Abstract
Pain is not a symptom generally associated with thalassaemia. However, providers have noted increasing patient reports of pain, creating an impetus for this prospective, observational assessment of pain in thalassaemia patients. The primary study goals were to assess pain prevalence, severity, location, and potential risk factors. This was a multicentre, prospective study of thalassaemia patients receiving care at 12 Thalassaemia Clinical Research Network (TCRN) sites. Pain was assessed using the Brief Pain Inventory (BPI). 252 thalassaemia patients ranging in age from 12 to 71 years (mean 28.8) were enrolled. Sixty-four percent reported experiencing pain during the last four weeks, 22% of whom reported pain on a daily basis. Ordinal regression analysis of pain ratings demonstrated significant (P < 0.001) correlation of increased age with increased pain, irrespective of diagnosis, transfusion status, gender, bone density, chelator type or iron overload. Eighty-one percent reported having pain for one year or longer and 31% reported pain for five or more years. Pain is a major cause of morbidity and an unrecognized problem for patients with thalassaemia. Age is the strongest predictor of frequency and severity. Little else is known about the aetiology and predictors of this pain syndrome.
Keywords: Thalassemia, Pain, Brief Pain Inventory
INTRODUCTION
Historically, the focus of care for patients with thalassaemia, particularly individuals with transfusion-dependent thalassaemia, has been to optimize haemoglobin levels and effective chelation therapy. The effectiveness of these strategies has increased the life spans of patients with thalassaemia. Patients are increasingly able to enjoy the activities of adulthood including raising children and building careers. Unfortunately, longer life spans have also revealed previously unidentified health issues. Pain has surfaced as a potential issue in thalassaemia. Over the last decade, clinicians have noted increasing reports of chronic pain among patients. These reports provided the impetus for a multi-centre, prospective, observational assessment of pain in patients with thalassaemia. This Assessment of Pain survey study was conducted by the Thalassemia Clinical Research Network (TCRN), which is a National Institutes of Health (NIH)/National Heart, Lung, and Blood Institute (NHLBI) funded network composed of five core thalassaemia centres in North America and their associated satellite sites.
There are a limited number of studies of pain in thalassaemia (Finsterbush, et al 1985, Pakbaz, et al 2005, Scalone, et al 2008, Trachtenberg, et al 2010, Vogiatzi, et al 2009) and no studies to date have been designed specifically to assess the prevalence and characteristics of pain in this population. Scalone et al (2008) identified 62% of thalassaemia patients on chelation reporting pain, although only 1% reported extreme pain. Pakbaz et al (2005) reported moderate pain in 21% of both transfusion-dependent (N=29) and transfusion-independent (N=19) thalassaemia patients (aged 14.6±7.5 years), with severe pain in 14% of transfusion-dependent and 5% of transfusion-independent patients. An analysis of SF-36 Quality of Life data, collected as part of the Thalassaemia Longitudinal Cohort (TLC), revealed a significant relationship between increasing age and pain (Trachtenberg, et al 2010). This study also found a trend between low bone mass and bodily pain reports, but it did not reach significance (Trachtenberg, et al 2010). An assessment of the relationship between bone and joint pain in 361 thalassaemia patients found no association between pain reports, bone mass and fractures (Vogiatzi, et al 2009).
This study is the first comprehensive assessment of pain in thalassaemia. The Assessment of Pain Study was designed to: (1) assess the prevalence and severity of pain in thalassaemia, (2) identify common pain sites, (3) describe current pain management practices in thalassaemia, (4) assess the impact of pain on functioning and well-being and (5) assess the association of pain with potential risk factors.
METHODS
The TCRN Pain study protocol was approved by the TCRN Data and Safety Monitoring Board and by the ethical review boards of all TCRN institutions. Informed consent, and assent in the case of a minor, was obtained from patients with all thalassaemia syndromes greater than 12 years of age. The lower age cut-off was chosen because this is an age where children can independently report pain and an age at which pain due to thalassaemia is unlikely.
Eligible thalassaemia syndromes were β-thalassaemia major, β-thalassaemia intermedia, haemoglobin H disease and E β-thalassaemia.
Eligible patients were stratified into three levels, chronically transfused patients (≥ 8 transfusions/year), intermittent (< 8 transfusions/year), or no transfusions in the last year. Study enrollment was achieved using a convenience sample of patients recruited by transfusion status during a scheduled clinic visit.
To assess pain, study participants completed a paper version of the Brief Pain Inventory (BPI) Survey at a clinic visit.
The BPI was selected to assess pain based on its wide use and demonstrated validity and reliability over multiple cultures and languages (Cleeland 2009, Dworkin, et al 2005). Although originally developed to assess cancer pain, the BPI has been found to be a reliable and valid instrument to assess chronic pain, including pain due to arthritis and lower back pain (Keller, et al 2004, Tan, et al 2004). The BPI received a Flesch-Kincaid grade level of 5.4, indicating that individuals with a fifth grade reading level understand the questions. The primary pain dimensions assessed by the BPI include: severity, location and interference with daily life. Participants rate their worst, least, and average pain over the last seven days, as well as their pain at the time of completing the survey. Participants also rate the level that pain interferes with activities of daily living in seven areas: general activity, mood, walking ability, work, relationships with people, sleep and enjoyment of life. Pain is rated on a 0 to 10 scale, with 0=no pain/interference and 10=pain as bad as you can imagine/complete interference. Patients reporting pain in the last seven days were queried about activities that increased and decreased pain. Patients also reported medications and non-pharmacologic therapies utilized to manage pain. Participants responded yes or no to taking medications from the following categories:
Non-Steroidal Anti-Inflammatory medications, such as Motrin, Advil, Celebrex.
Acetaminophen (Tylenol)
Short-acting narcotic, such as morphine, dilaudid, Vicodin, Tylenol with Codeine
Long-acting narcotic, such as Oxycotin, MS Cotin, Methadone.
In addition, the question, “How much bodily pain have you had during the past 4 weeks?” was added to the BPI to ensure pain reports were captured for patients who experience pain on a more intermittent basis.
Clinical and Laboratory Data from the TCRN Thalassaemia Longitudinal Cohort (TLC) Study
139 of the 252 patients enrolled in the Pain Survey concomitantly participated in the TLC study. A standardized protocol of clinical and laboratory data analysis was conducted (Kwiatkowski, et al 2012). Results closest to the date of pain study enrollment were entered, including serum ferritin (N=134), liver iron concentration (LIC) by magnetic resonance imaging (MRI), (R2), (superconducting quantum interference device, SQUID) (N=102), and ejection fraction and cardiac iron determined by MRI/T2* (n=94). Bone mineral density and vertebral fractures were determined on the lumbar spine, left proximal femur and whole body, utilizing Hologic QDR 4500 scanners and morphometric x-ray absorptiometry (MXA) (n=81). Iron chelation regimens used included the type and dose of chelator (n=139). Cardiac T2*, LIC, and serum ferritin were reported as averages over the prior year.
Statistical analysis
Descriptive statistics of survey questions are presented. Regression and logistic regression models for various measures of pain were fit. Age (as a categorical variable), gender, diagnosis, transfusion status, and marital status were considered as predictors. Due to a strong age effect, the p-values for the other predictors were age-adjusted. Participants with and without pain were compared in terms of iron overload, haemoglobin level, fracture history, chelator use and bone density using t-tests. Effect of chelator choice on pain was assessed by Fisher exact test and logistic regression controlling for age. In all analyses, significance was accepted for alpha ≤0.05.
RESULTS
Two hundred and fifty-two thalassaemia patients, ranging in age from 12–71 years (mean age 28.8+/−11.75) and receiving care at one of 12 thalassaemia centres across the United States and Canada participated in the study (Table I). Thirty-five percent of the TCRN total population participated in this study including: 47% β-thalassaemia major, 21% β-thalassaemia intermedia, 30% E β-thalassaemia and 12% α-thalassaemia of the total TCRN population by diagnosis. 54% of participants were female. Diagnoses included: β-thalassaemia (81%), E β-thalassaemia (11%), Haemoglobin H (6%) and other thalassaemia conditions (2%). 80% (n=201) of participants were chronically transfused (8+ transfusions in past year), 6% (n=14) intermittently transfused and 15% (n=37) had not been transfused in the past year.
Table I.
Participant Demographics by Transfusion Status
| Overall | Regularly transfused | Intermittently transfused | Not transfused in the past year | |
|---|---|---|---|---|
| n = 252 | n = 201 (80%) | n = 14 (6%) | n = 37 (15%) | |
| Age (years) | ||||
| Mean ± Standard deviation | 28.8 ± 11.8 | 28.2 ± 10.4 | 35.8 ± 14.4 | 29.5 ± 16.2 |
| Median (min - max) | 27 (12 – 71) | 27 (12 – 57) | 38.5 (12 – 55) | 25 (12 – 71) |
| Gender | ||||
| Male | 115 (46%) | 84 (42%) | 8 (57%) | 23 (62%) |
| Female | 137 (54%) | 117 (58%) | 6 (43%) | 14 (38%) |
| Diagnosis | ||||
| β-Thalassaemia | 204 (81%) | 182 (91%) | 8 (57%) | 14 (38%) |
| E-β-Thalassaemia | 28 (11%) | 16 (8%) | 4 (29%) | 8 (22%) |
| Haemoglobin H disorders | 16 (6%) | 0 (0%) | 2 (14%) | 14 (38%) |
| Other | 4 (2%) | 3 (1%) | 0 (0%) | 1 (3%) |
| Marital status | ||||
| Married/Relationship | 79 (31%) | 60 (30%) | 6 (43%) | 13 (35%) |
| Single/Separated/Divorced | 173 (69%) | 141 (70%) | 8 (57%) | 24 (65%) |
Note: Percentages may not add up to 100 due to rounding.
Sixty-four percent of the 252 participants reported experiencing pain during the last four weeks (Table II). Pain was reported in 31% of pediatric patients compared to 72% of patients aged 18 years and older (p< 0.001). The prevalence of pain dramatically increased in the young adult group and remained high in adults of all ages: 30% (age 12–17 years), 66% (age 18–24 years), 69% (age 25–34 years) and 79%(age ≥ 35 years). Daily pain was reported in 22% of the patients with pain.
Table II.
Pain Severity and Frequency Over the Past 4 Weeks by Age
| Age (years) | |||||||
|---|---|---|---|---|---|---|---|
| Overall | 12–17 | 18–24 | 25–34 | 35–44 | 45–71 | P value | |
| n = 252 | n = 50 | n = 56 | n = 61 | n = 61 | n = 24 | ||
| Severity of pain in past four weeks | <0.001 | ||||||
| None | 90 (36%) | 34 (68%) | 19 (35%) | 19 (31%) | 12 (20%) | 6 (25%) | |
| Very mild | 40 (16%) | 5 (10%) | 11 (20%) | 9 (15%) | 14 (23%) | 1 (4%) | |
| Mild | 51 (20%) | 8 (16%) | 14 (25%) | 15 (25%) | 9 (15%) | 5 (21%) | |
| Moderate | 48 (19%) | 3 (6%) | 8 (15%) | 13 (21%) | 16 (26%) | 8 (33%) | |
| Severe | 15 (6%) | 0 (0%) | 1 (2%) | 4 (7%) | 8 (13%) | 2 (8%) | |
| Very severe | 7 (3%) | 0 (0%) | 2 (4%) | 1 (2%) | 2 (3%) | 2 (8%) | |
| Missing/Unknown | 1 | 0 | 1 | 0 | 0 | 0 | |
| Frequency of pain in past 4 weeks | <0.001 | ||||||
| No pain | 90 (36%) | 34 (68%) | 19 (34%) | 19 (31%) | 12 (20%) | 6 (25%) | |
| Every day | 35 (14%) | 1 (2%) | 2 (4%) | 6 (10%) | 13 (21%) | 13 (54%) | |
| Once a week | 78 (31%) | 8 (16%) | 24 (43%) | 22 (36%) | 21 (34%) | 3 (13%) | |
| Once a month | 48 (19%) | 6 (12%) | 11 (20%) | 14 (23%) | 15 (25%) | 2 (8%) | |
| Missing/Unknown | 1 | 1 | 0 | 0 | 0 | 0 | |
| Frequency of pain medication in past four weeks | 0.10 | ||||||
| No pain | 90 (36%) | 34 (68%) | 19 (35%) | 19 (31%) | 12 (20%) | 6 (25%) | |
| Every day | 16 (6%) | 0 (0%) | 2 (4%) | 2 (3%) | 8 (13%) | 4 (17%) | |
| Once a week | 40 (16%) | 3 (6%) | 8 (14%) | 11 (18%) | 12 (20%) | 6 (25%) | |
| Once a month | 42 (17%) | 4 (8%) | 9 (16%) | 5 (8%) | 21 (34%) | 3 (13%) | |
| Never | 64 (25%) | 9 (18%) | 18 (32%) | 24 (39%) | 8 (13%) | 5 (21%) | |
| Pain in the past seven days | |||||||
| 93 (37%) | 4 (8%) | 19 (34%) | 22 (36%) | 34 (56%) | 14 (58%) | <0.001 | |
Note: Percentages may not add up to 100 due to rounding
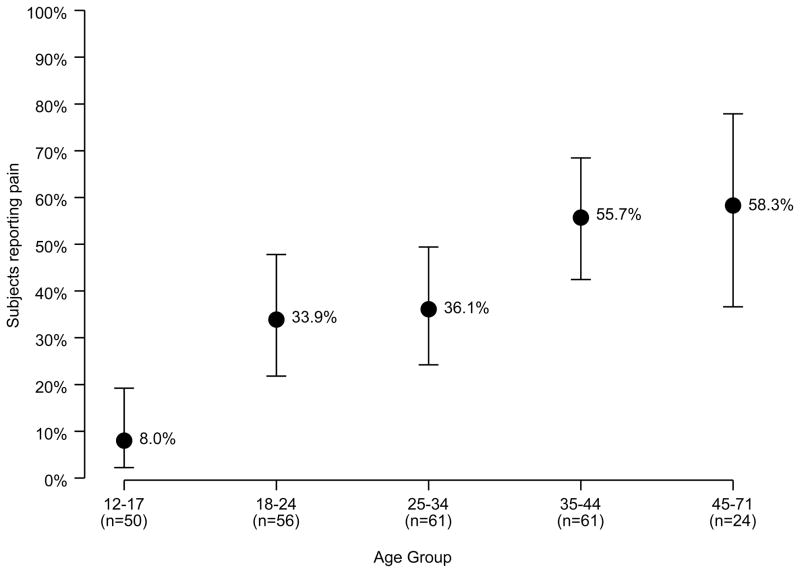
Thirty-seven percent of the 252 participants reported having pain in the last seven days. Only 8% of 12- to 17-year-olds reported pain in the last week, compared to 34% in the 18–24 age group and 56% for respondents 35–71 (Figure 1). Ordinal regression analysis of participant ratings of worst, least, and average pain over the last seven days demonstrated significant (p<0.001) correlation of increased age with increased pain across all categories, irrespective of diagnosis, transfusion status, and gender.
Figure 1.
Percentage of participants reporting pain in the last 7 days by age group (n=252)
Severity and duration of pain
Of the 93 participants who reported pain in the last seven days, the mean worst pain rating was 5.8±2.3 out of 10. Thirty-nine percent reported their worst pain as severe (7–10/10) while 42% rated their worst pain as moderate (4–6/10). Sixty-three percent of these participants rated their average pain over the last seven days to be at least moderate. Older patients were more likely to rate their pain in the past 7 days as moderate or severe (p<0.001). When adjusting for age, neither gender, diagnostic category, nor transfusion status were associated with pain severity ratings.
In the group of patients reporting pain in the last 7 days, 81% reported pain duration of one year or longer. Thirty-one percent reported experiencing pain for 5 or more years. These patients average age was 35.8 years, range 15–55 years.
Sites of pain
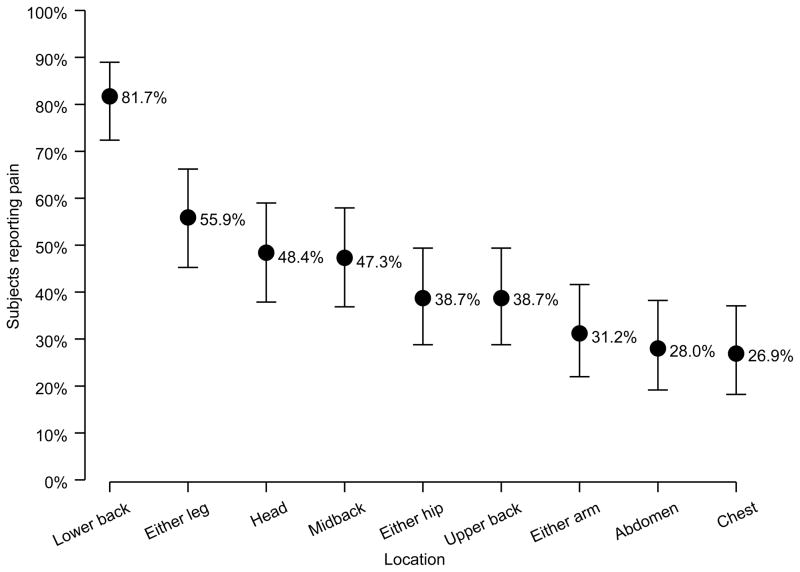
Patients with pain reported an average number of 4.0 sites of pain. The number of pain sites significantly increased with age (p=0.026), with 45- to 71-year-olds reporting an average of 4.5 pain sites, 2.0 more sites of pain than those aged 18–24 years. Of those reporting pain, 82% indicated lower back as a site of pain, followed by leg (56%), head (48%) and mid back (47%) (Figure 2). There was a trend towards increased pain in each site with age, with statistical significance reached for lower back (p= 0.036), arms (p=0.042), and hips (p=0.007). Although gender did not significantly influence the number of pain sites reported, females were significantly more likely to report head (30.7% vs. 10.2%, p<0.001) and upper back pain (25.4% vs. 8.0%, p=0.002). Otherwise, gender, marital status, diagnosis and transfusion status did not significantly influence pain sites.
Figure 2.
Percentage of participants reporting pain in the last 7 days by site (n=93)
Triggers of Pain and Pain management
Participants described their pain as aching (79%), throbbing (61%), sharp (50%), and tiring (55%). Twenty percent classified their pain as unbearable. Low haemoglobin was the most frequently cited reason for increased pain (45%), followed by standing (38%) and lifting (34%). Of the 162 patients that reported pain in the last 4 weeks, 60% reported taking a pain medication during that time period. Decreased pain was associated with pain medications (63%), blood transfusions (47%) and rest (43%). Nonsteroidal anti-inflammatory drugs were the most commonly used pain medication (52%), followed by acetaminophen (34%), short-acting opioids (17%), and long-acting opioids (8%). Twenty-five percent reported they received no pain relief from medications and/or non-pharmaceutical treatments and only 4% reported receiving complete relief with treatment. Eighteen percent of respondents felt they required stronger pain medications, despite the fact that 25% of patients experiencing pain utilized opioids for pain management.
Pain interference with life activities
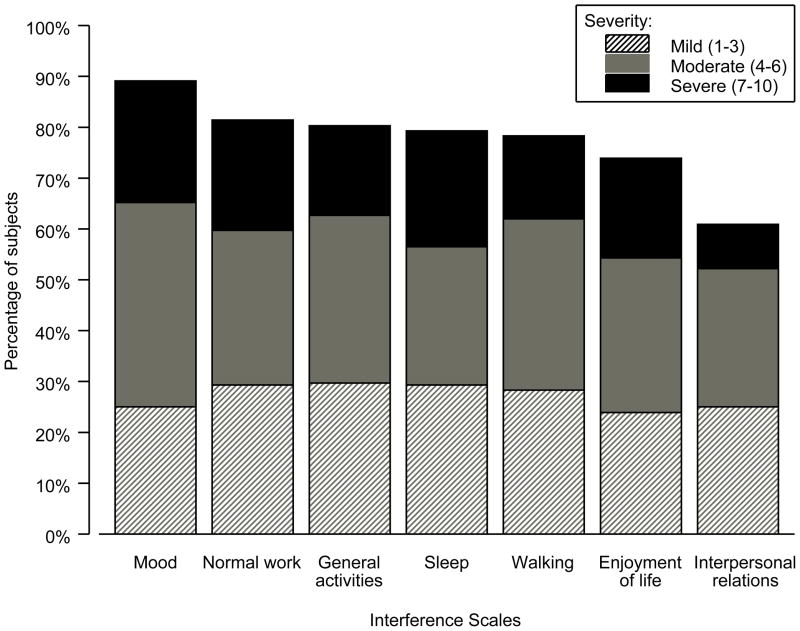
Across all measures of life activities (general activity, mood, walking ability, work, relationships with people, sleep and enjoyment of life), pain significantly correlated with increased interference (p≤0.005) controlling for age. Age also significantly correlated with increased interference (p≤0.002). Approximately two thirds of participants experiencing pain reported moderate to severe interference (rating of ≥4 out of 10) across all life activity dimensions. Mood and normal work (both outside the home and housework) were rated as the most impacted by pain, with interpersonal relationships rated as the least impacted (Figure 3). Females reported significantly more impact of pain on mood (p=0.025). Marital status trended toward significance (p=0.085) with participants who were divorced, separated, or unmarried in a relationship, reporting greater impact of pain on their relationships.
Figure 3.
Percentage of subjects reporting interference in the last 7 days by severity (n=93)
Association of pain reports with haemoglobin, iron overload, chelator use, fractures and bone density
No significant difference in average haemoglobin was found between participants who reported pain in the last seven days and those who were pain free (p=0.46). Measures of iron overload; T2*, LIC and serum ferritin, were compared for individuals who reported pain versus pain free participants. There was no significant difference in pain levels associated with iron measurements (mean serum ferritin p=0.50; mean LIC p=0.40; mean T2* p=0.87) (Table III).
Table III.
Pain Reports by Potential Pain Predictors
| No pain reported | Any type of pain reported | Age-adjusted P- value | |
|---|---|---|---|
| Haemoglobin, g/l (n=125) | 0.46 | ||
| Mean ± Standard Deviation | 98 ± 8 | 97 ± 11 | |
| Bone Health | |||
| Spine density z-scores | N=51 | N=30 | 0.009 |
| < −2.5 | 18 (35.3%) | 8 (26.7%) | |
| ≥ −2.5 but < −1.5 | 14 (27.5%) | 8 (26.7%) | |
| ≥ −1.5 | 19 (37.3%) | 14 (46.7%) | |
| Mean ± Standard Deviation | −2.05 ± 1.28 | −1.52 ± 1.57 | |
| Lifetime fractures (n=138) | N=80 | N=58 | 0.83 |
| Mean ± Standard Deviation | 0.69 ± 1.57 | 1.22 ± 1.52 | |
| Fractures in the past year (n=138) | N=80 | N=58 | 0.34 |
| Mean ± Standard Deviation | 0.06 ± 0.33 | 0.16 ± 0.59 | |
| Iron Status | |||
| LIC, mg/g dry weight | N=56 | N=46 | 0.40 |
| ≤ 10 | 32 (57%) | 29 (63%) | |
| >10 – <15 | 7 (13%) | 4 (9%) | |
| ≥ 15 | 17 (30%) | 13 (28%) | |
| Mean ± Standard Deviation | 13.5 ± 15.6 | 10.7 ± 10.3 | |
| Ferritin, μg/l | N=78 | N=56 | 0.50 |
| < 1,000 | 35 (44.9%) | 21 (37.5%) | |
| 1,000 – <3,000 | 29 (37.2%) | 28 (50.0%) | |
| ≥ 3,000 | 14 (17.9%) | 7 (12.5%) | |
| Mean ± Standard Deviation | 1821.2 ± 2095.9 | 1648.9 ± 1402.9 | |
| Cardiac T2*, ms | N=51 | N=43 | 0.87 |
| < 10 | 5 (21.7%) | 4 (22.2%) | |
| 10 – <20 | 11 (47.8%) | 8 (44.4%) | |
| ≥ 20 | 35 (68.6%) | 31 (72.1%) | |
| Mean ± Standard Deviation | 26.7 ± 12.4 | 28.7 ± 11.7 | |
| Chelator | N=80 | N=59 | 0.13 |
| None | 5 (6.3%) | 4 (6.8%) | |
| Deferoxamine | 8 (10.0%) | 15 (25.4%) | |
| Oral | 44 (55.0%) | 22 (37.3%) | |
| Deferoxamine + Oral | 23 (28.8%) | 18 (30.5%) | |
Note: P-values are from a logistic regression predicting any pain (yes/no) as a function of the variable of interest and including age (in years) as a predictor. LIC, liver iron concentration.
There was no association between iron chelator regimen, type, or administration (p=0.13). In addition, fracture history was not a predictor of pain (p=0.83). For individuals reporting pain over the last four weeks, no significant relationship was found between bone density and pain reports (p=0.77) Unexpectedly, individuals reporting pain over the last seven days were found to have significantly greater bone mass (p=0.009) compared to participants with no pain. An analysis was conducted to assess whether weight differences might have contributed to the finding that individuals reporting pain had higher bone densities than those reporting no pain. There was no significant difference in weight: mean weight in the no pain group was 54.4kg versus mean weight for the pain group 57.7kg (p= 0.95). Bisphosphinate use did not account for the discrepancy.
To assess for the impact of diagnosis on these findings, an analysis limited to participants with β-thalassaemia was conducted. Similar to the findings for the total pain study population, neither haemoglobin level (p=0.86), bone density (p=0.012), iron status: T2* (p=0.89), LIC (p=0.38), ferritin (p=0.64); or chelator use (p=0.20) were found to be significantly different between β-thalassaemia participants reporting pain over the last seven days and those who were pain free.
Study Limitations
Limitations of this study include the relatively small number of intermittently transfused patients. Thalassaemia intermedia patients were difficult to enroll because they are seen less regularly than transfusion-dependent patients. TLC data was not collected simultaneously with pain data and therefore the time difference may confound results. In addition, only a portion of study participants (55%) participated in both TLC and this study, with a limited number (n=81; 32%) contributing bone density data. The BPI has not been validated in thalassaemia.
DISCUSSION
Pain is prevalent among the surveyed thalassaemia patients, with most respondents reporting pain in the last 4 weeks and some reporting daily pain. Age was identified as the only independent predictor of pain (p=<0.001), with 57% of patients 35 years and older reporting pain in the last seven days versus 8% in the 12–18 years age group. Pain appears to develop earlier and be more severe than in the general population. In the general American population of individuals 20 years of age and older, 26% reported pain in the last four weeks. This compares to 73% of individuals with thalassaemia over 20 years of age (National Center for Health Statistics (U.S.) 2006). Pain severity ratings in patients with thalassaemia were high and comparable to individuals with neuromuscular diseases. Comparable BPI pain severity ratings found similar mean average pain levels between neuromuscular disorders (4.1/10) and thalassaemia (4.0/10) (Abe, et al 2008, Ende, et al 2006, Jensen, et al 2008). Mean worst pain ratings for patients with thalassaemia (6.6/10) approached those of cancer patients (8.3/10) (Chang, et al 2002). The comparison of pain ratings between neuromuscular, oncology and thalassaemia patients provides some perspective on pain severity in thalassaemia.
The duration of pain in patients with thalassaemia was prolonged, with 81% of patients aged 20 years and older reporting pain for one year or longer and 31% reporting pain for five or more years. Thalassaemia patients have increased pain duration in comparison to the general American population. The National Center for Health Statistics reported 42% of respondents aged 20 years and older reporting pain for one year or longer (National Center for Health Statistics (U.S.) 2006). Pain in thalassaemia is chronic with the number of pain sites increasing significantly with age.
The aetiology of pain in thalassaemia is unknown. Traditionally, pain in thalassaemia has been thought to be a result of low haemoglobin, low bone mass, and/or iron overload. This study found no significant relationship between average pretransfusion haemoglobin level and pain. To further assess the relationship between transfusion and pain, a pilot study has been conducted as part of this study and data is currently under analysis.
Low bone mass and consequent compression fractures have been frequently cited as a possible mechanism for pain in thalassaemia. However, there has been a surprising lack of association between bone mass and pain. This study found no association with pain and fracture history. Similarly, Vogiatzi et al (2009) reported that bone and joint pain were not associated with bone mass or fractures. Although there has been some association between pain and iron overload reported in hereditary haemachromatosis, this association was not observed in this study (Ines, et al 2001, Rihl and Kellner, 2004, von Kempis 2001).
Pain management in thalassaemia is inadequate. Pain appears to be under-treated, with 25% of participants reporting no relief with pain treatment. Limited pain relief is achieved despite the use of opiods. Depression, while not assessed in this study, is often associated with chronic pain. While anti-depressants are used in chronic pain, only 10% of patients reported taking anti-depressants.
As individuals with thalassaemia age, pain becomes more common, more severe and the number of sites of pain increase. Pain interferes with multiple dimensions of life, particularly mood and work. The results of this study support the need for regular pain assessment among all patients with thalassaemia, regardless of age, transfusion status, diagnosis or haemoglobin level. The data supports the need for research focused on pain mechanisms as well as improved strategies for pain management. As thalassaemia patients continue to enjoy a longer life-span, pain may become an even greater issue in this population.
Acknowledgments
This work was supported by the following NIH-NHLBI cooperative agreements: U01-HL65232 and NIH/NCRR UL1-RR-024134 to the Children’s Hospital of Philadelphia, U01-HL72291 and by Harvard Catalyst CTSC U-01RR025758 to Children’s Hospital, Boston, U01-HL65233 to University Health Network Toronto General Hospital, U01-HL65239 to Children’s Hospital & Research Center Oakland, U01-HL65244 and CTSC UL1-RR024996 to Weill Medical College of Cornell University, and U01-HL65238 to New England Research Institutes. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NHLBI.
The authors also wish to acknowledge the contributions of all the individuals with thalassaemia who participated in this study.
Appendix 1
The following institutions and researchers contributed to the Thalassemia Clinical Research Network Assessment of Pain Study data reported in this paper.
Children’s Hospital, Boston: Ellis Neufeld, MD, PhD, Jennifer Eile, NP, Research Nurse, Latoya Lashley, Study Coordinator; Satellites: Children’s Healthcare of Atlanta, Leann Schilling, MPH, Study Coordinator, Principal Investigator; Baylor College of Medicine, Bogden Dino, Study Coordinator. Weill Medical College of Cornell University: Dorothy Kleinert, RN, Research Nurse, Patricia Giardina, MD; The Children’s Hospital of Philadelphia: Alan Cohen, MD, Janet Kwiatkowski, MD, Marie Martin, RN, Research Nurse, Principal Investigator, Sage Green, Study Coordinator; Satellite: Children’s Memorial Hospital, Chicago, IL: Alexis Thompson, MD, Janice Beatty, RN, Research Nurse, Diane Calamaras, RN, CPNP, Research Nurse, Pauline Hess, Study Coordinator. Children’s Hospital at Oakland: Dru Haines, CPNP, Research Nurse, Principal Investigator, Olivia Oliveros, Study Coordinator, Elliott Vichinsky, MD; Satellites: Children’s Hospital of Los Angeles, Thomas Coates, MD, Principal Investigator, Susan Carson, CPNP, Research Nurse, Principal Investigator, Ani Dongelyan, Study Coordinator, Tatiana Hernandez, Study Coordinator; Children’s and Women’s Health Center of British Columbia, Jennifer Kies, Study Coordinator. Toronto General Hospital, Toronto, Ontario, Canada: Nancy Oliveri, MD, Cecilia Kim, BS, Study Coordinator; Satellite: Hospital for Sick Children: Manuela Merelles-Pulcini, RN, Study Coordinator. NHLBI oversight, Kathryn Hassell, MD. Data Coordinating Center: New England Research Institutes, Sonja McKinlay, PhD, Principal Investigator, Lisa Virzi, RN, MS, MBA, Project Director, Felicia Trachtenberg, PhD, Senior Statistician, Eric Gerstenberger, MS, Statistician.
Footnotes
A list of TCRN member institutions and staff appears in Appendix 1.
References
- Abe Y, Miyashita M, Ito N, Shirabi I, Momose Y, Ichikawa Y, Tsuji S, Kazuma K. Attitude of outpatients with neuromuscular diseases in Japan to pain and use of analgesics. J Neurol Sci. 2008;267:22–27. doi: 10.1016/j.jns.2007.09.027. [DOI] [PubMed] [Google Scholar]
- Chang VT, Hwang SS, Kasimis B. Longitudinal Documentation of cancer pain management outcomes: a pilot study at a VA medical Center. J Pain Symptom Manage. 2002;24:494–505. doi: 10.1016/s0885-3924(02)00516-x. [DOI] [PubMed] [Google Scholar]
- Cleeland C. The Brief Pain Inventory. User Guide. The University of Texas M. D. Anderson Cancer Center; Houston, TX, USA: 2009. p. 63. [Google Scholar]
- Dworkin RH, Turk DC, Farrar JT, Haythornthwaite JA, Jensen MP, Katz NP, Kerns RD, Stucki G, Allen RR, Bellamy N, Carr DB, Chandler J, Cowan P, Dionne R, Galer BS, Hertz S, Jadad AR, Kramer LD, Manning DC, Martin S, McCormick CG, McDermott MP, McGrath P, Quessy S, Rappaport BA, Robbins W, Robinson JP, Rothman M, Royal MA, Simon L, Stauffer JW, Stein W, Tollett J, Wernicke J, Witter J. Core outcome measures for chronic pain clinical trials: IMMPACT recommendations. Pain. 2005;113:9–19. doi: 10.1016/j.pain.2004.09.012. Immpact. [DOI] [PubMed] [Google Scholar]
- Ende DM, Osborne TL, Hanley MA, Jensen MP, Kraft GH. The scope and nature of pain in persons with multiple sclerosis. Mult Scler. 2006;12:629–638. doi: 10.1177/1352458506071346. [DOI] [PubMed] [Google Scholar]
- Finsterbush A, Ferber I, Mogle P. Lower limb pain in thalassemia. J Rheumatol. 1985;12:529–532. [PubMed] [Google Scholar]
- Ines LS, da Silva JA, Malcata AB, Porto AL. Arthropathy of genetic hemochromatosis: a major and distinctive manifestation of the disease. Clin Exp Rheumatol. 2001;19:98–102. [PubMed] [Google Scholar]
- Jensen MP, Hoffman AJ, Stoelb BL, Abresch RT, Carter GT, McDonald CM. Chronic pain in persons with myotonic dystrophy and facioscapulohumeral dystrophy. Arch Phys Med Rehabil. 2008;89:320–328. doi: 10.1016/j.apmr.2007.08.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller S, Bann CM, Dodd SL, Schein J, Mendoza TR, Cleeland CS. Validity of the brief pain inventory for use in documenting the outcomes of patients with noncancer pain. Clin J Pain. 2004;20:309–318. doi: 10.1097/00002508-200409000-00005. [DOI] [PubMed] [Google Scholar]
- Kwiatkowski JL, Kim HY, Thompson AA, Quinn CT, Mueller BU, Odame I, Giardina PJ, Vichinsky EP, Boudreaux JM, Cohen AR, Porter JB, Coates T, Olivieri NF, Neufeld EJ for the Thalassemia Clinical Research N. Chelation use and iron burden in North American and British thalassemia patients: a report from the Thalassemia Longitudinal Cohort. Blood. 2012;119:2746–2753. doi: 10.1182/blood-2011-04-344507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Center for Health Statistics (U.S.) Trends in the Health of Americans 2006 with Chartbook. U.S. Dept. of Health and Human Services, Centers for Disease Control and Prevention, National Center for Health Statistics; Hyattsville, MD, USA: 2006. [Google Scholar]
- Pakbaz Z, Treadwell M, Yamashita R. Quality of life in patients with thalassemia intermedia compared to thalassemia major. In: Vichinsky EP, editor. Cooley’s anemia: Eighth Symposium; Annals of the New York Academy of Sciences. New York Academy of Sciences; New York, N.Y: 2005. pp. 457–461. [DOI] [PubMed] [Google Scholar]
- Rihl M, Kellner H. Arthropathy of hereditary hemochromatosis. Z Rheumatol. 2004;63:22–29. doi: 10.1007/s00393-004-0563-x. [DOI] [PubMed] [Google Scholar]
- Scalone L, Mantovani LG, Krol M, Rofail D, Ravera S, Bisconte MG, Borgna-Pignatti C, Borsellino Z, Cianciulli P, Gallisai D, Prossomariti L, Stefano I, Cappellini MD. Costs, quality of life, treatment satisfaction and compliance in patients with beta-thalassemia major undergoing iron chelation therapy: the ITHACA study. Curr Med Res Opin. 2008;24:1905–1917. doi: 10.1185/03007990802160834. [DOI] [PubMed] [Google Scholar]
- Tan G, Jensen MP, Thornby JI, Shanti BF. Validation of the Brief Pain Inventory for chronic nonmalignant pain. J Pain. 2004;5:133–137. doi: 10.1016/j.jpain.2003.12.005. [DOI] [PubMed] [Google Scholar]
- Trachtenberg F, Foote D, Martin M, Carson S, Coates T, Beams O, Vega O, Merelles-Pulcini M, Giardina PJ, Kleinert DA, Kwiatkowski J, Thompson AA, Neufeld EJ, Schilling L, Thayalasuthan V, Pakbaz Z, Yamashita R Thalassemia Clinical Research N. Pain as an emergent issue in thalassemia. Am J Hematol. 2010;85:367–370. doi: 10.1002/ajh.21670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogiatzi MG, Macklin EA, Fung EB, Cheung AM, Vichinsky E, Olivieri N, Kirby M, Kwiatkowski JL, Cunningham M, Holm IA, Lane J, Schneider R, Fleisher M, Grady RW, Peterson CC, Giardina PJ Thalassemia Clinical Research, N. Bone disease in thalassemia: a frequent and still unresolved problem. J Bone Miner Res. 2009;24:543–557. doi: 10.1359/jbmr.080505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Kempis J. Arthropathy in hereditary hemochromatosis. Curr Opin Rheumatol. 2001;13:80–83. doi: 10.1097/00002281-200101000-00013. [DOI] [PubMed] [Google Scholar]