Summary
The conserved transcriptional regulator Heat Shock Factor 1 (Hsf1) is a key sensor of proteotoxic and other stress in the eukaryotic cytosol, yet its regulation is poorly understood. We surveyed Hsf1 activity in a genome-wide loss-of-function library in Saccaromyces cerevisiae as well as ~78,000 double mutants and found Hsf1 activity to be modulated by highly diverse stresses. These included disruption of a ribosome-bound complex we named the Ribosome Quality Control Complex (RQC) comprising the Ltn1 E3 ubiquitin ligase, two highly conserved but poorly characterized proteins (Tae2 and Rqc1), and Cdc48 and its cofactors. Electron microscopy and biochemical analyses revealed that the RQC forms a stable complex with 60S ribosomal subunits containing stalled polypeptides and triggers their degradation. A negative feedback loop regulates the RQC and Hsf1 senses an RQC-mediated translation stress signal distinctly from other stresses. Our work reveals the range of stresses Hsf1 monitors and elucidates a conserved cotranslational protein quality control mechanism.
Introduction
Proteins are monitored by quality control processes from the moment they emerge from the ribosome (Albanese et al., 2006; Craig et al., 2003; Mariappan et al., 2010) until they are ultimately targeted for degradation. For some proteins this timeline is abbreviated, as defective nascent polypeptides can be shunted to the proteasome before synthesis of the full-length protein is complete (Gottesman et al., 1998; Inada and Aiba, 2005; Moore and Sauer, 2007; Piatkov et al., 2012; Turner and Varshavsky, 2000; Yewdell et al., 1996). In yeast, cotranslational degradation processes target protein products from defective mRNA or aborted translation (Ito-Harashima et al., 2007). These processes employ conserved E3 ligases, including the Ccr4/Not complex (Dimitrova et al., 2009) and Listerin (Ltn1) (Bengtson and Joazeiro, 2010). Deletion of LTN1 results in accumulation of abortive translation products in yeast and a hypomorph of the mouse ortholog results in neurodegeneration (Chu et al., 2009), suggesting an important role for cotranslational degradation in higher eukaryotes. Despite these findings, the mechanisms by which protein quality is monitored during translation and how they integrate with the larger suite of protein quality control mechanisms remain poorly understood.
The central coordinator of eukaryotic protein quality control in the cytosol is the conserved transcription factor Heat Shock Factor (Hsf1). Hsf1 detects a diverse group of cellular stresses, including proteotoxic stress (Jolly et al., 1999), oxidative stress (Ahn and Thiele, 2003), and glucose starvation (Hahn and Thiele, 2004). Hsf1 modulates expression of a wide range of stress response genes, including those mediating protein folding and degradation, energy generation, chemical detoxification, and metabolic activities (Hahn et al., 2004). Hsf1 is essential for viability in budding yeast and absence of its ortholog in mice has a range of effects (Christians and Benjamin, 2006), including neurodegeneration and resistance to acquiring cancer (Dai et al., 2007), whereas overexpression leads to increased lifespan in worms (Hsu et al., 2003). Hsf1 is functionally conserved to the degree that a human-derived isoform complements HSF1 deletion in yeast (Liu et al., 1997).
Despite the identification of several regulators of Hsf1 and stresses sensed by Hsf1 (Ali et al., 1998; Hahn and Thiele, 2004; Westerheide et al., 2009; Xavier et al., 2000), a holistic understanding of the stresses that activate Hsf1 and the mechanisms that integrate them is lacking. We set out to study Hsf1 by developing a fluorescent reporter for Hsf1 activity and measuring its activation under a variety of genetic perturbations. We then systematically examined the functional relationship between these perturbations by measuring Hsf1 activity in roughly 78,000 double mutants.
Our analyses revealed a previously-uncharacterized stress response arising from disruption of translation-related genes and identified a ribosome-bound complex composed of Ltn1, the AAA ATPase Cdc48 (Stolz et al., 2011), and two poorly characterized yet conserved proteins (Tae2 and Ydr333C). This complex, which we term the Ribosome Quality Control Complex (RQC), plays at least two critical roles: to degrade stalled polypeptides, and to signal stress to Hsf1 via a pathway independent of and distinct from other stresses. RQC activity is tuned by a conserved autoregulatory loop. Our work clarifies a mechanism of eukaryotic cotranslational degradation and its integration into the larger suite of protein quality control processes.
Results
A Genome-Wide Screen Reveals that Hsf1 Senses Diverse Stress Conditions
We modeled our investigation of Hsf1 after an approach we developed to explore the unfolded protein response in the endoplasmic reticulum (ER) of budding yeast (Jonikas et al., 2009). There we used the synthetic genetic array strategy developed by Boone and colleagues (Tong et al., 2001) to cross a quantitative fluorescent reporter of ER protein folding state into a library of loss-of-function alleles. We then used synergistic phenotypes arising from pairwise combinations of alleles to organize genes into functional pathways (Jonikas et al., 2009). Similarly, in this work we created a fluorescent sensor of Hsf1 activity using a synthetic promoter containing four adjacent Hsf1 binding sites (called Heat Shock Elements or HSE) (Sorger and Pelham, 1987), and used this reporter to drive expression of GFP (Figure 1A). To internally control for sources of cell-to-cell variability that affect expression of all genes (e.g. cell size), we included a second reporter in which RFP is driven by the constitutive TEF2 promoter. Measured using a flow cytometry in a wild-type strain, our HSE reporter showed basal activation at 25°C that was 8-fold above autofluorescent background levels. We then introduced the reporter into a full genome library of loss-of-function alleles. The library included both deletions of non-essential genes (Giaever et al., 2002) and hypomorphic DAmP alleles of many essential genes (Breslow et al., 2008). We measured the Hsf1 activity in each loss-of-function background using a previously described high-throughput flow cytometry system (Jonikas et al., 2009; Newman et al., 2006).
Figure 1. A genome-wide screen reveals that Hsf1 senses diverse stress conditions.
(A) Schema for fluorescent Hsf1 reporter. An RFP driven by the TEF2 promoter and a GFP driven by a synthetic promoter with multiple Hsf1 binding sites were integrated in the URA3 locus of the yeast genome. (B) Independent crosses of reporter strain into 731 loss-of-function alleles (selected hits from full genome screen) shows allelic variation and reproducibility of the reporter system. (C) Selected categories of annotated functions from genome-wide screen. Red bars indicate number of strains below a standard deviation for selected categories. Genes beyond one standard deviation are labeled as space is available, with full results in Table S1. (D) Hsf1 and Msn2/4 activities with genome-wide library of alleles at steady state (25°C) and after 1 hour heat shock at 37°C. P-values from student’s t-test show enrichment for selected categories in each quadrant (delimited by 1 standard deviation from the median). (E) Effects of constitutively active hsf1 allele (hsf1*) and msn2 allele (msn2*) on Hsf1 and Msn2/4 activities at 25°C. Also see Tables S1 and S2 and Figure S1.
We found Hsf1 activity is modulated by disruption of many genes (Figure 1B, Table S1) from a large number of functional classes (Figure 1C). Prominent among the strong Hsf1 activators were ‘stressor’ alleles that would be predicted to increase the amount of misfolded protein in the cytosol, consistent with Hsf1’s role in monitoring cytosolic protein folding state. These stressors include deletion of chaperones, proteasomal components, and components of the ER translocon. Chaperones aid in the folding and disaggregation of proteins, however they may also play a more direct role in regulating Hsf1. For example, the chaperone Hsp90 directly inhibits Hsf1 under specific conditions (Ali et al., 1998). Loss of individual chaperones did not always increase Hsf1 activity, and in some cases decreased it. Most of these cases involved components that functioned in translation or the mitochondria (discussed below). Compromising the proteasome disrupts clearance of terminally ubiquitylated proteins, and also leads to strong activation of Hsf1. Similarly, disrupting translocation of proteins from the cytosol to the ER, either by blocking insertion of tail anchored proteins into the ER membrane or disrupting the translocon or alternative translocon (Figure 1C), increased Hsf1 activity. This is likely caused by accumulation of ER-destined proteins in the cytosol, which generally possess aggregation-prone hydrophobic transmembrane domains and signal sequences.
A separate class of perturbations that strongly affected Hsf1 activity is deletion alleles of chromatin-associated proteins. Loss of members of the Chromatin Assembly Complex (CAF-1), Histone Regulatory complex (HIR), or a copy of the histone H4 gene strongly upregulated Hsf1. By contrast, deleting members of the ADA or SAGA histone acetyltransferase complexes or the SWI/SNF nucleosome remodeling complex, which increase access to chromatin to make it more available to transcription factors, strongly decreased Hsf1 activity. These observations are consistent with previous reports highlighting SAGA’s and SWI/SNF’s roles in opening chromatin at targets of Hsf1 (Kremer and Gross, 2009; Zhao et al., 2005) and a general role for histone-mediated gene silencing at these target genes (Fritah et al., 2009), and therefore our GFP reporter.
Stressor Alleles Have Diverging Behavior When Subject to Different Conditions
Functional annotation of the hits from the fluorescent Hsf1 activity screen under normal growth conditions revealed that genes with differing functions had similar effects on Hsf1. For example, deleting either translation-related or mitochondrial genes lowered Hsf1 activity to similar degrees. To determine whether the Hsf1 phenotypes for different stressors behaved uniformly across environmental conditions, we performed a second genome-wide screen after a mild 1 hour heat shock at 37°C (Figure 1D). This second screen revealed that mitochondrial and translation alleles had different Hsf1 phenotypes, with strains harboring mitochondrial alleles less sensitive to heat shock. Alleles that increased Hsf1 activity also separated into two classes; hypoinducers were enriched for alleles inducing folding stress (chaperones and proteasomal components) and chromatin modifiers (Table S2), while the hyperinducers were enriched for positive regulators of the growth-promoting Protein Kinase A (PKA).
We wondered whether the four classes of strains identified by the temperature conditions had distinct underlying mechanisms for modulating Hsf1 activity. In yeast, PKA suppresses a general stress response mediated by the transcriptional regulators Msn2 and Msn4 (Gorner et al., 1998). To investigate the four groups we identified, we therefore introduced a GFP-based Msn2/4 reporter (designed similarly to our Hsf1 reporter), and performed additional genome-wide screens at 25°C and 37°C. These screens revealed that Msn2/4 activity was constitutively upregulated by mitochondrial alleles yet generally unperturbed by translation alleles (Figure 1D, S1). Alleles that hyperinduce Hsf1 upon heat stress suppressed Msn2/4 in the same condition, suggesting an inverse relationship between Hsf1 and Msn2 in some alleles. Supporting this, expression of a constitutively active Msn2 allele lowered Hsf1 activity (Figure 1E, S1). These observations underscore the potential of these two responses to act in concert to protect a cell from stress. Future work will elucidate whether communication between Hsf1-dependent and Msn2/4-dependent stress responses is direct, indirect, or mediated by PKA. These experiments reveal how exposing the library of strains to different conditions segregates them into distinct classes that likely differ in the mechanism by which they regulate Hsf1.
The Translation Stress Genetic Interaction Network Identifies Ltn1/Rqc1
To obtain a higher-resolution view of the different stresses affecting Hsf1, we chose approximately 700 alleles for follow-up study. We crossed 107 alleles with 731 alleles from the panel of deletions and hypomorphs used in our primary Hsf1 screens, yielding ~78,000 double mutants. These alleles were selected for their strong effect, inhibiting or activating, Hsf1. We then compared Hsf1 activity in each double mutant to what we would expect given the activities of the single mutants. These genetic interactions quantify the deviation between the actual and expected Hsf1 activity thus reveal specific functional relationships between genes (Figure 2A). The pattern of genetic interactions for a given gene provides a quantitative phenotype that can be used with hierarchical clustering to identify other genes with common functions (interaction map available at http://yeastquantitativegenetics.ucsf.edu:8000/DataBrowser).
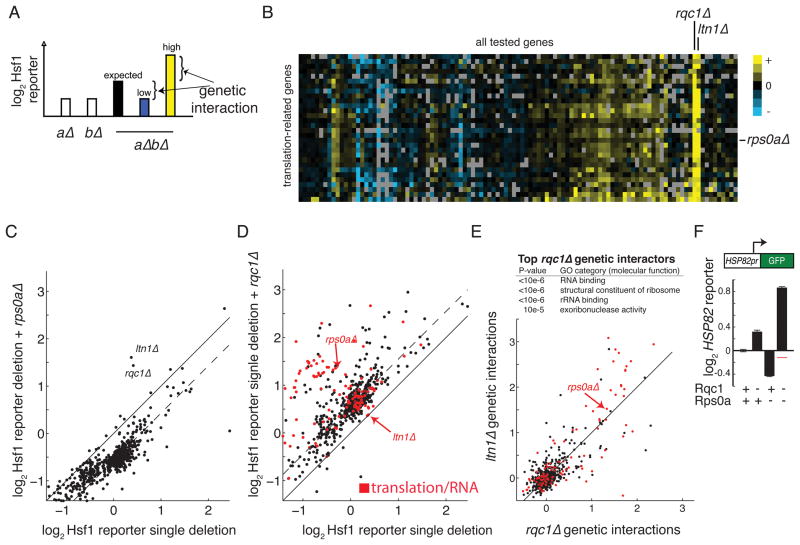
Figure 2. Translation stress identifies Ltn1 and Rqc1.
(A) Schematic diagram illustrating strategy for quantifying genetic interactions in double mutant strains. An expected value for the double mutant is first computed based on single mutant reporter levels. The expected value is then compared to the actual double mutant value to arrive at a genetic interaction score. Positive genetic interactions (double mutant is higher than expected) are colored in yellow while negative genetic interactions (double mutant is lower than expected) are colored in blue. (B) Genetic interactions corresponding to a set of translation-related genes that clustered together in a genetic interaction map. (C–D) Hsf1 activity levels in single and double mutant strains. The y-axis of each graph shows double mutant values for a common mutant (rps0aΔ or rqc1Δ) combined with diverse alleles. Single mutant values appear on the x-axis. (E) Comparison of Ltn1 and Rqc1 genetic interaction scores. Inset: Top enriched Gene Ontology (GO) categories of positive interactors with rqc1Δ from full genome screen (see Tables S1 an S3). (F) HSP82 reporter levels showing a positive genetic interaction between rqc1Δ and rps0aΔ (red line denotes expected value of double mutant). Translation and RNA related genes are marked in red in (D–E).
The genetic interaction map revealed numerous groups of similarly behaving alleles, and in many cases these clusters were made up of genes with related functional annotations. Among the largest of these was a translation-related group (Figures 2B). Notably, this cluster featured strong interactions with the poorly characterized but highly conserved protein Ydr333c, which we name here Ribosome Quality Control 1 (Rqc1), and the ribosome bound E3 ligase Ltn1 (Bengtson and Joazeiro, 2010; Fleischer et al., 2006). We show the full interactions for rps0aΔ, which encodes for a core component of the 40S ribosomal subunit as an example of translation-related gene (Figure 2C), and rqc1Δ (Figure 2D). ltn1Δ and rqc1Δ are clearly visible outliers, indicating strong genetic interactions with rps0aΔ. ltn1Δ and rqc1Δ had a highly similar interaction profile (Figure 2E). Measuring rqc1Δ interactions across the full set of mutant alleles revealed that its interactors were enriched for genes with translation, RNA-related, or proteasomal function (Figure 2C, E, Table S3). Activity levels of the HSP82 promoter, which includes an HSE sequence, mimicked the activity of our fluorescent reporter insofar as they showed a similar positive interaction between rqc1Δ and a translation allele (Figure 2F). Therefore our ltn1Δ and rqc1Δ interaction data are unlikely to be an artifact of our synthetic reporter system.
Rqc1/Ltn1 Are Members of a Larger Ribosome-Bound Complex (RQC)
To determine the roles of Ltn1 and Rqc1 in preventing translational stress, we sought to identify their binding partners. We therefore immunoprecipitated a functional version of Rqc1 (see Figure 4D below) that contained a C-terminal 3xFLAG epitope expressed from its endogenous genomic locus. A large complex co-purified with Rqc1, which we named the Ribosome Quality Control Complex (RQC) (Figures 3A–B, S2A–B). Identification of individual bands by mass spectrometry revealed Rqc1, Ltn1, and the conserved and poorly characterized protein Tae2. Based on coomassie staining intensity, Rqc1, Ltn1, and Tae2 appear nearly stochiometric. However, the most abundant band in the sample was Cdc48, an AAA+ ATPase protein characterized for its roles as a molecular force generator (Stolz et al., 2011). Cdc48 acts as a hexamer (Kondo et al., 1997), and its cofactors Ufd1 and Npl4 (Ye et al., 2001) were also present at lower amounts in the sample, consistent with the expected 1:6 stoichiometry. Also prominent in the IP were protein components of the 60S ribosomal subunit (36 out of 42 total (Klinge et al., 2011) 60S ribosomal proteins were identified). In marked contrast, only 4 members of the 40S ribosomal subunit components were detected (including Asc1, which appears later in this study) and in each case they were found in gel bands that predominantly contained other proteins and thus are likely substoichiometric.species. The material from the Rqc1 IP sedimented with 60S ribosomes on a sucrose gradient, despite the large excess of 80S in the input material to this IP (Figure 3C). However, since it is possible that the Rqc1 associates with polysomes that were lost during initial clarificaton steps, we also performed the IP using RNAase-digested input, which collapsed polysomes into monosomes. The 60S subunit similarly predominated in the resulting IP (Figure S2C–D). These results and subsequent electron microscopy analyses (see below) indicate that the RQC predominantly associates with 60S ribosomes although they does not exclude the possibility that it transiently associates with the 80S prior to subunit disassociation. Interestingly, K48-linked ubiquitin, a degradation signal, was found in each excised gel lane. IPs of a C-terminally FLAG-tagged Tae2 protein yielded results qualitatively similar to Rqc1 (Figures 3B, S2B).
Figure 4. Rqc1 levels are autoregulated by a conserved negative feedback loop.

(A) GFP levels in strains expressing a contranslationally degraded polybasic reporter subject to 10 hours cycloheximide treatment at the indicated concentration. (B) Ribosome footprint density at endogenous polybasic stretches (6 or greater K or R per 10 residues, N=103). (C) Conservation of Rqc1, with polybasic and TCF25 (Bateman et al., 2004) domains highlighted. (D) Assay showing the ability of Rqc1 alleles (WT, rqc1-FLAG, rqc1ala-FLAG, rqc1Δ) to act upon a model cotranslationally degraded substrate. (E) rqc1-FLAG and rqc1ala-FLAG protein levels in deletion strains. (F) Results of screen for regulators model polybasic substrate (full results in Table S1). tae2Δ and the four strongest hits labeled. (G) GFP and RFP levels of model polybasic substrate in selected hits from full-genome screen. Also see Table S4 and Figure S5.
Figure 3. Rqc1/Ltn1 organize a larger cotranslational quality control complex.
(A) Immunoprecipation (IP) of endogenous Rqc1 3xFLAG fusion protein viewed with Coomassie staining. Selected non-background bands identified by mass spectrometry are labeled (all identified non-background bands available in Figure S2) (B) Silver staining of Rqc1 and Tae2 IPs in selected deletion backgrounds and with cycloheximide (CHX) (100 ug/ml, added 2 min before harvesting). Below, western blot for Cdc48 in IPs along with quantified amounts (Cdc48/FLAG). (C) RNA absorbance (260 nm) of 10–50% sucrose gradient for input and output of Rqc1 IP. Each trace is independently scaled. (D) Class averages of particles selected from electron micrographs of negatively stained Rqc1-FLAG IP in WT, tae2Δ and ltn1Δ strain backgrounds. (E) Western blot of a cotranslationally degraded model substrate containing a polybasic region in selected genetic backgrounds. (F) GFP levels in samples from (E) measured using a flow cytometer and normalized to control. (G) Western blot for mono-ubiquitin in IP samples. Also see Figures S2–4.
To determine which components physically bound one another, we immunoprecipitated Rqc1 and Tae2 in strains harboring deletions of various RQC members. (Figure 3B). Both Rqc1 and Ltn1 were required for recruitment of Cdc48 and its cofactors to the RQC. Deletion of Ltn1’s RING domain also abolished this recruitment (Figure S2E–F) but did not prevent association of Ltn1 with the RQC. This suggests that Ltn1-mediated ubiquitylation is required for recruitment of Cdc48. Notably, inclusion of Ltn1, Rqc1, and Tae2 in the RQC was not abolished by deletion of other RQC components, suggesting that these players do not recruit each other or act as scaffolds for each other.
Structural Analysis of the RQC Complex
To obtain insight into the mechanism of RQC function, we utilized negative stain electron microscopy and image analysis to visualize the immunoprecipitated complex directly. Raw electron micrographs revealed abundant 60S ribosome particles associated with objects the size and shape of Cdc48 hexamers (Pye et al., 2007) (Figures 3D, S3A–B). Analysis of particles from ltn1Δ or tae2Δ strains, both of which lead to loss of Cdc48 in the RQC IPs, provided additional support for the assignment of this density to Cdc48. Two-dimensional clustering, alignment, and averaging of both WT and the ltn1Δ or tae2Δ particles revealed additional density both adjacent and distal to the putative Cdc48 density which likely correspond to other RQC factors (Figure 3D, S3A, see Figure S3C for a gallery of raw particles). These observations are consistent with the biochemical data indicating that Cdc48 binding to 60S ribosomal subunits is mediated by the RQC. Furthermore, the well-defined orientation of Cdc48 and other RQC members on the ribosome makes the RQC an excellent candidate for future structural and mechanistic biochemical studies.
The RQC Targets Nascent Polypeptides for Proteasomal Degradation
Ltn1 and Tae2 have both been previously reported to mediate degradation of protein products translated from a reporter mRNA purposely designed without a stop codon (Alamgir et al., 2010; Bengtson and Joazeiro, 2010). Translation of poly-A tails leads to production of poly-lysine tracts, which mark transcripts as non-stop and trigger their destruction of the RNA via non-stop decay (Inada and Aiba, 2005) and the nascent chain via the proteasome (Ito-Harashima et al., 2007). It was further discovered that any strong polybasic tract embedded in the middle of a model substrate could trigger its destruction, and therefore that electrostatic charge was sufficient to engage the cotranslational quality control system (Dimitrova et al., 2009). To study the roles of the RQC in cotranslational degradation, we designed a similar reporter containing a polybasic tract (12 arginines) sandwiched between GFP and RFP (Figure 3E). Deleting RQC1 stabilized GFP to levels similar to those found in ltn1Δ mutants (Figures 3E, F). TAE2 deletion had a smaller, yet still significant effect. Both Rqc1 and Cdc48 co-IPed with a non-stop substrate that had previously been found to co-IP with Ltn1 (Figure S4) (Bengtson and Joazeiro, 2010). Together, these observations strongly implicate the RQC as a key player in cotranslational degradation, consistent with Ltn1’s established role as the E3 ligase responsible for ubiquitylation of several model cotranslational degradation substrates (Bengtson and Joazeiro, 2010). To determine whether Ltn1 is required for ubiquitylation of all RQC substrates, we probed our immunoprecipitated samples with an anti-ubiquitin antibody and observed a considerable ubitquitin signal whose presence was dependant on Ltn1, but not other RQC components (Figure 3G). The presence of K48-linked ubiquitin in our mass-spec analyses was also Ltn1-dependent. Therefore Ltn1 is likely the primary E3 ligase used by the RQC. In additional to stalled polypeptides, the ubiquitylated substrates might also include components of the RQC and the ribosome itself.
Evidence that the RQC Acts Upon Nascent Chains Arising From Stalled Translation
We next sought to determine which types of translation failures recruit the RQC. Inada and colleagues previously proposed that cotranslational degradation of substrates containing polybasic tracts begins with detection of stalled ribosomes (Dimitrova et al., 2009). Following in vitro studies by Deutsch and colleagues (Lu and Deutsch, 2008), they proposed that the stalling is caused by electrostatic affinity between the positively charged residues of the polybasic tract and the negatively charged nucleotides of the ribosome exit tunnel. We reasoned that if the RQC generally acts upon the products of stalled translation, treatment with drugs that stall translation elongation would increase the load of RQC substrates. In this scenario, the high amount of substrates would present a challenge unlikely to be met by the relatively rare RQC, whose core components, Ltn1 and Rqc1, were reported to exist at less than 1000 molecules per cell (Ghaemmaghami et al., 2003). Therefore under conditions of high translational stalling, the model predicts that reporter substrates will escape RQC detection and degradation. To test this hypothesis, we titrated the translational inhibitor cycloheximide, which inhibits the translocation step of translation and thus causes stalling (Schneider-Poetsch et al., 2010) and measured its effect on the polybasic reporter. Consistent with our model, low amounts of cycloheximide fully stabilized the GFP translation fragment of our polybasic reporter (Figure 4A), while high amounts of cycloheximide shut down all translation. Cycloheximide had no effect on an ltn1Δ strain, consistent with drug-induced GFP stabilization occurring through the RQC pathway. Interestingly, increasing the load of RQC substrates by addition of cycloheximide led to increased levels of Cdc48 in the Rqc1 immunoprecipitate (Figure 3B). This data as well as co-IP of Cdc48 with an RQC substrate (Figure S4) suggest that Cdc48 is instrumental in degradation of RQC substrates (see Discussion). Addition of cycloheximide also activated Hsf1 signaling in rqc1Δ and ltn1Δ mutants, again implicating the RQC in addressing cellular needs that arise with stalled translation (Figure S5A). The mechanism by which the RQC recognizes and binds stalled ribosomes remains a central question.
Endogenous Polybasic Sequences Cause Translational Stalling
To determine the effect of polybasic sequences on translation in vivo, we performed a genome-wide database search for polybasic stretches made up of six or more lysines or arginines in a window of 10 amino acids (Table S4). We then used ribosome profiling (Ingolia et al., 2009), to determine whether polybasic stretches cause ribosomal pauses. Consistent with the stalling hypothesis, we observed a peak in ribosome occupancy ~8 amino acids following the start of the polybasic regions, a length which places the regions mostly within the ribosome exit channel (Figure 4B). This provides experimental support to the electrostatic model proposed previously (Lu and Deutsch, 2008). Taken together with the observed degradation of the polybasic reporter, our results suggest that the RQC may generally act upon the endogenous products of stalled translation.
Rqc1 Levels are Autoregulated by a Negative Feedback Loop
Prominent on the list of putative RQC substrates (compiled informatically based on charge) was Rqc1, which contains a polybasic sequence near its N terminus (Figure 4C). This polybasic region was conserved in all identified orthologs (Figure 4C). To assess the functional role of Rqc1’s polybasic region, we created a mutant FLAG-tagged Rqc1 allele in which the polybasic tract was replaced with neutrally-charged alanine residues. Both this allele and a FLAG-tagged version of the wild-type allele were able to mediate degradation of the polybasic reporter (Figure 4D). Strikingly, relative to wild-type protein, the Rqc1 alanine mutant was ~4.5-fold more abundant in cellular extracts (Figure 4E). Interestingly, we also found that inhibiting the RQC by deleting LTN1 or TAE2 also increased wild-type Rqc1 levels but did not further increase the levels of the rqc1 alanine mutant. These observations argue that the RQC regulates Rqc1 levels via its polybasic tract. QPCR analysis established that the increases in Rqc1 protein levels in the alanine mutant and ltn1Δ backgrounds was not due to an increase in levels of Rqc1’s mRNA levels (Figure S5B). Defining the extent to which Rqc1 regulation is mediated through cotranslational or posttranslational control will be addressed in future studies.
To identify other factors that mediate cotranslational stalling and degradation, we performed a full genome screen that monitored levels of our polybasic fluorescent reporter (Figure 4F, Table S1). The screen identified Ltn1, Rqc1, Asc1, and the ribosome-bound, RING domain containing protein Hel2 as the factors that most strongly increased the steady-state levels of the polybasic construct. Similarly to what has been reported for ASC1 deletion (Kuroha et al., 2010), deleting HEL2 stabilized production of the full-length construct, bypassing ubiquitylation and degradation by the RQC possibly by preventing RNA cleavage following polypeptide-mediated stalling of translation (Figure 4G). Therefore, Asc1 and Hel2 are factors for polypeptide quality control in the RQC pathway but apparently at an upstream stage. Deletion of any of these top hits also upregulated levels of Rqc1 (Figure 4E). Taken together, these observations indicate that Rqc1 is regulated by a negative feedback mechanism mediated by the RQC. This autoregulatory loop may dynamically balance Rqc1 levels to match the load of stalled translation products and its existence suggests that cells benefit from precisely tuning RQC activity (although we did not observe an obvious growth defect in strains with increased Rqc1 levels). A model of limited RQC activity is consistent with the low levels of RQC components compared to the number of ribosomes and the relative ease in saturating the RQC system by addition of cycloheximide.
Tae2 Monitors Translation Stress and Signals to Hsf1
Deleting RQC1 or LTN1 induced Hsf1 and this is what led us to the RQC complex. Curiously however, deleting the gene encoding another core RQC member, Tae2, did not induce Hsf1 (Figure 5A). This result is consistent with three distinct models: 1) Ltn1 and Rqc1 perform Tae2-independent functions that are responsible for Hsf1 induction, 2) deletion of TAE2 alleviates the defect in cotranslational degradation caused by loss of Ltn1/Rqc1, and 3) deletion of TAE2 blocks the signal from the RQC to Hsf1. To distinguish between these possibilities, we examined cotranslational degradation and Hsf1 induction in tae2Δ strains in combination with either ltn1Δ or rqc1Δ. Deleting TAE2 abolished activation of Hsf1, even in ltn1Δ rps0aΔ and rqc1Δ rps0aΔ strains that ordinarily hyperinduce Hsf1 (Figure 5A). By contrast, levels of the polybasic reporter were elevated in all of these strains, indicating that the underlying defect in cotranslational degradation was not repaired by deleting TAE2 (Figure 5B, S6). These data suggest that a Tae2-dependent signaling process conveys translation stress to Hsf1. Critically, deleting TAE2 did not prevent Hsf1 induction in strains mutated for genes encoding chaperones, proteasomal components, or chromatin modifiers (Figure 5C), indicating that Tae2 is not involved in signaling general cytosolic folding stresses. Rather, the translation stress signal is independent of other Hsf1-inducing stresses, and uniquely requires Tae2.
Figure 5. Tae2 is responsible for a distinct Hsf1 branch that monitors translation stress.
(A) Hsf1 activity in deletions strains for the RQC and a ribosomal subunit causing synergistic activation of Hsf1. (B) GFP levels of the cotranslationally degraded reporter construct in selected strain backgrounds from (A). (C) Effect of TAE2 deletion on Hsf1 activity arising from non-translation stresses. Also see Figure S6.
Hsf1 Senses Translation Stress Distinctly From Other Cellular Stresses
We reasoned that if there were a specific “ privileged” signal propagated from stalled ribosomes via Tae2 to Hsf1, then it might be possible to isolate alleles of Hsf1 that have a specific defect in sensing this signal. We therefore used error-prone PCR to generate a library of 290 mutant hsf1 alleles and crossed them into ltn1Δ rps0aΔ and rqc1Δ rps0aΔ strains, which strongly activate the translation stress pathway. These sensitized backgrounds allowed us to identify specific hsf1 alleles that interfere with the RQC-mediated translation stress response (Figure 6A–B). In order to determine whether any hsf1 alleles specifically interacted with the translation stress pathway as opposed to Hsf1 signaling in general, we crossed them into 24 strains that highly modulated Hsf1 activity (Figure 6C, interaction map temporarily located at http://jacumba.ucsf.edu:8080/~onn/CellSubmission/). We did not find hsf1 alleles that mimicked the phenotype of tae2Δ, suppressing RQC-mediated but not other stress signaling. However, several HSF1 alleles had highly specific interactions with the RQC-mediated translation stress pathway (Figure 6C) resulting in hyperactivation of the Hsf1 reporter. All of these alleles contained mutations in the 3′ end of the region encoding the Hsf1 DNA-binding domain. To determine whether mutating this domain was sufficient to modulate Hsf1 sensing of translation stress, we generated two point-mutants in this region. Each of these yielded an hsf1 allele that strongly interacts with the translation stress pathway (Figure 6D). These data further support our model in which translation stress is specifically communicated to Hsf1 independently of other stresses and suggests it may act via Hsf1’s DNA-binding domain.
Figure 6. Hsf1 senses translation stress distinctly from other cellular stresses.
(A) Schematic diagram illustrating genetic interactions between loss-of-function alleles and mutant Hsf1 alleles. Procedure is identical to that of Figure 2A except interactions are computed between hsf1 mutants and loss of function alleles (or pairs of alleles, as are shown in (B)). (B) Genetic interactions between hsf1 mutants and double mutant alleles activating translation stress signaling. Each point represents one hsf1 mutant. Strong positive interactors (1) and negative interactors (2) are circled. (C) Genetic interactions between hsf1 mutants circled in (B) and loss-of-function alleles having strong effect on Hsf1 activity. The hierarchical clustering tree shown was calculated from the full set of 290 hsf1 mutants, not just the six shown. (D) Hsf1 reporter levels showing genetic interactions between mutations to a region altered in each member of the positive interacting group (I246N and G244V) and genetic backgrounds inducing translation stress. Red marks denote expected values and deviations from these reveal genetic interactions.
Discussion
Our work reveals that Hsf1 monitors a much larger breadth of cellular insults than had been appreciated, including a novel stress response that monitors the translational status of the cell at the ribosome. In addition, we identified the RQC, a multiprotein system responsible for both communicating this stress signal to Hsf1 and disposing of stalled nascent peptides via the ubiquitin-proteasome system.
Our analysis also comprehensively identified perturbations that induce Hsf1 in a manner consistent with previous descriptions of Hsf1 function. These include deletion of genes encoding chaperones, proteasomal components, or the machinery that translocates protein to the ER. Nonetheless we identified a large number of genes whose loss induces Hsf1 without any obvious connection to proteotoxicity. These included chromatin-related factors and a diverse set of genes whose common theme is their correlation with high PKA activity. A second group of Hsf1-inducing mutations comprised a group of genes required for maintenance of lipid homeostasis (CHO2, INO2, INO4, KCS1, PHO80, PHO85, PHO88). Additionally, we identified a class of poorly characterized, conserved genes whose mutation strongly induces our Hsf1 reporter, and whose genetic interactions resemble those of the Hsp90-family chaperone encoded by HSC82 (HGH1, YPL225W, AIM29; Figures 1C and Table S1). Notably, HGH1 and HSC82 mutants had highly similar genetic interactions with hsf1 mutants (Figure 6C), indicating an especially tight connection with Hsp90. Finally, we found an intimate connection between translation and Hsf1 activity, which led to the identification of the RQC.
RQC substrates include nascent proteins that contain polybasic tracts, which we found to stall translation in vivo. However, the RQC appears to monitor a broader class of translational stalls. Deletion of ASC1, a gene required for premature translation termination and cotranslational degradation of the polybasic reporter (Kuroha et al., 2010), does not reduce levels of ubiquitin on the RQC and actually increases recruitment of Cdc48 (Figure 3B). These results argue that the RQC also mediates ubiquitylation of substrates that do not require ASC1. Presumably, these substrates do not contain polybasic tracts. Indeed, induction of stalls by treating cultures with cycloheximide both stabilized expression of our polybasic reporter and recruited additional Cdc48 to the RQC. These observations support a model in which widespread translational pausing exhausts the ability of the RQC to meet demand, so that a fraction of aborted translation products escape ubiquitylation and degradation. It is therefore possible that the RQC plays a more general role in disposing of partially synthesized products of stalled translation. Consistent with this, loss of Ltn1 leads to stabilization of a range of stalled model polypeptides (R. Matsuda, T. Inada, personal communication).
The RQC component Cdc48, a hexameric force-generating ATPase, may provide the mechanical force that dislodges the poly-ubiquitylated nascent peptides from the exit channel of the 60S ribosome and/or help deliver them to the proteasome. Cdc48 may also assist in removing other RQC components from the ribosome or in degradation of ribosomes that have produced defective polypeptides. All of these activities are consistent with Cdc48’s roles in extracting poly-ubiquitylated substrates through pores in the ER (Bays et al., 2001) and mitochondria (Heo et al., 2010) before they are targeted for degradation by the proteasome. In this way Cdc48 would be analogous to another AAA+ ATPase, the bacterial ClpXP, which degrades nascent peptides from aborted translation (Gottesman et al., 1998). Rqc1, the E3 ubiquitin ligase Ltn1, and ubiquitin are all required for Cdc48 recruitment, consistent with a late-acting, ubiquitin-dependent role for Cdc48. Electron micrographs of RQC-bound ribosomes illustate that Cdc48 associates with 60S ribosome in a single or limited number of orientations, suggesting a highly specific mechanism of action.
Taken together with previous studies, our findings suggest a model in which translational stalling both recruits the RQC and causes dissociation of the ribosome into 40S and 60S subunits (Figure 7), although we cannot at this stage distinguish whether initial recruitment of the RQC occurs prior to or after subunit dissociation. The initial recognition step of stalled polypeptides appears to require Asc1 and Hel2 for some substrates (e.g. those that contain polybasic tracts) and these factors may mediate RNA cleavage. The nascent polypeptide is then ubiquitylated, perhaps extracted from the 60S subunit by Cdc48, and degraded by the proteasome. How RQC initially recognizes stalled ribosomes, given its low abundance relative to ribosomes, is a fascinating question, which should be informed by structural studies of the RQC-ribosome complex.
Figure 7. Schematic model for RQC-mediated degradation of nascent chains.
The 80S ribosome stalls during translation (left panel) and, for polybasic substrates (++++ symbol), this is recognized by Asc1 and Hel2 leading to translation termination and possibly RNA cleavage. Ltn1, Rqc1, and Tae2 are then recruited and the 40S subunit disassociates (the order of these events remains to be determined). Ltn1 then ubiquitylates the nascent chain (second panel) leading to recruitment of Cdc48 and its cofactors Npl4 and Ufd1 (third panel). In addition, to its role in substrate ubiquitylation, Tae2 signals translation stress to Hsf1. Levels of Rqc1 are downregulated by the activity of the RQC leading to a negative feedback loop controlling overall activity of the pathway.
Our work also uncovered a translation-stress signaling pathway from ribosome to Hsf1 that was wholly dependent on the RQC member Tae2 (Figure 7). We were able to modulate the cell’s ability to sense this stress both by disrupting the signal emanating from the RQC or mutating the terminal sensor, Hsf1. This pathway did not modulate the cells response to other types of stress (e.g. misfolded protein, deletion of chromatin modifiers). Thus, multiple independent pathways may activate Hsf1, allowing it to respond to different stresses independently. Consistent with this notion, the translation stress pathway had unique genetic interactions with Hsf1 mutants.
The possibility that Ltn1 defects may similarly lead to constitutive Hsf1 induction in mammalian cells raises the question of whether the neurodegenerative effect of mutating murine Ltn1 is caused by its direct effect on cotranslational quality control or the indirect effects of constitutive stress signaling. Regardless, the negative feedback loop regulating Rqc1 levels through an absolutely conserved polybasic region underscores the need to finely tune activity of the RQC system. We anticipate that this study’s holistic view of stresses and responses will find use in understanding the cell’s multiple protein quality control systems and the pathologies that occur when any of them is compromised.
Experimental Procedures
Full descriptions of experiments are included in Extended Experimental Procedures. Briefly, fluorescent Hsf1 and Msn2/4 reporter strains were made from a spore from Y8091, a derivative of S288C (Tong et al., 2001). Hsf1 and Msn2/4 were integrated genomically at the URA3 locus as detailed in Figure S7. Using the Synthetic Genetic Array strategy (Tong et al., 2001), reporter strains were mated to each of approximately 6000 strains each containing a loss of function allele. IPs using 3xFLAG epitope strains were grown at 30 °C to OD600 1.6 in YEPD and then washed in 4 °C water before freezing in liquid nitrogen and subsequent mechanical lysis in liquid nitrogen-cooled conditions. Final elution was done by addition of 3xFLAG peptide. For EM analysis, the immuno-precipitated complex was adsorbed to glow discharged carbon-coated copper grids, stained with uranyl formate, and finally viewed at 42000x nominal magnification. All fluorescent measurements were performed at 25°C at log phase in synthetic complete media using a Beckman Dickenson LSR II flow cytometer. Fluorescence values were computed as the median internally-normalized values (GFP/RFP for Hsf1 and Msn2/4 reporters, GFP/sidescatter and RFP/sidescatter for polybasic reporter and HSC82pr reporter) for each well. Values were then normalized to WT and log2 scores computed, so that the wild type strain had value 0. All error bars denote standard error between duplicate wells on the same multiwell plate, or, in the case of Western blots, independent experiments. Genetic interaction scores between alleles were computed as the actual double mutant activity (log2 fold basal units) minus the sum of the two single activities (log2 fold basal units).
Supplementary Material
Highlights.
Comprehensive characterization of the stresses sensed by Hsf1
Characterization of a ribosome-bound complex that targets ribosomes stalled at translation
An autoregulatory loop regulates activity of the complex
Discovery of a translation-stress signaling pathway from the ribosome to Hsf1
Acknowledgments
We thank the members of the Weissman lab, M. Bassik, E. Boydston, L. Gilbert, A. Grenninger, C. Jan, M. Jonikas, M. Kampmann, Y. Liu, W. Luddington, M. McKeon, E. Oh, C. Reiger, M. Smith, N. Stern-Ginossar, and P. Walter, for their valuable help with the manuscript and input on the project, T. Becker and R. Beckmann for help with EM, M. Larson and B. Toyama for help with graphic design, and M. Szeto for help in creating the fluorescent reporters. O.B. was supported by a Helen Hay Whitney postdoctoral fellowship. This work was funded by the Howard Hughes Medical Institute (to J.S.W.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahn SG, Thiele DJ. Redox regulation of mammalian heat shock factor 1 is essential for Hsp gene activation and protection from stress. Genes Dev. 2003;17:516–528. doi: 10.1101/gad.1044503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alamgir M, Erukova V, Jessulat M, Azizi A, Golshani A. Chemical-genetic profile analysis of five inhibitory compounds in yeast. BMC Chem Biol. 2010;10:6. doi: 10.1186/1472-6769-10-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albanese V, Yam AY, Baughman J, Parnot C, Frydman J. Systems analyses reveal two chaperone networks with distinct functions in eukaryotic cells. Cell. 2006;124:75–88. doi: 10.1016/j.cell.2005.11.039. [DOI] [PubMed] [Google Scholar]
- Ali A, Bharadwaj S, O’Carroll R, Ovsenek N. HSP90 interacts with and regulates the activity of heat shock factor 1 in Xenopus oocytes. Mol Cell Biol. 1998;18:4949–4960. doi: 10.1128/mcb.18.9.4949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bateman A, Coin L, Durbin R, Finn RD, Hollich V, Griffiths-Jones S, Khanna A, Marshall M, Moxon S, Sonnhammer EL, et al. The Pfam protein families database. Nucleic Acids Res. 2004;32:D138–141. doi: 10.1093/nar/gkh121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bays NW, Wilhovsky SK, Goradia A, Hodgkiss-Harlow K, Hampton RY. HRD4/NPL4 is required for the proteasomal processing of ubiquitinated ER proteins. Mol Biol Cell. 2001;12:4114–4128. doi: 10.1091/mbc.12.12.4114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bengtson MH, Joazeiro CA. Role of a ribosome-associated E3 ubiquitin ligase in protein quality control. Nature. 2010;467:470–473. doi: 10.1038/nature09371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breslow DK, Cameron DM, Collins SR, Schuldiner M, Stewart-Ornstein J, Newman HW, Braun S, Madhani HD, Krogan NJ, Weissman JS. A comprehensive strategy enabling high-resolution functional analysis of the yeast genome. Nat Methods. 2008;5:711–718. doi: 10.1038/nmeth.1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christians ES, Benjamin IJ. Heat shock response: lessons from mouse knockouts. Handb Exp Pharmacol. 2006:139–152. doi: 10.1007/3-540-29717-0_6. [DOI] [PubMed] [Google Scholar]
- Chu J, Hong NA, Masuda CA, Jenkins BV, Nelms KA, Goodnow CC, Glynne RJ, Wu H, Masliah E, Joazeiro CA, et al. A mouse forward genetics screen identifies LISTERIN as an E3 ubiquitin ligase involved in neurodegeneration. Proc Natl Acad Sci U S A. 2009;106:2097–2103. doi: 10.1073/pnas.0812819106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig EA, Eisenman HC, Hundley HA. Ribosome-tethered molecular chaperones: the first line of defense against protein misfolding? Curr Opin Microbiol. 2003;6:157–162. doi: 10.1016/s1369-5274(03)00030-4. [DOI] [PubMed] [Google Scholar]
- Dai C, Whitesell L, Rogers AB, Lindquist S. Heat shock factor 1 is a powerful multifaceted modifier of carcinogenesis. Cell. 2007;130:1005–1018. doi: 10.1016/j.cell.2007.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimitrova LN, Kuroha K, Tatematsu T, Inada T. Nascent peptide-dependent translation arrest leads to Not4p-mediated protein degradation by the proteasome. J Biol Chem. 2009;284:10343–10352. doi: 10.1074/jbc.M808840200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleischer TC, Weaver CM, McAfee KJ, Jennings JL, Link AJ. Systematic identification and functional screens of uncharacterized proteins associated with eukaryotic ribosomal complexes. Genes Dev. 2006;20:1294–1307. doi: 10.1101/gad.1422006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritah S, Col E, Boyault C, Govin J, Sadoul K, Chiocca S, Christians E, Khochbin S, Jolly C, Vourc’h C. Heat-shock factor 1 controls genome-wide acetylation in heat-shocked cells. Mol Biol Cell. 2009;20:4976–4984. doi: 10.1091/mbc.E09-04-0295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghaemmaghami S, Huh WK, Bower K, Howson RW, Belle A, Dephoure N, O’Shea EK, Weissman JS. Global analysis of protein expression in yeast. Nature. 2003;425:737–741. doi: 10.1038/nature02046. [DOI] [PubMed] [Google Scholar]
- Giaever G, Chu AM, Ni L, Connelly C, Riles L, Veronneau S, Dow S, Lucau-Danila A, Anderson K, Andre B, et al. Functional profiling of the Saccharomyces cerevisiae genome. Nature. 2002;418:387–391. doi: 10.1038/nature00935. [DOI] [PubMed] [Google Scholar]
- Gorner W, Durchschlag E, Martinez-Pastor MT, Estruch F, Ammerer G, Hamilton B, Ruis H, Schuller C. Nuclear localization of the C2H2 zinc finger protein Msn2p is regulated by stress and protein kinase A activity. Genes Dev. 1998;12:586–597. doi: 10.1101/gad.12.4.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottesman S, Roche E, Zhou Y, Sauer RT. The ClpXP and ClpAP proteases degrade proteins with carboxy-terminal peptide tails added by the SsrA-tagging system. Genes & development. 1998;12:1338–1347. doi: 10.1101/gad.12.9.1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn JS, Hu Z, Thiele DJ, Iyer VR. Genome-wide analysis of the biology of stress responses through heat shock transcription factor. Mol Cell Biol. 2004;24:5249–5256. doi: 10.1128/MCB.24.12.5249-5256.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn JS, Thiele DJ. Activation of the Saccharomyces cerevisiae heat shock transcription factor under glucose starvation conditions by Snf1 protein kinase. J Biol Chem. 2004;279:5169–5176. doi: 10.1074/jbc.M311005200. [DOI] [PubMed] [Google Scholar]
- Heo JM, Livnat-Levanon N, Taylor EB, Jones KT, Dephoure N, Ring J, Xie J, Brodsky JL, Madeo F, Gygi SP, et al. A stress-responsive system for mitochondrial protein degradation. Mol Cell. 2010;40:465–480. doi: 10.1016/j.molcel.2010.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu AL, Murphy CT, Kenyon C. Regulation of aging and age-related disease by DAF-16 and heat-shock factor. Science. 2003;300:1142–1145. doi: 10.1126/science.1083701. [DOI] [PubMed] [Google Scholar]
- Inada T, Aiba H. Translation of aberrant mRNAs lacking a termination codon or with a shortened 3′-UTR is repressed after initiation in yeast. EMBO J. 2005;24:1584–1595. doi: 10.1038/sj.emboj.7600636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingolia NT, Ghaemmaghami S, Newman JR, Weissman JS. Genome-wide analysis in vivo of translation with nucleotide resolution using ribosome profiling. Science. 2009;324:218–223. doi: 10.1126/science.1168978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito-Harashima S, Kuroha K, Tatematsu T, Inada T. Translation of the poly(A) tail plays crucial roles in nonstop mRNA surveillance via translation repression and protein destabilization by proteasome in yeast. Genes Dev. 2007;21:519–524. doi: 10.1101/gad.1490207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolly C, Usson Y, Morimoto RI. Rapid and reversible relocalization of heat shock factor 1 within seconds to nuclear stress granules. Proc Natl Acad Sci U S A. 1999;96:6769–6774. doi: 10.1073/pnas.96.12.6769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonikas MC, Collins SR, Denic V, Oh E, Quan EM, Schmid V, Weibezahn J, Schwappach B, Walter P, Weissman JS, et al. Comprehensive characterization of genes required for protein folding in the endoplasmic reticulum. Science. 2009;323:1693–1697. doi: 10.1126/science.1167983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klinge S, Voigts-Hoffmann F, Leibundgut M, Arpagaus S, Ban N. Crystal structure of the eukaryotic 60S ribosomal subunit in complex with initiation factor 6. Science. 2011;334:941–948. doi: 10.1126/science.1211204. [DOI] [PubMed] [Google Scholar]
- Kondo H, Rabouille C, Newman R, Levine TP, Pappin D, Freemont P, Warren G. p47 is a cofactor for p97-mediated membrane fusion. Nature. 1997;388:75–78. doi: 10.1038/40411. [DOI] [PubMed] [Google Scholar]
- Kremer SB, Gross DS. SAGA and Rpd3 chromatin modification complexes dynamically regulate heat shock gene structure and expression. J Biol Chem. 2009;284:32914–32931. doi: 10.1074/jbc.M109.058610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuroha K, Akamatsu M, Dimitrova L, Ito T, Kato Y, Shirahige K, Inada T. Receptor for activated C kinase 1 stimulates nascent polypeptide-dependent translation arrest. EMBO Rep. 2010;11:956–961. doi: 10.1038/embor.2010.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu XD, Liu PC, Santoro N, Thiele DJ. Conservation of a stress response: human heat shock transcription factors functionally substitute for yeast HSF. EMBO J. 1997;16:6466–6477. doi: 10.1093/emboj/16.21.6466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J, Deutsch C. Electrostatics in the ribosomal tunnel modulate chain elongation rates. J Mol Biol. 2008;384:73–86. doi: 10.1016/j.jmb.2008.08.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariappan M, Li X, Stefanovic S, Sharma A, Mateja A, Keenan RJ, Hegde RS. A ribosome-associating factor chaperones tail-anchored membrane proteins. Nature. 2010;466:1120–1124. doi: 10.1038/nature09296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore SD, Sauer RT. The tmRNA system for translational surveillance and ribosome rescue. Annu Rev Biochem. 2007;76:101–124. doi: 10.1146/annurev.biochem.75.103004.142733. [DOI] [PubMed] [Google Scholar]
- Newman JR, Ghaemmaghami S, Ihmels J, Breslow DK, Noble M, DeRisi JL, Weissman JS. Single-cell proteomic analysis of S. cerevisiae reveals the architecture of biological noise. Nature. 2006;441:840–846. doi: 10.1038/nature04785. [DOI] [PubMed] [Google Scholar]
- Piatkov KI, Brower CS, Varshavsky A. The N-end rule pathway counteracts cell death by destroying proapoptotic protein fragments. Proc Natl Acad Sci U S A. 2012;109:E1839–1847. doi: 10.1073/pnas.1207786109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pye VE, Beuron F, Keetch CA, McKeown C, Robinson CV, Meyer HH, Zhang X, Freemont PS. Structural insights into the p97-Ufd1-Npl4 complex. Proc Natl Acad Sci U S A. 2007;104:467–472. doi: 10.1073/pnas.0603408104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider-Poetsch T, Ju J, Eyler DE, Dang Y, Bhat S, Merrick WC, Green R, Shen B, Liu JO. Inhibition of eukaryotic translation elongation by cycloheximide and lactimidomycin. Nat Chem Biol. 2010;6:209–217. doi: 10.1038/nchembio.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorger PK, Pelham HR. Purification and characterization of a heat-shock element binding protein from yeast. EMBO J. 1987;6:3035–3041. doi: 10.1002/j.1460-2075.1987.tb02609.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolz A, Hilt W, Buchberger A, Wolf DH. Cdc48: a power machine in protein degradation. Trends Biochem Sci. 2011;36:515–523. doi: 10.1016/j.tibs.2011.06.001. [DOI] [PubMed] [Google Scholar]
- Tong AH, Evangelista M, Parsons AB, Xu H, Bader GD, Page N, Robinson M, Raghibizadeh S, Hogue CW, Bussey H, et al. Systematic genetic analysis with ordered arrays of yeast deletion mutants. Science. 2001;294:2364–2368. doi: 10.1126/science.1065810. [DOI] [PubMed] [Google Scholar]
- Turner GC, Varshavsky A. Detecting and measuring cotranslational protein degradation in vivo. Science. 2000;289:2117–2120. doi: 10.1126/science.289.5487.2117. [DOI] [PubMed] [Google Scholar]
- Westerheide SD, Anckar J, Stevens SM, Jr, Sistonen L, Morimoto RI. Stress-inducible regulation of heat shock factor 1 by the deacetylase SIRT1. Science. 2009;323:1063–1066. doi: 10.1126/science.1165946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xavier IJ, Mercier PA, McLoughlin CM, Ali A, Woodgett JR, Ovsenek N. Glycogen synthase kinase 3beta negatively regulates both DNA-binding and transcriptional activities of heat shock factor 1. J Biol Chem. 2000;275:29147–29152. doi: 10.1074/jbc.M002169200. [DOI] [PubMed] [Google Scholar]
- Ye Y, Meyer HH, Rapoport TA. The AAA ATPase Cdc48/p97 and its partners transport proteins from the ER into the cytosol. Nature. 2001;414:652–656. doi: 10.1038/414652a. [DOI] [PubMed] [Google Scholar]
- Yewdell JW, Anton LC, Bennink JR. Defective ribosomal products (DRiPs): a major source of antigenic peptides for MHC class I molecules? J Immunol. 1996;157:1823–1826. [PubMed] [Google Scholar]
- Zhao J, Herrera-Diaz J, Gross DS. Domain-wide displacement of histones by activated heat shock factor occurs independently of Swi/Snf and is not correlated with RNA polymerase II density. Mol Cell Biol. 2005;25:8985–8999. doi: 10.1128/MCB.25.20.8985-8999.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.