Abstract
Background
Epidemiologic studies suggest that physical activity reduces breast cancer risk by 20-40%. However, prior studies have relied on measures of self-report.
Methods
In a population-based case-control study, we evaluated accelerometer measures of active and sedentary behavior in relation to breast cancer among 996 incident cases and 1,164 controls, residents of Warsaw, Poland (2000-2003), who wore an accelerometer for seven days. Accelerometer values were averaged across valid wear days and summarized as overall activity (counts [ct]/minute/day) and in minutes spent in sedentary behavior (0-99 ct/min) and light (100-759 ct/min) and moderate-to-vigorous (760+ ct/min) activity. Odds ratios (OR) and 95% confidence intervals (CI) were estimated using unconditional logistic regression.
Results
Comparing women in the highest quartile (Q4) of activity to those in the lowest (Q1), time spent in moderate-to-vigorous activity was inversely associated with breast cancer odds after adjustment for known risk factors, sedentary behavior and wear time (ORQ4vsQ1:0.39, 95%CI: 0.27-0.56; P-trend<.0001). Sedentary time was positively associated with breast cancer, independent of moderate-to-vigorous activity (ORQ4vsQ1:1.81, 95%CI: 1.26-2.60; P-trend=0.001). Light activity was not associated with breast cancer in multivariable models including both moderate-to-vigorous activity and sedentary behavior.
Conclusions
Our findings support an inverse association between accelerometer-based measures of moderate-to-vigorous physical activity and breast cancer while also suggesting potential increases in risk with sedentary time.
Keywords: Breast cancer, physical activity, sedentary behavior, objective measures
Introduction
Epidemiologic studies suggest that increased physical activity reduces breast cancer risk [1-3], with risk reductions ranging from 20 to 40%, depending on the study design, population, and intensity of activity. Protective associations with increased activity have been observed for both pre- and postmenopausal breast cancer risk with more consistent associations noted among studies of postmenopausal women (reviewed in [4]). While numerous studies have evaluated physical activity in relation to breast cancer, few studies have considered the potential independent role of sedentary behavior on risk [5-6], and moreover, prior studies have relied solely on self-reported measures of physical activity and sedentary behavior.
The influence of both active and sedentary behavior on breast cancer risk is biologically plausible. Sedentary behavior, characterized by behaviors that require sitting or lying down and only low energy expenditure [7], has been independently associated with breast cancer risk factors, including increased body mass index (BMI) and biomarkers of metabolic dysfunction and inflammation [8-11]. These biological pathways are implicated in breast carcinogenesis and are also hypothesized mechanisms for the association between physical activity and breast cancer [12]. Despite this biologic rationale, only two studies have evaluated the relation between self-reported sedentary behaviors and breast cancer risk [5-6], with null findings reported. However, self-reported sedentary time may lead to potential measurement error, biasing results toward a null association.
Accelerometer-based measures of physical activity and sedentary time may improve the assessment of these behaviors by objectively quantifying the duration and intensity of a range of activities. In a population-based case-control study, we evaluated accelerometer-based measures of active and sedentary behavior in relation to breast cancer.
Methods
Details of the NCI Polish Breast Cancer Case-Control Study have been described elsewhere [13-14]. In brief, the NCI Polish Study is a population-based case-control study conducted among women 20-74 years of age, residing in Warsaw and Łódź, Poland from 2000 to 2003. The accelerometer component of the NCI Polish Study was restricted to Warsaw. Newly diagnosed cytologically or histologically confirmed in situ or invasive breast cancers were identified using a rapid identification system and the Warsaw cancer registry. Information on tumor size, grade and axillary node status was ascertained from surgical pathology reports; hormone receptor status was ascertained via immunohistochemistry. Tumor characteristics were independently confirmed by the study pathologist. Treatment information was ascertained from medical records and surgical pathology forms. Controls were randomly selected from the Polish Electronic System, a database with demographic information from all Polish residents, and were frequency matched to cases in five year age categories. Among Warsaw participants, 76% of eligible cases and 69% of eligible controls agreed to participate in the interview-based questionnaire. The study protocol was reviewed and approved by Institutional Review Boards at the U.S. National Cancer Institute (NCI) and the participating Polish institutions.
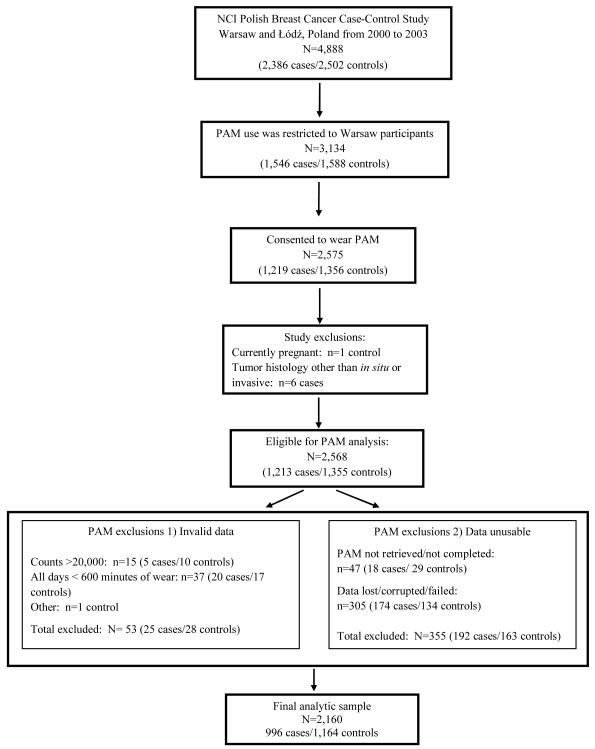
Risk factors assessed via an interview-administered questionnaire included demographic information, occupational history, medical and reproductive history, hormone use, and lifestyle factors. Height and weight were measured by study nurses. Women participating from the Warsaw site (n=1,546 cases/1,588 controls) were also asked to wear an accelerometer on their waist for seven days and to complete a daily log to document monitor wear. Participants were instructed to wear the monitor during waking hours and to remove it while sleeping or engaging in activities such as bathing or swimming. Participants were also asked to record when the monitor was removed and to provide the reason. On average, cases initiated their accelerometer wear time 60 days post-diagnosis. Seventy nine percent (n=1,219) of cases and 85% of controls (n=1,356) consented to wearing an accelerometer. Of those, women currently pregnant (n=1 control) and cases with histologies other than in situ or invasive breast carcinoma (n=6 cases) were excluded, resulting in a total of 1,213 cases and 1,355 controls eligible for this analysis (Figure 1).
Figure 1.
Physical Activity Monitor (PAM) Results in the NCI Polish Breast Cancer Case-Control Study
Data Collection
Accelerometer Measures
The accelerometer (Actigraph 7164; Actigraph, LLC, Fort Walton Beach, Florida) measures bodily movement on a minute-by-minute basis and stores this information in the form of an “activity count” that reflects the duration and intensity of ambulatory activities [15]. Physical activity and sedentary behavior were summarized by the number of minutes per day spent sedentary (0-99 counts) or in light (100-759 counts) and moderate-to-vigorous (760+ counts) activity. Overall activity was summarized by total counts per day (ct/min/d). These count cut-points were selected based on prior studies [15-16]. We employed standard data reduction procedures [16] to determine monitor wear time and implement quality control procedures to exclude invalid days of observation with fewer than 10 hours of monitor wear or evidence of monitor malfunction. All summary measures were averaged across valid days of wear. The exclusions made during the processing and cleaning of the monitor data are detailed in Figure 1. Excluded were women with unusable monitor data (n=192 cases/163 controls), excessive monitor counts (>20,000 ct/min, n=5 cases/10 controls), <10 hours of wear on all days (n=20 cases/17 controls), or other invalid data (n=1 control). All women included in this analysis had at least one valid day of wear (10 hours of wear). Thus, the final study population includes 996 incident breast cancer cases and 1,164 controls.
Statistical Analysis
Median levels of each accelerometer measure were compared by case-control status using the Kruskal-Wallis test. Spearman rank correlation coefficients were used to assess correlations between accelerometer measures. Accelerometer-based measures were categorized based on the quartile (Q) distribution among the controls. Unconditional logistic regression was used to estimate odds ratios (ORs) and 95% confidence intervals (CIs). Minimally adjusted models included age (continuous), BMI (<25, 25-29, ≥30 kg/m2, unknown) and wear time (minutes) as covariates. Fully adjusted multivariable models (MV) included additional adjustment for the following breast cancer risk factors: education (< than high school, high school/some college/professional training, college graduate, unknown), current smoker (yes/no), age at menarche (≤ 12, 13-14, ≥ 15, unknown), number of full term births (0, 1, 2, 3+), family history of breast cancer among 1st degree female relatives (yes/no), history of screening mammography (yes/no/unknown), history of benign breast disease (yes/no/unknown), and a combined variable for menopausal status/age at menopause (premenopausal and postmenopausal/age at menopause <45, 45-49, 50-54, 55+, unknown). Oral contraceptive and hormone therapy use were not included in the final models as addition of these covariates did not alter risk estimates nor were they associated with accelerometer measures. Time spent in sedentary and active behavior was modeled (1) individually and (2) mutually adjusted for one another in order to test for independent associations. Wald tests of trend were performed using the midpoint for each category of the accelerometer measure. In sensitivity analyses, the above models were restricted to women with ≥ 3 days of valid wear.
Stratified analyses were conducted to assess whether the association between accelerometer measures and breast cancer risk varied by BMI (<25, 25-29, ≥30 kg/m2) or menopausal status (premenopausal, postmenopausal). Interactions between accelerometer measures and these factors were tested using multiparameter Wald tests. We performed separate polytomous logistic regression models, restricting to invasive tumors, to assess whether the association between accelerometer measures and breast cancer varied by tumor size (≤ 2 cm, > 2 cm, unknown), tumor grade (well/moderately differentiated, poorly differentiated, unknown), axillary node metastasis (positive, negative, unknown), estrogen receptor (ER) status (positive, negative, unknown) and progesterone receptor (PR) status (positive, negative, unknown). These polytomous models were adjusted only for age, BMI, and wear time. Heterogeneity of exposure-disease ORs was evaluated using logistic regression restricted to cases [17] with the relevant tumor characteristic as the outcome and the accelerometer measure as the exposure.
To evaluate potential treatment effects, we created an indicator variable to account for the timing of monitor wear in relation to the timing of treatment (i.e. whether the monitor was worn prior to or after surgery or other treatments). Other treatments included chemotherapy, radiotherapy or hormone therapy. Given the variability in timing of monitor wear, we categorized women according to whether the monitor was worn prior to surgery and other treatments, after surgery but prior to other treatments, after surgery and other treatments, surgery date unknown, and treatment date unknown. Separate logistic regression models were performed, restricting the cases by the indicator variables listed above.
Similar estimates were observed when in situ cases (n=76) were excluded; thus, we present the results from the analyses including all breast cancers. All statistical analyses were performed using SAS version 9.2. Probability values <0.05 were considered statistically significant.
Results
The distribution of breast cancer risk factors by case-control status is presented in Table 1. The majority of the subjects were 50 years of age or older and postmenopausal. Compared with controls, cases had younger ages at menarche (≤ 12 y), more education, later ages at a first birth and more frequent family histories of breast cancer.
Table 1.
Descriptive characteristics of Warsaw participants in the Polish Breast Cancer Case-Control Study, 2000-2003 (n=2,160)
| Characteristic | Cases n=996 n(%) |
Controls n=1,164 n(%) |
|---|---|---|
| Age (y) | ||
| 25-49 | 305 (30.6) | 345 (29.6) |
| 50-75 | 691 (69.4) | 819 (70.4) |
| Education | ||
| Less than high school | 201 (20.2) | 354 (30.4) |
| High school, some college or professional training |
480 (48.2) | 570 (49.0) |
| College graduate | 309 (31.0) | 233 (20.0) |
| Age at first menstrual period (y) | ||
| ≤ 12 | 256 (25.7) | 245 (21.0) |
| 13-14 | 522 (52.4) | 595 (51.1) |
| ≥ 15 | 211 (21.2) | 309 (26.5) |
| Menopausal status | ||
| Premenopausal | 250 (25.1) | 376 (32.3) |
| Postmenopausal | 746 (74.9) | 788 (67.7) |
| Age at menopause*(y) | ||
| < 45 | 80 (10.7) | 102 (12.9) |
| 45-49 | 207 (27.7) | 256 (32.5) |
| 50-54 | 325 (43.6) | 300 (38.1) |
| 55+ | 86 (11.5) | 102 (12.9) |
| Number of full term births | ||
| Nulliparous | 154 (15.5) | 139 (11.9) |
| 1 | 338 (33.9) | 345 (29.6) |
| 2 | 403 (40.5) | 513 (44.1) |
| 3+ | 101 (10.1) | 167 (14.3) |
| Age at first full term birth (y) | ||
| Nulliparous | 154 (15.5) | 139 (11.9) |
| <20 | 75 (7.5) | 117 (10.1) |
| 20-24 | 384 (38.6) | 515 (44.2) |
| 25-30 | 242 (24.3) | 272 (23.4) |
| >30 | 141 (14.2) | 121 (10.4) |
| Family history of breast cancer† | ||
| Yes | 101 (10.1) | 74 (6.4) |
| No | 895 (89.9) | 1,090 (93.6) |
| History of benign breast disease | ||
| Yes | 102 (10.2) | 78 (6.7) |
| No | 869 (87.2) | 1,070 (91.9) |
| Ever had a screening mammogram | ||
| Yes | 665 (66.8) | 666 (57.2) |
| No | 318 (31.9) | 489 (42.0) |
| Current Smoker | ||
| Yes | 166 (16.6) | 314 (27.0) |
| No | 669 (67.2) | 734 (63.0) |
| Current body mass index (BMI) (kg/m2) | ||
| <25 | 483 (48.5) | 493 (42.4) |
| 25-29 | 352 (35.3) | 424 (36.4) |
| ≥ 30 | 148 (14.9) | 227 (19.5) |
Note:Percentages may not sum to 100 due to missing values.
Among postmenopausal women
Family history in first degree female relatives
The distribution of accelerometer measures among cases and controls is presented in Supplementary Table 1. Cases and controls wore the monitor for approximately five valid days (mean (SD): [5.2 (1.5)] and [5.5 (1.4)], respectively), and only 7% of cases and 4% of controls wore the monitor less than 3 valid days. Overall activity levels, measured by average counts per day (ct/day), were lower among cases [261.6 (188.3, 341.8); median (25th, 75th)] than controls [317.7 (244.8, 400.6)] (p-value<.0001) and on average, cases spent more time sedentary (average ct/day) than controls [485.2 (424.9, 542.2) and 457.8 (396.6, 542.1), respectively; p-value<0.0001]. Among both cases and controls, light activity was positively correlated with moderate-to-vigorous activity (r=0.52 and 0.39, respectively (p-value<.0001)) and both light and moderate-to-vigorous activity were inversely correlated with sedentary time ((light activity: r=−0.46 and −0.45, respectively (p-value<.0001); moderate-to-vigorous activity: r=−0.49 and −0.53, respectively (p-value<.0001) (Supplementary Table 2)).
Table 2 summarizes the association of breast cancer with each individual accelerometer measure. Results from MV models were similar to those from analyses adjusted only for age, BMI, and monitor wear time; thus, estimates from the parsimonious models are described hereafter. When comparing women in the highest vs. lowest quartile of overall activity counts, overall activity was inversely associated with breast cancer (ORQ4vsQ1=0.30; 95% CI: 0.23, 0.39; p-trend<0.0001). Time spent in both light (OR=0.45; 95% CI: 0.34, 0.59; p-trend<0.0001) and moderate-to-vigorous (OR=0.27; 95% CI: 0.20, 0.35; p-trend<0.0001) intensity activities were statistically significantly associated with reduced odds of breast cancer. A significant increase in breast cancer was associated with increasing quartiles of sedentary time such that the highest quartile of sedentary time had 3.54 times the odds of breast cancer compared with those in the lowest quartile (95% CI: 2.68, 4.68; p-trend<0.0001).
Table 2.
Relation between accelerometer-measured active and sedentary behavior and breast cancer
| Measures modeled individually |
Measures modeled simultaneously |
|||||
|---|---|---|---|---|---|---|
| Accelerometer-based measures* |
Cases (n=996) |
Controls (n=1,164) |
Age,BMI& wear time adjusted OR1 (95% CI) |
Multivariable Adjusted OR2 (95% CI) |
Age, BMI & wear time adjusted OR3 (95% CI) |
Age, BMI & wear time adjusted OR4 (95% CI) |
| Overall activity, total counts per day |
||||||
| ≤ 244.79 | 443 | 291 | 1.00 | 1.00 | n/a | n/a |
| 244.80 – 317.72 | 246 | 291 | 0.54 (0.43, 0.68) | 0.52 (0.41, 0.66) | ||
| 317.73-400.62 | 163 | 291 | 0.35 (0.27, 0.45) | 0.33 (0.25, 0.44) | ||
| ≥ 400.63 | 144 | 291 | 0.30 (0.23, 0.39) | 0.29 (0.22, 0.38) | ||
| P-trend | <.0001 | <.0001 | ||||
| Light, min/day | ||||||
| ≤ 225.12 | 384 | 291 | 1.00 | 1.00 | 1.00 | |
| 225.13-265.77 | 272 | 291 | 0.73 (0.58, 0.92) | 0.71 (0.56, 0.90) | n/a | 0.95 (0.72, 1.26) |
| 265.78-305.41 | 189 | 291 | 0.53 (0.41, 0.68) | 0.56 (0.43, 0.72) | 0.88 (0.63, 1.23) | |
| ≥ 305.42 | 151 | 291 | 0.45 (0.34, 0.59) | 0.47 (0.35, 0.63) | 0.87 (0.57, 1.33) | |
| P-trend | <.0001 | <.0001 | 0.23 | |||
| Moderate-to-vigorous, min/day |
||||||
| ≤ 65.0 | 449 | 292 | 1.00 | 1.00 | 1.00 | 1.00 |
| 65.10 - 95.58 | 260 | 290 | 0.56 (0.44, 0.71) | 0.53 (0.41, 0.67) | 0.63 (0.50, 0.81) | 0.59 (0.45, 0.76) |
| 95.59 - 128.24 | 162 | 291 | 0.34 (0.26, 0.44) | 0.32 (0.24, 0.41) | 0.42 (0.31, 0.56) | 0.38 (0.27, 0.51) |
| ≥ 128.25 | 124 | 291 | 0.27 (0.20, 0.35) | 0.26 (0.20, 0.35) | 0.39 (0.27, 0.56) | 0.35 (0.24, 0.52) |
| P-trend | <.0001 | <.0001 | <.0001 | <.0001 | ||
| Sedentary, min/day | ||||||
| ≤ 396.60 | 153 | 291 | 1.00 | 1.00 | 1.00 | 1.00 |
| 396.61-457.74 | 234 | 291 | 1.69 (1.30, 2.21) | 1.64 (1.24, 2.18) | 1.32 (0.99, 1.76) | 1.21 (0.87, 1.68) |
| 457.75-524.10 | 273 | 291 | 2.25 (1.72, 2.94) | 2.13 (1.61, 2.82) | 1.48 (1.08, 2.03) | 1.26 (0.85, 1.88) |
| ≥ 524.11 | 336 | 291 | 3.54 (2.68, 4.68) | 3.45 (2.57, 4.61) | 1.81 (1.26, 2.60) | 1.48 (0.88, 2.49) |
| P-trend | <.0001 | <.0001 | 0.0012 | 0.14 | ||
Overall activity determined by total counts per day averaged across valid days of wear; time spent sedentary or in light and moderate-to-vigorous activity calculated as the average minutes per day across valid days of wear. Categories determined by the quartile distribution among controls; behavior defined by the following counts: sedentary (0-99 counts), overall activity (100+ counts), light (100-759 counts), and moderate-to-vigorous (760+ counts); data for moderate-to-vigorous activity missing for one case.
Age and BMI adjusted models also include adjustment for wear time.
Multivariable models adjusted for the following: age, BMI, education, current smoking status, age at menarche, number of full term births, breast cancer family history, previous screening mammography, history of benign breast disease, menopausal status/age at menopause combination variable, and wear time.
Models include time spent in moderate-to-vigorous and sedentary behavior.
Models include time spent in light, moderate-to-vigorous, and sedentary behavior.
After mutual adjustment for sedentary time and moderate-to-vigorous activity (Table 2), increased time spent in moderate-to-vigorous activity remained inversely associated with breast cancer (ORQ4vsQ1=0.39; 95% CI: 0.27, 0.56). Associations with sedentary time attenuated with further adjustment for time spent in moderate-to-vigorous activity but still remained positively associated with risk (ORQ4vsQ1=1.81; 95% CI: 1.26, 2.60). Additional adjustment for light activity further attenuated the positive association with sedentary behavior (ORQ4vsQ1=1.48; 95% CI: 0.88, 2.49) while estimates for moderate-to-vigorous activity (ORQ4vsQ1=0.35; 95% CI: 0.24, 0.52) remained relatively unchanged. Sensitivity analyses, restricting to women with ≥ 3 valid days of wear, yielded similar results as those presented in Table 2 (data not shown).
The inverse associations observed with moderate-to-vigorous activity in the overall analyses (Table 2) were observed across all models, regardless of whether the monitor was worn pre- or post-surgery and other treatments (Table 3). When cases were restricted to those who wore the monitor prior to surgery and other treatments, increased time spent in moderate-to-vigorous activity was associated with approximately a 60% reduction in breast cancer odds (ORQ4vsQ1=0.43; 95% CI: 0.22, 0.86), similar to the overall results. The association between sedentary time and breast cancer varied by timing of monitor wear in relation to treatment. Among women who wore the monitor prior to surgery and other treatments, we observed no evidence of a positive association with sedentary behavior which is in contrast to the finding observed in analyses restricting cases to women who wore the monitor after surgery (Table 3).
Table 3.
Independent associations of moderate-to-vigorous activity and sedentary time with breast cancer odds, by timing of treatment1
| Timing of Monitor Wear in Relation to Treatment (No. of cases) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||
| Average minutes per day* |
Controls (N=1,164) |
Cases | Monitor worn prior to surgery & other treatments (N=141) |
Cases | Monitor worn after surgery but prior to other treatments (N=230) |
Cases | Monitor worn after surgery & other treatments (N=381) |
Cases | Surgery date unknown (N=118) |
Cases | Treatment date unknown (N=105) |
| Moderate-to- vigorous, min/day |
|||||||||||
| ≤ 65.0 | 292 | 47 | 1.00 | 135 | 1.00 | 155 | 1.00 | 49 | 1.00 | 55 | 1.00 |
| 65.10 - 95.58 | 290 | 38 | 0.72 (0.44, 1.17) | 50 | 0.41 (0.27, 0.60) | 113 | 0.83 (0.60, 1.16) | 29 | 0.62 (0.37, 1.06) | 24 | 0.54 (0.31, 0.95) |
| 95.59 - 128.24 | 291 | 31 | 0.56 (0.32, 0.98) | 24 | 0.22 (0.13, 0.37) | 70 | 0.56 (0.37, 0.83) | 19 | 0.44 (0.23, 0.83) | 13 | 0.37 (0.18, 0.77) |
| ≥ 128.25 | 291 | 24 | 0.43 (0.22, 0.86) | 21 | 0.25 (0.13, 0.47) | 43 | 0.42 (0.25, 0.70) | 21 | 0.55 (0.26, 1.17) | 13 | 0.49 (0.21, 1.17) |
|
| |||||||||||
|
| |||||||||||
| Sedentary, min/day | |||||||||||
| ≤ 396.60 | 291 | 24 | 1.00 | 24 | 1.00 | 63 | 1.00 | 24 | 1.00 | 15 | 1.00 |
| 396.61 - 457.74 | 291 | 41 | 1.37 (0.78, 2.42) | 50 | 1.52 (0.86, 2.68) | 86 | 1.22 (0.82, 1.84) | 26 | 1.07 (0.56, 2.02) | 21 | 1.38 (0.64, 2.94) |
| 457.75 - 524.10 | 291 | 43 | 1.30 (0.70, 2.39) | 65 | 1.85 (1.02, 3.34) | 96 | 1.36 (0.88, 2.12) | 35 | 1.41 (0.72, 2.75) | 31 | 1.95 (0.88, 4.31) |
| ≥ 524.11 | 291 | 33 | 0.78 (0.37, 1.63) | 91 | 2.40 (1.26, 4.57) | 136 | 2.25 (1.37, 3.70) | 33 | 1.37 (0.63, 3.00) | 38 | 2.52 (1.05, 6.07) |
Time spent sedentary or in moderate-to-vigorous activity calculated as the average minutes per day across valid days of wear. Categories determined by the quartile distribution among controls; behavior defined by the following counts: sedentary (0-99 counts) and moderate-to-vigorous (760+ counts); data for moderate-to-vigorous activity missing for one case.
Models adjusted for the following: age, BMI, and wear time. Cases who wore the monitor prior to surgery and after other treatments were excluded from these analyses (n=21) due to small numbers across accelerometer categories.
In analyses stratified by tumor characteristics, reduced odds were observed for all tumor types with increasing moderate-to-vigorous activity (Table 4). No statistical differences by tumor size, tumor grade, nodal status or hormone receptor status were observed (p-heterogeneity >0.25) in models with simultaneous adjustment for moderate-to-vigorous activity and sedentary behavior. However, the positive association between sedentary time and breast cancer appeared weaker for those with node negative than positive tumors (respective ORQ4vsQ1=1.50; 95% CI: 0.96, 2.34 vs. 2.45; 95% CI: 1.41, 4.23; p-heterogeneity=0.41). In analyses stratified by BMI and menopausal status (Table 5), we observed no effect modification by menopausal status of associations with either sedentary time or moderate-to-vigorous activity. However, associations between sedentary time and breast cancer risk varied by BMI (p-interaction=0.005), with relationships restricted to overweight women (BMI25-30 kg/m2: ORQ4 vs Q1= 3.13; 95% CI: 1.63-6.01) (p-trend=0.0006). Estimates among women with a BMI ≥ 30 kg/m2 were suggestive of a positive association with increased time spent sedentary, although not statistically significant.
Table 4.
Independent associations of moderate-to-vigorous activity and sedentary time in relation to breast cancer odds, by tumor characteristics1
| Accelerometer measures (average minutes/day)* | ||||||||
|---|---|---|---|---|---|---|---|---|
| Tumor characteristics (among invasive cases, n=919) |
Moderate-to-vigorous Activity (≤ 65.0 as referent) |
Sedentary Time (≤ 396.6 as referent) |
||||||
|
| ||||||||
| 65.10 - 95.58 | 95.59 - 128.24 | ≥ 128.25 | P-value 2 |
396.61-
457.74 |
457.75 - 524.10 | ≥ 524.11 | P-value 2 | |
|
| ||||||||
| Tumor size | ||||||||
| ≤ 2 cm | 0.58 (0.41, 0.81) | 0.31 (0.20, 0.48) | 0.28 (0.17, 0.48) | 1.47 (0.95, 2.27) | 1.62 (1.02, 2.58) | 2.06 (1.22, 3.47) | ||
| > 2 cm | 0.66 (0.48, 0.91) | 0.47 (0.33, 0.69) | 0.41 (0.25, 0.65) | 1.26 (0.86, 1.83) | 1.36 (0.90, 2.04) | 1.65 (1.04, 2.63) | ||
| Unknown | 0.71 (0.44, 1.14) | 0.47 (0.26, 0.85) | 0.56 (0.28, 1.12) | 0.94 | 1.34 (0.74, 2.42) | 1.72 (0.92, 3.20) | 1.78 (0.87, 3.66) | 0.94 |
|
| ||||||||
|
Tumor grade
(differentiation) |
||||||||
| Well/moderate | 0.68 (0.51, 0.90) | 0.43 (0.30, 0.60) | 0.39 (0.26, 0.59) | 1.37 (0.98, 1.93) | 1.46 (1.01, 2.11) | 1.92 (1.26, 2.91) | ||
| Poor | 0.47 (0.30, 0.74) | 0.38 (0.22, 0.63) | 0.24 (0.12, 0.49) | 1.10 (0.63, 1.92) | 1.32 (0.74, 2.36) | 1.52 (0.79, 2.94) | ||
| Unknown | 0.67 (0.41, 1.08) | 0.37 (0.20, 0.69) | 0.54 (0.27, 1.10) | 0.69 | 1.53 (0.82, 2.83) | 1.95 (1.01, 3.73) | 1.76 (0.82, 3.75) | 0.63 |
|
| ||||||||
| Nodal Status | ||||||||
| Positive | 0.66 (0.47, 0.94) | 0.46 (0.30, 0.70) | 0.38 (0.22, 0.66) | 1.76 (1.12, 2.77) | 2.05 (1.26, 3.32) | 2.45 (1.41, 4.23) | ||
| Negative | 0.62 (0.46, 0.84) | 0.36 (0.25, 0.53) | 0.33 (0.21, 0.52) | 1.11 (0.77, 1.60) | 1.16 (0.78, 1.71) | 1.50 (0.96, 2.34) | ||
| Unknown | 0.61 (0.36, 1.02) | 0.48 (0.26, 0.88) | 0.57 (0.27, 1.20) | 0.78 | 1.44 (0.76, 2.72) | 1.92 (0.98, 3.75) | 1.87 (0.86, 4.06) | 0.41 |
|
| ||||||||
| ER | ||||||||
| Positive | 0.66 (0.50, 0.88) | 0.35 (0.24, 0.50) | 0.41 (0.26, 0.62) | 1.42 (1.00, 2.03) | 1.59 (1.08, 2.33) | 1.95 (1.26, 3.02) | ||
| Negative | 0.62 (0.42, 0.91) | 0.48 (0.31, 0.77) | 0.38 (0.21, 0.69) | 1.34 (0.84, 2.13) | 1.40 (0.85, 2.33) | 1.87 (1.05, 3.33) | ||
| Unknown | 0.55 (0.32, 0.93) | 0.53 (0.30, 0.96) | 0.28 (0.12, 0.63) | 0.94 | 1.07 (0.54, 2.11) | 1.42 (0.71, 2.82) | 1.29 (0.59, 2.85) | 0.67 |
| PR | ||||||||
| Positive | 0.60 (0.43, 0.82) | 0.34 (0.23, 0.51) | 0.44 (0.28, 0.70) | 1.51 (1.03, 2.22) | 1.53 (1.01, 2.33) | 1.97 (1.22, 3.17) | ||
| Negative | 0.71 (0.51, 0.98) | 0.42 (0.28, 0.63) | 0.34 (0.21, 0.57) | 1.26 (0.84, 1.89) | 1.48 (0.96, 2.29) | 1.80 (1.10, 2.95) | ||
| Unknown | 0.55 (0.33, 0.92) | 0.58 (0.33, 1.03) | 0.29 (0.13, 0.65) | 0.25 | 1.08 (0.55, 2.13) | 1.48 (0.75, 2.94) | 1.43 (0.66, 3.12) | 0.92 |
|
| ||||||||
Time spent sedentary or in moderate-to-vigorous activity calculated as the average minutes per day across valid days of wear. Categories determined by the quartile distribution among controls; behavior defined by the following counts: sedentary (0-99 counts) and moderate-to-vigorous (760+ counts); data for moderate-to-vigorous activity missing for one case.
Multinomial models adjusted for age, BMI, and wear time; each model includes all controls and the specific invasive cases of interest.
P for heterogeneity
Table 5.
Independent effects of moderate-to-vigorous activity and sedentary time on breast cancer odds, by menopausal status and body mass index (BMI)†
| Average minutes per day* |
Menopausal Status |
BMI (kg/m2)** |
|||
|---|---|---|---|---|---|
| Premenopausal cases/controls (250/376) |
Postmenopausal cases/controls (746/788) |
< 25 cases/controls (473/485) |
25-30 cases/controls (348/416) |
≥ 30 cases/controls (148/221) |
|
| Moderate-to-vigorous, min/day |
|||||
| ≤ 65.09 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| 65.10 - 95.58 | 0.42 (0.25, 0.71) | 0.62 (0.46, 0.84) | 0.43 (0.28, 0.64) | 0.72 (0.46, 1.14) | 0.66 (0.34, 1.27) |
| 95.59 - 128.24 | 0.30 (0.17, 0.53) | 0.36 (0.25, 0.53) | 0.30 (0.19, 0.47) | 0.36 (0.22, 0.62) | 0.48 (0.20, 1.14) |
| ≥128.25 | 0.27 (0.13, 0.54) | 0.36 (0.23, 0.57) | 0.19 (0.11, 0.35) | 0.50 (0.27, 0.94) | 0.36 (0.13, 1.03) |
| P-trend | 0.0003 | <.0001 | <.0001 | 0.005 | 0.04 |
| P-interaction | 0.96 | 0.53 | |||
| Sedentary, min/day | |||||
| ≤ 396.60 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| 396.61 - 457.74 | 1.42 (0.81, 2.49) | 1.27 (0.88, 1.84) | 0.94 (0.59, 1.49) | 1.65 (0.97, 2.78) | 1.66 (0.73, 3.78) |
| 457.75 - 524.10 | 1.46 (0.79, 2.73) | 1.42 (0.96, 2.10) | 0.69 (0.41, 1.15) | 2.18 (1.26, 3.78) | 2.17 (0.93, 5.06) |
| ≥ 524.11 | 2.10 (1.00, 4.42) | 1.63 (1.04, 2.55) | 0.88 (0.48, 1.59) | 3.13 (1.63, 6.01) | 2.18 (0.85, 5.62) |
| P-trend | 0.07 | 0.03 | 0.60 | 0.0006 | 0.11 |
| P-interaction | 0.37 | 0.005 | |||
Time spent sedentary or in moderate-to-vigorous activity calculated as the average minutes per day across valid days of wear. Categories determined by the quartile distribution among controls; behavior defined by the following counts: sedentary (0-99 counts) and moderate-to-vigorous (760+ counts); data for moderate-to-vigorous activity missing for one case. Models include time spent in moderate-to-vigorous and sedentary behavior.
Multivariable models adjusted for the following: age, BMI, education, current smoking status, age at menarche, number of full term births,breast cancer family history, previous screening mammogram, history of benign breast disease, menopausal status/age at menopause combination variable, and wear time. BMI and menopausal status excluded from their respective models.
BMI analyses excluded those with missing BMI values; unknown categories for age at menarche and education were also excluded due to too few numbers.
Discussion
Within this population-based case-control study, our findings suggest that accelerometer-measured physical activity is inversely associated with breast cancer risk, with strong reductions associated with increasing moderate-to-vigorous activity, after accounting for time spent sedentary. Furthermore, independent of the amount of time spent in moderate-to-vigorous activity, increased time spent sedentary appeared associated with increased odds of breast cancer.
Notwithstanding our results, we cannot dismiss the potential for treatment and disease status to have influenced our findings. To address the effects of treatment, we conducted sensitivity analyses based on whether cases wore the monitor prior to or only after surgery and/or other treatments. While the timing of treatment may have affected both active and sedentary behavior among the cases, the estimates for moderate-to-vigorous activity were relatively consistent regardless of when the monitor was worn. In contrast, we saw no evidence of an association between sedentary time and breast cancer when cases were restricted to those who wore the monitor prior to surgery and other treatments, suggesting that the increased odds associated with sedentary behavior may be a reflection of diseased subjects becoming more sedentary after treatment.
Studies of self-reported physical activity and breast cancer have evaluated whether observed associations vary by hormone receptor status, with inconsistent findings reported [4]. We also examined associations by tumor characteristics to disentangle the potential influence disease status may have had on the behaviors of cases and to assess potential tumor heterogeneity in estimates. Inverse associations for moderate-to-vigorous activity remained relatively consistent across tumor characteristics. Although we found no statistical heterogeneity in the relations of sedentary behavior to breast cancer according to tumor size, tumor grade, and hormone receptor status, those with node positive tumors may have spent more time sedentary, possibly reflecting more severe diagnoses and/or treatment modalities. Establishing temporality is difficult within the context of a case-control design; however, the lack of statistical heterogeneity in associations by tumor characteristics suggests that associations are not entirely explained by disease severity.
Despite these issues, our findings are consistent with the hypothesis that sedentary behavior is independently associated with adverse health outcomes, even among those physically active [7]. This notion has largely been evaluated in the field of mortality [18] and cardiovascular disease [19]. However, to date, there is limited information on the association between sedentary behavior and breast cancer risk [5-6,20]. In contrast to our findings, results from a cohort study conducted within the NIH-AARP Diet and Health Study [5] and a case-control study conducted in India [6] suggest no association between self-reported sedentary time, measured as time spent watching television/playing video games or sitting at work, and breast cancer risk. Given the limitations of self-reported measures, one cannot rule out the potential for measurement error to have biased their results toward the null. Within our study, on average, cases and controls spent 60% and 55%, respectively, of their time in behaviors of low energy expenditure. These estimates of objectively measured sedentary time are similar to those estimated for women of comparable ages in the National Health and Nutrition Survey (NHANES) population [16,21]. Our findings suggest that studies of pre-diagnostic self-report and accelerometer-based measures of sedentary behavior are warranted, particularly in light of the high levels of sedentary behavior in which most adults engage on a daily basis.
In terms of relationships of breast cancer with objective measures of physical activity, we observed strong inverse associations with overall activity, and with both light and moderate-to-vigorous intensity activity. These findings are consistent with the majority of prior studies of self-reported physical activity, including a prior report from the NCI Polish Breast Cancer Study [13], which have observed risk reductions ranging from 25-30% depending on the type of activity [22]. However, as the magnitude of associations for moderate-to-vigorous activity were stronger (i.e. 60-70% reductions) for accelerometer-measured, than what is generally seen for self-reported, activity our findings may reflect improved precision of time spent in activity when accelerometer-based exposure assessments are employed. Nonetheless, one cannot exclude the possibility that the risk reductions observed in our study may also, in part, be explained by reserve causation. Thus, these findings require replication in prospective studies with objective measures of pre-diagnostic activity.
Objective measures of active and sedentary time have been evaluated in cross-sectional studies of cardio-metabolic biomarkers [11] and breast cancer risk factors [9]. Increased activity, as measured objectively, was inversely associated with breast cancer risk factors, including C-reactive protein, insulin, BMI, and waist-to-hip ratio [9]. These studies validate the use of accelerometer measures in breast cancer studies and lend support for a biological rationale underlying associations between objective measures and breast cancer.
Given the multiple, inter-related biological mechanisms potentially underlying associations with active and sedentary behavior, we assessed whether these behaviors may have differential associations among subgroups defined by menopausal status and BMI. Results from studies of self-reported physical activity have suggested stronger, more consistent risk reductions among postmenopausal versus premenopausal women [3]. However, in our analysis, associations with objectively measured sedentary time or moderate-to-vigorous activity did not differ by menopausal status, a finding consistent with that from the prior analysis of self-reported measures in this study [13]. With respect to BMI, we observed stronger positive associations with increased sedentary time among overweight women. Results from studies of self-reported sedentary behavior are limited but suggest no potential effect modification by BMI [4].
Although accelerometers may increase the precision and range of exposure, there are also limitations to this measure. Accelerometers capture a wide range of ambulatory activities, including walking, yet activities such as swimming, cycling and load carrying are not always measured well by the accelerometer [15]. It is possible that women diagnosed with breast cancer may choose to engage in lower impact activities, such as swimming or cycling; however, this potential bias seems unlikely in our population as only 3.6% of women reported removing their accelerometer for either swimming or cycling activities (n=77). Of these, 19 were breast cancer cases. Although the cut-points we used to distinguish between time spent in sedentary behavior or in light and moderate-to-vigorous activity were based on calibration studies [15-16], our estimates of time spent in each of these behaviors were dependent on assigned cut-points. While the mean values (minutes/day) may vary if different cut-points were used, our overall findings for active and sedentary behavior in this report would not be affected.
In addition to limitations inherent to both the accelerometer and the case-control study design, an underlying assumption of this analysis is that post-diagnostic active and sedentary behavior serves as a proxy for pre-diagnostic behavior among these women. However, it is possible that cases may have altered their physical activity patterns after diagnosis and/or treatment. Irwin et al. examined physical activity levels pre- and post-breast cancer diagnosis among a cohort of women, overall, and in relation to treatment type [23]. On average, physical activity levels decreased by 11% after a breast cancer diagnosis, with greater decreases observed among women undergoing radiation or chemotherapy versus surgery alone [23]. Although we could not directly assess changes pre- and post-diagnosis, the range of time spent in both active and sedentary behavior was similar for cases and controls. If cases increased their sedentary time due to the nature of their diagnosis, one would expect to see much larger differences in the range of sedentary time by case-control status; nevertheless, we cannot rule out the possibility that bias introduced by disease status may have resulted in overestimation of observed effects of both active and sedentary behaviors.
The balance between an ideal timing of exposure assessment, often gained by prospective study designs, and optimizing exposure measurement with the use of an objective tool is a challenge. However, the innovative application of accelerometers in this large, population-based case-control study provided an objective alternative to approximate usual activity. Participants wore the monitor for an average of five valid days (of the seven days requested), with similar compliance by both cases and controls; this is in line with reported average days of wear from previous studies in NHANES [16]. Additionally, we were able to assess the independent associations of different types of behavior. Furthermore, our study offered a wide range of exposure information, a high participation rate, and a large number of cases and controls, which afforded the opportunity to assess potential differences by subgroups and tumor characteristics and to capture a wide range of behaviors.
In summary, in this first study to assess objective measures of both active and sedentary behavior in relation to breast cancer, our findings support a reduction in the odds of breast cancer with increased light and moderate-to-vigorous activity. Our results confirm those of prior studies of self-report suggesting beneficial risk reductions for breast cancer with increased activity and support current international public health guidelines [24-25] recommending that women engage regularly in moderate-to-vigorous intensity activity for breast cancer risk reduction. Additionally, our results are suggestive of potential increases in breast cancer with increasing time spent sedentary; however, given the limitations inherent to the case-control design, these findings should be cautiously interpreted. Future breast cancer studies are warranted utilizing objective measures of active and sedentary behavior.
Supplementary Material
Supplementary Table 1. Distribution of accelerometer measures by case-control status
Supplementary Table 2. Spearman rank correlation coefficients (rs) for accelerometer measures by case-control status
Acknowledgements
We thank Michael Stagner and Pei Chao for their work on study and data management (IMS, Silver Spring, MD); physicians, pathologists, nurses, and interviewers from participating centers in Poland for their efforts in the field. We also thank the participants of the Polish Study for their contributions to this study, Dr. Bill Anderson for his statistical consultation, Dr. Mark Sherman for his independent evaluation of histopathological information, and Drs. Montserrat Garcia-Closas and Mark Sherman for contributions to study design.
Participating centers in Poland Cancer Center and M. Skodowska-Curie Institute of Oncology in Warsaw: Departments of Epidemiology (Coordinating center: Dr Jolanta Lissowska, Mrs Alicja Bardin-Mikolajczak, Dr Witold Zatonski), Breast Cancer Treatment and Reconstruction (Drs Edward Towpik and Jerzy Giermek), Departments of Surgical Oncology (Dr Pawel Kukawski), Pathology (Drs Grzegorz Rymkiewicz, Marcin Ligaj, Joanna Baran’ska, Agnieszka Turowicz, Włodzimierz Olszewski).
Polish Oncological Foundation in Warsaw: Pathology (Drs Dorota Mazepa-Sikora, Włodzimierz Olszewski).
Nofer Institute of Occupational Medicine in Łódz’: (Drs Neonila Szeszenia-D˛browska, Beata Peplonska).
Medical University in Łódz’: Oncology Clinic (Drs Arkadiusz Jeziorski, Janusz Piekarski), and Pathology Department (Drs Radzislaw Kordek, Grazyna Pasz-Walczak, Robert Kubiak, Dorota Kupnicka, Boguslaw Olborski).
Community Copernicus Hospital in Łódz’: Department of Surgical Oncology (Drs Zbigniew Morawiec and Mariusz Pawlak).
Polish Mother’s Health Memorial Hospital in Łódz’: Departments Surgical Oncology and Breast Diseases (Drs Marcin Faflik, Magdalena Baklinska, Marek Zadrozny, Boguslaw Westfal) and Clinical Pathomorphology (Drs Stanislaw Lukaszek, Andrzej Kulig).
Grant Support Polish Breast Cancer Study was supported by the Intramural Research Program of the National Cancer Institute, Department of Health and Human Services, USA.
Abbreviations
- BMI
body mass index
- CI
confidence interval
- Ct
counts
- ER
estrogen receptor
- MV
multivariable
- NCI
US National Cancer Institute
- OR
odds ratio
- PR
progesterone receptor
- Q
quartile
- SD
standard deviation
Footnotes
Conflict of Interest The authors declare they have no conflicts of interest.
References
- 1.Lynch BM, Neilson HK, Friedenreich CM. Physical activity and breast cancer prevention. Recent Results Cancer Res. 2011;186:13–42. doi: 10.1007/978-3-642-04231-7_2. doi:10.1007/978-3-642-04231-7_2. [DOI] [PubMed] [Google Scholar]
- 2.Friedenreich CM. Physical activity and breast cancer: review of the epidemiologic evidence and biologic mechanisms. Recent Results Cancer Res. 2011;188:125–139. doi: 10.1007/978-3-642-10858-7_11. doi:10.1007/978-3-642-10858-7_11. [DOI] [PubMed] [Google Scholar]
- 3.Monninkhof EM, Elias SG, Vlems FA, van der Tweel I, Schuit AJ, Voskuil DW, van Leeuwen FE. Physical activity and breast cancer: a systematic review. Epidemiology. 2007;18(1):137–157. doi: 10.1097/01.ede.0000251167.75581.98. doi:10.1097/01.ede.0000251167.75581.98. [DOI] [PubMed] [Google Scholar]
- 4.Friedenreich CM, Neilson HK, Lynch BM. State of the epidemiological evidence on physical activity and cancer prevention. Eur J Cancer. 2010;46(14):2593–2604. doi: 10.1016/j.ejca.2010.07.028. doi:S0959-8049(10)00706-9 [pii] 10.1016/j.ejca.2010.07.028. [DOI] [PubMed] [Google Scholar]
- 5.George SM, Irwin ML, Matthews CE, Mayne ST, Gail MH, Moore SC, Albanes D, Ballard-Barbash R, Hollenbeck AR, Schatzkin A, Leitzmann MF. Beyond recreational physical activity: examining occupational and household activity, transportation activity, and sedentary behavior in relation to postmenopausal breast cancer risk. Am J Public Health. 2010;100(11):2288–2295. doi: 10.2105/AJPH.2009.180828. doi:AJPH.2009.180828 [pii] 10.2105/AJPH.2009.180828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mathew A, Gajalakshmi V, Rajan B, Kanimozhi VC, Brennan P, Binukumar BP, Boffetta P. Physical activity levels among urban and rural women in south India and the risk of breast cancer: a case-control study. European Journal of Cancer Prevention. 2009;18(5):368–376. doi: 10.1097/CEJ.0b013e32832e1c46. doi:Doi10.1097/Cej.0b013e32832e1c46. [DOI] [PubMed] [Google Scholar]
- 7.Owen N, Healy GN, Matthews CE, Dunstan DW. Too much sitting: the population health science of sedentary behavior. Exerc Sport Sci Rev. 2010;38(3):105–113. doi: 10.1097/JES.0b013e3181e373a2. doi:10.1097/JES.0b013e3181e373a200003677-201007000-00003 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ford ES, Li C, Zhao G, Pearson WS, Tsai J, Churilla JR. Sedentary behavior, physical activity, and concentrations of insulin among US adults. Metabolism. 2010;59(9):1268–1275. doi: 10.1016/j.metabol.2009.11.020. doi:S0026-0495(09)00503-4 [pii] 10.1016/j.metabol.2009.11.020. [DOI] [PubMed] [Google Scholar]
- 9.Lynch BM, Friedenreich CM, Winkler EA, Healy GN, Vallance JK, Eakin EG, Owen N. Associations of objectively assessed physical activity and sedentary time with biomarkers of breast cancer risk in postmenopausal women: findings from NHANES (2003-2006) Breast Cancer Res Treat. 2011 doi: 10.1007/s10549-011-1559-2. doi:10.1007/s10549-011-1559-2. [DOI] [PubMed] [Google Scholar]
- 10.Ford ES, Kohl HW, 3rd, Mokdad AH, Ajani UA. Sedentary behavior, physical activity, and the metabolic syndrome among U.S. adults. Obes Res. 2005;13(3):608–614. doi: 10.1038/oby.2005.65. doi:13/3/608 [pii] 10.1038/oby.2005.65. [DOI] [PubMed] [Google Scholar]
- 11.Healy GN, Matthews CE, Dunstan DW, Winkler EA, Owen N. Sedentary time and cardio-metabolic biomarkers in US adults: NHANES 2003-06. Eur Heart J. 2011;32(5):590–597. doi: 10.1093/eurheartj/ehq451. doi:ehq451 [pii] 10.1093/eurheartj/ehq451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Neilson HK, Friedenreich CM, Brockton NT, Millikan RC. Physical activity and postmenopausal breast cancer: proposed biologic mechanisms and areas for future research. Cancer Epidemiol Biomarkers Prev. 2009;18(1):11–27. doi: 10.1158/1055-9965.EPI-08-0756. doi:18/1/11 [pii] 10.1158/1055-9965.EPI-08-0756. [DOI] [PubMed] [Google Scholar]
- 13.Peplonska B, Lissowska J, Hartman TJ, Szeszenia-Dabrowska N, Blair A, Zatonski W, Sherman ME, Garcia-Closas M, Brinton LA. Adulthood lifetime physical activity and breast cancer. Epidemiology. 2008;19(2):226–236. doi: 10.1097/EDE.0b013e3181633bfb. doi:10.1097/EDE.0b013e3181633bfb 00001648-200803000-00011 [pii] [DOI] [PubMed] [Google Scholar]
- 14.Garcia-Closas M, Brinton LA, Lissowska J, Chatterjee N, Peplonska B, Anderson WF, Szeszenia-Dabrowska N, Bardin-Mikolajczak A, Zatonski W, Blair A, Kalaylioglu Z, Rymkiewicz G, Mazepa-Sikora D, Kordek R, Lukaszek S, Sherman ME. Established breast cancer risk factors by clinically important tumour characteristics. Br J Cancer. 2006;95(1):123–129. doi: 10.1038/sj.bjc.6603207. doi:6603207 [pii] 10.1038/sj.bjc.6603207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matthews CE. Calibration of Accelerometer Output for Adults. Medicine & Science in Sports & Exercise. 2005;37(11):S512–S522. doi: 10.1249/01.mss.0000185659.11982.3d. [DOI] [PubMed] [Google Scholar]
- 16.Matthews CE, Chen KY, Freedson PS, Buchowski MS, Beech BM, Pate RR, Troiano RP. Amount of time spent in sedentary behaviors in the United States, 2003-2004. Am J Epidemiol. 2008;167(7):875–881. doi: 10.1093/aje/kwm390. doi:kwm390 [pii] 10.1093/aje/kwm390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Begg CB, Zhang ZF. Statistical analysis of molecular epidemiology studies employing case-series. Cancer Epidemiol Biomarkers Prev. 1994;3(2):173–175. [PubMed] [Google Scholar]
- 18.Patel AV, Bernstein L, Deka A, Feigelson HS, Campbell PT, Gapstur SM, Colditz GA, Thun MJ. Leisure time spent sitting in relation to total mortality in a prospective cohort of US adults. Am J Epidemiol. 2010;172(4):419–429. doi: 10.1093/aje/kwq155. doi:kwq155 [pii] 10.1093/aje/kwq155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Healy GN, Dunstan DW, Salmon J, Shaw JE, Zimmet PZ, Owen N. Television time and continuous metabolic risk in physically active adults. Med Sci Sports Exerc. 2008;40(4):639–645. doi: 10.1249/MSS.0b013e3181607421. doi:10.1249/MSS.0b013e3181607421. [DOI] [PubMed] [Google Scholar]
- 20.Lynch BM. Sedentary behavior and cancer: a systematic review of the literature and proposed biological mechanisms. Cancer Epidemiol Biomarkers Prev. 2010;19(11):2691–2709. doi: 10.1158/1055-9965.EPI-10-0815. doi:1055-9965.EPI-10-0815 [pii] 10.1158/1055-9965.EPI-10-0815. [DOI] [PubMed] [Google Scholar]
- 21.Lynch BM, Dunstan DW, Healy GN, Winkler E, Eakin E, Owen N. Objectively measured physical activity and sedentary time of breast cancer survivors, and associations with adiposity: findings from NHANES (2003-2006) Cancer Causes Control. 2010;21(2):283–288. doi: 10.1007/s10552-009-9460-6. doi:10.1007/s10552-009-9460-6. [DOI] [PubMed] [Google Scholar]
- 22.Friedenreich CM, Cust AE. Physical activity and breast cancer risk: impact of timing, type and dose of activity and population subgroup effects. Br J Sports Med. 2008;42(8):636–647. doi: 10.1136/bjsm.2006.029132. doi:bjsm.2006.029132 [pii] 10.1136/bjsm.2006.029132. [DOI] [PubMed] [Google Scholar]
- 23.Irwin ML, Crumley D, McTiernan A, Bernstein L, Baumgartner R, Gilliland FD, Kriska A, Ballard-Barbash R. Physical activity levels before and after a diagnosis of breast carcinoma - The health, eating, activity, and lifestyle (HEAL) study. Cancer. 2003;97(7):1746–1757. doi: 10.1002/cncr.11227. doi:Doi 10.1002/Cncr.11227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kushi LH, Byers T, Doyle C, Bandera EV, McCullough M, McTiernan A, Gansler T, Andrews KS, Thun MJ. American Cancer Society Guidelines on Nutrition and Physical Activity for cancer prevention: reducing the risk of cancer with healthy food choices and physical activity. CA Cancer J Clin. 2006;56(5):254–281. doi: 10.3322/canjclin.56.5.254. quiz 313-254. doi:56/5/254 [pii] [DOI] [PubMed] [Google Scholar]
- 25.Vainio H, Kaaks R, Bianchini F. Weight control and physical activity in cancer prevention: international evaluation of the evidence. Eur J Cancer Prev. 2002;11(Suppl 2):S94–100. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1. Distribution of accelerometer measures by case-control status
Supplementary Table 2. Spearman rank correlation coefficients (rs) for accelerometer measures by case-control status