gal-T1-Y289L and the ketone derivative of the galactose, while the wild-type enzyme cannot transfer the galactose with the C2 ketone moiety. We also show that the modifications occur only at the N-glycan chains of the glycoproteins, as indicated by PNGase F treatment.
The results of the transfer of 3H-labeled GalNAc by β4Gal-T1-Y289L to several linear and N-linked branched oligosaccharides concur with the results obtained by the MALDI mass spectral analysis. Also, the results obtained by the chemoenzymatic method are consistent with the MALDI mass spectral analysis. The efficiency of transfer of GalNAc to various linear oligosaccharide acceptors was nearly 100%. The length of the oligosaccharide acceptor did not affect the transfer efficiency of GalNAc. However, when branched oligosaccharide acceptors are used in the enzymatic assay, the efficiency of transfer of GalNAc is reduced. Comparison of the MALDI profiles before and after addition of GalNAc and C2 keto galactose indicated that only one molecule of GalNAc is transferred to one of the two GlcNAc residues available for the transfer on the pentasaccharide, GlcNAcβ1,2-Manα1,6-(GlcNAcβ1,2-Manα1,3)-Man and the heptasaccharide tetrapeptide, Arg-[GlcNAcβ1,2-Manα1,6-(GlcNAcβ1,2-Manα1,3)-Manβ1,4-GlcNAcβ1,4-GlcNAcβ]-Asn-Glu-Gly. Both enzymatic and MALDI analysis showed that GalNAc is transferred to only 50% of the available GlcNAc residues in the branched structures. This may be explained by the results of a previous work from our laboratory using kinetic and crystallographic analysis, which showed that, at lower concentrations, the 1,2–1,6-arm of a biantennary N-glycan is a preferred antenna for galactosylation by β4Gal-T1 (27). In contrast, at higher concentrations, the 1,2–1,3-arm of a biantennary N-glycan is a preferred antenna for galactosylation (unpublished results).
When the acceptor is ovalbumin, the transfer efficiency is reduced to 37%. This can be explained by the known structural variability of N-glycans on ovalbumin preparations as has been previously observed (29). The largest ovalbumin structure found by MALDI mass spectral analysis was a galactosylated hybrid glycan of composition (Man)3(GlcNAc)7(GalNAc)1 (2154.1 m/z; see Figure 3B).
Having demonstrated the labeling of ovalbumin by enzymatic and MALDI mass analysis, we explored the sensitivity of the chemoenzymatic approach for the detection of N-linked modifications on ovalbumin. This chemoenzymatic method enabled the detection of N-linked modifications within minutes using as little as 5 ng of ovalbumin and 10 ng of IgG1. This study also shows the possibility of modifying the single N-linked glycosylation site at Asn297 on the Fc domain of IgG1. Although some glycosylation may occur outside the Fc domain, this is usually a rare event (31). The glycans that are linked to Asn297 of IgG1 have been characterized on the basis of the number of terminal galactoses into three groups, IgG1-G2, IgG1-G1, and IgG1-G0 (32). In the IgG1-G0, the glycans do not have terminal galactose residues, but instead have terminal GlcNAc residues, as in the heptasaccharide tetrapeptide used in this study. Only 5% to 14% of the total serum IgG1 is fully sialylated (32, 33), and generally, IgG1-G2, IgG1-G1, and IgG1-G0 fractions are present in 16%, 35%, and 35% of the total IgG glycan pool, respectively (32). Here, we show that the C2 keto galactose moiety can be transferred from its UDP derivative to the free GlcNAc residues on the N-linked glycan chains of the IgG1 molecules. The transfer was followed by coupling the ketone handle to an aminooxy biotin, which was then detected by a sensitive chemiluminescence assay (21).
The method of coupling a target agent to a carrier protein via glycan chains, as described here using ovalbumin and IgG1 as model systems, offers an advantage over other cross-linking methods. Here, the target agent is linked in a site-directed manner, only where the carbohydrate is attached to the glycoprotein, as in the IgG1 molecule at the Fc domain, away from the antigen binding site. The problem common to previous approaches using monoclonal antibodies for immunotherapy is the lack of specificity of the reactions, resulting in heterologous labeling and a decrease in the antibody affinity for the antigen.
The currently described method also represents a sensitive and effective approach to monitor the glycosylation of therapeutic glycoproteins and monoclonal antibodies. The potential of glycosyltransferase mutants to produce glycoconjugates carrying sugar moieties with reactive groups may be a benefit to the glycotargeting of drugs to their site of action. The role of glycotargeting in the internalization of drugs has been established by numerous studies (34, 35) since the discovery of the asialoglycoprotein receptor (36); however, much of its potential remains unexplored. Although a great number of pharmaceutical agents are discovered each year, the clinical application of these is many times hindered because of failure to reach the site of action. Our approach using mutant glycosyltransferases to transfer chemically reactive sugar residues for linking of other molecules via specific glycan chains has the potential for development of an efficient drug delivery system. A word of caution is that there is still the challenge to up-scale the production of chimeric proteins needed in pharmaceutical settings.
ACKNOWLEDGMENT
We thank Drs. Dimiter S. Dimitrov, Mei-Yun Zhang, and Yang Feng from the Nanobiology program, CCR, NCI, for providing IgG1samples, and Dr. Maria Manzoni from the Structural Glycobiology Section, CCR, NCI, for the critical reading of the manuscript. This project has been funded in whole or in part with federal funds from the National Cancer Institute, National Institutes of Health, under contract N01-CO-12400. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products or organizations imply endorsement by the U.S. Government. This Research was supported in part by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research.
LITERATURE CITED
- 1.Lowe JB. Glycan-dependent leukocyte adhesion and recruitment in inflammation. Curr. Opin. Cell. Biol. 2003;15:531–538. doi: 10.1016/j.ceb.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 2.Talbot P, Shur BD, Myles DG. Cell adhesion and fertilization: steps in oocyte transport, sperm-zona pellucida interactions, and sperm-egg fusion. Biol. Reprod. 2003;68:1–9. doi: 10.1095/biolreprod.102.007856. [DOI] [PubMed] [Google Scholar]
- 3.Roth S, McGuire EJ, Roseman S. Evidence for cell-surface glycosyltransferases. Their potential role in cellular recognition. J. Cell Biol. 1971;51:536–547. doi: 10.1083/jcb.51.2.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hakamori S The glycosynapse. Proc. Natl. Acad. Sci. U.S.A. 2002;99:225–232. doi: 10.1073/pnas.012540899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hakomori S. Carbohydrate-to carbohydrate interaction, through glycosynapse, as a basis of cell recognition and membrane organization. Glycoconjugate J. 2004;21:125–137. doi: 10.1023/B:GLYC.0000044844.95878.cf. [DOI] [PubMed] [Google Scholar]
- 6.Rudd PM, Elliott T, Cresswell P, Wilson IA, Dwek RA. Glycosylation and the immune system. Science. 2001;291:2370–2376. doi: 10.1126/science.291.5512.2370. [DOI] [PubMed] [Google Scholar]
- 7.Sharon N, Lis H. History of lectins: from hemagglutinins to biological recognition molecules. Glycobiology. 2004;14:53R–62R. doi: 10.1093/glycob/cwh122. [DOI] [PubMed] [Google Scholar]
- 8.Bucior I, Burger MM. Carbohydrate-carbohydrate interaction as a major force initiating cell-cell recognition. Glycoconjugate J. 2004;21:111–123. doi: 10.1023/B:GLYC.0000044843.72595.7d. [DOI] [PubMed] [Google Scholar]
- 9.Gornik O, Dumic J, Flogel M, Lauc G. Glycoscience: a new frontier in rational drug design. Acta Pharm. 2006;56:19–30. [PubMed] [Google Scholar]
- 10.Raman R, Sasisekharan V, Sasisekharan R. Structural insights into biological roles of protein-glycosaminoglycan interactions. Chem. Biol. 2005;12:267–277. doi: 10.1016/j.chembiol.2004.11.020. [DOI] [PubMed] [Google Scholar]
- 11.Lowe JB, Marth JD. A genetic approach to mammalian glycan function. Annu. Rev. Biochem. 2003;72:643–691. doi: 10.1146/annurev.biochem.72.121801.161809. [DOI] [PubMed] [Google Scholar]
- 12.Qasba PK, Ramakrishnan B, Boeggeman E. Substrate-induced conformational changes in glycosyltransferases. Trends Biochem. Sci. 2005;30:53–62. doi: 10.1016/j.tibs.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 13.Amado M, Almeida R, Schwientek T, Clausen H. Identification and characterization of large galactosyltransferase gene families: galactosyltransferases for all functions. Biochim. Biophys. Acta. 1999;1473:35–53. doi: 10.1016/s0304-4165(99)00168-3. [DOI] [PubMed] [Google Scholar]
- 14.Hennet T. The galactosyltransferase family. Cell. Mol. Life Sci. 2002;59:1081–1095. doi: 10.1007/s00018-002-8489-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ramakrishnan B, Qasba PK. Structure-based design of beta-1,4-galactosyltransferase-I (beta 4Gal-T1) with equally efficient N-acetylgalactosaminyltransferase activity: point mutation broadens beta 4Gal-T1 donor specificity. J. Biol. Chem. 2002;277:20833–20839. doi: 10.1074/jbc.M111183200. [DOI] [PubMed] [Google Scholar]
- 16.Ramakrishnan B, Boeggeman E, Ramasamy V, Qasba PK. Structure and catalytic cycle of beta-1,4-galactosyltransferrase. Curr. Opin. Struct. Biol. 2004;14:593–600. doi: 10.1016/j.sbi.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 17.Marcus SL, Polakowski R, Seto NOL, Leinala E, Borisova S, Blancher A, Roubinet F, Evans SV, Palcic MM. A single point mutation reverses the donor specificity of human blood group B-synthesizing galactosyltransferase. J. Biol. Chem. 2003;278:12403–12405. doi: 10.1074/jbc.M212002200. [DOI] [PubMed] [Google Scholar]
- 18.Pedersen LC, Darden TA, Negishi M. Crystal structure of β1,3 glucuronyltransferase I in complex with active donor substrate UDP-GlcUA. J. Biol. Chem. 2002;277:21869–21873. doi: 10.1074/jbc.M112343200. [DOI] [PubMed] [Google Scholar]
- 19.Kakuda S, Shiba T, Ishiguro M, Tagawa H, Oka S, Kajihara Y, Kawasaki T, Wakatsuki S, Kato R. Structural basis for acceptor substrate recognition of a human glucuronyltransferase, GlcAT-P, an enzyme critical in the biosynthesis of the carbohydrate epitope HNK-1. J. Biol. Chem. 2004;279:22693–22703. doi: 10.1074/jbc.M400622200. [DOI] [PubMed] [Google Scholar]
- 20.Torres CR, Hart GW. Topography and polypeptide distribution of terminal N-acetylglucosamine residues on the surfaces of intact lymphocytes. J. Biol. Chem. 1984;259:3308–3317. [PubMed] [Google Scholar]
- 21.Khidekel N, Arndt S, Lamarre-Vincent N, Lippert A, Poulin-Kerstien KG, Ramakrishnan B, Qasba PK, Hsieh-Wilson LC. A chemoenzymatic approach toward the rapid and sensitive detection of O-GlcNAc posttranslational modifications. J. Am. Chem. Soc. 2003;125:16162–16163. doi: 10.1021/ja038545r. [DOI] [PubMed] [Google Scholar]
- 22.Qasba PK, Ramakrishnan B, Boeggeman E. Mutant glycosyltransferases assist in the development of a targeted drug delivery system and contrast agents for MRI. AAPS J. 2006;8:E190–E195. doi: 10.1208/aapsj080123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boeggeman EE, Balaji PV, Sethi N, Masibay AS, Qasba PK. Expression of deletion constructs of bovine β1,4-galactosyltransferase in Escherichia coli: importance of Cys134 for its activity. Protein Eng. 1993;6:779–785. doi: 10.1093/protein/6.7.779. [DOI] [PubMed] [Google Scholar]
- 24.Ramakrishnan B, Shah PS, Qasba PK. Alpha-Lactalbumin (α-LA stimulates milk beta-1,4-galactosyltransferase I (beta 4Gal-T1) to transfer glucose from UDP-glucose to N-acetylglucosamine. Crystal structure of beta 4Gal-T1 × α-LA complex with UDP-Glc. J. Biol. Chem. 2001;276:37665–37671. doi: 10.1074/jbc.M102458200. [DOI] [PubMed] [Google Scholar]
- 25.Boeggeman E, Ramakrishnan B, Qasba PK. The N-terminal stem region of bovine and human β1,4-galactosyltransferase I increases the in vitro folding efficiency of their catalytic domain from inclusion bodies. Protein Expression Purif. 2003;30:219–220. doi: 10.1016/s1046-5928(03)00093-7. [DOI] [PubMed] [Google Scholar]
- 26.Boeggeman E, Qasba PK. Studies on the metal binding sites in the catalytic domain of β1,4-galactosyltransferase. Glycobiology. 2002;12:395–407. doi: 10.1093/glycob/cwf045. [DOI] [PubMed] [Google Scholar]
- 27.Ramasamy V, Ramakrishnan B, Boeggeman E, Ratner DM, Seeberger PH, Qasba PK. Oligosaccharide preferences of beta1,4-galactosyltransferase-I: crystal structures of Met340His mutant of human beta1,4-galactosyltransferase-I with a pentasaccharide and trisaccharides of the N-glycan moiety. J. Mol. Biol. 2005;353:53–67. doi: 10.1016/j.jmb.2005.07.050. [DOI] [PubMed] [Google Scholar]
- 28.Powell JT, Brew K. A comparison of the interactions of galactosyltransferase with a glycopropein substrate (ovalbumin) and with α-lactalbumin. J. Biol. Chem. 1976;251:3653–3663. [PubMed] [Google Scholar]
- 29.Harvey DJ, Wing DR, Kuster B, Wilson IBH. Composition of N-linked carbohydrates from ovalbumin and co-purified glycoproteins. J. Am. Soc. Mass Spectrom. 2000;11:654–571. doi: 10.1016/S1044-0305(00)00122-7. [DOI] [PubMed] [Google Scholar]
- 30.Ramakrishnan B, Boeggeman E, Qasba PK. Mutation of arginine 228 to lysine enhances the glucosyltransferase activity of bovine beta-1,4-galactosyltransferase I. Biochemistry. 2005;44:3202–3210. doi: 10.1021/bi0479454. [DOI] [PubMed] [Google Scholar]
- 31.Parekh RB, Dwek RA, Sutton BJ, Fernandes DL, Leung A, Stanworth D, Rademacher TW, Mizuochi T, Taniguchi T, Matsuta K, Takeuchi F, Nagano Y, Miyamoto T, Kobata A. Association of rheumatoid arthritis and primary osteoarthritis with changes in the glycosylation pattern of total serum IgG. Nature (London) 1985;316:452–457. doi: 10.1038/316452a0. [DOI] [PubMed] [Google Scholar]
- 32.Arnold JN, Dwek RA, Rudd PM, Sim RB. Mannan binding lectin and its interaction with immunogloblulins in health and in disease. Immunol. Lett. 2006;106:103–110. doi: 10.1016/j.imlet.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 33.Kaneko Y, Nimmerjahn F, Ravetch JV. Anti-inflammatory activity of immunoglobulin G resulting from Fc sialylation. Science. 2006;313:670–673. doi: 10.1126/science.1129594. [DOI] [PubMed] [Google Scholar]
- 34.Rogers JC, Kornfeld S. Hepatic uptake of proteins coupled to fetuin glycopeptide. Biochem. Biophys. Res. Commun. 1971;45:1461–1467. doi: 10.1016/0006-291x(71)90462-1. [DOI] [PubMed] [Google Scholar]
- 35.Hangeland JJ, Levis JT, Lee YC, Tso POP. Cell-type specific and ligand specific enhancement of cellular uptake of oligodeoxynucleoside methylphosphonates covalently linked with a neoglycopeptide, YEE (ah-GalNAc) Bioconjugate Chem. 1995;6:695–701. doi: 10.1021/bc00036a006. [DOI] [PubMed] [Google Scholar]
- 36.Tanabe T, Pricer WE, Jr, Ashwell G. Subcellular membrane topology and turnover of a rat hepatic binding protein specific for asialoglycoproteins. J. Biol. Chem. 1979;254:1038–1043. [PubMed] [Google Scholar]