Abstract
Micro-/nanoscale technologies such as lithographic techniques and microfluidics offer promising avenues to revolutionalize the fields of tissue engineering, drug discovery, diagnostics and personalized medicine. Microfabrication techniques are being explored for drug delivery applications due to their ability to combine several features such as precise shape and size into a single drug delivery vehicle. They also offer to create unique asymmetrical features incorporated into single or multiple reservoir systems maximizing contact area with the intestinal lining. Combined with intelligent materials, such microfabricated platforms can be designed to be bioadhesive and stimuli-responsive. Apart from drug delivery devices, microfabrication technologies offer exciting opportunities to create biomimetic gastrointestinal tract models incorporating physiological cell types, flow patterns and brush-border like structures. Here we review the recent developments in this field with a focus on the applications of microfabrication in the development of oral drug delivery devices and biomimetic gastrointestinal tract models that can be used to evaluate the drug delivery efficacy.
Keywords: Microfabrication, Microdevices, Microfluidics, Oral drug delivery, Oral absorption, In vitro models
1. Introduction
Oral delivery remains the preferred route of drug administration due to its non-invasive nature and improved patient compliance. With advances in molecular biology and biotechnology, the repertoire of therapeutic compounds has extended beyond small molecular weight compounds to include proteins, peptides, large biological macromolecules, supramolecular structures, nanomedicine, and even whole cells. However, many of these biological therapeutics have poor oral bioavailability due to formidable barriers posed by gastrointestinal (GI) tract and are therefore rarely used for oral drug delivery. Oral administration of such compounds with improved bioavailability requires effective drug delivery systems before successful use in humans. The design of such systems requires an understanding of intestinal physiology and the mucosal microenvironment.
The GI tract acts as a compartmentalized mechano-chemical processing center for ingested food. Its lining also acts as a formidable barrier to pathogens and the byproducts of digestion. Nutrients extracted from food are absorbed primarily in the upper small intestine through specific transport pathways. The high surface area and plethora of absorptive mechanisms available in the small intestine make it the primary target site for enhancements in drug absorption. The wall of the intestine, known as the brush border, is covered primarily with epithelium organized into circular folds. Molecular transport across this epithelium occurs through two routes, either between cells (paracytosis) or through the cells (transcytosis) [1, 2](Fig. 1). Transcytosis can be further delineated into either carrier mediated, diffusive, or endocytotic transport. A small fraction, <1% of the brush border possess lymphatic properties. These discrete areas are known as Peyer’s patches. The lumen of the small intestine also contains pancreatic enzymes, bile salts, and a thick mucus layer. Such typical physiology of GI tract leads to poor bioavailability due to several factors including low mucosal permeability and/or degradation of a drug before absorption.
Fig. 1.
Schematic of the structure of the epithelium. Molecules can be transported across the epithelial barrier by passive diffusion through transcellular or paracellular pathways, or actively transported across by membrane-derived vesicles or membrane bound carriers. Uptake can also occur due to adsorptive endocytosis via clathrin-coated pits and vesicles, uid phase endocytosis, and phagocytosis induced by M cell antigen sampling. Reproduced with permission from ref. [123].
Therapeutic agents may be classified in four classes (Biopharmaceutics Classification System (BCS) according to their permeability and solubility [3]. Therapeutic agents such as proteins, DNA or RNA are considered BCS class III compounds, highly soluble yet poorly permeable. These compounds are generally the most susceptible to degradation in the GI tract. The transport of larger compounds, such as nanoparticles, can be restricted by the layer of mucus that lines the GI tract. Furthermore, the epithelial lining of the intestine has poor non-specific permeability for most water soluble macromolecules. The transport of small molecular weight compounds can be limited by poor mucosal permeability and solubility, such as BCS class IV compounds. Up to 70% of new molecular entities currently under development have poor aqueous solubility [4].
Several controlled drug delivery strategies have been proposed to overcome barriers to oral drug absorption of peptide, protein and macromolecular drugs. These include modification of intestinal epithelium through the use of permeation enhancers, modification of drug itself to improve its permeability/solubility characters, use of protease inhibitors to curb the proteolytic degradation, encapsulation techniques using micro/nanoparticles, and use of intelligent polymers and hydrogels. These approaches have been reviewed in detail in various articles [1, 2, 5–8].
Another emerging approach in the field of oral drug delivery is the use of microfabricated systems. Microfabrication techniques were originally developed in the microelectronics industry. Microelectronic process engineering is a discipline that developed due to the rapid growth of the integrated circuit (IC) industry. Traditionally, microelectromechanical systems (MEMS) have been used to produce functional devices on the micron scale, such as sensors, switches, filters, and gears, from silicon, the dominant material used throughout the IC industry. Micro/nanofabrication techniques have enabled development of miniaturized diagnostic tools and high throughput screening assays for drug discovery and tissue engineering [9–13]. Although in their infancy, microfabrication technologies are being explored for oral drug delivery. They have significant potential to overcome some of the barriers of oral drug delivery through fabrication of asymmetrical devices with precise control over size and shape. Apart from drug delivery devices, microfabrication approaches can also enhance the field of oral drug delivery by designing biomimetic in vitro GI tract model systems that can aid in better prediction of drug absorption in vivo. In this review, we will focus on the general methods of microfabrication and their applications in the development of oral drug delivery system and in vitro cell culture models that can be used to evaluate the drug delivery efficacy.
2. Microfabrication methods
Numerous lithographic techniques can be used for the preparation of microfabricated devices and fundamentals of these methods can be found in the review by Xia et al [14]. In general, microfabricated devices are manufactured by the repeated application of unit process steps such as thin-film deposition, photolithography, and etching. In a simplified process, a two-dimensional (2D) feature layout is created utilizing computer-aided design (CAD) software and printed into a photomask. The pattern is transferred to a substrate via photolithography. During photolithography, a substrate is coated with a photosensitive polymer layer and exposed to UV light from a mask aligner through the photomask. During development, photoresist crosslinked to the substrate surface remains, while uncrosslinked or cleaved photoresist areas are washed away. While standard photolithography has a resolution of approximately 0.5–1 μm, it is possible to achieve sub-100 nm using methods such as E-beam, ion beam, and dip-pen lithography. The pattern can then be transferred into the substrate material using a wet or dry etch process. Though typically performed on silicon or glass, other materials, including polymers, are amenable to this fabrication process.
Hard patterned substrates (such as silicon) can be used as a master mold in the process known as soft lithography to create a robust inverse replica in a soft elastomer poly(dimethyl siloxane) (PDMS). Given the fidelity of this process, both the master mold and PDMS mold can be used for a large number of replication rounds. Therefore, by utilizing microfabrication processes such as soft lithography in combination with various replica molding techniques, such as spin-assisted solvent casting, micromolding, hot embossing, and nanotemplating, devices can be structured precisely, simply, rapidly, into biocompatible materials at low-cost and without the further use of specialized microfabrication equipment [14, 15].
3. Applications in drug delivery
Over the past several years, microfabrication technology has been applied to the successful development of a variety of health care-related products. Although research on microfabricated devices for biomedical applications, particularly in diagnostics, has rapidly expanded in recent years, relatively few researchers have concentrated on therapeutic applications of microfabrication technology, such as drug delivery. However, the use of microtechnology offers a number of advantages which may revolutionize the field of controlled release. These include localized delivery, delivery on demand, controlled release kinetics, programmable dosing, sequential delivery, and diagnostic feedback dispensing. Several review articles are available regarding trends in microfabricated systems for drug delivery, with a few examples outlined here [16–20].
3.1 Programmable Drug Delivery
Microfabrication technology has been used to fabricate programmable devices as a new class of controlled release systems for drug delivery. These devices are particularly intriguing due to their small size, potential for integration with microelectronics and their ability to store and release chemicals on demand [21, 22]. The first experimental demonstration of a microchip with potential application in drug delivery was described by Santini et al [23]. The ultimate goal of this approach is to develop a microfabricated device devoid of moving parts, but with the ability to store and release multiple chemical substances [21, 23–25]. Drugs stored within reservoirs were sealed either with active or passive coatings. For example, in the original work the reservoirs were sealed with a thin layer of gold and released upon application of an applied electric potential that dissolved the coating. Subsequent version also included fully degradable polymeric systems with plastic seals.
Other approaches can also be used to sense and release drugs. For example, the use of “artificial muscle,” a chemo-mechanical actuator, valves in conjunction with silicon micromachined drug release structures can render a microchip responsive to a patient’s therapeutic requirements and deliver certain amount of a drug in response to a biological stimulus [21]. By electroplating these polymers onto electrodes, reservoirs can be opened or closed, and the drug compound released or retained, via the swelling and shrinking processes of the polymer system in response to electrochemical actuation [21].
Programmed drug delivery can also be achieved by incorporation of microscale pumps to deliver drug solutions, or suspensions containing solid formulations. The advance of microfabrication for microfluidics has enabled fluid handling devices which can dispense nano- to picoliter volumes. Piezo-actuated pumps, shape-memory alloy actuated pumps, and electrokinetic pumps based on electrophoretic and electro-osmotic mechanisms offer a wide range of methods for precision delivery of drugs [19, 26]. Additionally, electronically controlled devices provide for precise control of delivery volumes, multiple therapeutics, and timed sequence programmable flow [27].
3.2 Particulate Systems
As their scale decreases, microfabricated devices can be delivered by ingestion (<1 mm), intra-tissue injection (<200 μm), inhalation (<100 μm) or released into circulation (<10 μm) [18]. Microfabrication methods, because of their ability to control microarchitecture and feature size, have been used successfully to develop novel nano/microparticles for applications in drug delivery. Silicon particles have been used as multistage drug delivery systems [28] and for intravenous delivery [29]. Several methods, including soft lithography [30–33], particle replication in non-wetting templates (PRINT) [34, 35], hydrogel templating [36, 37], imprint lithography [38], and in situ photopolymerization in microfluidic channels [39] have been developed to prepare homogeneous particles in polymers. Microstructures with complex geometries can influence anisotropic interactions with biomolecules and cells [35, 40, 41].
4. Microfabricated Oral Drug Delivery Systems
Microfabrication also offers great opportunities to enhance the oral delivery of pharmaceuticals by allowing for precise control over shape, size, and geometry of delivery devices. Microfabricated devices can also increase drug loading capacities and provide better control over drug release. One approach for inducing greater levels of absorption and stability at the intestinal epithelium is the use of a multilayered patch system. Patches are designed with layers of thin, flexible membranes: an impermeable backing, a drug reservoir, a rate-controlling membrane, and an adhesive. When the patch is applied, the drug begins to flow through the skin into the bloodstream at a rate regulated by the membrane, pre-programmed to keep the drug at an effective level. These properties are ideal for oral dosage forms intended for delivery to the intestinal mucosa. Microfabricated patch systems designed for oral drug delivery are capable of three main functions: (i) bioadhesive properties for retention of the dosage form, (ii) release drug in a controlled fashion, and (iii) provide unidirectional release towards the intestinal epithelium [42].
4.1 Micropatch Fabrication
The size of orally delivered particles has a great impact on their transit through the GI tract. Larger particles can get trapped in the mucus layer protecting the epithelium, resulting a relatively short residence time. Certain types smaller micro- and nanoscale particles are known to permeate the epithelium [43], but the uptake is largely restricted to Peyer’s patches, which take up a small fraction of the brush border and lead to lymphoid tissue. This pathway carries the risk of toxic accumulation and poor biodistribution. Microfabricated patch systems are alternative to standard particulate delivery systems, such as microspheres. They are designed small enough to travel in between intestinal villi, maximizing the large absorptive surface area of the intestinal folds, but wide enough to prevent cell uptake. In contrast to particulate systems, micropatches are designed flat and thin to maximize contact area with the intestinal lining. At the same time, this flat design minimizes the side areas exposed to the constant flow of liquids through the intestine. The devices can be microfabricated to incorporate single or multiple drug reservoirs which can be loaded with any number of drugs/biomolecules of interest. These reservoirs, unlike multi-directional release from a spherical delivery system, allow for unidirectional release of the drug. Furthermore, regions of the device can be surface modified in order to incorporate cell targeting mechanisms which localize the vehicle at a specific site of action. Modification of microspheres is performed uniformly over the entire surface area, which increases instability and may induce rolling when exposed to flow. However, selective surface modification on only the reservoir side allows micropatches to stably anchor in an orientation which permits the released drug to follow the shortest diffusional pathway towards the intestinal epithelium. Fabrication processes for creating oral micropatches have been developed based on standard MEMS fabrication techniques including photolithography, etching, and thin film deposition, as well as soft lithography [20, 33, 42, 44–47].
Standard materials such as porous silicon [44] and silicon oxide [45] have been successfully used for microfabricated-based drug delivery systems. Although silicon and glass are the materials of choice for electronic and mechanical devices, it is not clear if these materials are necessarily appropriate for all applications in biology and medicine [48]. Polymers allow for shorter fabrication times and potential large scale fabrication of complex drug delivery vehicles. In one such demonstration, poly(methyl methacrylate) (PMMA) microdevices were fabricated using an off-wafer process [49, 50]. Microdevices were also fabricated from SU-8, a chemically amplified, epoxy-based negative photoresist typically used for producing ultra-thick resist layers. The use of SU-8 as a device material eliminates the need of a secondary patterning material and the dry etch procedure. Instead, multi-level processing can be used to create features in multiple layers. Repeated, aligned photolithography was used to define the backing, reservoir, and supplementary feature layers [46, 47, 51, 52]. Asymmetrical microparticles were also fabricated from biodegradable polymers poly(DL-lactide-co-glycolide) (PLGA) and gelatin using soft lithographic techniques [46]. In this manner, several batches of asymmetrical microparticles may be generated from a single master. The height of the resulting devices is determined by the height of the features in the PDMS master and the concentration of polymer in solution. Lateral resolution is determined by the features of the PDMS master and the solvents used. Guan et al. were able to combine methods of dip-coating, microcontact hot-printing and soft lithography to produce microdevices containing single-reservoir and multiple-reservoir systems as well as sustained release microcapsule depots in PLGA (Fig. 3A, B, C) [32].
Fig. 3.
A) Single-reservoir microdevices released in water showing asymmetrical plate-like geometry with ring-shaped microwell in the center for drug loading; B) Multiple-reservoir microdevices released in water, each containing 14 closed reservoirs surrounded by a number of open reservoirs; C) Microcapsule microdevices made from PLGA as sustained release depots; D) Self-folding polymeric microdevice with enhanced mucoadhesion for transmucosal drug delivery, E) Folded microdevices grabbing onto pig intestinal mucosa with stable adhesion even after water rinsing. Reprinted with permission from [32].
4.2 Micropatch Loading
Micropatch systems offer some advantages in drug loading over conventional solid dosage forms. They can contain reservoirs which can be loaded by microinjection with pico- to nanoliters of a polymeric solution. Water quickly evaporates from these reservoirs leaving behind the drug contained in a timed-release polymer plug. Intimate contact between the micropatch and intestinal epithelium would provide a short diffusion distance, potentially negating the need for excipients to aid in dissolution. Using a specific type of polymer reservoir would predetermine the time and rate of release of drug from the reservoir; for example, a hydrogel that swells in response to a specific pH, solvent or temperature or a polymer with a known dissolution rate. Different polymers with various dissolution rates could then be used in separate reservoirs to obtain controlled release of several compounds [45]. By capitalizing on surface-liquid interactions, it is also possible to utilize discontinuous de-wetting as method for bulk filling of reservoirs [32].
Micropatch reservoirs can also be filled using photolithography. For example, after microdevice development, a photosensitive hydrogel precursor solution, consisting of a crosslinker, photoinitiator, poly(ethylene glycol) dimethacrylate (PEGDMA) and drug, can be spun into the empty reservoirs. Using a mask aligner, only the hydrogel within the reservoir is exposed to UV light and subsequently developed in water and isopropanol. Using repeated application and crosslinking, microdevice reservoirs can be loaded with multilayered hydrogels for sequential delivery of therapeutics (Fig. 2) [51, 52]. In the case of soft lithographic methods for fabrication of particles, drugs may be added to the prepolymer solution and incorporated into the device during the transfer process [46].
Fig. 2.
A) Schematic of fabrication of multi-layer poly(ethylene glycol methacrylate)-laden SU-8 microdevice; B) A uorescent micrograph composite of a layered hydrogel prepared with DNP-BSA, FITC-BSA and Texas red-BSA (from the outermost layer to the innermost). The grey dotted-line box highlights the reservoir area and the red dotted-line box the outer area of the microdevice; C) A uorescent micrograph of each individual lter for the labeled BSA is presented for three unique hydrogel- lled microdevices. Reprinted with permission from [124].
4.3 Bioadhesion
As an alternate way to overcome short gut residence times and poor mucosal contact, increasing interest has been placed on bioadhesive systems that can delay the transit and prolong their residence at a specific site of delivery, thus enhancing the drug absorption process [53, 54]. These mucoadhesive devices can protect the drug during the absorption process in addition to protecting it on its route to the delivery site. This in turn increases the drug concentration gradient due to intense contact. The general concepts and mechanisms of mucoadhesion have been reviewed in detail [54–56]. In general, bioadhesion can be achieved through chemical or physical approaches.
4.3.1. Chemical approaches
The use of adhesion promoters such as linear or tethered polymer chains to promote bioadhesion during oral drug delivery has been well documented [57–62]. Mucoadhesion depends largely on the structure of the synthetic polymers used in controlled release applications and has been reviewed by Serra et al [55]. Another strategy utilizing immobilization of lectin as targeting molecules for enhanced adhesion and specificity to intestinal epithelial models has been previously described [63–67]. Post-fabrication chemical modification may be performed to immobilize bioactive targeting molecules to the surface of microdevices [45, 47, 49, 50, 52].
In vitro studies were performed using the Caco-2 cell line to measure the cytoadhesive properties of lectin-conjugated microdevices. The binding characteristics of microdevices modified with two types of lectin (tomato, known to agglutinate Caco-2 cells, and peanut, an unrelated lectin non-specific to Caco-2 cells) were observed as a function of time. Although both lectin conjugates produce a higher degree of binding (approximately 2–4 fold greater) than the pristine microdevices, a marked difference still remained between the peanut and tomato lectin conjugates (approximately 2-fold) [49, 50]. Tomato-lectin modified microdevices and microspheres were also studied in order to compare the effect of a tailored microdevice shape versus a traditional spherical shape on adhesion stability. It was found that the percent of microdevices remained consistently bound (~68% of total applied) over consecutive washes while microspheres significantly decreased to approximately 17% [33]. Although microspheres have a larger surface area, or more importantly a larger lectin-modified surface area, than the micropatch systems, microspheres appeared to be less stable when subject to consecutive removal. The stability may be in part associated with the small fraction of the surface area which is directly in contact and anchoring to the cell monolayer at any given time. This suggests that the larger modified contact area of the flat micropatch device may provide a more stable interface. Additionally, studies have shown that these micropatch systems promote stable adhesion in the presence of mucin [42], as well as under shear flow conditions [52].
4.3.2. Physical approaches
Using microfabrication techniques, microdevice bodies can be designed to contain precisely shaped microneedles and microposts. These features may allow for the particle to more firmly adhere to the mucosa, potentially increasing drug permeability.
Microneedle systems were originally developed as an approach to enhance the poor permeability of the skin by creating microscale conduits for transport across the stratum corneum for transdermal drug delivery [68]. The microfabrication of microneedles that are long and robust enough to penetrate this layer of skin, but short enough to avoid stimulating nerves, has the potential to make transdermal delivery of drugs more effective [68–70]. Microneedle platforms have been fabricated in silicon, and also transferred into biodegradable carboxymethylcellulose, amylopectin, poly(lactic acid), poly(glycolic acid), and PLGA [71–74]. Tapered, needle-like structures have also been scaled down into the submicron range to provide adhesion and drug delivery in wet environments for potential applications in surgical, wound, and internal bandage systems [75]. Microneedles, in combination with infusion methods such as pressurized reservoirs and electrically controlled systems, have also been utilized for drug delivery [76–78]. Furthermore, by modifying needle dimension and design to incorporate multiple channels and ports, optimized microhypodermic needles and microprobes have also been developed for cellular, local tissue, or systemic delivery [70, 79].
The same microneedle/micropost design principles can be applied to oral drug delivery to increase the retention time of the microfabricated devices in the GIT. Using microfabrication techniques, oral microdevices can be designed to contain precisely shaped microposts/microneedles. These features may penetrate the mucus layer leading to anchorage of the particles/microdevices. For example, Guan et al [31, 32] used similar approach to fabricate a bilayered system of a poly(EGMA-co-EGDMA) and crosslinked chitosan microparticles with self-folding arms. It is expected that by penetrating into the mucus layer, the arms may anchor microparticles, providing increased resistance to surface erosion of the mucus layer [32]. This mechanism may also provide a means to “grab” the intestinal villi, also potentially leading to a longer retention time of the device (Fig. 3D, E). In addition, the presence of microposts on oral microdevices may shed mucosa to increase the uptake of compounds into the blood vessels of the submucosa [46]. Combined with the current chemically driven targeting mechanism, these microposts may provide a mechanically driven controlled release feature [46].
Another physical method to enhance bioadhesion is the use of a particularly promising class of gecko-inspired or nanostructure-based adhesives. Under nano-adhesive conditions, as the number of adhesive elements per surface area increases, the surface area to volume ratio increases and van der Waals adhesion is predicted to increase [80, 81]. The microvilli present on the surface of the mucosal epithelia dramatically increase its surface area. Therefore, by creating a nanostructured microdevice to target the microvilli-coated intestinal epithelium, it may be possible to generate strong bioadhesive forces due to geometric features alone. A standard vapor-liquid-solid method for synthesizing silicon nanowires on flat wafer surfaces has been used in order to achieve growth of size-specific nanowires on microdevice surfaces [82]. Nanowire-coated devices were found to adhere to Caco-2 cells at a frequency five times greater than non-coated devices under static conditions, and when tested under flow conditions, a median survival shear (the shear at which 50% of the devices detach) of 9.15 dynes/ cm2 was reported. Additionally, devices both chemically (tomato lectin) and physically (nanowire) modified for bioadhesion adhered as well or slightly better than unmodified devices under static conditions. However, under flow conditions and in the presence of mucin, these dually modified devices were found to be disadvantageous in terms of adhesion, with a median survival shear of 3.60 dynes/cm2. As lectins bind to both cells and mucus, adding a mucin layer introduces competition between these elements for binding to the lectin-modified nanowires, which may explain reduced adhesion. Therefore, geometry-based adhesion may offer distinct advantages over mucoadhesive chemistry in terms of mucosal tissue adhesion.
5. Microfabricated in vitro models
Development of physiologically relevant three-dimensional (3D) in vitro models is another area where microfabrication can advance the field of oral drug delivery. The drug development process is a long and expensive process with only one out of ten drug candidates in clinical trial reaching final FDA approval stage. The number of new molecular entities that are approved by the FDA is also declining with 53 approved in 1996 and only 19 approved in 2010 [83]. The main reason for this low success rate is poor prediction of drug efficacy and toxicity in preclinical testing. The current drug testing paradigm is based on 2D cell monolayers and in vivo animal models before clinical trials in humans. Although 2D cell monolayer-based assays are routinely used for drug efficacy and toxicity testing, such systems often fail to recapitulate microenvironmental context and in vivo biological complexity. Such systems are static and do not mimic the exchange of metabolites between the tissues, physiological shear stress, fluid flow dynamics as experienced by cells in vivo [84]. In vivo animal models allow testing drug distribution, efficacy and toxicity under physiological conditions; however, there are differences in animal and human physiology making extrapolation of animal data to human difficult. Animal studies are also expensive, time consuming and ethically controversial. Better in vitro model systems are necessary to accurately predict drug efficacy and toxicity. Microfabrication approaches have been proposed recently to develop physiologically relevant, in vitro 3D tissue models to reduce or replace animal studies including ‘organ-on-a-chip’ [85] ‘body-on-a-chip’ [86], ‘lung-on-a-chip’ [87] and ‘perfused multi-well liver tissue’ [88].
Conventional approaches to study drug absorption across the intestinal mucosa are classified into in vivo, in situ and in vitro models and have been reviewed elsewhere [89]. The most accepted and widely used in vitro absorption model consists of Caco-2 cells seeded on a polycarbonate membrane in a transwell device [1, 90]. When cultured as a monolayer, Caco-2 cells differentiate to form brush border microvilli on the upper side of the monolayer and contain both tight junctions and brush border associated enzymes. In this model, test compounds are added on the apical side of the Caco-2 cell monolayer, and compounds penetrating the cell are monitored at the basolateral side of the monolayer. Although used successfully to model oral drug absorption [91], this model still has limitations. For example, low permeabilities have been observed in vitro compared to in vivo data for the drugs that are transported through paracellular transport route [92]. Similarly, compounds with low solubility/ dissolution may have less absorption than that predicted by the Caco-2 system since oral absorption may be limited by low solubility. In addition, Caco-2 monolayers are planar in geometry, and does not accurately represent the brush border topography. Also, the use of a static monoculture neglects the influence of mucus-secreting goblet cells, and peristalsis on drug absorption. Furthermore, these monolayers cannot be used to predict the bioavailability of compounds susceptible to hepatic first pass clearance.
Microfabricated devices can potentially better model the GI surface topography and can also be used to better control the mechanics of cell-cell and cell-substrate interactions. Current efforts in this field can be divided into three approaches: i) engineered intestinal tissues; ii) microfluidic-based approaches and iii) microscale cell culture analogs (μCCA).
5.1. Engineered intestinal tissues
The small intestinal epithelium consists of an epithelial monolayer of enterocytes, goblet cells, and Peyer’s patches resting on a basement membrane. The absorptive surface area is enhanced through the topographical arrangement of this monolayer into nger-like projections (villi) and well-like invaginations (crypts). In addition to enhancing surface area, this spatial arrangement also dictates cell behavior[93]. Wang and co-workers used simple microfabrication approaches to create biomimetic crypt-like microarchitecture on polymer substrates [93]. Caco-2 cells seeded on such substrates showed higher metabolic activity and lower alkaline phosphatase activity compared to the flat substrates signifying influence of topography on cell phenotype. In a follow-up study, authors patterned type I collagen membrane using soft lithography to study the synergistic effect of crypt-like topography and extracellular matrix (ECM) proteins (fibronectin and laminin) on Caco-2 adhesion, proliferation, differentiation and tight junction formation [94]. It was found that crypt-like topography had short term effects on cell phenotype whereas substrate chemistry (ECM protein coating) had more prominent and long term effects on intestinal epithelial cell behaviors. Insight from such studies can be useful in developing biomimetic in vitro intestinal models for drug absorption.
Gunawan et al [95] created immobilized physiological protein gradients (laminin and collagen type I) similar to those found in small intestinal crypts using microfluidic gradient generator. Results revealed region-specific expression of p27 (pro-differentiation marker) and proliferating cell nuclear antigen (PCNA; proliferative cells), markers that are linked to the cell cycle progression, when intestinal epithelial cells were cultured on immobilized counter-gradients of laminin and collagen I. Such studies are necessary to understand the role of various ECM proteins on the intestinal epithelial renewal along the crypt-villus axis.
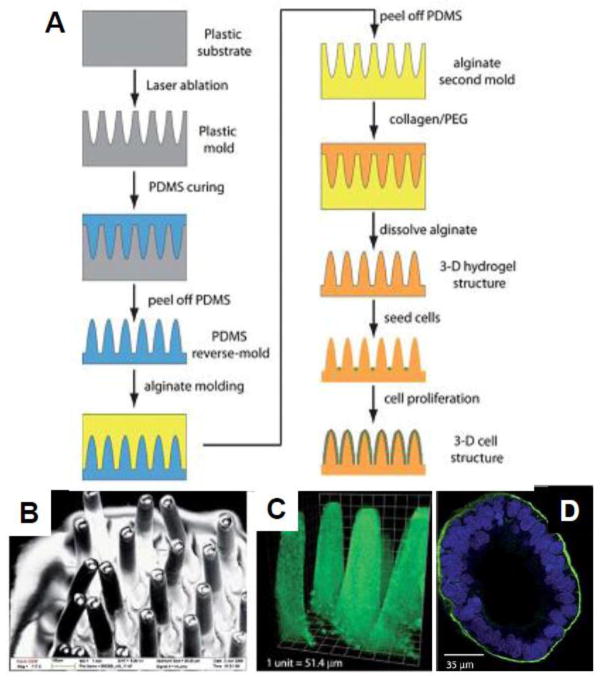
Another emerging approach to engineer biomimetic GI tract models is the use of hydrogels. Hydrogels have attracted great attention for 3D cell cultures since they mimic the ECM and can be easily modified to generate tailored microenvironments [96–100]. Additionally, hydrogels are amenable to various micromolding and soft lithographic techniques for drug delivery [31, 32] as well as tissue engineering applications [101, 102]. Recently, Sung et al developed a biomimetic GI tract model using laser ablation combined with sacrificial molding in microscale collagen hydrogels mimicking actual density and the size of human intestinal villi (Fig. 4) [103]. Caco-2 cells seeded onto the structure covered the whole structure in three weeks resembling finger-like intestinal villi covered with epithelial cells. Thus, microfabrication approaches can be used to recapitulate in vivo microenvironment to construct physiologically realistic in vitro models of intestinal villi that can improve the predictability of drug absorption studies.
Fig. 4.
Tissue engineering approach to create in vivo-like microenvironments; A) Schematic of fabrication process of crypt-like microstructures. First plastic mold is created by laser ablation, from which the PDMS reverse-mold is created. The alginate second mold is made from the PDMS, and dissolved after the nal hydrogel structure is made. B) SEM image of the PDMS villi structure; C) Confocal microscope image of the collagen scaffold showing crypt-like topography. D) Confocal x-y image of Caco-2 cells on the collagen scaffold, stained for actin (green) and nucleic acid (blue). Reprinted with permission from Sung [103].
5.2. Microfluidic based approaches
The field of microfluidics is gaining popularity in drug discovery, development [11] and personalized biomolecular diagnostics [10] due to their ability to provide fluid flow in the physiological range. Micro uidic devices also require minimal samples and reagents, and promote the effective use of space, while providing potential for multiple assays and processes in a single device for high-throughput assays [104]. Moreover, micro uidics offers spatial and temporal fluidic control in a biomimetic environment enabling long-term cell culture and differentiation [10]. Thus, microfluidics is becoming integral part of the cell-based assays to predict oral drug absorption.
For realistic prediction of oral drug bioavailability, an in vitro model should incorporate all major physiological obstacles to the drugs entry into systemic circulation. These include the transport properties of the epithelium, liver metabolism, and the vascular transport that links them. Transwell co-culture models have been designed to include apical caco-2 monolayers along with hepatocytes in the basolateral compartment [105, 106]. However, these models use large liquid to cell ratios and devoid of circulation of medium. To overcome these limitations, perfused co-culture system can be designed using microfluidic-based approaches.
To generate a biomimetic microenvironment for drug absorption studies, a perfused co-cultures system was designed using microfluidics that enhanced cytochrome P450 (CYP) 1A1/2 activity [107]. In another study, Mahler et al co-cultured mucous secreting HT29-MTX goblet-like cells with Caco-2 cells to mimic intestinal cell populations and HepG2/C3A cell line as liver cell populations [108, 109]. Presence of HT-29 cells resulted in Caco-2 cell layer covered with mucus when cultured in physiologically realistic ratios [109].
Microfluidics has been used to design a bioreactor system with physiologically meaningful ow conditions to study various epithelial cell transport processes [104, 110, 111]. Ferrell and co-workers[112] fabricated a bilayer micro uidic system with integrated transepithelial electrical resistance (TEER) measurement electrodes to evaluate kidney epithelial cells under physiologically relevant uid ow conditions. The apical and basolateral uidic chambers were connected via a transparent microporous membrane. The top chamber contained micro uidic channels to perfuse the apical surface of the cells whereas the bottom chamber acted as a reservoir for transport across the cell layer and provides support for the membrane. TEER electrodes were integrated into the device to monitor cell growth and evaluate cell–cell tight junction integrity in real time. Such bioreactors can be easily integrated with perfused co-culture systems that closely mimic GI epithelial barriers along with first pass metabolism described above.
Kimura et al have developed a micro uidic device embedded with a stirrer-based micropump to create on-chip perfusion, and an optical ber connection for on-line uorescence detection for drug screening and toxicity testing (Fig. 5) [113]. In another study, a microfluidic device containing microhole arrays was fabricated to reduce the Caco-2 culture time [114]. In vivo permeabilities in the human and rat intestine are highly correlated with those measured by the micro uidic device. However, the limitation of the device is that tight cell junctions are not formed since single cells are trapped in each microhole for a short period. Consequently, this system cannot be applicable for drugs transported through tight junctions; however, it can still be used for drugs that are transported passively or actively with the aid of transport proteins [114].
Fig. 5.
Microfluidic-based approaches to create perfused in vitro models for drug absorption; i) Fabrication process of the micro uidic device. (a) Prepolymer of PDMS poured over an SU-8 structure; (b) the PDMS structure is peeled from the mold master; (c) coating CYTOP inside of microchannel; (d) the semipermeable membrane and magnetic stir-bar are placed on the PDMS layer for assembly; (e) PDMS layers are bonded. ii) Schematic illustration of the integrated micro uidic device. Caco-2 cells are cultured only on the semipermeable membrane in the AP side culture chamber. The stir-bar is driven by motor- controlled permanent magnets beneath the device. iii) Photograph of the micro uidic device. Reprinted with permission from [113].
5.3. Microscale cell culture analogs (μCCA)
A drug’s absorption, distribution, metabolism, and excretion (ADME) is a result of interaction between various cells, tissues and organs that are interconnected by vasculature. Physiologically based pharmacokinetic (PBPK) mathematical models that describe an organism as a set of interconnected tissue or organ compartments based on vasculature have been designed to calculate the time-dependent distribution of a drug in various tissues [115]. An important advancement in the field of drug screening is to integrate multiple miniaturized organ model systems into a single device to recapitulate the potential interaction between different organs in determining the drug’s ADME. The concept of microscale cell culture analog (μCCA) is a physical representation of PBPK model where different cell types are cultured in small chambers interconnected by fluidic channels [116]. Such systems offer versatile in vitro models to study drug’s biotransformation, and interaction between different tissues in determining drug’s response (both efficacy and toxicity). Fabrication and applications of macroscopic and microscopic CCAs have been reviewed in detail in recent reviews [84, 86, 117].
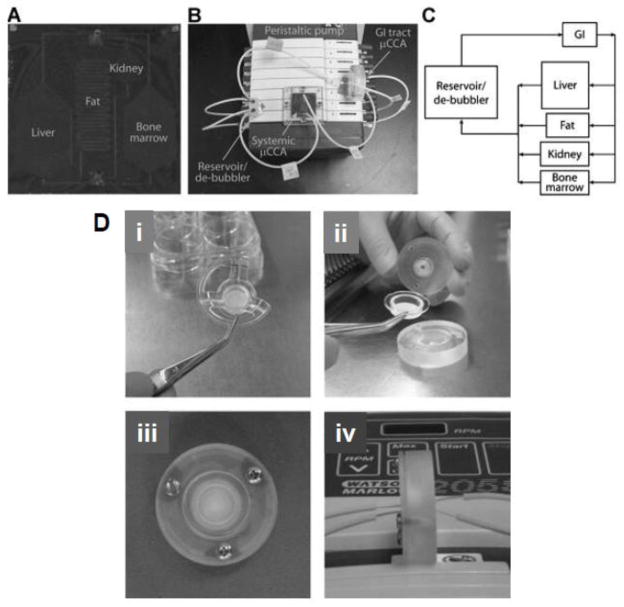
Most of the previous work with μCCAs was done to mimic intravenous administration of a compound as the drug was added directly into the circulating culture medium [118–121]. More recently, GI tract μCCAs have been developed that include digestion, a mucus layer, and physiologically realistic cell populations to determine oral bioavailability of drugs (Fig. 6) [108, 122]. The GI tract μCCA was used together with a systemic μCCA to demonstrate absorption, distribution, metabolism and toxicity of a widely used analgesic and antipyretic drug, acetaminophen [108]. The authors showed that acetaminophen was absorbed and metabolized by GI cells, then circulated to the liver cell compartment. Liver cells were capable of metabolizing the drug into reactive metabolite resulting in a dose-dependent toxicity to the liver cells [108].
Fig. 6.
Gastrointestinal tract on a chip to predict ADME after oral drug administration; A) Image of the systemic μCCA containing liver, kidney, bone marrow, and fat chamber. The channels connecting compartments were 100 mm deep. The other poorly and well-perfused tissues were represented by the external de-bubbler, which was a 200 μL reservoir. B) Image of the systemic and GI tract μCCA experimental set-up. C) A schematic of the ow pattern in the μCCA system. D) GI tract μCCA device and assembly. i) The Snapwell membrane; ii) The Snapwell membrane being placed in between the top and bottom pieces of the GI tract μCCA; iii) The top of the assembled GI tract μCCA; iv) The inlets and outlets on the apical and basolateral sides of the assembled GI tract. Reprinted with permission from [108].
Combination of microfabrication with microfluidics has allowed precise control over microscale structures. In addition, the ability to pattern physiologically relevant cell types, as well as to manipulate geometry of the substrate in 3D and flow patterns / hydrodynamic shear stress in the physiological range upon the cells takes us one step closer to creating whole-body-on-a-chip for efficient screening of drug efficacy and toxicity. Reduction in the amount of sample, spatiotemporal fluidic control, easy fabrication and reduced cost makes it more attractive for high throughput drug screening and can further reduce the cost of drug development if integrated earlier in the drug development process. Potentially, such systems can be used as an alternative to animal models in drug screening.
6. Conclusions
Microfabrication techniques have been adapted to create physiologically relevant materials and devices that mimic the scale cells experience in vivo and have found wide biomedical applications, including drug delivery, tissue engineering and biosensing. In this short review, we have mainly focused on applying microfabrication techniques for oral drug delivery applications. Micromachining allows for control over particle size, shape, aspect ratio, and surface features, which can be engineered to overcome the barriers associated with oral delivery. For example, the microfabricated oral drug delivery system can be manufactured to have increased contact with the intestinal wall, while minimizing shear disturbances and allowing for unidirectional drug release from a protected reservoir to enhance their retention in the body. This is an exciting emerging field; however, we should acknowledge it is still in its infancy. Most microfabricated oral drug delivery systems have only been tested mostly with in vitro or ex vivo models. In vivo considerations such as mechanical forces, biodistribution and removal from the body have had limited study with regards to microfabricated implants. In addition to further study in animal models, application in an in vivo environment would also require further consideration of material construction and biodegradability. Furthermore, affordable methods for manufacturing scale-up will need to be developed in order for these “proof-of-concept” microfabricated devices for oral drug delivery to move towards commercial reality.
Acknowledgments
AK acknowledges funding from the National Institutes of Health (EB009196; DE019024; EB007249; HL099073; AR057837), the National Science Foundation CAREER Award (DMR0847287) and the Office of Naval Research Young Investigator Award. NAP acknowledges funding from the National Institutes of Health (EB000246-18 and a Physical Science-Oncology Centers U54 grant), from the National Science Foundation (DGE-03-33080, CBE-10-33746), the Bill and Melinda Gates Foundation, and the Pratt Foundation. SS is grateful for System-based Consortium for Organ Design and Engineering (SysCODE) postdoctoral training fellowship. OZF acknowledges the support of a UNCF-Merck Science Initiative postdoctoral fellowship. Portions of the work cited here and performed by SLT were funded by National Aeronautics and Space Administration Graduate Student Research Program Fellowship, Center for the Integration of Medicine and Innovative Technology, and National Institutes of Health EB002687. Q. X. acknowledges the startup funding from Tufts University and Tufts FRAC award. We thank Dr. Akhilesh Gaharwar for his comments and suggestion to improve this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Peppas NA, Carr DA. Impact of absorption and transport on intelligent therapeutics and nanoscale delivery of protein therapeutic agents. Chemical Engineering Science. 2009;64:4553–4565. doi: 10.1016/j.ces.2009.04.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goldberg M, Gomez-Orellana I. Challenges for the oral delivery of macromolecules. Nat Rev Drug Discov. 2003;2:289–295. doi: 10.1038/nrd1067. [DOI] [PubMed] [Google Scholar]
- 3.Martinez MN, Amidon GL. A mechanistic approach to understanding the factors affecting drug absorption: A review of fundamentals. Journal of Clinical Pharmacology. 2002;42:620–643. doi: 10.1177/00970002042006005. [DOI] [PubMed] [Google Scholar]
- 4.Hauss DJ. Oral lipid-based formulations. Adv Drug Deliv Rev. 2007;59:667–676. doi: 10.1016/j.addr.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 5.Morishita M, Peppas NA. Is the oral route possible for peptide and protein drug delivery. Drug Discovery Today. 2006;11:905–910. doi: 10.1016/j.drudis.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 6.Peppas NA, Wood KM, Blanchette JO. Hydrogels for oral delivery of therapeutic proteins. Expert Opinion on Biological Therapy. 2004;4:881–887. doi: 10.1517/14712598.4.6.881. [DOI] [PubMed] [Google Scholar]
- 7.Liechty WB, Kryscio DR, Slaughter BV, Peppas NA. Polymers for Drug Delivery Systems. Annual Review of Chemical and Biomolecular Engineering. 2010;1:149–173. doi: 10.1146/annurev-chembioeng-073009-100847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wong JE, Gaharwar AK, Müller-Schulte D, Bahadur D, Richtering W. Dual-stimuli responsive PNiPAM microgel achieved via layer-by-layer assembly: Magnetic and thermoresponsive. Journal of Colloid and Interface Science. 2008;324:47–54. doi: 10.1016/j.jcis.2008.05.024. [DOI] [PubMed] [Google Scholar]
- 9.Siegel RA, Gu YD, Lei M, Baldi A, Nuxoll EE, Ziaie B. Hard and soft micro- and nanofabrication: An integrated approach to hydrogel-based biosensing and drug delivery. Journal of Controlled Release. 2010;141:303–313. doi: 10.1016/j.jconrel.2009.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shaw KJ, Birch C, Hughes EM, Jakes AD, Greenman J, Haswell SJ. Microsystems for personalized biomolecular diagnostics. Engineering in Life Sciences. 2011;11:121–132. [Google Scholar]
- 11.Kang L, Chung BG, Langer R, Khademhosseini A. Microfluidics for drug discovery and development: From target selection to product lifecycle management. Drug Discovery Today. 2008;13:1–13. doi: 10.1016/j.drudis.2007.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chung BG, Kang LF, Khademhosseini A. Micro- and nanoscale technologies for tissue engineering and drug discovery applications. Expert Opinion on Drug Discovery. 2007;2:1653–1668. doi: 10.1517/17460441.2.12.1653. [DOI] [PubMed] [Google Scholar]
- 13.Caldorera-Moore M, Peppas NA. Micro- and nanotechnologies for intelligent and responsive biomaterial-based medical systems. Advanced Drug Delivery Reviews. 2009;61:1391–1401. doi: 10.1016/j.addr.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xia Y, Rogers JA, Paul KE, Whitesides GM. Unconventional Methods for Fabricating and Patterning Nanostructures. Chem Rev. 1999;99:1823–1848. doi: 10.1021/cr980002q. [DOI] [PubMed] [Google Scholar]
- 15.Qin D, Xia YN, Whitesides GM. Soft lithography for micro- and nanoscale patterning. Nature Protocols. 2010;5:491–502. doi: 10.1038/nprot.2009.234. [DOI] [PubMed] [Google Scholar]
- 16.Ainslie KM, Desai TA. Microfabricated implants for applications in therapeutic delivery, tissue engineering, and biosensing. Lab on a Chip. 2008;8:1864–1878. doi: 10.1039/b806446f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hilt JZ, Peppas NA. Microfabricated drug delivery devices. Int J Pharm. 2005;306:15–23. doi: 10.1016/j.ijpharm.2005.09.022. [DOI] [PubMed] [Google Scholar]
- 18.LaVan DA, McGuire T, Langer R. Small-scale systems for in vivo drug delivery. Nat Biotechnol. 2003;21:1184–1191. doi: 10.1038/nbt876. [DOI] [PubMed] [Google Scholar]
- 19.Staples M, Daniel K, Cima MJ, Langer R. Application of micro- and nano-electromechanical devices to drug delivery. Pharmaceutical Research. 2006;23:847–863. doi: 10.1007/s11095-006-9906-4. [DOI] [PubMed] [Google Scholar]
- 20.Tao SL, Desai TA. Microfabricated drug delivery systems: from particles to pores. Advanced Drug Delivery Reviews. 2003;55:315–328. doi: 10.1016/s0169-409x(02)00227-2. [DOI] [PubMed] [Google Scholar]
- 21.Santini JT, Jr, Richards AC, Scheidt RA, Cima MJ, Langer RS. Microchip technology in drug delivery. Ann Med. 2000;32:377–379. doi: 10.3109/07853890008995941. [DOI] [PubMed] [Google Scholar]
- 22.Staples M. Microchips and controlled-release drug reservoirs. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2010;2:400–417. doi: 10.1002/wnan.93. [DOI] [PubMed] [Google Scholar]
- 23.Santini JT, Jr, Cima MJ, Langer R. A controlled-release microchip. Nature. 1999;397:335–338. doi: 10.1038/16898. [DOI] [PubMed] [Google Scholar]
- 24.Grayson AC, Voskerician G, Lynn A, Anderson JM, Cima MJ, Langer R. Differential degradation rates in vivo and in vitro of biocompatible poly(lactic acid) and poly(glycolic acid) homo- and co-polymers for a polymeric drug-delivery microchip. J Biomater Sci Polym Ed. 2004;15:1281–1304. doi: 10.1163/1568562041959991. [DOI] [PubMed] [Google Scholar]
- 25.Richards Grayson AC, Choi IS, Tyler BM, Wang PP, Brem H, Cima MJ, Langer R. Multi-pulse drug delivery from a resorbable polymeric microchip device. Nat Mater. 2003;2:767–772. doi: 10.1038/nmat998. [DOI] [PubMed] [Google Scholar]
- 26.Amirouche F, Zhou Y, Johnson T. Current micropump technologies and their biomedical applications. Microsystems Technology. 2009;15:647–666. [Google Scholar]
- 27.Sewell WF, Borenstein JT, Chen Z, Fiering J, Handzel O, Holmboe M, Kim ES, Kujawa SG, McKenna MJ, Mescher MM, Murphy B, Swan EE, Peppi M, Tao S. Development of a microfluidics-based intracochlear drug delivery device. Audiol Neurootol. 2009;14:411–422. doi: 10.1159/000241898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tasciotti E, Liu X, Bhavane R, Plant K, Leonard AD, Price BK, Cheng MM, Decuzzi P, Tour JM, Robertson F, Ferrari M. Mesoporous silicon particles as a multistage delivery system for imaging and therapeutic applications. Nat Nanotechnol. 2008;3:151–157. doi: 10.1038/nnano.2008.34. [DOI] [PubMed] [Google Scholar]
- 29.Martin FJ, Melnik K, West T, Shapiro J, Cohen M, Boiarski AA, Ferrari M. Acute toxicity of intravenously administered microfabricated silicon dioxide drug delivery particles in mice: preliminary findings. Drugs R D. 2005;6:71–81. doi: 10.2165/00126839-200506020-00002. [DOI] [PubMed] [Google Scholar]
- 30.Guan J, Ferrell N, James Lee L, Hansford DJ. Fabrication of polymeric microparticles for drug delivery by soft lithography. Biomaterials. 2006;27:4034–4041. doi: 10.1016/j.biomaterials.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 31.Guan J, He H, Hansford DJ, Lee LJ. Self-folding of three-dimensional hydrogel microstructures. J Phys Chem B. 2005;109:23134–23137. doi: 10.1021/jp054341g. [DOI] [PubMed] [Google Scholar]
- 32.Guan J, He H, Lee LJ, Hansford DJ. Fabrication of particulate reservoir-containing, capsulelike, and self-folding polymer microstructures for drug delivery. Small. 2007;3:412–418. doi: 10.1002/smll.200600240. [DOI] [PubMed] [Google Scholar]
- 33.Tao SL, Desai TA. Micromachined devices: The impact of controlled geometry from cell-targeting to bioavailability. Journal of Controlled Release. 2005;109:127–138. doi: 10.1016/j.jconrel.2005.09.019. [DOI] [PubMed] [Google Scholar]
- 34.Canelas DA, Herlihy KP, DeSimone JM. Top-down particle fabrication: control of size and shape for diagnostic imaging and drug delivery. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2009;1:391–404. doi: 10.1002/wnan.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gratton SE, Ropp PA, Pohlhaus PD, Luft JC, Madden VJ, Napier ME, DeSimone JM. The effect of particle design on cellular internalization pathways. Proc Natl Acad Sci U S A. 2008;105:11613–11618. doi: 10.1073/pnas.0801763105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Acharya G, Shin CS, McDermott M, Mishra H, Park H, Kwon IC, Park K. The hydrogel template method for fabrication of homogeneous nano/microparticles. Journal of Controlled Release. 2010;141:314–319. doi: 10.1016/j.jconrel.2009.09.032. [DOI] [PubMed] [Google Scholar]
- 37.Acharya G, McDermott M, Shin SJ, Park H, Park K. Hydrogel templates for the fabrication of homogeneous polymer microparticles. Methods Mol Biol. 2011;726:179–185. doi: 10.1007/978-1-61779-052-2_12. [DOI] [PubMed] [Google Scholar]
- 38.Glangchai LC, Caldorera-Moore M, Shi L, Roy K. Nanoimprint lithography based fabrication of shape-specific, enzymatically-triggered smart nanoparticles. J Control Release. 2008;125:263–272. doi: 10.1016/j.jconrel.2007.10.021. [DOI] [PubMed] [Google Scholar]
- 39.Dendukuri D, Pregibon DC, Collins J, Hatton TA, Doyle PS. Continuous-flow lithography for high-throughput microparticle synthesis. Nat Mater. 2006;5:365–369. doi: 10.1038/nmat1617. [DOI] [PubMed] [Google Scholar]
- 40.Champion JA, Katare YK, Mitragotri S. Making polymeric micro- and nanoparticles of complex shapes. Proc Natl Acad Sci U S A. 2007;104:11901–11904. doi: 10.1073/pnas.0705326104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Champion JA, Mitragotri S. Shape induced inhibition of phagocytosis of polymer particles. Pharm Res. 2009;26:244–249. doi: 10.1007/s11095-008-9626-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tao SL, Desai TA. Gastrointestinal patch systems for oral drug delivery. Drug Discov Today. 2005;10:909–915. doi: 10.1016/S1359-6446(05)03489-6. [DOI] [PubMed] [Google Scholar]
- 43.Lai SK, Wang YY, Hanes J. Mucus-penetrating nanoparticles for drug and gene delivery to mucosal tissues. Adv Drug Deliv Rev. 2009;61:158–171. doi: 10.1016/j.addr.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Foraker AB, Walczak RJ, Cohen MH, Boiarski TA, Grove CF, Swaan PW. Microfabricated porous silicon particles enhance paracellular delivery of insulin across intestinal Caco-2 cell monolayers. Pharm Res. 2003;20:110–116. doi: 10.1023/a:1022211127890. [DOI] [PubMed] [Google Scholar]
- 45.Ahmed A, Bonner C, Desai TA. Bioadhesive microdevices with multiple reservoirs: a new platform for oral drug delivery. Journal of Controlled Release. 2002;81:291–306. doi: 10.1016/s0168-3659(02)00074-3. [DOI] [PubMed] [Google Scholar]
- 46.Tao SL, Desai TA. Microfabrication of multilayer, asymmetric, polymeric devices for drug delivery. Advanced Materials. 2005;17:1625–1630. [Google Scholar]
- 47.Tao SL, Popat K, Desai TA. Off-wafer fabrication and surface modification of asymmetric 3D SU-8 microparticles. Nat Protoc. 2006;1:3153–3158. doi: 10.1038/nprot.2006.451. [DOI] [PubMed] [Google Scholar]
- 48.Quake SR, Scherer A. From micro- to nanofabrication with soft materials. Science. 2000;290:1536–1540. doi: 10.1126/science.290.5496.1536. [DOI] [PubMed] [Google Scholar]
- 49.Tao SL, Lubeley MW, Desai TA. Bioadhesive poly(methyl methacrylate) microdevices for controlled drug delivery. Journal of Controlled Release. 2003;88:215–228. doi: 10.1016/s0168-3659(03)00005-1. [DOI] [PubMed] [Google Scholar]
- 50.Tao SL, Lubeley MW, Desai TA. Synthesis of cytoadhesive poly(methylmethacrylate) for applications in targeted drug delivery. J Biomed Mater Res A. 2003;67:369–375. doi: 10.1002/jbm.a.10047. [DOI] [PubMed] [Google Scholar]
- 51.Ainslie KM, Kraning CM, Desai TA. Microfabrication of an asymmetric, multilayered microdevice for controlled release of orally delivered therapeutics. Lab on a Chip. 2008;8:1042–1047. doi: 10.1039/b800604k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ainslie KM, Lowe RD, Beaudette TT, Petty L, Bachelder EM, Desai TA. Microfabricated devices for enhanced bioadhesive drug delivery: attachment to and small-molecule release through a cell monolayer under flow. Small. 2009;5:2857–2863. doi: 10.1002/smll.200901254. [DOI] [PubMed] [Google Scholar]
- 53.Peppas NA. Devices based on intelligent biopolymers for oral protein delivery. International Journal of Pharmaceutics. 2004;277:11–17. doi: 10.1016/j.ijpharm.2003.03.001. [DOI] [PubMed] [Google Scholar]
- 54.Huang YB, Leobandung W, Foss A, Peppas NA. Molecular aspects of muco- and bioadhesion: Tethered structures and site-specific surfaces. Journal of Controlled Release. 2000;65:63–71. doi: 10.1016/s0168-3659(99)00233-3. [DOI] [PubMed] [Google Scholar]
- 55.Serra L, Domenech J, Peppas NA. Engineering design and molecular dynamics of mucoadhesive drug delivery systems as targeting agents. Eur J Pharm Biopharm. 2009;71:519–528. doi: 10.1016/j.ejpb.2008.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Peppas NA, Little MD, Huang Y. Bioadhesive Controlled Release Systems. In: Wise DL, Brannon-Peppas L, Klibanov AM, Langer RL, Mikos AG, Peppas NA, Trantolo DJ, Wnek GE, Yaszemski MJ, editors. Handbook of Pharmaceutical Controlled Release Technology. Dekker; New York, NY: 2000. pp. 255–269. [Google Scholar]
- 57.Morishita M, Goto T, Peppas NA, Joseph JI, Torjman MC, Munsick C, Nakamura K, Yamagata T, Takayama K, Lowman AM. Mucosal insulin delivery systems based on complexation polymer hydrogels: effect of particle size on insulin enteral absorption. Journal of Controlled Release. 2004;97:115–124. doi: 10.1016/j.jconrel.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 58.Wood KM, Stone GM, Peppas NA. The effect of complexation hydrogels on insulin transport in intestinal epithelial cell models. Acta Biomaterialia. 2010;6:48–56. doi: 10.1016/j.actbio.2009.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ichikawa H, Peppas NA. Novel complexation hydrogels for oral peptide delivery: In vitro evaluation of their cytocompatibility and insulin-transport enhancing effects using Caco-2 cell monolayers. Journal of Biomedical Materials Research Part A. 2003;67A:609–617. doi: 10.1002/jbm.a.10128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Morishita M, Goto T, Nakamura K, Lowman AM, Takayama K, Peppas NA. Novel oral insulin delivery systems based on complexation polymer hydrogels: Single and multiple administration studies in type 1 and 2 diabetic rats. Journal of Controlled Release. 2006;110:587–594. doi: 10.1016/j.jconrel.2005.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Morishita M, Goto M, Takayama K, Peppas NA. Oral insulin delivery systems based on complexation polymer hydrogels. Journal of Drug Delivery Science and Technology. 2006;16:19–24. [Google Scholar]
- 62.Kamei N, Morishita M, Chiba H, Kavimandan NJ, Peppas NA, Takayama K. Complexation hydrogels for intestinal delivery of interferon beta and calcitonin. Journal of Controlled Release. 2009;134:98–102. doi: 10.1016/j.jconrel.2008.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lehr CM. Lectin-mediated drug delivery: the second generation of bioadhesives. J Control Release. 2000;65:19–29. doi: 10.1016/s0168-3659(99)00228-x. [DOI] [PubMed] [Google Scholar]
- 64.Naisbett B, Woodley J. Binding of tomato lectin to the intestinal mucosa and its potential for oral drug delivery. Biochem Soc Trans. 1990;18:879–880. doi: 10.1042/bst0180879a. [DOI] [PubMed] [Google Scholar]
- 65.Wirth M, Kneuer C, Lehr CM, Gabor F. Lectin-mediated drug delivery: discrimination between cytoadhesion and cytoinvasion and evidence for lysosomal accumulation of wheat germ agglutinin in the Caco-2 model. J Drug Target. 2002;10:439–448. doi: 10.1080/1061186021000038300. [DOI] [PubMed] [Google Scholar]
- 66.Wood KM, Stone G, Peppas NA. Lectin functionalized complexation hydrogels for oral protein delivery. J Control Release. 2006;116:e66–68. doi: 10.1016/j.jconrel.2006.09.053. [DOI] [PubMed] [Google Scholar]
- 67.Wood KM, Stone GM, Peppas NA. Wheat germ agglutinin functionalized complexation hydrogels for oral insulin delivery. Biomacromolecules. 2008;9:1293–1298. doi: 10.1021/bm701274p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Henry S, McAllister DV, Allen MG, Prausnitz MR. Microfabricated microneedles: a novel approach to transdermal drug delivery. J Pharm Sci. 1998;87:922–925. doi: 10.1021/js980042+. [DOI] [PubMed] [Google Scholar]
- 69.Kaushik S, Hord AH, Denson DD, McAllister DV, Smitra S, Allen MG, Prausnitz MR. Lack of pain associated with microfabricated microneedles. Anesth Analg. 2001;92:502–504. doi: 10.1097/00000539-200102000-00041. [DOI] [PubMed] [Google Scholar]
- 70.McAllister DV, Allen MG, Prausnitz MR. Microfabricated microneedles for gene and drug delivery. Annu Rev Biomed Eng. 2000;2:289–313. doi: 10.1146/annurev.bioeng.2.1.289. [DOI] [PubMed] [Google Scholar]
- 71.Lee JW, Park JH, Prausnitz MR. Dissolving microneedles for transdermal drug delivery. Biomaterials. 2008;29:2113–2124. doi: 10.1016/j.biomaterials.2007.12.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Park JH, Allen MG, Prausnitz MR. Biodegradable polymer microneedles: fabrication, mechanics and transdermal drug delivery. Conf Proc IEEE Eng Med Biol Soc. 2004;4:2654–2657. doi: 10.1109/IEMBS.2004.1403761. [DOI] [PubMed] [Google Scholar]
- 73.Park JH, Allen MG, Prausnitz MR. Biodegradable polymer microneedles: fabrication, mechanics and transdermal drug delivery. J Control Release. 2005;104:51–66. doi: 10.1016/j.jconrel.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 74.Park JH, Allen MG, Prausnitz MR. Polymer microneedles for controlled-release drug delivery. Pharm Res. 2006;23:1008–1019. doi: 10.1007/s11095-006-0028-9. [DOI] [PubMed] [Google Scholar]
- 75.Mahdavi A, Ferreira L, Sundback C, Nichol JW, Chan EP, Carter DJ, Bettinger CJ, Patanavanich S, Chignozha L, Ben-Joseph E, Galakatos A, Pryor H, Pomerantseva I, Masiakos PT, Faquin W, Zumbuehl A, Hong S, Borenstein J, Vacanti J, Langer R, Karp JM. A biodegradable and biocompatible gecko-inspired tissue adhesive. Proc Natl Acad Sci U S A. 2008;105:2307–2312. doi: 10.1073/pnas.0712117105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Martanto W, Moore JS, Couse T, Prausnitz MR. Mechanism of fluid infusion during microneedle insertion and retraction. J Control Release. 2006;112:357–361. doi: 10.1016/j.jconrel.2006.02.017. [DOI] [PubMed] [Google Scholar]
- 77.Martanto W, Moore JS, Kashlan O, Kamath R, Wang PM, O’Neal JM, Prausnitz MR. Microinfusion using hollow microneedles. Pharm Res. 2006;23:104–113. doi: 10.1007/s11095-005-8498-8. [DOI] [PubMed] [Google Scholar]
- 78.Roxhed N, Samel B, Nordquist L, Griss P, Stemme G. Painless drug delivery through microneedle-based transdermal patches featuring active infusion. IEEE Trans Biomed Eng. 2008;55:1063–1071. doi: 10.1109/TBME.2007.906492. [DOI] [PubMed] [Google Scholar]
- 79.Donnelly RF, Raj Singh TR, Woolfson AD. Microneedle-based drug delivery systems: microfabrication, drug delivery, and safety. Drug Deliv. 2010;17:187–207. doi: 10.3109/10717541003667798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Arzt E, Gorb S, Spolenak R. From micro to nano contacts in biological attachment devices. Proc Natl Acad Sci U S A. 2003;100:10603–10606. doi: 10.1073/pnas.1534701100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Spolenak R, Gorb S, Arzt E. Adhesion design maps for bio-inspired attachment systems. Acta Biomater. 2005;1:5–13. doi: 10.1016/j.actbio.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 82.Fischer KE, Aleman BJ, Tao SL, Hugh Daniels R, Li EM, Bunger MD, Nagaraj G, Singh P, Zettl A, Desai TA. Biomimetic nanowire coatings for next generation adhesive drug delivery systems. Nano Lett. 2009;9:716–720. doi: 10.1021/nl803219f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mullard A. 2010 FDA drug approvals. Nature Reviews Drug Discovery. 2011;10:82–85. doi: 10.1038/nrd3370. [DOI] [PubMed] [Google Scholar]
- 84.Park TH, Shuler ML. Integration of Cell Culture and Microfabrication Technology. Biotechnology Progress. 2003;19:243–253. doi: 10.1021/bp020143k. [DOI] [PubMed] [Google Scholar]
- 85.Moraes C, Mehta G, Lesher-Perez S, Takayama S. Organs-on-a-Chip: A Focus on Compartmentalized Microdevices. Annals of Biomedical Engineering. 2011:1–17. doi: 10.1007/s10439-011-0455-6. [DOI] [PubMed] [Google Scholar]
- 86.Esch MB, King TL, Shuler ML. The Role of Body-on-a-Chip Devices in Drug and Toxicity Studies. Annual Review of Biomedical Engineering. 2011;13 doi: 10.1146/annurev-bioeng-071910-124629. null. [DOI] [PubMed] [Google Scholar]
- 87.Huh D, Matthews BD, Mammoto A, Montoya-Zavala M, Hsin HY, Ingber DE. Reconstituting Organ-Level Lung Functions on a Chip. Science. 2010;328:1662–1668. doi: 10.1126/science.1188302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Domansky K, Inman W, Serdy J, Dash A, Lim MHM, Griffith LG. Perfused multiwell plate for 3D liver tissue engineering. Lab on a Chip. 2010;10:51–58. doi: 10.1039/b913221j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Le Ferrec E, Chesne C, Artusson P, Brayden D, Fabre G, Gires P, Guillou F, Rousset M, Rubas W, Scarino ML. In vitro models of the intestinal barrier - The report and recommendations of ECVAM Workshop 46. Atla-Alternatives to Laboratory Animals. 2001;29:649–668. doi: 10.1177/026119290102900604. [DOI] [PubMed] [Google Scholar]
- 90.Gan LSL, Thakker DR. Applications of the Caco-2 model in the design and development of orally active drugs: elucidation of biochemical and physical barriers posed by the intestinal epithelium. Advanced Drug Delivery Reviews. 1997;23:77–98. [Google Scholar]
- 91.Yazdanian M, Glynn SL, Wright JL, Hawi A. Correlating Partitioning and Caco-2 Cell Permeability of Structurally Diverse Small Molecular Weight Compounds. Pharmaceutical Research. 1998;15:1490–1494. doi: 10.1023/a:1011930411574. [DOI] [PubMed] [Google Scholar]
- 92.Artursson P. Epithelial transport of drugs in cell-culture.1. A model for studying the passive diffusion of drugs over intestinal absorptive (Caco-2) cells. Journal of Pharmaceutical Sciences. 1990;79:476–482. doi: 10.1002/jps.2600790604. [DOI] [PubMed] [Google Scholar]
- 93.Wang L, Murthy SK, Fowle WH, Barabino GA, Carrier RL. Influence of micro-well biomimetic topography on intestinal epithelial Caco-2 cell phenotype. Biomaterials. 2009;30:6825–6834. doi: 10.1016/j.biomaterials.2009.08.046. [DOI] [PubMed] [Google Scholar]
- 94.Wang L, Murthy SK, Barabino GA, Carrier RL. Synergic effects of crypt-like topography and ECM proteins on intestinal cell behavior in collagen based membranes. Biomaterials. 2010;31:7586–7598. doi: 10.1016/j.biomaterials.2010.06.036. [DOI] [PubMed] [Google Scholar]
- 95.Gunawan RC, Choban ER, Conour JE, Silvestre J, Schook LB, Gaskins HR, Leckband DE, Kenis PJA. Regiospecific Control of Protein Expression in Cells Cultured on Two-Component Counter Gradients of Extracellular Matrix Proteins. Langmuir. 2005;21:3061–3068. doi: 10.1021/la048303k. [DOI] [PubMed] [Google Scholar]
- 96.Slaughter BV, Khurshid SS, Fisher OZ, Khademhosseini A, Peppas NA. Hydrogels in Regenerative Medicine. Advanced Materials. 2009;21:3307–3329. doi: 10.1002/adma.200802106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sant S, Hancock MJ, Donnelly JP, Iyer D, Khademhosseini A. Biomimetic gradient hydrogels for tissue engineering. The Canadian Journal of Chemical Engineering. 2010;88:899–911. doi: 10.1002/cjce.20411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lutolf MP. Spotlight on hydrogels. Nature Materials. 2009;8:451–453. doi: 10.1038/nmat2458. [DOI] [PubMed] [Google Scholar]
- 99.Peppas NA, Huang Y, Torres-Lugo M, Ward JH, Zhang J. Physicochemical, foundations and structural design of hydrogels in medicine and biology. Annual Review of Biomedical Engineering. 2000;2:9–29. doi: 10.1146/annurev.bioeng.2.1.9. [DOI] [PubMed] [Google Scholar]
- 100.Peppas NA, Hilt JZ, Khademhosseini A, Langer R. Hydrogels in biology and medicine: From molecular principles to bionanotechnology. Advanced Materials. 2006;18:1345–1360. [Google Scholar]
- 101.Nichol JW, Koshy ST, Bae H, Hwang CM, Yamanlar S, Khademhosseini A. Cell-laden microengineered gelatin methacrylate hydrogels. Biomaterials. 2010;31:5536–5544. doi: 10.1016/j.biomaterials.2010.03.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Aubin H, Nichol JW, Hutson CB, Bae H, Sieminski AL, Cropek DM, Akhyari P, Khademhosseini A. Directed 3D cell alignment and elongation in microengineered hydrogels. Biomaterials. 2010;31:6941–6951. doi: 10.1016/j.biomaterials.2010.05.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sung JH, Yu J, Luo D, Shuler ML, March JC. Microscale 3-D hydrogel scaffold for biomimetic gastrointestinal (GI) tract model. Lab on a Chip. 2011;11:389–392. doi: 10.1039/c0lc00273a. [DOI] [PubMed] [Google Scholar]
- 104.Imura Y, Asano Y, Sato K, Yoshimura E. A Microfluidic System to Evaluate Intestinal Absorption. Analytical Sciences. 2009;25:1403–1407. doi: 10.2116/analsci.25.1403. [DOI] [PubMed] [Google Scholar]
- 105.Choi SH, Nishikawa M, Sakoda A, Sakai Y. Feasibility of a simple double-layered coculture system incorporating metabolic processes of the intestine and liver tissue: application to the analysis of benzo a pyrene toxicity. Toxicology in Vitro. 2004;18:393–402. doi: 10.1016/j.tiv.2003.09.010. [DOI] [PubMed] [Google Scholar]
- 106.Lau YY, Chen Y-H, Liu T-t, Li C, Cui X, White RE, Cheng K-C. Evaluation of a novel in vitro caco-2 hepatocyte hybrid system for predicting in vivo oral bioavailability. Drug Metabolism and Disposition. 2004;32:937–942. [PubMed] [Google Scholar]
- 107.Choi SH, Fukuda O, Sakoda A, Sakai Y. Enhanced cytochrome P450 capacities of Caco-2 and Hep G2 cells in new coculture system under the static and perfused conditions: evidence for possible organ-to-organ interactions against exogenous stimuli. Materials Science and Engineering: C. 2004;24:333–339. [Google Scholar]
- 108.Mahler GJ, Esch MB, Glahn RP, Shuler ML. Characterization of a Gastrointestinal Tract Microscale Cell Culture Analog Used to Predict Drug Toxicity. Biotechnology and Bioengineering. 2009;104:193–205. doi: 10.1002/bit.22366. [DOI] [PubMed] [Google Scholar]
- 109.Mahler GJ, Shuler ML, Glahn RP. Characterization of Caco-2 and HT29-MTX cocultures in an in vitro digestion/cell culture model used to predict iron bioavailability. Journal of Nutritional Biochemistry. 2009;20:494–502. doi: 10.1016/j.jnutbio.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 110.Jang KJ, Suh KY. A multi-layer microfluidic device for efficient culture and analysis of renal tubular cells. Lab on a Chip. 2010;10:36–42. doi: 10.1039/b907515a. [DOI] [PubMed] [Google Scholar]
- 111.Jang KJ, Cho HS, Kang DH, Bae WG, Kwon TH, Suh KY. Fluid-shear-stress-induced translocation of aquaporin-2 and reorganization of actin cytoskeleton in renal tubular epithelial cells. Integrative Biology. 2011;3:134–141. doi: 10.1039/c0ib00018c. [DOI] [PubMed] [Google Scholar]
- 112.Ferrell N, Desai RR, Fleischman AJ, Roy S, Humes HD, Fissell WH. A microfluidic bioreactor with integrated transepithelial electrical resistance (TEER) measurement electrodes for evaluation of renal epithelial cells. Biotechnology and Bioengineering. 2010;107:707–716. doi: 10.1002/bit.22835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kimura H, Yamamoto T, Sakai H, Sakai Y, Fujii T. An integrated microfluidic system for long-term perfusion culture and on-line monitoring of intestinal tissue models. Lab on a Chip. 2008;8:741–746. doi: 10.1039/b717091b. [DOI] [PubMed] [Google Scholar]
- 114.Yeon JH, Park JK. Drug Permeability Assay Using Microhole-Trapped Cells in a Microfluidic Device. Analytical Chemistry. 2009;81:1944–1951. doi: 10.1021/ac802351w. [DOI] [PubMed] [Google Scholar]
- 115.Brown RP, Delp MD, Lindstedt SL, Rhomberg LR, Beliles RP. Physiological parameter values for physiologically based pharmacokinetic models. Toxicology and Industrial Health. 1997;13:407–484. doi: 10.1177/074823379701300401. [DOI] [PubMed] [Google Scholar]
- 116.Khamsi R. Labs on a chip Meet the stripped down rat. Nature. 2005;435:12–13. doi: 10.1038/435012a. [DOI] [PubMed] [Google Scholar]
- 117.Sung JH, Shuler ML. In vitro microscale systems for systematic drug toxicity study. Bioprocess and Biosystems Engineering. 2010;33:5–19. doi: 10.1007/s00449-009-0369-y. [DOI] [PubMed] [Google Scholar]
- 118.Sin A, Chin KC, Jamil MF, Kostov Y, Rao G, Shuler ML. The design and fabrication of three-chamber microscale cell culture analog devices with integrated dissolved oxygen sensors. Biotechnology Progress. 2004;20:338–345. doi: 10.1021/bp034077d. [DOI] [PubMed] [Google Scholar]
- 119.Tatosian DA, Shuler ML. A Novel System for Evaluation of Drug Mixtures for Potential Efficacy in Treating Multidrug Resistant Cancers. Biotechnology and Bioengineering. 2009;103:187–198. doi: 10.1002/bit.22219. [DOI] [PubMed] [Google Scholar]
- 120.Viravaidya K, Shuler ML. Incorporation of 3T3-L1 cells to mimic bioaccumulation in a microscale cell culture analog device for toxicity studies. Biotechnology Progress. 2004;20:590–597. doi: 10.1021/bp034238d. [DOI] [PubMed] [Google Scholar]
- 121.Viravaidya K, Sin A, Shuler ML. Development of a microscale cell culture analog to probe naphthalene toxicity. Biotechnology Progress. 2004;20:316–323. doi: 10.1021/bp0341996. [DOI] [PubMed] [Google Scholar]
- 122.McAuliffe GJ, Chang JY, Glahn RP, Shuler ML. Development of a Gastrointestinal Tract Microscale Cell Culture Analog to Predict Drug Transport. Molecular & Cellular Biomechanics. 2008;5 (print)|1556–5300(electronic) [PubMed] [Google Scholar]
- 123.Tao SL, Desai TA. Microdevices for Oral Drug Delivery. In: Ferrari M, Desai T, Bhatia S, editors. BioMEMS and Biomedical Nanotechnology. Springer; US: 2007. pp. 237–261. [Google Scholar]
- 124.Ainslie KM, Kraning CM, Desai TA. Microfabrication of an asymmetric, multi-layered microdevice for controlled release of orally delivered therapeutics. Lab Chip. 2008;8:1042–1047. doi: 10.1039/b800604k. [DOI] [PMC free article] [PubMed] [Google Scholar]