Abstract
Previous studies have shown that preconditioning rats with a non-pressor dose of angiotensin II (AngII) sensitizes the pressor response produced by later treatment with a higher dose of AngII and that AngII and aldosterone (Aldo) can modulate each other’s pressor effects through actions involving the central nervous system (CNS). The current studies tested whether Aldo can cross-sensitize the pressor actions of AngII to enhance hypertension by employing an Induction-Delay-Expression (I-D-E) experimental design. Male rats were implanted for telemetered BP recording. During I, sub-pressor doses of either sc or icv Aldo were delivered for 1 week. Rats were then rested for 1 week (D) to assure that any exogenous Aldo was metabolized. After this, AngII was given sc for 2 weeks (E).
During I and D Aldo had no sustained effect on BP. However, during E AngII-induced hypertension was greater in the groups receiving sc or icv Aldo during I in comparison to those groups receiving vehicle. Central administration of mineralocorticoid receptor antagonist blocked sensitization. Brain tissue collected at the end of D and E showed increased mRNA expression of several renin-angiotensin-aldosterone system components in cardiovascular-related forebrain regions of cross-sensitized rats. Cultured subfornical organ neurons preincubated with Aldo displayed greater increases in [Ca2+]i after AngII, and there was greater Fra-like immunoreactivity present at the end of E in cardiovascular-related forebrain structures. Taken together, these results indicate that Aldo pretreatment cross-sensitizes the development of AngII-induced hypertension probably by mechanisms that involve the CNS.
Keywords: Aldosterone, Angiotensin II, Sensitization, Blood Pressure, Brain Renin-Angiotensin-Aldosterone System Expression
Introduction
Systemic and brain renin-angiotensin-aldosterone systems (RAAS) play critical roles in the regulation of blood pressure (BP) and body fluid homeostasis. In excess, the primary effectors of RAAS, angiotensin II (AngII) and aldosterone (Aldo), exert similar direct and indirect adverse effects on various body tissues and organs. The interactions and synergism between Aldo and AngII have been studied both in the periphery and central nervous system (CNS).1,2 We and others have demonstrated that central infusion of a mineralocorticoid receptor (MR) blocker or Aldo synthase (AS) inhibitor attenuates hypertension induced by systemic AngII administration. Conversely, central blockade of angiotensin type 1 receptors (AT1-R) attenuates Aldo/salt-induced hypertension.3,4 Moreover, icv infusions of low doses of AngII increase hypothalamic Aldo fourfold, whereas subcutaneous infusion of the same dose has no effect on plasma or hypothalamic Aldo levels in rats.4 In rats icv infusions of Aldo cause sympathetic hyperactivity, hypertension and increased angiotensin-converting enzyme (ACE) and AT1-R mRNA expression in the hypothalamus.5 Blockade of MRs in the CNS by icv infusion of an MR antagonist prevents sympathetic hyperactivity in Dahl S rats during high salt intake,6 and attenuated the up-regulation of AT1-R and ACE in the hypothalamus in rats with heart failure.7
The sensitization of sodium appetite (i.e., the ingestion of salty substances) and thirst (water drinking) has been associated with the central interactions of Aldo and AngII.8,9 Epstein and colleagues demonstrated that a low dose of deoxycorticosterone acetate (DOCA) pretreatment regimen, which by itself does not produce a salt appetite, enhances the actions of AngII so that a subsequent, subthreshold icv dose of AngII triggers a robust sodium appetite.10 ectrophysiological studies also indicate that forebrain regions, such as median preoptic nucleus (MnPO), contain neurons that rapidly sensitize in response to AngII when animals are preconditioned with Aldo.11 Our previous work demonstrated that a sub-pressor preconditioning dose of AngII can act on the brain and sensitize the hypertensive response to a later AngII treatment, and that increased expression of AS and MR in forebrain cardiovascular control regions are likely to mediate this enhanced pressor effect.12 Such results prompted us to hypothesize that in addition to AngII, Aldo may also have the capacity to cross-sensitize the actions of AngII to enhance the hypertensive response to the octapeptide. To test this hypothesis we conducted BP recording studies using a two-infusion protocol. This involved a period of induction (I) when a sub-pressor dose of Aldo was first infused either peripherally or centrally. Then after a period of delay (D), a second AngII infusion was given to test the expression (E) of hypertension. To gain further insight into the mechanisms underlying Aldo induced cross-sensitization, additional cellular and molecular studies were also conducted.
Methods
What follows is a brief summary of the experimental protocols. A detailed description of key methods can be found in an expanded Methods section in the online Data Supplement (available at http:/hyper.ahajournals.org).
Animals
Sixty-one male Sprague-Dawley rats (10–12 weeks old, Harlan) were used. They were housed in temperature- and light-controlled animal quarters and were provided with rat chow (7013 NIH-31 modified rat diet, 0.25% NaCl) ad libitum. All experiments were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals, and were approved by The University of Iowa Animal Care and Use Committee.
Experimental Protocol
The present studies followed an I-D-E experimental design as described previously.12 During I, a sub-pressor dose of Aldo or vehicle was delivered subcutaneously (sc, 750 ng/h) or centrally (icv, 10 ng/h) by osmotic minipump (model 2001, Alzet) for 1 week. To assure that any exogenous Aldo was metabolized, the rats then rested for 1 week (D). After this time, a second pump (model 2002, Alzet) was implanted to deliver AngII (120 ng/kg/min) for 2 weeks (E). Rats were randomly assigned to one of six groups: 1) I with Aldo plus E with saline (I-Aldo+E-S, n=4), 2) I with saline plus E with AngII (I-S+E-AngII, n=5), 3) I with Aldo plus E with AngII (I-Aldo+E-AngII, n=6), 4) I with Aldo and icv MR antagonist (RU28,318, 1.1µg/h) plus E with AngII (I-Aldo/icv RU28318+E-AngII, n=6), 5) I with icv saline plus E with AngII (I-icv S+E-AngII, n=4), and 6) I with icv Aldo plus E with AngII (I-icv Aldo+E-AngII, n=6). Brains were harvested at the end of E for analyses of tissue mRNA expression. The microdissected tissue samples for mRNA expression contained the paraventricular hypothalamic nuclei (PVN) or a set of structures lying along the lamina terminalis (LT), including the subfornical organ (SFO), MnPO and organum vasculosum. Two additional control (saline, n=5) and experimental (subpressor dose of Aldo, n=5) groups received identical I and D procedures but had their brains collected at the end of D for analysis of mRNA expression. Likewise, four additional groups including one control (saline) and three experimental groups (same treatments as group 1–3 without physiological studies) were performed for assessments of Fos-related antigen immunoreactivity (Fra-IR, n=4 per group).
Physiological Studies
Under Ketamine-xylazine anethesia, rats were chronically instrumented with telemetry probes (TA11PA-C40; DSI) placed in the femoral artery for continuous monitoring of mean arterial pressure (MAP) and heart rate (HR), as described previously.3,12 Beginning seven days after recovery from surgery, MAP and HR data collection was initiated. To study the cross-sensitizing effect of Aldo on AngII-induced hypertension, the first osmotic pump containing saline or Aldo and the second pump containing saline or AngII were implanted on the back of rats under isoflurane anesthesia.
Measurement of mRNA Expression in the LT and PVN
Total RNA was isolated from LT and PVN tissue samples using Trizol method (Invitrogen). Total RNA was reverse transcribed using random hexamers following the manufacturer’s instructions (Applied Biosystems). cDNA was amplified and analyzed using a C1000 thermocycler system (Bio-Rad). Changes in mRNA expression levels were normalized to GAPDH levels and calculated using the ΔΔCt method. Results are expressed as relative fold change, mean of fold change ± SE.
Fluorescent immunohistochemistry
Immunohistochemical studies were performed to assess neuronal activation in the SFO and PVN. Expression of Fra-like activity was used as an indicator of chronic neuronal activation. Brain sections were incubated with a rabbit polyclonal anti-Fos antibody (K-25, 1:1000, Santa Cruz) for 72 h at 4 °C. After being thoroughly washed with PBS, sections were incubated with Cy™2-conjugated AffiniPure donkey anti-rabbit IgG (1:100, Jackson) for 2 h at room temperature. Fluorescence was then identified using confocal microscopy.
Measurement of intracellular calcium ([Ca2+]i) in cultured SFO neurons
Experiments were performed in SFO cells after three days in culture. Cells were loaded with Fluo-4, an indicator of [Ca2+]i, and Fluo-4 fluorescence was imaged using confocal microscopy and then was quantified using Fluoview 5 (Olympus, Japan) analysis software. To explore the effect of Aldo on AngII-induced [Ca2+]i, cells were incubated overnight with Aldo (10 nM) prior to an acute application of AngII (100 nM). The percentage changes of Fluo-4 fluorescence intensity after AngII application were expressed relative to fluorescence of vehicle treated neurons
Data Analysis
Baseline MAP and HR data were collected for 5 days and then for 28 consecutive days throughout I, D and E. MAP and HR are presented as mean daily values averaged from daytime and nighttime measurements. Difference scores for MAP and HR were calculated for each animal based on the mean of the 5-day baseline subtracted from the mean of the final 5 days of treatment. One-way ANOVAs for the experimental groups were then conducted on the means of calculated difference scores. After establishing a significant ANOVA, post-hoc analyses were performed with Tukey multiple comparison tests between pairs of mean change scores.
The atlas of Paxinos and Watson was used to define regions of interest to evaluate Fra-IR in the SFO and PVN. Statistical evaluation of Fra-IR counts was performed by one-way ANOVA and Tukey tests. The same statistical methods were used to analyze the differences in mRNA expression of brain RAAS components in the groups that had previously received Aldo during I vs. the vehicle treated group, and in Fluo-4 fluorescence intensity response to AngII in the neurons with or without overnight incubation with Aldo.
Results
The Effect of Aldo-induced Sensitization during I on MAP and HR Induced by AngII during E
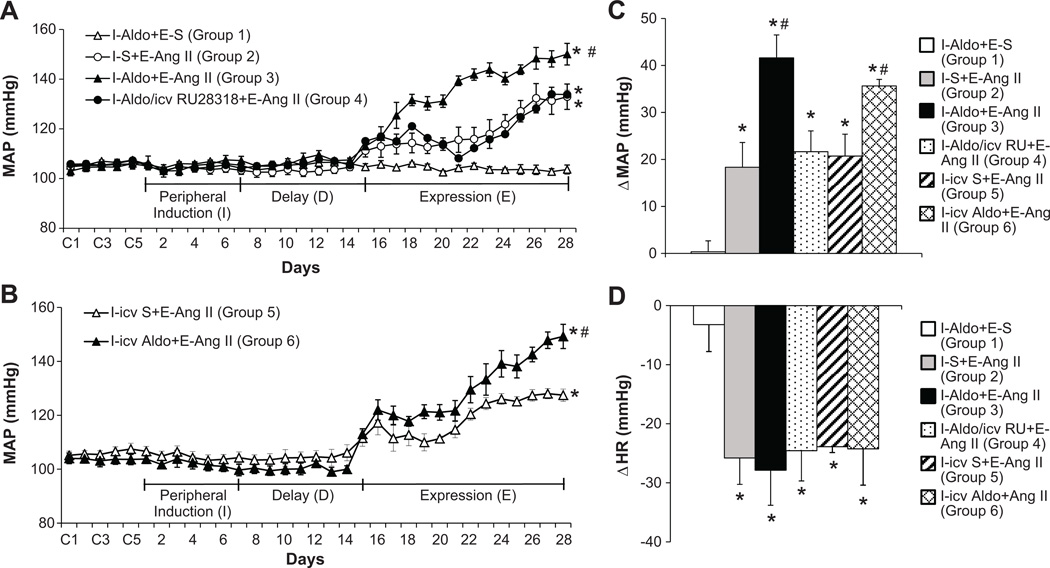
During I and D, the sc sub-pressor dose of Aldo had no effect on MAP. However, over the course of E, AngII induced a greater increase in MAP in the rats that received Aldo (Δ41.6±4.9 mmHg, p<0.05, Fig. 1A, 1C) during I as compared to the rats treated with saline (Δ19.8±4.1 mmHg) during I. This augmentation of the pressor effect induced by AngII was attenuated by concurrent icv infusions of the MR antagonist RU28318 along with the sc Aldo during I (Δ21.7±4.4 mmHg, p<0.05).
Figure 1.
Augmented pressor effects induced by AngII during the expression (E) period in rats after peripheral (Fig 1A) or central (Fig 1B) treatment with a subpressor dose of Aldo during the induction (I) period. This effect was attenuated by central blockade of MR. Figures 1C and 1D show the changes in MAP and HR after infusion of AngII during E in all groups. I-S = peripheral treatment with saline during I; I-Aldo = peripheral treatment with Aldo during I; I-Aldo/icv RU = peripheral treatment with Aldo plus central treatment with RU28318 (RU) during I; I-icv S = central treatment with saline during I; I-icv Aldo = central treatment with Aldo during I; E-S = peripheral treatment with saline during E; E-AngII = peripheral treatment with a pressor dose of AngII during E. (* p<0.05 vs. baseline or I–Aldo+E-S, # p<0.05 vs. I-S+E-AngII, I-icv S+E-AngII or I-Aldo/icv RU+E-AngII).
To confirm that the effect of Aldo-induced sensitization was through its actions on the central nervous system (CNS), a low dose of Aldo, which had no effects on BP, was infused icv for 1 week during I. This icv infusion of the low dose of Aldo also enhanced the pressor effects induced by a subsequent AngII (Δ35.6±1.4 mmHg, p<0.05) during E as compared to that of icv saline treated rats (Δ21.5±3.8 mmHg, Figs 1B and 1C).
AngII infusion during E produced a significant decrease in HR in all groups (p <0.05). However, the fall in HR during AngII treatment was similar in all groups (Fig 1D).
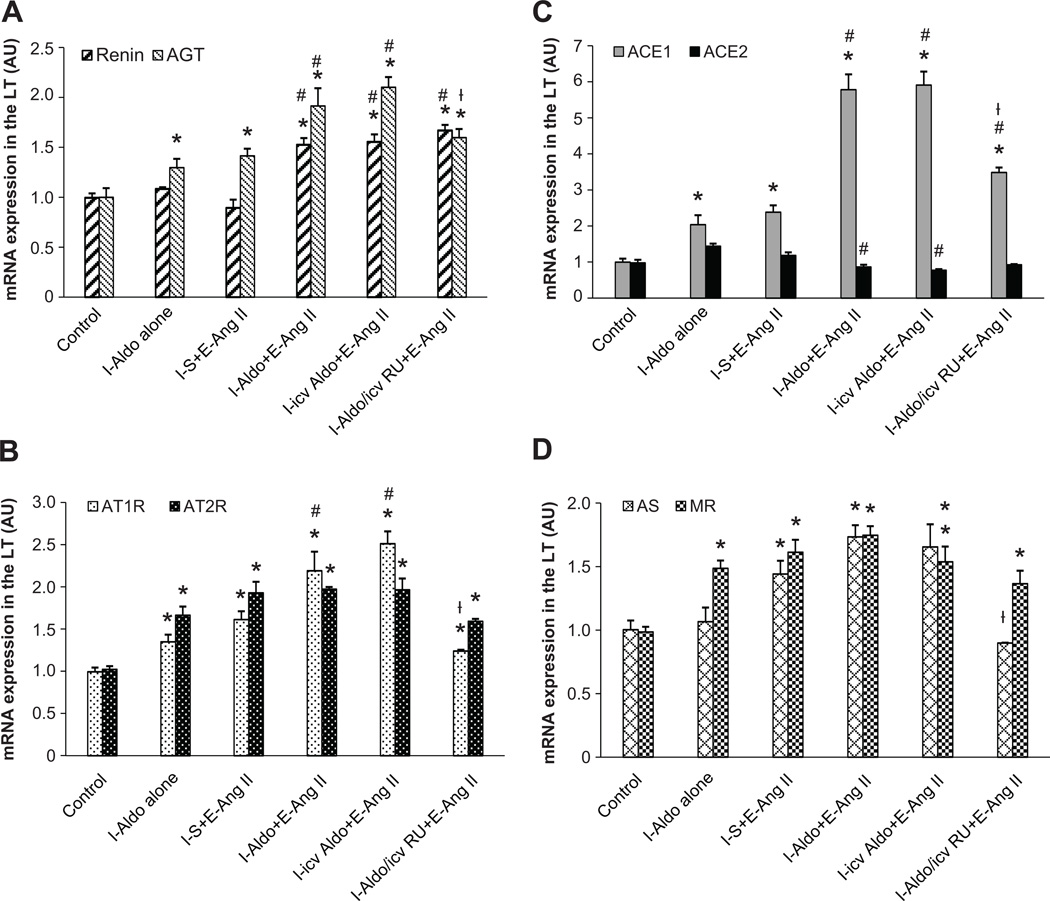
The Effect of AngII Infusions on the mRNA Expression of RAAS Components in the LT
In LT tissue collected at the end of D, a sub-pressor dose of Aldo during I induced a significant increase in the mRNA expression of AGT, AT1-R, AT2-R, ACE1, and mineralocorticoid receptor (MR) in the LT when compared with controls (p<0.05). The expression of renin, ACE2 and AS in the LT was not higher after D (p>0.05, Fig 2).
Figure 2.
AngII infusion induced greater increases in mRNA expression of AGT (Fig 2A), AT1-R (Fig 2B), and ACE1 (Fig 2C) in the LT of rats receiving a sub-pressor dose Aldo as compared with those receiving saline during induction (I). Central infusion of MR antagonist blocked these effects. E, expression; S, saline; RU, RU28318. (* p<0.05 vs. control, # p<0.05 vs. I-Aldo alone or I-S+E-AngII, ┼ p<0.05 vs. I-(icv)Aldo+E-AngII).
At the end of E, AngII induced a similar increase in the mRNA expression of RAAS components in the rats treated with saline during I, with an additional significant increase in AS (p<0.05). However, the AngII infusion during E resulted in greater increases in mRNA expression of renin, AGT, AT1-R, and ACE1 in the LT of the group that received either sc or icv Aldo during I (p<0.05). In contrast, ACE2 mRNA expression was significantly reduced (p<0.05). Central infusion of the MR antagonist during I blocked these augmented effects on mRNA expression (Fig 2).
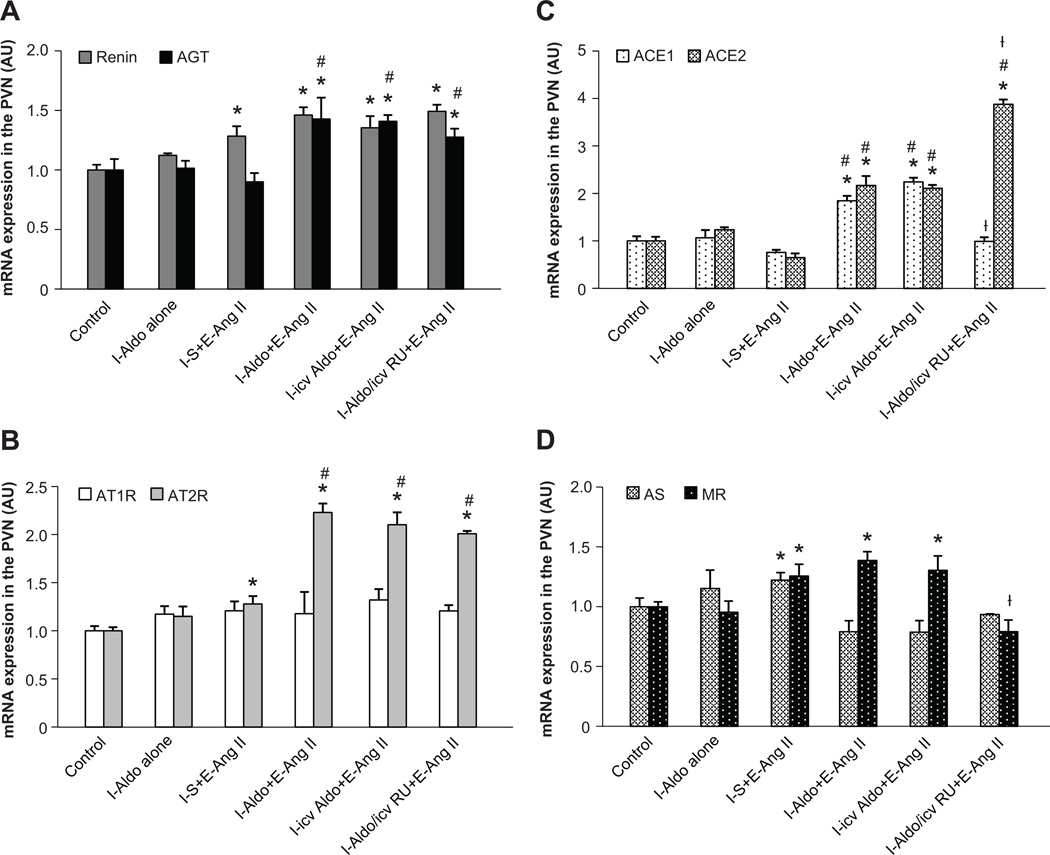
The Effect of AngII Infusions on mRNA Expression of RAAS Components in the PVN
The sub-pressor dose of Aldo administered during I had no effect on mRNA expression of any of the RAAS components studied in the PVN at the end of D. At the end of E, AngII induced only a slight, but significant increase in the mRNA expression of renin, AT2-R, AS and MR in the PVN of the animals that received saline during I (p<o.05, Fig 3). However, the sub-pressor dose of Aldo delivered peripherally or centrally during I enhanced AngII-induced mRNA expression in AGT, AT2-R, ACE1 and ACE2 during E (p<0.05), while the mRNA expression of renin and MR remained elevated above the control condition, but not significantly higher than the animals that received saline during I followed by AngII during E. Central infusion of the MR antagonist during I enhanced ACE2 mRNA expression, but blocked the increased expression in ACE1 and MR, and had no effect on increased expression of renin, AGT and AT2-R produced by the infusion of AngII (Fig 3).
Figure 3.
AngII infusion induced greater increases in mRNA expression of AGT (Fig 3A), AT2-R (Fig 3B), ACE1 and ACE2 (Fig 2C) in the PVN of rats receiving a sub-pressor dose of Aldo as compared with those receiving saline during induction (I). E, expression; S, saline; RU, RU28318. (* p<0.05 vs. control, # p<0.05 vs. I-Aldo alone or I-S+E-AngII, ┼ p<0.05 vs. I-(icv)Aldo+E-AngII).
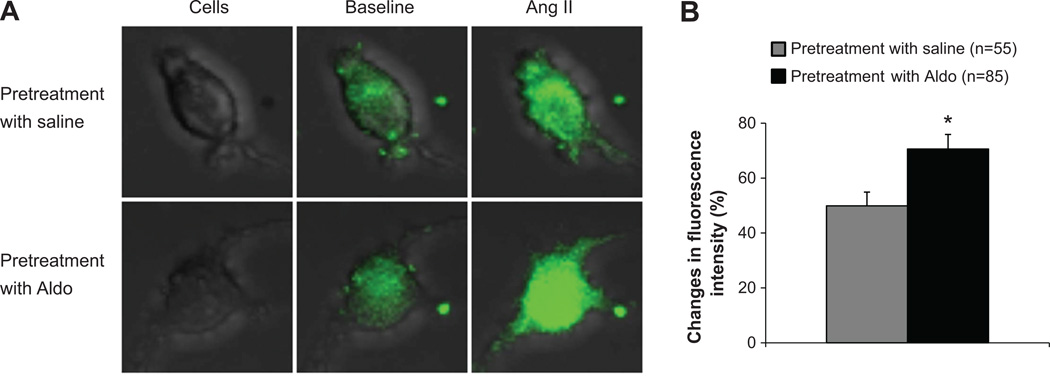
The Effect of Pretreatment with Aldo on AngII-induced [Ca2+]i in Cultured SFO Neurons
Figure 4A shows representative bright field and Fluo-4 fluorescent images of cultured SFO neurons with or without AngII and Aldo treatments. Acute application of AngII increased Fluo-4 intensity in SFO neurons by 49.9±5.0% (n=55, p<0.05, Fig 4B). Overnight pre-incubation of Aldo augmented the AngII-induced increase in Fluo-4 intensity in SFO neurons (70.6±5.3%, n=85, p<0.05, Fig 4B). Moreover, pretreatment with Aldo also significantly increased the number of SFO neurons sensitive to acute application of AngII when compared with SFO neurons pretreated with saline (57.4% vs.48.7%).
Figure 4.
Overnight pre-incubation of Aldo augmented AngII-induced increase in [Ca2+]i in SFO neurons. Figure 4A shows representative bright field and Fluo-4 fluorescent images of cultured SFO neurons with or without AngII and Aldo treatment. Fig 4B summarizes changes in fluorescence intensity when treated with AngII in SFO neurons with or without Aldo pre-incubation. (* p<0.05 vs. pretreatment with saline).
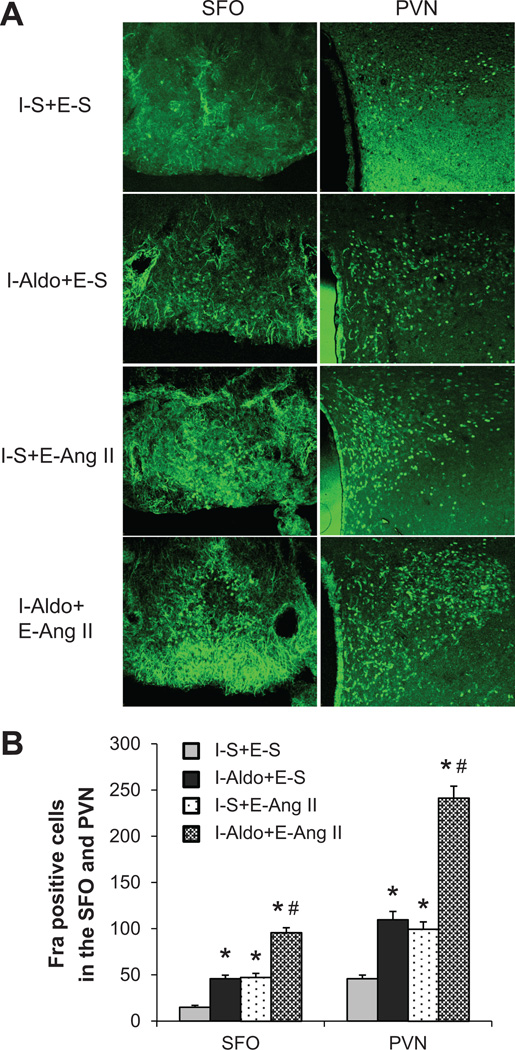
The Effect of AngII Infusion on Neuronal Activation in the SFO and PVN of Rats Treated with Saline or a Subpressor Dose of Aldo during I
A subpressor dose of Aldo during I induced significant (p <0.05) increases in Fra-IR in both the SFO (45.8±6.8 cells) and PVN (109.8±9.8 cells) compared with sparse labeling seen in these structures in the animals receiving saline during I and E (SFO 15.0±2.0 cells, PVN 45.8±7.4 cells). At the end of E, AngII induced a similar increase in Fra-IR in both the SFO (47.3±4.4 cells) and PVN (99.4±7.9 cells) of the rats that received saline during I. A sub-pressor dose of Aldo given during I augmented Fra-IR in the SFO (95.7±10.4 cells, p<0.05) and PVN (241.3±13.0 cells, p<0.05) after AngII infusion during E (Fig 5).
Figure 5.
Treatment with a sub-pressor dose of Aldo during induction (I) enhanced subsequent AngII-induced Fra expression (indicating neuronal excitation) in the SFO and PVN as compared to those treated with saline during I. Representative fluorescence microscopic images of sections from all groups of rats (Fig 5A). Fig 5B. Quantification of Fra positive neurons in the SFO and PVN of each group. E, expression; S, saline. (* p<0.05 vs. I-S+E-S, # p<0.05 vs. I-Aldo+E-S or I-S+E-AngII)
Discussion
Previous studies from our laboratory have shown that AngII and Aldo interact in the CNS in a mutually cooperative manner to induce hypertension3 and that low doses of AngII acting in the brain sensitizes the hypertensive response to a subsequently administered, slow pressor dose of AngII.12 The present studies investigated whether a conditioning treatment with Aldo acts to enhance the hypertensinogenic effects of AngII and whether this is mediated through the CNS. The major results of these studies provide evidence indicating that prior systemic treatment with a sub-pressor dose of Aldo cross-sensitizes the pressor actions of AngII. Aldo-AngII cross-sensitization can also be produced when Aldo is administered icv. This observation, plus the demonstration that MR antagonist administered icv during I blocked the cross-sensitizing action of systemic Aldo, indicates that activation of MR is necessary for Aldo-AngII cross-sensitization and that the sensitization process is likely to involve the CNS. Furthermore, the cross-sensitizing action of Aldo is associated with increased neuronal activation as demonstrated by elevated levels of immunoreactivity for immediate early gene protein (i.e., Fra-like fluorescence) and elevated mRNA expression of components of the brain RAAS in the LT and PVN. Also studies of SFO neurons demonstrated that conditioning cultured cells with Aldo enhanced the neuronal responses to AngII. Taken together, these and previous results12 indicate that sub-pressor levels of either circulating Aldo or AngII can induce long-term changes in CNS function that increase the vulnerability to hypertension.
Forebrain structures located along the LT and hypothalamic nuclei, particularly the PVN, play important roles in the long-term regulation of BP and body fluid homeostasis.13 Many forebrain regions have been shown to contain both MR and AT1-R,14,15 and such a close proximity of these receptors raises the possibility that there are common cellular sites of interaction between mineralocorticoids and AngII. Using iontophoretic and electrophysiological techniques, Thornton and colleagues investigated in vivo synergistic actions produced by peripheral priming doses of DOCA followed at a later time by centrally administered AngII. They found that DOCA pretreatment elicited a long-term enhancement of MnPO neuronal activity and increased the sensitivity to AngII.11 In in vitro studies investigating the interactions of Aldo and AngII on vascular smooth muscle cells, pretreatment with Aldo decreased the threshold for increases in [Ca2+]i elicited by the octapeptide.16 Employing a similar in vitro strategy in the present studies, we found that cultures of SFO neurons conditioned with Aldo displayed an increased number of neurons responsive to AngII and that these neurons showed a greater increase in [Ca2+]i in response to AngII. This finding is consistent with the observations of others11 and the idea that Aldo and AngII can act on the same forebrain neurons to synergize and cross-sensitize each other’s actions.
All components of the RAAS involving both Aldo and AngII synthesis and receptors are expressed within the CNS.17,18 In the periphery renin production is generally considered to be rate limiting for the RAAS. However, the regulation of AGT, ACE and the number and affinity of AT-R are also important for regulating activity of this system.18,19 Increased renin and AGT expression in the brain results in an increase in AngII production, which is associated with elevated thirst and salt intake.20 Aldo has also been shown to upregulate AGT through an AngII-dependent pathway to enhance ischemia-induced neovascularization.21 In our current investigations where Aldo was delivered during I, LT tissue collected at the end of D showed elevated expression of ACE1, AT1-R and MR mRNAs. In addition to the elevated expression of these three mRNAs, Aldo pretreatment also increased AGT expression in the LT. Interestingly, at the end of D there were no changes in expression of mRNA for components of the RAAS in the PVN with Aldo given during I. These results are consistent with our previous study using a low dose of AngII as the sensitizing pretreatment.12 Taken together, these observations suggest that there are LT RAAS components that are common mediators of sensitization/cross sensitization processes. The capacity of AngII or Aldo to mutually up-regulate their receptors may be indicative of actions within central positive feed-forward systems which are responsible for sensitization/cross-sensitization and which can accelerate the onset and rate of development of hypertension.
Pretreatment with Aldo not only increased expression of several RAAS pressor-related mRNAs in the LT after D, but also enhanced renin, AGT, AT1-R and ACE1 expression in the LT and AGT, and ACE1 expression in the PVN in I-Aldo+E-AngII rats. Because BP was higher in I-Aldo+E-ANGII rats as compared to I-S+E-ANGII animals, it also cannot be ruled out that the observed increase in mRNA expression was not due to the increased elevation of BP. However, it seems reasonable to consider that there was an accelerated increase in the activity of the brain RAAS as a result of Aldo cross-sensitization. One explanation for the apparent enhancement of the hypertensive response is the possibility that the act of terminating Aldo administration itself might actually be the cause of sensitization. However, this does not seem to be as parsimonious as the interpretation that it was the initial exposure to Aldo during I that induced persistent CNS molecular and structural changes. This view is one that is most consistent with sensitization studies in other fields of neuroscience (drug addiction, learning and memory, long term potentiation, pain, enhancement of salt appetite, etc.). The simple reason we chose the experimental design of “removing” Aldo at the end of the induction period was to be assured that we had eliminated the possibility that exogenous Aldo was still available at the time E began.
The complete set of enzymes for the synthetic cascade of Aldo has been documented to be present in the brain by demonstrations of mRNA expression and of AS activity.13 Peripheral or icv administration of either MR antagonists or specific AS inhibitors significantly attenuates AngII-induced kidney injury and hypertension, suggesting that an increase in the local synthesis of Aldo acting on brain MR can generate increased AT1-R.3,4,22 Although such functional studies indicate that increased endogenous brain Aldo and MR might enhance the pressor effects of AngII, previous studies did not report evidence of increased AS expression or elevated Aldo.4,23 These prior studies attempted to find increased AS expression by isolating mRNA from the entire hypothalamus. This procedure may have resulted in diluting AS mRNA because within the entire hypothalamus there may be only a small amount of AS localized to specific nuclei. In the present studies we microdissected the PVN from the entire hypothalamus and in addition collected the LT region in order to obtain greater concentrations of critical mRNA from key tissues, thereby permitting better quantification of mRNA expression. We found that Aldo given during I induced a small increase in MR expression in the LT when measured at the end of D, and that there were marked increases in AS and MR mRNA expression in both the LT and PVN in I-S+E-AngII treated rats. Interestingly I-Aldo+E-AngII animals did not evidence increased AS or MR expression beyond that seen in I-S+E-AngII treated rats.
Recently identified components of the RAAS, ACE2 and Ang(1–7)/Mas-R, have been shown to have important roles in the control of BP and sympathetic activity associated with their pressor attenuating actions.24 Xia and colleagues reported that AT1-R in the brain of chronically hypertensive mice overexpressing hRen and hAGT have reduced ACE2 activity, but not ACE2 expression.25 Aldo has also been shown to down-regulate ACE2 expression in rat cardiocytes and in the kidney.26,27 MR blockade increases ACE 2 activity and expression in congestive heart failure patients and in experimental animals.28 In the present study, we found that Aldo alone or systemic AngII infusion by itself did not affect ACE2 expression in the LT or PVN. However, in I-Aldo+E-AngII treated rats there was a decrease in ACE2 expression in the LT, but an increase in the PVN. This dissociation of ACE2 mRNA expression in the LT versus PVN indicates that there is site specific regulation of ACE2 in cardiovascular-related forebrain regions and that this may represent a shift in homeostatic equilibrium when transitioning from a normotensive to a prohypertensive state. The increased ACE2 expression in the PVN may reflect the response of counter regulatory mechanisms attempting to correct the enhanced AngII-induced hypertension. Previous studies have shown that blockade of endogenous Ang(1–7) in the PVN reduced renal sympathetic tone,29 and that overexpression of ACE2 in brain reduced BP and counteracted most of the other typical actions of AngII.30
Similar to the effects on ACE2 expression, there was enhanced AT2-R expression in the PVN in response to systemic AngII infusion following sensitization with Aldo. These results are consistent with our recent studies on AngII sensitization.12 AT2-R have been proposed to act as functional antagonists to counter regulate the effects of AngII on AT1-R.31 Either overexpression or selective activation of central AT2-R decreased BP and plasma norepinephrine levels probably by reducing sympathetic outflow.32,33 The enhanced central AT2-R expression seen in the present study might reflect activation of inhibitory mechanisms that buffer the actions of AngII acting on AT1-R. However, recent studies have shown that AT2-R is involved in AngII-stimulated Aldo release. In rat adrenal glomerulosa or in human adrenals with an Aldo-producing adenoma, AT2-R agonists increased Aldo secretion and this response was completely inhibited by the AT2-R antagonist, PD123319.34,35 If there is a similar relationship in the brain between AT2-R and Aldo, enhanced AT2-R expression might be expected to increase brain Aldo production to facilitate increased BP. Further studies will be necessary to clarify the roles of AngII/AT2-R and ACE2/Ang(1–7)/Mas-R in Aldo and AngII sensitization/cross-sensitization processes.
Perspectives
Both earlier studies and the present experiments demonstrate that preconditioning with non-pressor infusions of either AngII or Aldo can sensitize/cross-sensitize the hypertensinogenic actions of AngII. These findings indicate that the AngII sensitizing and Aldo cross-sensitizing effects are sustained and are associated with maintained changes in the expression of “pressor” components of the brain RAAS. In particular ACE1, AT1-R and AS expression are increased in structures of the LT. Such enduring preconditioning effects must be mediated by some form of neuroplasticity which involves a cascade of cellular and molecular events in the coupling between I and E. Further experiments will be performed to determine the role of some particular factors (e.g. brain-derived neurotropic factors) or pathways in the neuroplasticity, which mediates the sensitizing effects induced by Aldo or AngII.
Since circulating Aldo can access many brain regions, and evidence indicates that Aldo may be synthesized de novo in the CNS, increased mineralocorticoid levels in the brain may lead to generation of a hypertensinogenic viscous cycle by accelerating the activation of pressor components of the central RAAS. Regardless of the source of Aldo, the application of MR blockers with systemic and central actions in conjunction with AngII antagonists provides an important rationale for a strategy to reduce the AngII and Aldo CNS sensitizing/cross-sensitizing effects that may contribute to and accelerate the onset and progression of hypertension.
Supplementary Material
Novelty and Significance.
What is New?
These studies demonstrate that exposure to systemic or central, non-pressor doses of Aldo is capable of sensitizing hypertensinogenic responses to AngII.
What is Relevant?
The demonstration of cross-sensitization between the central actions of Aldo and AngII indicates that CNS neuroplasticity is likely to play an important role in the pathogenesis and progression of some forms of hypertension.
Summary
The study indicates that Aldo acts on the brain to cross-sensitize the hypertensive response to AngII and that cross-sensitization is associated with maintained altered expression of RAAS within components of a forebrain cardiovascular control network.
Acknowledgments
Sources of Funding
This work was supported by the NIH grants HL-14388, HL-98207, and MH-80241.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: None
References
- 1.Huang BS, Leenen FH. Mineralocorticoid actions in the brain and hypertension. Curr Hypertens Rep. 2011;13:214–220. doi: 10.1007/s11906-011-0192-0. [DOI] [PubMed] [Google Scholar]
- 2.Min LJ, Mogi M, Iwai M, Horiuchi M. Signaling mechanisms of angiotensin II in regulating vascular senescence. Ageing Res Rev. 2009;8:113–121. doi: 10.1016/j.arr.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 3.Xue B, Beltz TG, Yu Y, Guo F, Gomez-Sanchez CE, Hay M, Johnson AK. Central interactions of aldosterone and angiotensin II in aldosterone- and angiotensin-induced hypertension. Am J Physiol Heart Circ Physiol. 2011;300:H555–H564. doi: 10.1152/ajpheart.00847.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang BS, Ahmadi S, Ahmad M, White RA, Leenen FH. Central neuronal activation and pressor responses induced by circulating ANGII: role of the brain aldosterone-"ouabain" pathway. Am J Physiol Heart Circ Physiol. 2010;299:H422–H430. doi: 10.1152/ajpheart.00256.2010. [DOI] [PubMed] [Google Scholar]
- 5.Zhang ZH, Yu Y, Kang YM, Wei SG, Felder RB. Aldosterone acts centrally to increase brain renin-angiotensin system activity and oxidative stress in normal rats. Am J Physiol Heart Circ Physiol. 2008;294:H1067–H1074. doi: 10.1152/ajpheart.01131.2007. [DOI] [PubMed] [Google Scholar]
- 6.Huang BS, White RA, Jeng AY, Leenen FH. Role of central nervous system aldosterone synthase and mineralocorticoid receptors in salt-induced hypertension in Dahl salt-sensitive rats. Am J Physiol Regul Integr Comp Physiol. 2009;296:R994–R1000. doi: 10.1152/ajpregu.90903.2008. [DOI] [PubMed] [Google Scholar]
- 7.Yu Y, Wei SG, Zhang ZH, Gomez-Sanchez E, Weiss RM, Felder RB. Does aldosterone upregulate the brain renin-angiotensin system in rats with heart failure? Hypertension. 2008;51:727–733. doi: 10.1161/HYPERTENSIONAHA.107.099796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sakai RR, Fine WB, Epstein AN, Frankmann SP. Salt appetite is enhanced by one prior episode of sodium depletion in the rat. Behav Neurosci. 1987;101:724–731. doi: 10.1037//0735-7044.101.5.724. [DOI] [PubMed] [Google Scholar]
- 9.Moellenhoff E, Blume A, Culman J, Chatterjee B, Herdegen T, Lebrun C, Unger T. Effect of repetitive icv injections of ANGII on c-Fos and AT(1)-receptor expression in the rat brain. Am J Physiol Regul Integr Comp Physiol. 2001;280:R1095–R1104. doi: 10.1152/ajpregu.2001.280.4.R1095. [DOI] [PubMed] [Google Scholar]
- 10.Zhang DM, Stellar E, Epstein AN. Together intracranial angiotensin and systemic mineralocorticoid produce avidity for salt in the rat. Physiol Behav. 1984;32:677–681. doi: 10.1016/0031-9384(84)90325-1. [DOI] [PubMed] [Google Scholar]
- 11.Thornton SN, Nicolaidis S. Long-term mineralocorticoid-induced changes in rat neuron properties plus interaction of aldosterone and ANGII. Am J Physiol. 1994;266:R564–R571. doi: 10.1152/ajpregu.1994.266.2.R564. [DOI] [PubMed] [Google Scholar]
- 12.Xue B, Zhang Z, Johnson RF, Johnson AK. Sensitization of slow pressor angiotensin II (AngII)-initiated hypertension: induction of sensitization by prior AngII treatment. Hypertension. 2012;59:459–466. doi: 10.1161/HYPERTENSIONAHA.111.185116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johnson AK, Gross PM. Sensory circumventricular organs and brain homeostatic pathways. FASEB J. 1993;7:678–686. doi: 10.1096/fasebj.7.8.8500693. [DOI] [PubMed] [Google Scholar]
- 14.Gomez-Sanchez EP, Gomez-Sanchez CE. Central regulation of blood pressure by the mineralocorticoid receptor. Mol Cell Endocrinol. 2012;350:289–298. doi: 10.1016/j.mce.2011.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lenkei Z, Palkovits M, Corvol P, Llorens-Cortes C. Distribution of angiotensin type-1 receptor messenger RNA expression in the adult rat brain. Neuroscience. 1998;82:827–841. doi: 10.1016/s0306-4522(97)00328-x. [DOI] [PubMed] [Google Scholar]
- 16.Wehling M, Neylon CB, Fullerton M, Bobik A, Funder JW. Nongenomic effects of aldosterone on intracellular Ca2+ in vascular smooth muscle cells. Circ Res. 1995;76:973–979. doi: 10.1161/01.res.76.6.973. [DOI] [PubMed] [Google Scholar]
- 17.Gomez-Sanchez EP, Ahmad N, Romero DG, Gomez-Sanchez CE. Is aldosterone synthesized within the rat brain? Am J Physiol Endocrinol Metab. 2005;288:E342–E346. doi: 10.1152/ajpendo.00355.2004. [DOI] [PubMed] [Google Scholar]
- 18.Lavoie JL, Sigmund CD. Minireview: overview of the renin-angiotensin system- an endocrine and paracrine system. Endocrinology. 2003;144:2179–2183. doi: 10.1210/en.2003-0150. [DOI] [PubMed] [Google Scholar]
- 19.Bohlender J, Ménard J, Ganten D, Luft FC. Angiotensinogen concentrations and renin clearance : implications for blood pressure regulation. Hypertension. 2000;35:780–786. doi: 10.1161/01.hyp.35.3.780. [DOI] [PubMed] [Google Scholar]
- 20.Sakai K, Agassandian K, Morimoto S, Sinnayah P, Cassell MD, Davisson RL, Sigmund CD. Local production of angiotensin II in the subfornical organ causes elevated drinking. J Clin Invest. 2007;117:1088–1095. doi: 10.1172/JCI31242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Michel F, Ambroisine ML, Duriez M, Delcayre C, Levy BI, Silvestre JS. Aldosterone enhances ischemia-induced neovascularization through angiotensin II-dependent pathway. Circulation. 2004;109:1933–1937. doi: 10.1161/01.CIR.0000127112.36796.9B. [DOI] [PubMed] [Google Scholar]
- 22.Lea WB, Kwak ES, Luther JM, Fowler SM, Wang Z, Ma J, Fogo AB, Brown NJ. Aldosterone antagonism or synthase inhibition reduces end-organ damage induced by treatment with angiotensin and high salt. Kidney Int. 2009;75:936–944. doi: 10.1038/ki.2009.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ye P, Kenyon CJ, Mackenzie SM, Nichol K, Seckl JR, Fraser R, Connell JM, Davies E. Effects of ACTH, dexamethasone, and adrenalectomy on 11beta-hydroxylase (CYP11B1) and aldosterone synthase (CYP11B2) gene expression in the rat central nervous system. J Endocrinol. 2008;196:305–311. doi: 10.1677/JOE-07-0439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu P, Sriramula S, Lazartigues E. ACE2/ANG-(1–7)/Mas pathway in the brain: the axis of good. Am J Physiol Regul Integr Comp Physiol. 2011;300:R804–R817. doi: 10.1152/ajpregu.00222.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xia H, Feng Y, Obr TD, Hickman PJ, Lazartigues E. Angiotensin II type 1 receptor-mediated reduction of angiotensin-converting enzyme 2 activity in the brain impairs baroreflex function in hypertensive mice. Hypertension. 2009;53:210–216. doi: 10.1161/HYPERTENSIONAHA.108.123844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yamamuro M, Yoshimura M, Nakayama M, Abe K, Sumida H, Sugiyama S, Saito Y, Nakao K, Yasue H, Ogawa H. Aldosterone, but not angiotensin II, reduces angiotensin converting enzyme 2 gene expression levels in cultured neonatal rat cardiomyocytes. Circ J. 2008;72:1346–1350. doi: 10.1253/circj.72.1346. [DOI] [PubMed] [Google Scholar]
- 27.Fukuda S, Horimai C, Harada K, Wakamatsu T, Fukasawa H, Muto S, Itai A, Hayashi M. Aldosterone-induced kidney injury is mediated by NFκB activation. Clin Exp Nephrol. 2011;15:41–49. doi: 10.1007/s10157-010-0373-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Keidar S, Gamliel-Lazarovich A, Kaplan M, Pavlotzky E, Hamoud S, Hayek T, Karry R, Abassi Z. Mineralocorticoid receptor blocker increases angiotensin-converting enzyme 2 activity in congestive heart failure patients. Circ Res. 2005;97:946–953. doi: 10.1161/01.RES.0000187500.24964.7A. [DOI] [PubMed] [Google Scholar]
- 29.Gomes da Silva AQ, Sousa dos Santos RA, Peliky Fontes MA. Blockade of endogenous angiotensin-(1–7) in the hypothalamic paraventricular nucleus reduces renal sympathetic tone. Hypertension. 2005;46:341–348. doi: 10.1161/01.HYP.0000179216.04357.49. [DOI] [PubMed] [Google Scholar]
- 30.Feng Y, Xia H, Cai Y, Halabi CM, Becker LK, Santos RA, Speth RC, Sigmund CD. Lazartigues E: Brain-selective overexpression of human Angiotensin-converting enzyme type 2 attenuates neurogenic hypertension. Circ Res. 2010;106:373–382. doi: 10.1161/CIRCRESAHA.109.208645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Masaki H, Kurihara T, Yamaki A, Inomata N, Nozawa Y, Mori Y, Murasawa S, Kizima K, Maruyama K, Horiuchi M, Dzau VJ, Takahashi H, Iwasaka T, Inada M, Matsubara H. Cardiac-specific overexpression of angiotensin II AT2 receptor causes attenuated response to AT1 receptor-mediated pressor and chronotropic effects. J Clin Invest. 1998;101:527–535. doi: 10.1172/JCI1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gao J, Zhang H, Le KD, Chao J, Gao L. Activation of central angiotensin type 2 receptors suppresses norepinephrine excretion and blood pressure in conscious rats. Am J Hypertens. 2011;24:724–730. doi: 10.1038/ajh.2011.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gao L, Wang W, Wang W, Li H, Sumners C, Zucker IH. Effects of angiotensin type 2 receptor overexpression in the rostral ventrolateral medulla on blood pressure and urine excretion in normal Rats. Hypertension. 2008;51:521–527. doi: 10.1161/HYPERTENSIONAHA.107.101717. [DOI] [PubMed] [Google Scholar]
- 34.Yatabe J, Yoneda M, Yatabe MS, Watanabe T, Felder RA, Jose PA, Sanada H. Angiotensin III stimulates aldosterone secretion from adrenal gland partially via angiotensin II type 2 receptor but not angiotensin II type 1 receptor. Endocrinology. 2011;152:1582–1588. doi: 10.1210/en.2010-1070. [DOI] [PubMed] [Google Scholar]
- 35.Tanabe A, Naruse M, Naruse K, Yoshimoto T, Tanaka M, Mishina N, Imaki T, Sugaya K, Miyazaki H, Demura H. Angiotensin II receptor subtype in human adrenal glands. Nippon Rinsho. 1999;57:1042–1048. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.