Abstract
Historically, bipolar disorder and schizophrenia have been considered distinct disorders with different etiologies. Growing evidence suggests that overlapping genetic influences contribute to risk for these disorders and that each disease is genetically heterogeneous. Using cluster analytic methods, we empirically identified homogeneous subgroups of patients, their relatives, and controls based on distinct neurophysiologic profiles. Seven phenotypes were collected from two independent cohorts at two institutions. K-means clustering was used to identify neurophysiologic profiles. In the analysis of all participants, three distinct profiles emerged: “globally impaired”, “sensory processing”, and “high cognitive”. In a secondary analysis, restricted to patients only, we observed a similar clustering into three profiles. The neurophysiological profiles of the SZ and BPD patients did not support the DSM diagnostic distinction between these two disorders. Smokers in the globally impaired group smoked significantly more cigarettes than those in the sensory processing or high cognitive groups. Our results suggest that empirical analyses of neurophysiological phenotypes can identify potentially biologically relevant homogenous subgroups independent of diagnostic boundaries. We hypothesize that each neurophysiology subgroup may share similar genotypic profiles, which may increase statistical power to detect genetic risk factors.
Keywords: bipolar disorder, schizophrenia, K-means clustering, neurophysiologic profiles
1. INTRODUCTION
Historically, bipolar disorder (BPD) and schizophrenia (SCZ) have been considered distinct nosological entities, with each disorder thought to have a different etiology and pathogenesis. This distinction, known as the “Kraepelinian dichotomy,” (Kraepelin, 1919) has persisted in the current version of the DSM diagnostic classification system. The validity of maintaining such a distinction between SCZ and BPD has been called into question (Craddock et al., 2005). Both disorders are phenotypically and genetically complex, and the clinical boundaries between them can often be blurred (Craddock et al., 2005). Psychosis, for example, is a core feature of SCZ and is common in BPD. Individuals within either diagnostic category typically show highly complex and heterogeneous clinical, behavioral and neurocognitive profiles (Gottesman and Gould, 2003; Kremen et al., 2004).
Growing evidence from epidemiology (Lichtenstein et al., 2009), molecular genetics (Craddock et al., 2005) and cognitive neuroscience (Thaker, 2008) suggests that partially overlapping genetic influences contribute to risk for SCZ and BPD, and that each disease is genetically heterogeneous (Harrison and Weinberger, 2005). Recent genome-wide association analyses (GWAS) support the ideas that multiple genes influence risk for both SCZ and BPD and that there is overlap between the genes that contribute to risk for each disorder (Green et al., 2009). Consistent with such shared genetic susceptibility, several neurophysiological and cognitive endophenotypes have been observed in patients with both disorders (Hall et al., 2008; Muir et al., 1991; O'Donnell et al., 2004a; Salisbury et al., 1999; Spencer et al., 2008b) and in their clinically unaffected relatives (Hall et al., 2007; Shenton et al., 1989; Solovay et al., 1987). On the other hand, each disorder is also associated with functional impairments and genetic risk factors that are relatively specific (Benes, 2010; Hall et al., 2009a; Javitt et al., 2008a; O'Donnell et al., 2004a; Salisbury et al., 1998).
The phenotypic and genetic heterogeneity within a diagnostic category and the phenotypic and genetic overlap between diagnostic categories suggest that stratifying individuals on the basis of diagnosis may not optimally identify homogeneous subgroups or be the most powerful strategy in genetic association studies. Even the distinction between affected cases and unaffected controls may not be straightforward at a biological level. Subjects traditionally classified as unaffected, such as some relatives and controls, typically possess highly complex and heterogeneous behavioral and neurocognitive profiles, just as individuals with a diagnosis do. For example, a significant proportion of unaffected relatives of SCZ or BPD exhibit neurophysiological and/or cognitive traits that are associated with the diseases (e.g., endophenotypes) (Freedman et al., 2000; Gottesman and Gould, 2003; Matthysse et al., 1992; Turetsky et al., 2007). Among unrelated control subjects, some may exhibit neurocognitive phenotypes that are indistinguishable from those observed in subgroups of SCZ or BPD patients. It has been proposed that an observed discontinuity in affection status is, in fact, the result of arbitrarily classifying people by kind rather than by degree, and that there is a continuously distributed genotype underlies an artificially dichotomized phenotype, as suggested by a “liability threshold model” (Falconer and Mackay, 1996; Neale and Kendler, 1995). That is, both affected and unaffected individuals may be part of the same distribution of liability for the disorder. Unaffected individuals may carry susceptibility genes without manifesting clinical symptoms due to low penetrance or failure to exceed a critical threshold of genetic risk factors. Hence, classifying individuals based on empirically derived neurophysiological profiles can potentially identify biologically relevant homogenous subgroups independent of clinical diagnosis or affection status. More phenotypically homogeneous groups, in turn, may share similar genotype profiles leading to increased statistical power to detect genetic risk factors (Allison et al., 1998).
In this study, we explored the use of an unsupervised cluster analytical approach to extract neurophysiological profiles in patients with DSM-IV diagnoses of SCZ, schizoaffective [SA], or BPD, their unaffected relatives, and control subjects. Two independent datasets, each having the same neurophysiological phenotypes, were collected at two research institutions. The various domains of brain function ranged from the early pre-attentive stage of information processing to higher complex cognitive processes, and included P50 sensory gating, the early auditory gamma band response, mismatch negativity (MMN), and the N1, P2, and P3 ERP components. P50 sensory gating was used to measure inhibitory mechanisms thought to be crucial for protecting the brain from information overload (Freedman et al., 1991). Sensory gating deficit has been proposed as an endophenotype for both SCZ and BPD (Hall et al., 2007; Schulze et al., 2007). EAGBR was used to assess basic brain functions associated with auditory perception (Javitt et al., 2008a). Both SCZ and BPD patients show reduced early evoked GBR (Hall et al., 2011b; Hall et al., 2009b; Leicht et al., 2010; O'Donnell et al., 2004a; Roach and Mathalon, 2008), although this finding has not been confirmed in all studies (Gallinat et al., 2004; Spencer et al., 2008a). Early sensory processing at the level of auditory cortex was assessed with the N1 ERP (Salisbury et al., 2010) and MMN (Salisbury et al., 2007a; Salisbury et al., 2002). Reduced N1 and MMN ERPs were found in SCZ but not in patients with BPD (Hall et al., 2009a; Salisbury et al., 2010; Salisbury et al., 2007a), although some studies found reduced MMN in both disorders (Jahshan et al., 2012; Kaur et al., 2012). Higher-order cognitive processes associated with attention, working memory, and speed of information processing were assessed by the P2 and P3 ERP components (Donchin and Coles, 1988). Patients with both disorders have impaired central P3 ERPs but P2 ERP deficit has been documented in patients with SCZ not with BPD (O'Donnell et al., 2004b).
The primary goal was to examine whether neurophysiologic profiles could be defined that capture underlying phenotypic structure across diagnostic groups. Cluster analysis was used to empirically identify homogeneous subgroups of individuals who share similar neurophysiological profiles, regardless of diagnostic and affection status. We then compared clinical/demographic features of the profiles. We also conducted a secondary analysis restricted to the patient groups to examine whether neurophysiologic profiles support the DSM diagnostic distinction between SCZ and BPD.
2. Materials and methods
Subjects
Two independent samples were collected at two research institutions. The first sample was obtained from McLean Hospital and had a total of 120 individuals (Hall et al., 2010). Participants included 60 individuals with diagnosis of either SCZ (n = 20), SA (n = 30), or psychotic BPD (n = 10), 25 of their non-psychotic first-degree relatives (10 SCZ, 14 SA, 1 BPD), and 35 unrelated control participants with no family history of psychosis. Only relatives who did not meet diagnostic criteria for a lifetime diagnosis of psychotic disorder, BPD without psychotic features, or a SZ spectrum personality disorder were included in this study. Controls met the same inclusion criteria as relatives and also did not have a first- or second-degree relative with a history of psychosis, psychiatric hospitalization, or suicide. All SCZ and SA patients except two were taking antipsychotic medication at the time of testing. SZ and SA patients did not differ in mean daily dose in chlorpromazine (CPZ) equivalents (SZ: 676.5mg [SD= 570]; SA: 571.4mg [SD=427], P=.50). Of the BPD patients, one was unmedicated. Three were on a single mood stabilizer and others were on combinations of mood stabilizers, antipsychotics and antidepressants. Patients in both samples were sufficiently stable to participate on an outpatient basis. This study was approved by the McLean Hospital Institutional Review Board. The second sample was obtained from the Maudsley Hospital at the Institute of Psychiatry, London (Hall et al., 2007; Hall et al., 2008). A total of 349 subjects were included in this cohort, consisting of 39 SCZ patients (15 pairs of identical [MZ] twins concordant for SCZ, and 9 SCZ from MZ twins discordant for SCZ), 9 unaffected co-twins of SCZ, 58 psychotic BPD patients (6 pairs of MZ twins concordant for BPD, 10 BPD from MZ twin pairs discordant for BPD, 36 BPD patients from 30 families), 48 non-psychotic first-degree relatives, and 195 control participants (46 MZ twins pairs, 32 DZ twin pairs, and 39 unrelated singletons. Relatives and controls in the Maudsley sample met the same inclusion criteria mentioned above. All SCZ patients were taking antipsychotic medication (mean CPZ equivalent = 643.2mg [SD= 392]). Of the BPD patients, fourteen had been unmedicated for at least four weeks. Nine were on a single mood stabilizer and the others were on combinations of mood stabilizers, antipsychotics and antidepressants. The study was approved by the U.K. Multi-Centre Research Ethics Committee. Written informed consent was obtained from all participants. Demographic characteristics of the two samples are presented in Table 1.
Table 1.
Socio-demographic Characteristics of Subject Groups
| Samples | McLean | Maudsley | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| SCZ 1 Patients |
SCZ Relatives |
BPD 2 Patients |
BPD Relatives |
Controls | SCZ Patients |
SCZ Relatives |
BPD Patients |
BPD Relatives |
Controls | |
| (n=29) | (n=16) | (n=31) | (n=9) | (n=35) | (n=39) | (n=9) | (n=58) | (n=48) | (n=195) | |
|
Age, yrs |
43.4 | 51.8 | 45.0 | 51.0 | 37.9 | 36.5 | 31.6 | 42.4 | 42.8 | 37.3 |
| (11.3) | (10.8) | (9.7) | (15.8) | (14.2) | (10.9) | (9.3) | (11.8) | (11.9) | (12.1) | |
| 21–63 | 29–66 | 25–61 | 20–65 | 19–61 | 22–61 | 23–52 | 22–61 | 21–61 | 18–60 | |
|
Female, N (%) |
11 (37.9) |
14 (87.5) |
10 (32.3) |
8 (88.9) |
20 (57.1) |
10 (25.6) |
4 (44.4) |
38 (65.5) |
23 (47.9) |
136 (69.7) |
|
Age of onset |
25.4 (8.2) |
N/A | 23.3 (6.3) |
N/A | N/A | 22.2 (8.2) |
N/A | 22.1 (6.3) |
N/A | N/A |
|
Education, yrs |
14.5 (2.3) |
16.2 (2.8) |
14.5 (2.0) |
15.8 (2.7) |
14.4 (2.5) |
13.1 (2.7) |
14.0 (2.7) |
14.3 (3.2) |
15.1 (3.0) |
14.8 (2.4) |
|
Current smoker, N (%) |
6 (25.0) |
2 (13.3) |
12 (46.1) |
0 (0) | 4 (11.8) |
9 (25.7) |
2 (28.6) |
19 (33.3) |
9 (19.5) |
36 (18.4) |
|
No. cigarettes/ day, if smoker |
30.3 (19.6) |
7.5 (2.1) |
19.8 (10.5) |
0 (0) |
5.8 (4.9) |
20.7 (12.4) |
5.5 (6.4) |
19.1 (12.2) |
8.9 (4.7) |
9.7 (5.2) |
Note: Values are means (SD) unless otherwise indicated.
Sample includes patients with schizophrenia (SCZ) or schizoaffective (SA) depressed type.
Sample includes patients with bipolar disorder (BPD) or SA bipolar type.
Clinical assessments
Detailed structured diagnostic interviews were performed for all participants using the Structured Clinical Interview for DSM-IV (SCID), the Schedule for Affective Disorders and Schizophrenia – Lifetime Version (SADS-L; (Spitzer and Endicott, 1978)), or the Schedule for Clinical Assessment in Neuropsychiatry (SCAN) version 2.1. In the Maudsley sample, information regarding family history of psychiatric disorders in family members was collected from each participant using the Family Interview for Genetic Studies and from medical records. In the McLean sample, psychiatric diagnosis information was obtained from each participant about other members of the immediate and extended families using the Family Informant Schedule & Criteria (FISC)(Mannuzza et al., 1985). The following exclusion criteria applied to all participants: lack of fluency in English, history of serious head trauma or organic brain disease, history of substance abuse or dependence during the preceding 12 months or previous chronic dependence, and hearing loss verified by audiometry. All participants had an estimated verbal IQ of 80 or greater based on the vocabulary subtest of the Wechsler Adult Intelligence Scale.
Phenotypic measures
All participants completed the following tasks: 1) an auditory dual-click paradigm for eliciting P50 sensory gating and the early auditory gamma band response (EAGBR) to the S1 stimulus; 2) an auditory “odd-ball” paradigm for eliciting the N1, P2, and P3 ERP components; 3) an ignore MMN odd-ball task, with either both pitch and duration deviants (McLean sample) or only duration deviants (Maudsley sample). Subjects were not allowed to smoke a minimum of 40 minutes before data collection.
McLean Hospital--The EEG was recorded with Neuroscan Synamp amplifiers (0.01–100 Hz, 500 Hz digitization rate) with sintered Ag/AgCl electrodes in an electrode cap at 60 scalp sites, the nose tip, and the left mastoid, referenced to the right mastoid. The forehead (AFz) served as ground. Bipolar vertical and horizontal electro-oculograms were recorded from electrodes above and below the right eye (VEOG) and at the left and right outer canthi (HEOG). Electrode impedances were below 5 kΏ.
Maudsley Hospital---The EEG was recorded on a Nihon Kohden PV-441A machine (0.03–120 Hz, 500 Hz digitization) using silver/silver chloride electrodes from 16 scalp sites according to the 10/20 International System (Jasper, 1958). All stimuli were generated and presented using the Neuroscan STIM system. The forehead (AFz) served as ground and the reference was on the left mastoid. Bipolar vertical and horizontal electro-oculograms were recorded from electrodes above and below the left eye (VEOG) and at the left and right outer canthi (HEOG). Electrode impedances were below 6 kΏ.
Dual-Click paradigm
McLean sample---160 pairs of identical click stimuli (5-ms duration; 2-ms rise/fall; 500-ms interclick interval; 10-s inter-trial interval) were presented in 4 blocks (40 pairs per block). Stimulus intensity was adjusted to 50 dB above each individual’s hearing threshold, producing a stimulus at a sound level of 80dB . Maudsley Sample---120 pairs of identical click stimuli (5-ms duration; 2-ms rise/fall; 500-ms inter-click interval; 10-s inter-trial interval) were presented in 4 blocks (30 pairs per block). Stimulus intensity was adjusted to 43 dB above each individual’s hearing threshold, producing a stimulus at the identical a sound level of 80dB.
P50 Sensory Gating. Identical signal processing procedures were applied to McLean and Maudsley samples to extract P50 ERP waves. Signal processing was performed off-line using NEUROSCAN software (4.3). EEG signals were segmented into epochs (–100 to 400 ms), filtered (1-Hz high-pass filter), and corrected for baseline values using the 100-ms pre-stimulus interval. Epochs with activity exceeding 35 µV in the Cz or electro-oculography channel between 0 and 75 ms post-stimulus were automatically rejected. Epochs were averaged separately for the S1 and S2 waveforms, digitally filtered (10-Hz high-pass filter), and smoothed (by using a 7-point moving average applied twice). P50 event-related potentials are reported at the Cz site. For the S1 response, the most prominent peak 40–80 ms post-stimulus was selected as the P50 peak. The preceding negative trough was used to calculate the amplitude. For the S2 response, the positive peak with the latency closest to that of the conditioning P50 peak was selected, and its amplitude was determined as for the S1 wave. P50 sensory gating was calculated as (S2/S1)×100 (Hall et al., 2006a; Hall et al., 2010).
Evoked Gamma Band Response (GBR) to S1 stimuli. Signal processing was performed off-line using Brain Vision Analyzer software (Brain Products, Munich, Germany). EEG signals were first filtered between 10 and 80 Hz, segmented into epochs from −100 to 400 ms relative to stimulus onset, and then baseline corrected using the 100-ms pre-stimulus interval. Epochs containing artifacts ±50 µV at Fz, Cz, or Pz were then removed. Time-frequency analysis was computed using Matlab in McLean sample (Hall et al., 2010) and Excel in Maudsley sample (Hall et al., 2011a).
Auditory Oddball paradigm
McLean sample---400 binaural tones (73 dB; 50-msec duration, 5 ms rise/fall times); 15% target tones (1500 Hz) and 85% standard tones (1000 Hz) were presented. Participants were instructed to silently count target tones. Maudsley Sample---400 binaural tones (80 dB; 20-msec duration, 5 ms rise/fall times); 20% target tones (1500 Hz) and 80% standard tones (1000 Hz) were presented. Participants pressed a button in response to target tones (Hall et al., 2009a; Hall et al., 2006b).
P300 ERP components--- Signal processing was performed off-line using Brain Vision Analyzer software in McLean sample and NEUROSCAN software (4.3) in Maudsley sample. In both samples, the EEG data were segmented into epochs (Maudsley: –100 to 800 ms; McLean: −100 to 1000 ms) relative to stimulus onset, zero phase-shift digital low-pass filtered at 8.5Hz (24 dB/Oct) and baseline corrected using the 100-ms pre-stimulus interval. Eye-blink artifacts were corrected by using the default method available from the software. Epochs containing artifact >50 µV at the F7, F8, Fp1, or Fp2 site were removed. Separate average waves for target and standard tones were calculated. P300 amplitude and latency components were measured from the average wave for target tones at the Pz site between 280 and 600 ms (Hall et al., 2009a; Salisbury et al., 1999).
N1P2 ERP components--- The same signal processing procedures were applied to extract N1 and P2 ERP waves in each dataset. Signal processing was performed off-line using Brain Vision Analyzer software. EEG signals were digital low-pass filtered at 20Hz (24 dB/Oct). Eye-blink artifacts were corrected by using the default method available from the software. The EEG data were segmented into epochs from −100 to 1000 ms relative to stimulus onset and baseline corrected using the 100-ms pre-stimulus interval. Epochs containing artifact >50 µV at F7, F8, Fp1, or Fp2 site were removed. Peak N1 amplitude was automatically detected as the most negative point from 50 to 200 ms at Cz. Peak P2 amplitude was automatically detected as the most positive point from 150 to 300 ms at Cz (Salisbury et al., 2010).
2.1.1. MMN paradigm
McLean sample--- A total of 800 binaural 75-dB tones (3 per second), 80% standard (50 msec, 1000 Hz, 5-msec rise/fall time), 10% pitch deviant (1200 Hz) and 10% duration deviant (100 msec, 10-msec rise/fall time) were presented. During the task, subjects sat 1.2 m from a monitor that displayed a checkerboard with green and red squares. Subjects were instructed to ignore the tones and to make a keypad response each time the squares reversed colors asynchronously. Maudsley Sample---A total of 1200 binaural 80-dB, 1000-Hz tones (inter-stimulus interval=0.3 sec), 85% standard (25 msec, 1000 Hz, 5-msec rise/fall time) and 15% duration deviant (50 msec, 10-msec rise/fall time) were presented. Subjects were instructed to ignore the tones and focus their eyes on a picture located directly in front of them (Hall et al., 2009a; Hall et al., 2006b).
Signal processing was performed off-line using Brain Vision Analyzer software in McLean sample and NEUROSCAN software (4.3) in Maudsley sample. In both samples, the EEG data were segmented into epochs (–100 to 300 ms) relative to stimulus onset, filtered at 20 Hz (24 dB/Oct) (McLean) and 30 Hz (24 dB/Oct) (Maudsley), and baseline corrected using the 100-ms pre-stimulus interval.
Eye-blink artifacts were corrected by using the default method available from the software. Activity exceeding ±50 µV at Fp1, Fp2, F7, or F8 was considered artifact and was rejected. Both duration and pitch mismatch negativities were extracted separately by subtracting the averaged waveforms for the standard stimuli from those for the deviant stimuli. Mismatch negativity amplitude was measured at Fz from 100 to 200 milliseconds (Salisbury et al., 2007b). To be consistent with the data available on the Maudsley sample, only duration MMN data in the McLean sample were included in the analysis.
Cluster and Statistical Analyses
The same seven variables were included in the analyses in each dataset: P50 sensory gating, EAGBR to S1 stimuli, P3 amplitude, P3 latency, P2 amplitude, N1 amplitude, and duration MMN amplitude. Individuals were clustered using the K-means algorithm (Hartigan and Wong, 1979) implemented in JMP (version 8.0, SAS Institute Inc; www.statsoft.com/textbook/stcluan.html). The K-means algorithm was used in this study because the K-means algorithm has no distributional assumption and produces crisp non-hierarchical and non-overlapping clusters, which facilitates the interpretation of the findings (Hartigan and Wong, 1979). Since this study aimed to examine whether neurophysiologic profiles supported the DSM distinction among SCZ, BPD, and unaffected diagnosis, the number of clusters was initially hypothesized as 3, corresponding to the number of subject groups. In order to verify that three was an appropriate number of clusters, we used the method of v-fold cross-validation to empirically estimate the optimal number of clusters in each dataset (Hill and Lewicki, 2007). The cross validation algorithm suggested that the optimal number of clusters in each dataset was three (see Supplement). Cluster analysis was performed on each dataset separately.
Two different clustering analyses were used to answer two separate research questions. The first, and primary, research question was to examine whether distinct neurophysiologic profiles could be identified independent of diagnosis. We included all participants (patients, relatives, and controls) in the analysis to empirically identify homogeneous subgroups of individuals who share similar neurophysiological profiles, regardless of diagnostic and affection status. This assumption-free analytic strategy is an objective way to identify homogenous subgroups of individuals because it relies solely on the observed neurophysiological data to empirically derive distinct profiles for classifying individuals and does not assume that unaffected individuals are a homogenous group.
The second question addressed by this study was whether the neurophysiological profiles of the SZ and BPD patients supported the DSM diagnostic distinction between these two disorders. For this purpose, we restricted our analysis to the patient samples only. This analysis allowed us to derive the neurophysiological profiles found in the patient groups, to compare the clinical features associated with each profile, and to examine the proportion of patients with diagnoses of SZ or BPD with each profiles. If the neurophysiological profiles supported the DSM distinction between SZ and BPD, one would expect a significantly higher proportion of SCZ patients to be classified in one profile and a significantly higher proportion of BPD patients to be classified in another profile. Finally, we explored the degree of concordance between the two clustering analyses with respect to the patient groups. Prior to the cluster analyses, scores for each variable were converted to standardized z-scores. Missing scores in each of the 7 variables varied between 0% and 3% in the McLean dataset and 0%-5% in the IOP dataset. Missing scores for an individual were imputed using the mean value of his or her diagnostic group.
To compare clinical/demographic features and ERP variables between each profile, we used logistic or linear regression analyses estimating standard errors (SEs) that are robust against non-independence of observations from individuals within families (clusters) and against departures from normality assumptions (STATA version10; Stata Corp., College Station, TX). Gender and age were included as covariates. A Bonferonni corrected p value (p < 0.017, correction for 3 post-hoc comparisons) was used as the threshold for statistical significance.
3. RESULTS
3.1. Clustering analysis of all participants
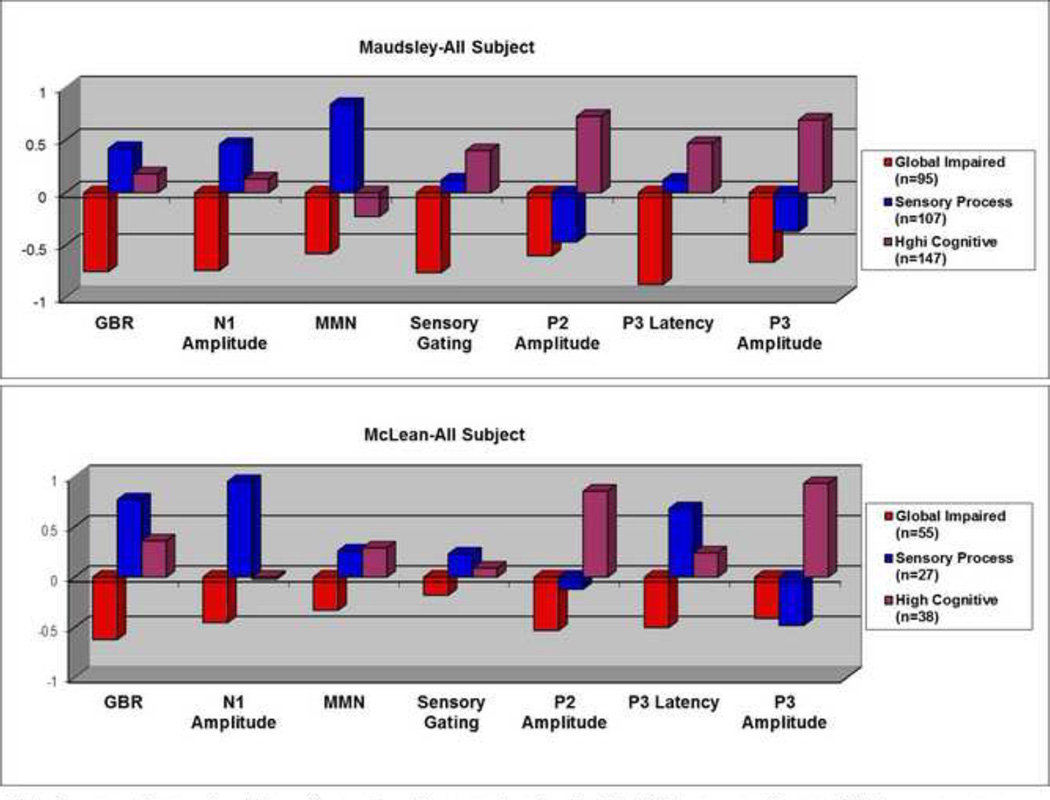
In the analysis of all participants, the overall pattern of neurophysiological profiles between the McLean and Maudsley samples was similar, Specifically, when profiles of all three clusters were compared to each other, one group of individuals (Maudsley: n=95; McLean: n=55) exhibited functional abnormalities on all measures. This group was termed the “globally impaired” group (Figure 1). A second group of individuals exhibited a neurophysiological profile that had the largest EAGBR and N1 responses across both datasets (Maudsley: n=107; McLean: n=27) (Figure 1). As the cognitive functions tapped by these measures are related to early stages of sensory registration and processing, and sensory memory, this cluster was labeled as the “sensory processing” group. The third group of individuals (Maudsley: n=147; McLean: n=38) exhibited a neurophysiological profile that showed the largest P2 and P3 ERP responses across both datasets (Figure 1). Both P2 and P3 responses are associated with higher cognitive processes. Hence, this cluster was labelled as the “high cognitive” group.
Figure 1.
Neurophysiological Profiles derived from All Participant Analysis
Note. For ease of comparison, the positive and negative score signs for the N1, MMN, sensory gating, and P3 latency measures were reversed so that positive values represent better performance and negative values represent worse performance.
In both samples, significantly higher proportions of SZ and BPD patients were classified in the impaired groups than in the sensory or high cognitive groups (Ps<0.001, Table 2). In the Maudsley sample, a significantly larger proportion of controls was classified in the sensory processing or the high cognitive groups than in the impaired group (both Ps<0.001, Table 2). The proportion of relatives did not differ significantly in the three clusters (Ps>0.05, Table 2). In the McLean sample, a significantly larger proportion of controls was classified in the high cognitive than in the globally impaired group (P=0.01, Table 2). A significantly larger proportion of relatives was classified in the sensory processing or the high cognitive groups than in the impaired group (both Ps<0.05, Table 2).
Table 2.
Neurophysiologic Profiles of All Subjects Across Diagnostic Groups
| Group Row % |
Cluster 1 (globally impaired) |
Cluster 2 (sensory processing) |
Cluster 3 (high- cognitive) |
Total | |
|---|---|---|---|---|---|
|
McLean Sample (n=120) |
Controls | 11 31% |
7 20% |
17 49% |
35 |
| SCZ Patients | 20 69% |
3 10% |
6 21% |
29 | |
| BPD Patients | 18 58% |
7 23% |
6 19% |
31 | |
| Relatives of Patients |
6 24% |
10 40% |
9 36% |
16 | |
| Total | 55 | 27 | 38 | 120 | |
|
Maudsley Sample (n=349) |
Controls | 23 12% |
64 33% |
108 55% |
195 |
| SCZ Patients | 26 67% |
5 13% |
8 20% |
39 | |
| BPD Patients | 28 48% |
15 26% |
15 26% |
58 | |
| Relatives of Patients |
18 32% |
23 40% |
16 28% |
9 | |
| Total | 95 | 107 | 147 | 349 | |
3.2. Clustering analysis of patient participants
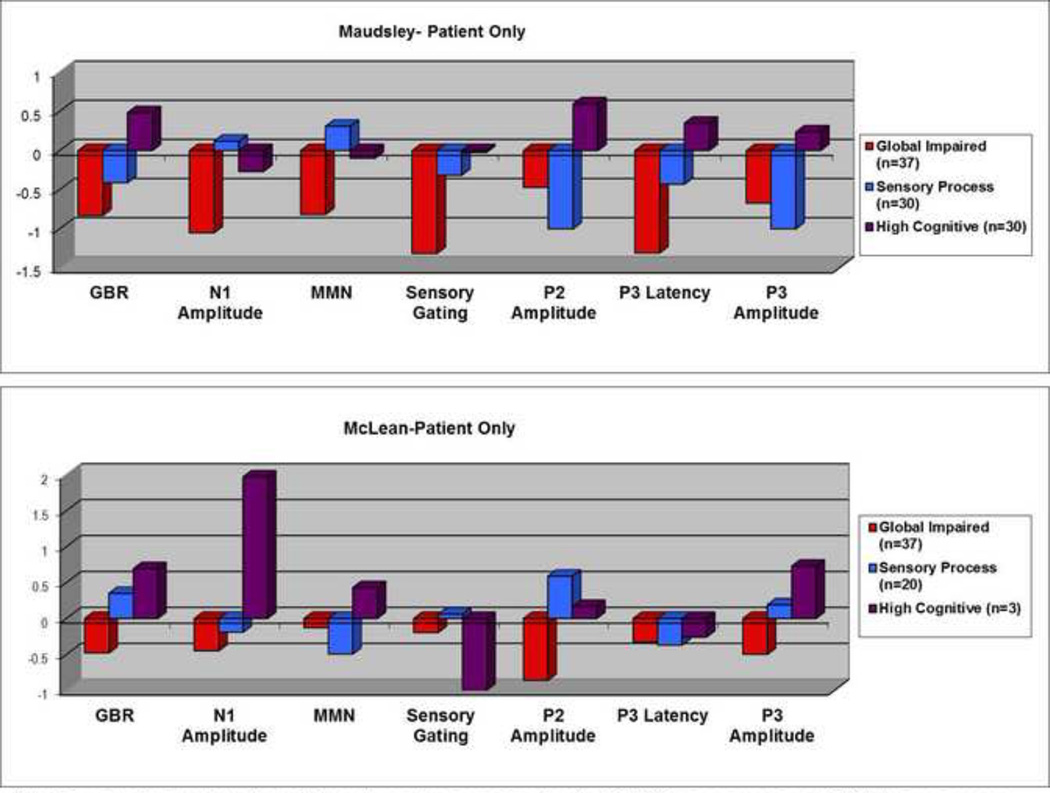
In the analysis of patient subjects, 37 in the Maudsley sample exhibited functional abnormalities, performing poorly on all measures (Figure 2 top). 20 of SCZ and 17 of BPD patients were classified in this group (Table 3). In the McLean sample, 37 patients also exhibited functional abnormalities, performing poorly on all measures (Figure 2 button). 17 of SCZ and 20 of BPD patients were classified in this group (Table 3). This “impaired” profile corresponded closely to the “global impaired” cluster. Thirty patients (8 of SCZ and 22 of BPD patients) in the Maudsley sample were clustered into a second group that exhibited the largest N1 and MMN responses but the smallest P2 and P3 activities (Table 3 & Figure 2).This profile corresponded to the “sensory processing” cluster. In the McLean sample twenty patients (12 of SCZ and 8 of BPD) were included into the “sensory processing” cluster, but these individuals had the best sensory gating and P2 responses (Table 3 & Figure 2). The remaining thirty patients in the Maudsley sample (11 of SCZ and 19 of BPD patients) were classified in the “high cognitive” cluster. In relation to the other two profiles, patients in this cluster had a profile showing the greatest responses in sensory gating, P3 latency, EAGBR, P2, and P3 amplitude measures (Table 3 & Figure 2 Top). Only three patients, all BPD, in the McLean sample were classified in this group. In both samples the proportion of SCZ and BPD patients classified in the “high cognitive” group was not significantly different.
Figure 2.
Neurophysiological Profiles derived from Patient-Only Analysis
Note: For ease of comparison, the positive and negative score signs for the N1, MMN, sensory gating, and P3 latency measures were reversed so that positive values represent better performance and negative values represent worse performance.
Table 3.
Neurophysiologic Profiles of Patient Subjects Across Diagnostic Groups
| Group Row % |
Cluster 1 (globally impaired) |
Cluster 2 (sensory processing) |
Cluster 3 (high cognitive) |
Total | |
|---|---|---|---|---|---|
|
McLean Sample (n=60) |
SCZ Patients |
17 59% |
12 41% |
0 0% |
29 |
|
BPD Patients |
20 64% |
8 26% |
3 10% |
31 | |
| Total | 37 | 20 | 3 | 60 | |
|
Maudsley Sample (n=97) |
SCZ Patients |
20 51% |
8 20% |
11 28% |
39 |
|
BPD Patients |
17 29% |
22 38% |
19 33% |
58 | |
| Total | 37 | 30 | 30 | 97 | |
3.3. Consistency between full sample and patient subsample clustering
In the Maudsley sample, concordance between patients classified in the “globally impaired” profile in the full sample analysis and in the patient-only analysis was 100% and .81%, respectively. Concordance between the two analyses for the “high cognitive” profiles was 77% and 33% in the Maudsley and McLean sample, respectively. Concordance for the “sensory processing” profiles was 47% and 45% in the Maudsley and McLean sample, respectively.
3.4. Differences among cluster groups
Table 4 presents summary statistics for demographic and clinical variables as a function of cluster, for all participants (top) and for the patient-only sample (bottom) in each dataset. Of note, across both datasets, individuals in the high cognitive group were significantly younger than those in the globally impaired group. In both datasets, smokers in the globally impaired group smoked significantly more cigarettes than those in the high sensory or high cognitive groups.
Table 4.
Demographic and Clinical Characteristics of Subjects with Globally Impaired, Sensory Processing, and High Cognitive Profiles
| All Participants | ||||||
|---|---|---|---|---|---|---|
| McLean Sample | Maudsley Sample | |||||
| Variable | Globally Impaired |
Sensory Processing |
High Cognitive |
Globally Impaired |
Sensory Processing |
High Cognitive |
| (n=55) | (n=27) | (n=38) | (n=95) | (n=107) | (n=147) | |
| Age, yrs | 46.2 a (110) |
46.3 (12.5) |
38.9 (14.6) |
43.4 a,b (12.3) |
37.4 (12.1) |
36.5 (11.1) |
|
Female, N (%) |
39 a,b (68%) |
8 (14%) |
10 (17.5%) |
50 (24.5%) |
59 (28%) |
102 (48%) |
|
Thought Disorder |
20.8 a (17.1) |
8.2 (8.2) |
12.6 (10.6) |
N/A | N/A | N/A |
|
Education, yrs |
14.2 (2.3) |
15.4 (2.6) |
15.2 (2.3) |
14.1 (3.0) |
14.9 (2.6) |
14.6 (2.6) |
|
Current smoker, N (%) |
17 a (71%) |
5 (21%) |
2 (8%) |
15 b (20%) |
36 c (48%) |
24 (32%) |
|
No. cigarettes/ day, if smoker |
22.8 a (15.4) |
9.8 (6.4) |
10.5 (13.4) |
18.8 a,b (11.5) |
12.2 (8.4) |
11.2 (9.3) |
| Patients | ||||||
| McLean Sample | Maudsley Sample | |||||
|
Globally Impaired |
Sensory Processing |
High Cognitive |
Globally Impaired |
Sensory Processing |
High Cognitive |
|
| (n=37) | (n=20) | (n=3) | (n=37) | (n=30) | (n=30) | |
| Age, yrs | 43.6 (10.6) |
45.2 (11.1) |
45.7 (3.9) |
44.2 a (11.4) |
37.9 (12.7) |
36.9 (9.9) |
|
Female, N (%) |
8 (38%) |
12 c (57%) |
1 (5%) |
16 (33%) |
15 (31%) |
17 (35%) |
|
Age of onset |
23.1 (5.9) | 27.6 (8.8) | 18.3 (1.2) | 24.6 (9.0) | 20.3 (5.1) | 20.9 (5.0) |
|
Duration of illness |
20.5 (10.7) |
17.6 (11.4) | 27.4 (3.7) | 19.7 (10.8) |
16.8 (11.8) | 16.1 (9.0) |
|
Thought Disorder |
23.8 a (18.5) |
16.0 (11.4) |
2.7 (2.5) |
N/A | N/A | N/A |
| BPRS | 45.2 (13.2) |
42.9 (17.3) | 41 (5.6) | N/A | N/A | N/A |
|
Education, yrs |
14.0 (2.0) | 15.4 (2.0) | 15.3 (3.0) | 13.3 (3.1) | 15.0 (3.3) | 13.3 (2.6) |
|
CPZ equivalente |
644.1 (478) |
618.0 (560) | 525 (106) | 591.7 (286) |
786.4 (458) | 645.4 (317) |
|
Current smoker, N (%) |
14 a,b (78%) |
3 c (17%) |
1 (5%) |
7 (25%) |
13 (46%) |
8 (29%) |
|
No. cigarettes/ day, if smoker |
25.3 (14.6) |
21.5 (12.3) |
20 (n/a) |
21.6 (12.9) |
19.2 (12.5) |
18.4 (12.1) |
Significant differences (p<0.017) between impaired and high cognitive groups.
Significant differences (p<0.017) between impaired and sensory processing groups.
Significant differences (p<0.017) between high cognitive and sensory processing groups.
Among patients, those in the McLean impaired group exhibited significantly higher amounts of thought disorder than those of the other two groups, who did not differ from each other (Impaired: mean=23.8 SD=12.3; Intermediate: mean=16.0 SD=11.4; Preserved: mean=2.7 SD=2.5, Table 4). The proportion of smokers was highest in the impaired group (78%) and lowest in the preserved group (5%). In both samples there was a trend for individuals in the impaired group to smoke the most cigarettes compared with the other two groups (Table 4).
2. DISCUSSION
The primary goal of this study was to examine whether distinct neurophysiologic profiles could be identified independent of diagnosis and clinical affection status. Using cluster analysis, three neurophysiologically distinct groups were identified and the overall ERP pattern of the profiles was similar between the two cohorts (Figure 1). In particular, a subgroup of individuals in both cohorts, labeled “globally impaired”, exhibited functional abnormalities on all measures compared with subjects in the other two clusters. In addition, both datasets identified a second group of individuals, labeled “sensory processing”, who performed best, relative to individuals in the other two clusters, on measures corresponding to early stages of sensory stimulus registration and processing (i.e., GBR and N1). A third group of individuals, labeled “high cognitive”, performed the best on tasks that probe higher cognitive function (i.e.,P2 and P3 amplitude). On the other hand, we found that two ERP measures, sensory gating and P3 latency, had an inconsistent pattern across datasets. Two possible explanations for this inconsistency are the small sample size of the McLean dataset and lower sensitivity of sensory gating and P3 latency measures compared with other ERP measures in separating high cognitive from sensory processing individuals.
In this study, seven neurophysiological phenotypes, each of which evaluated different brain information processing functions, were assessed in each participant. P50 sensory gating provides a measure of sensory inhibition and reflects the individual’s ability to filter out repetitive stimuli in order to minimize information overload (Freedman et al., 1991). EAGBR assesses basic brain functions associated with auditory perception (Javitt et al., 2008b). The N1 ERP reflects early sensory processing at the level of auditory cortex. MMN is generated by an automatic cortical change-detection process whereby the brain detects a difference between the current auditory input and the regularity of the immediately preceding auditory input (Naatanen, 1992). The MMN may be part of alerting and survival mechanisms that detect unusual and possibly dangerous events in the environment (Tiitinen et al., 1994). The P200 and P300 explore higher levels of cognitive processing, including sustained attention, speed of information processing and working memory (Donchin and Coles, 1988).
Comparison of the two clustering analyses (all subjects vs. patients-only) indicated there was high concordance between the two “globally impaired” groups. These results support the existence of a robust subgroup of patients with an impaired neurophysiological profile. In both datasets “globally impaired” individuals performed significantly worse than “high cognitive” individuals on the majority of ERP measures. “Globally impaired” individuals also performed significantly worse than “sensory processing” individuals on the majority of ERP measures (Supplementary Table S1). We hypothesize that patients with the impaired profile may be more similar in terms of underlying neurobiology and specific genetic risk factors. Consistent with this hypothesis, Wessman and colleagues (Wessman et al., 2009) used a cluster analytic technique to identify subgroups of individuals in Finnish pedigrees segregating SCZ who shared similar clinical symptoms and cognitive deficits, and incorporated these empirically derived phenotypes into a genetic association study. They found two subgroups of patients, one with pervasive cognitive deficits and the other with preserved cognitive capacity. A significant association was found between individuals in the deficit group, but not those with preserved cognitive function, and a putative risk variant of DTNBP1. In another example, Hallmayer and colleagues (Hallmayer et al., 2005) stratified a large SCZ family cohort into families with cognitive deficits and those without. The 6p25-22 linkage region was significantly associated in families that showed cognitive deficits (LOD score of 3.32 at marker D6S309), but not in families that showed preserved cognitive ability (LOD score of −2.12). These results collectively support the utility of using profile-based phenotypes to identify homogenous subgroups of individuals that may be informative for genetic studies.
Our results also indicate that the empirically derived “globally impaired” group is not restricted to clinically affected individuals. 12%–33% of RelSCZ, 31%–44% of RelBPD, as well as 12%–31% of controls were classified having impaired neurophysiologic profile as well. These observations are consistent with a liability threshold model, which assumes that common psychiatric diseases reflect the influence of many genes of individually small effect and that both affected and unaffected individuals are part of the same distribution of liability to the disorder. Purcell and colleagues have shown that SCZ involves hundreds or even thousands of common genetic variants and that risk genes for SCZ overlap with BPD (Purcell et al., 2009). We hypothesize that unaffected individuals with the impaired neurophysiologic profile may carry a larger proportion of risk genotypes than unaffected individuals in the other two groups, but below a threshold for clinical expression.
The second goal of the study was to examine whether neurophysiologic profiles among SZ and BPD patients support the DSM diagnostic distinction between these two disorders. To address this question, we restricted our analysis to the patient sample only. Our results indicate that SCZ from BPD patients did not have distinct neurophysiological profiles (Table 3). One reason may be that we restricted our sample of BPD subjects to those with psychotic features. The overlapping neurophysiological profiles observed in the SCZ and BPD groups may therefore reflect the fact that the neurobiology of BPD with psychosis is similar to that of the schizophrenia spectrum. In both samples patients in each profile did not differ significantly in mean age of onset, duration of illness, medication dosage, or symptom severity, suggesting that the observed neurophysiological profiles are unlikely due to these illness-related factors (Supplementary Table S1). However, patients in the McLean impaired group had higher amounts of thought disorder than those of the other two groups, who did not differ from each other. Unfortunately, thought disorder data were not available in the Maudsley sample. Of note, the similar results obtained in the McLean sample, which included SA patients, and in the Maudsley sample, which did not, indicate that the SCZ and BPD have overlapping profiles independent of whether SA patients are included.
Previous studies have suggested that neurophysiologic profiling may be useful for identifying phenotypic subgroups within diagnostic categories. For example, Turetsky and colleagues reported a study including multiple neurophysiological measures in which SCZ patient deficits loaded onto two independent information processing deficits: one associated with early sensory processing and the other denoting a disturbance of higher-order cognitive processes (Turetsky et al., 2009). These authors concluded that SCZ patients are heterogeneous and that profile based analysis may be an alternative for identifying homogeneous subgroups of individuals.
In both datasets, smokers in the globally impaired group smoked significantly more cigarettes than those in the high sensory or high cognitive groups (Table 5). A similar trend was also found in the patient-only analysis. It has been suggested that smoking, particularly in the mentally ill population, may be a form of self-medication to treat an underlying biological pathology or to reduce the side effects of medications (Leonard et al., 2001). Nicotine transiently enhances early sensory responses (Crawford et al., 2002), normalizes auditory P50 sensory gating deficits in SCZ, and improves cognitive function on attention (Lohr and Flynn, 1992) and working memory tasks (Jacobsen et al., 2004). Our observations that impaired individuals, regardless of affection status, exhibited poor neurophysiological profile and smoked the most cigarettes, are consistent with the self-medication hypothesis. Nicotine administration changes the expression of multiple genes and smoking behavior has been associated with variants at the alpha 7 nicotinic receptor locus (CHRNA7) (Leonard et al., 2001; Mexal et al., 2009).
This study has a number of limitations. First, the sample size in the McLean dataset was relatively modest and only 3 patients were classified in the “high cognitive” group, limiting the interpretability of that cluster. Second, the components of the “high cognitive” and the “sensory processing” profiles were inconsistent in the two datsets. To assess the possibility that the Maudsley sample may have been less heterogeneous than the McLean sample (because it was compos of twins), we performed additional k-means clustering, restricting the analysis to only one member of each twin pair. The patterns of neurophysiological profiles were very similar in the full sample and the reduced sample. Thus, the few differences observed between the two cohorts are likely due to the smaller sample size of the McLean cohort. Replication in a larger independent sample will be important in substantiating our findings. Third, the clustering method is unable to account for shared variance that may be present in analyses that included related individuals. Across both datasets, individuals in the high cognitive group were significantly younger than those in the globally impaired group. However, after effects of age and sex were removed, group differences in each ERP measure remained unchanged (Supplementary Table S1). In the patient only analysis, age effects were observed in the Maudsley sample but not in the McLean sample. Similarly, group differences in each ERP measure remained unchanged after age and sex effects were removed (Supplementary Table S1).
In summary, in independent cohorts of patients with BPD and SCZ, we found that neurophysiological profiling was able to identify three subgroups of individuals. These results suggest that empirical analyses of neurophysiological phenotypes can identify potentially biologically relevant homogenous subgroups independent diagnostic boundaries. We hypothesize that each of the homogeneous neurophysiology subgroups may share similar genotype profiles, which may increase statistical power to detect genetic risk factors.
Supplementary Material
Acknowledgements
Funding Sources: This work was supported by the Rappaport Mental Health Research Scholar Award, McLean Hospital to M-HH, NARSAD Sidney R. Baer, Jr. Foundation Awards to M-HH and DLL, grants from the Essel Foundation to DLL, and from the National Institute of Mental Health [1K01MH086714 to M-HH, 5R01MH071523 to DLL, MH58704 to DFS, and MH079799 to JWS].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Allison DB, Thiel B, St Jean P, Elston RC, Infante MC, Schork NJ. Multiple phenotype modeling in gene-mapping studies of quantitative traits: power advantages. The American Journal of Human Genetics. 1998;63:1190–1201. doi: 10.1086/302038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benes FM. Regulation of cell cycle and DNA repair in post-mitotic GABA neurons in psychotic disorders. Neuropharmacology. 2010;60:1232–1242. doi: 10.1016/j.neuropharm.2010.12.011. [DOI] [PubMed] [Google Scholar]
- Craddock N, O'Donovan MC, Owen MJ. The genetics of schizophrenia and bipolar disorder: dissecting psychosis. Journal of Medical Genetics. 2005;42:193–204. doi: 10.1136/jmg.2005.030718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford HJ, McClain-Furmanski D, Castagnoli N, Jr, Castagnoli K. Enhancement of auditory sensory gating and stimulus-bound gamma band (40 Hz) oscillations in heavy tobacco smokers. Neuroscience Letter. 2002;317:151–155. doi: 10.1016/s0304-3940(01)02454-5. [DOI] [PubMed] [Google Scholar]
- Donchin E, Coles MGH. Is the P300 Component a Manifestation of Context Updating. Behavioral and Brain Sciences. 1988;11:357–374. [Google Scholar]
- Falconer DS, Mackay TFC. Introduction to quantitative genetics. 4th ed. Harlow: Longman; 1996. [Google Scholar]
- Freedman R, Adams CE, Adler LE, Bickford PC, Gault J, Harris JG, Nagamoto HT, Olincy A, Ross RG, Stevens KE, Waldo M, Leonard S. Inhibitory neurophysiological deficit as a phenotype for genetic investigation of schizophrenia. American Journal of Medical Genetics. 2000;97:58–64. doi: 10.1002/(sici)1096-8628(200021)97:1<58::aid-ajmg8>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Freedman R, Waldo M, Bickford-Wimer P, Nagamoto H. Elementary neuronal dysfunctions in schizophrenia. Schizophrenia Research. 1991;4:233–243. doi: 10.1016/0920-9964(91)90035-p. [DOI] [PubMed] [Google Scholar]
- Gallinat J, Winterer G, Herrmann CS, Senkowski D. Reduced oscillatory gammaband responses in unmedicated schizophrenic patients indicate impaired frontal network processing. Clinical Neurophysiology. 2004;115:1863–1874. doi: 10.1016/j.clinph.2004.03.013. [DOI] [PubMed] [Google Scholar]
- Gottesman II, Gould TD. The endophenotype concept in psychiatry: etymology and strategic intentions. American Journal of Psychiatry. 2003;160:636–645. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- Green EK, Grozeva D, Jones I, Jones L, Kirov G, Caesar S, Gordon-Smith K, Fraser C, Forty L, Russell E, Hamshere ML, Moskvina V, Nikolov I, Farmer A, McGuffin P, Holmans PA, Owen MJ, O'Donovan MC, Craddock N. The bipolar disorder risk allele at CACNA1C also confers risk of recurrent major depression and of schizophrenia. Molecular Psychiatry. 2009;15:1016–1022. doi: 10.1038/mp.2009.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall M-H, Rijsdijk FV, Picchioni M, Schulze K, Ettinger U, Toulopoulou T, Murray R, Sham P. Substantial Shared Genetic Influences on Schizophrenia and Event- Related Potentials. American Journal of Psychiatry. 2007;164:804–812. doi: 10.1176/ajp.2007.164.5.804. [DOI] [PubMed] [Google Scholar]
- Hall M-H, Schulze K, Bramon E, Murray R, Sham P, Rijsdijk FV. Genetic Overlap Between P300, P50 and Duration Mismatch Negativity. American Journal of Medical Genetics. 2006a;141:336–343. doi: 10.1002/ajmg.b.30318. [DOI] [PubMed] [Google Scholar]
- Hall MH, Schulze K, Rijsdijk F, Kalidindi S, McDonald C, Bramon E, Murray RM, Sham P. Are auditory P300 and duration MMN heritable and putative endophenotypes of psychotic bipolar disorder? A Maudsley Bipolar Twin and Family Study. Psychological Medicine. 2009a;39:1277–1287. doi: 10.1017/S0033291709005261. [DOI] [PubMed] [Google Scholar]
- Hall MH, Schulze K, Rijsdijk F, Picchioni M, Ettinger U, Bramon E, Freedman R, Murray RM, Sham P. Heritability and Reliability of P300, P50 and Duration Mismatch Negativity. Behavior Genetics. 2006b;36:845–857. doi: 10.1007/s10519-006-9091-6. [DOI] [PubMed] [Google Scholar]
- Hall MH, Schulze K, Sham P, Kalidindi S, McDonald C, Bramon E, Levy DL, Murray RM, Rijsdijk F. Further evidence for shared genetic effects between psychotic bipolar disorder and P50 suppression: A combined twin and family study. American Journal of Medical Genetics. 2008;147B:619–627. doi: 10.1002/ajmg.b.30653. [DOI] [PubMed] [Google Scholar]
- Hall MH, Spencer KM, Schulze K, McDonald C, Kalidindi S, Kravariti E, Kane F, Murray RM, Bramon E, Sham P, Rijsdijk F. The Genetic and Environmental Influences of Event- Related Gamma Oscillations on Bipolar Disorder. Bipolar Disorder. 2011a;I13:260–271. doi: 10.1111/j.1399-5618.2011.00925.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall MH, Spencer KM, Schulze K, McDonald C, Kalidindi S, Kravariti E, Kane F, Murray RM, Bramon E, Sham P, Rijsdijk F. The Genetic and Environmental Influences of Event- Related Gamma Oscillations on Bipolar Disorder. Bipolar Disorder. 2011b;13:260–271. doi: 10.1111/j.1399-5618.2011.00925.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall MH, Taylor G, Salisbury DF, Levy DL. Sensory Gating Event-Related Potentials and Oscillations in Schizophrenia Patients and Their Unaffected Relatives. Schizophrenia Bulletin. 2011;37:1187–1199. doi: 10.1093/schbul/sbq027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall MH, Taylor G, Sham P, Schulze K, Rijsdijk F, Picchioni M, Toulopoulou T, Ettinger U, Bramon E, Murray RM, Salisbury DF. The Early Auditory Gamma-Band Response Is Heritable and a Putative Endophenotype of Schizophrenia. Schizophrenia Bulletin. 2009b doi: 10.1093/schbul/sbp134. Epub 2009 Nov 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallmayer JF, Kalaydjieva L, Badcock J, Dragovic M, Howell S, Michie PT, Rock D, Vile D, Williams R, Corder EH, Hollingsworth K, Jablensky A. Genetic evidence for a distinct subtype of schizophrenia characterized by pervasive cognitive deficit. The American Journal of Human Genetics. 2005;77:468–476. doi: 10.1086/432816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison PJ, Weinberger DR. Schizophrenia genes, gene expression, and neuropathology: on the matter of their convergence. Molecular Psychiatry. 2005;10:40–68. doi: 10.1038/sj.mp.4001558. image 45. [DOI] [PubMed] [Google Scholar]
- Hartigan JA, Wong MA. A K-means clustering algorithm. Applied Statistics. 1979;28:100- –108. [Google Scholar]
- Hill T, Lewicki P. STATISTICS Methods and Applications. Tulsa: StatSoft; 2007. [Google Scholar]
- Jacobsen LK, D'Souza DC, Mencl WE, Pugh KR, Skudlarski P, Krystal JH. Nicotine effects on brain function and functional connectivity in schizophrenia. Biol Psychiatry. 2004;55:850–858. doi: 10.1016/j.biopsych.2003.12.023. [DOI] [PubMed] [Google Scholar]
- Jahshan C, Wynn JK, Mathis KI, Altshuler LL, Glahn DC, Green MF. Crossdiagnostic comparison of duration mismatch negativity and P3a in bipolar disorder and schizophrenia. Bipolar Disord. 2012;14:239–248. doi: 10.1111/j.1399-5618.2012.01008.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasper H. Report to the committee on methods of clinical examination in electroencephalography. Electroencephalography and clinical neurophysiology. 1958;10:371–375. [Google Scholar]
- Javitt DC, Spencer KM, Thaker GK, Winterer G, Hajos M. Neurophysiological biomarkers for drug development in schizophrenia. Nature Reviews Drug Discovery. 2008a;7:68–83. doi: 10.1038/nrd2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javitt DC, Spencer KM, Thaker GK, Winterer G, Hajos M. Neurophysiological biomarkers for drug development in schizophrenia. Nat Rev Drug Discov. 2008b;7:68–83. doi: 10.1038/nrd2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur M, Battisti RA, Lagopoulos J, Philip PB, Hickie IB, Hermens DF. Neurophysiological biomarkers support bipolar-spectrum disorders within psychosis cluster. Journal of psychiatry & neuroscience : JPN. 2012;37:110081. doi: 10.1503/jpn.110081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraepelin E. Dementia Praecox and Paraphrenia. Chicago: Chicago Medical Book; 1919. [Google Scholar]
- Kremen WS, Seidman LJ, Faraone SV, Toomey R, Tsuang MT. Heterogeneity of schizophrenia: a study of individual neuropsychological profiles. Schizophrenia Research. 2004;71:307–321. doi: 10.1016/j.schres.2004.02.022. [DOI] [PubMed] [Google Scholar]
- Leicht G, Kirsch V, Giegling I, Karch S, Hantschk I, Moller HJ, Pogarell O, Hegerl U, Rujescu D, Mulert C. Reduced early auditory evoked gamma-band response in patients with schizophrenia. Biol Psychiatry. 2010;67:224–231. doi: 10.1016/j.biopsych.2009.07.033. [DOI] [PubMed] [Google Scholar]
- Leonard S, Adler LE, Benhammou K, Berger R, Breese CR, Drebing C, Gault J, Lee MJ, Logel J, Olincy A, Ross RG, Stevens K, Sullivan B, Vianzon R, Virnich DE, Waldo M, Walton K, Freedman R. Smoking and mental illness. Pharmacol Biochem Behav. 2001;70:561–570. doi: 10.1016/s0091-3057(01)00677-3. [DOI] [PubMed] [Google Scholar]
- Lichtenstein P, Yip BH, Bjork C, Pawitan Y, Cannon TD, Sullivan PF, Hultman CM. Common genetic determinants of schizophrenia and bipolar disorder in Swedish families: a population-based study. Lancet. 2009;373:234–239. doi: 10.1016/S0140-6736(09)60072-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohr JB, Flynn K. Smoking and schizophrenia. Schizophrenia Research. 1992;8:93–102. doi: 10.1016/0920-9964(92)90024-y. [DOI] [PubMed] [Google Scholar]
- Mannuzza S, Fryer A, Endicott J, Klein D. NY State Psychiatric Institute. New York: Anxiety Disorders Clinic; 1985. Family informant schedule and criteria (FISC) [Google Scholar]
- Matthysse S, Levy DL, Kinney D, Deutsch C, Lajonchere C, Yurgelun-Todd D, Woods B, Holzman PS. Gene expression in mental illness: a navigation chart to future progress. J Psychiatr Res. 1992;26:461–473. doi: 10.1016/0022-3956(92)90046-q. [DOI] [PubMed] [Google Scholar]
- Mexal S, Berger R, Logel J, Ross RG, Freedman R, Leonard S. Differential regulation of alpha7 nicotinic receptor gene (CHRNA7) expression in schizophrenic smokers. J Mol Neurosci. 2009;40:185–195. doi: 10.1007/s12031-009-9233-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muir WJ, St Clair DM, Blackwood DH. Long-latency auditory event-related potentials in schizophrenia and in bipolar and unipolar affective disorder. Psychol Med. 1991;21:867–879. doi: 10.1017/s003329170002986x. [DOI] [PubMed] [Google Scholar]
- Naatanen R. Attention and Brain Function. Hillsdale, NJ: Erlbaum; 1992. [Google Scholar]
- Neale MC, Kendler KS. Models of comorbidity for multifactorial disorders. The American Journal of Human Genetics. 1995;57:935–953. [PMC free article] [PubMed] [Google Scholar]
- O'Donnell BF, Hetrick WP, Vohs JL, Krishnan GP, Carroll CA, Shekhar A. Neural synchronization deficits to auditory stimulation in bipolar disorder. Neuroreport. 2004a;15:1369–1372. doi: 10.1097/01.wnr.0000127348.64681.b2. [DOI] [PubMed] [Google Scholar]
- O'Donnell BF, Vohs JL, Hetrick WP, Carroll CA, Shekhar A. Auditory eventrelated potential abnormalities in bipolar disorder and schizophrenia. International Journal of Psychophysiology. 2004b;53:45–55. doi: 10.1016/j.ijpsycho.2004.02.001. [DOI] [PubMed] [Google Scholar]
- Purcell SM, Wray NR, Stone JL, Visscher PM, O'Donovan MC, Sullivan PF, Sklar P. Common polygenic variation contributes to risk of schizophrenia and bipolar disorder. Nature. 2009;460:748–752. doi: 10.1038/nature08185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roach BJ, Mathalon DH. Event-related EEG time-frequency analysis: an overview of measures and an analysis of early gamma band phase locking in schizophrenia. Schizophrenia Bulletin. 2008;34:907–926. doi: 10.1093/schbul/sbn093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salisbury DF, Collins KC, McCarley RW. Reductions in the N1 and P2 auditory event-related potentials in first-hospitalized and chronic schizophrenia. Schizophrenia Bulletin. 2010;36:991–1000. doi: 10.1093/schbul/sbp003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salisbury DF, Kuroki N, Kasai K, Shenton ME, McCarley RW. Progressive and interrelated functional and structural evidence of post-onset brain reduction in schizophrenia. Archives of General Psychiatry. 2007a;64:521–529. doi: 10.1001/archpsyc.64.5.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salisbury DF, Kuroki N, Kasai K, Shenton ME, McCarley RW. Progressive and interrelated functional and structural evidence of post-onset brain reduction in schizophrenia. Archives of General Psychiatry. 2007b;64:521–529. doi: 10.1001/archpsyc.64.5.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salisbury DF, Shenton ME, Griggs CB, Bonner-Jackson A, McCarley RW. Mismatch negativity in chronic schizophrenia and first-episode schizophrenia. Archives of General Psychiatry. 2002;59:686–694. doi: 10.1001/archpsyc.59.8.686. [DOI] [PubMed] [Google Scholar]
- Salisbury DF, Shenton ME, McCarley RW. P300 topography differs in schizophrenia and manic psychosis. Biological Psychiatry. 1999;45:98–106. doi: 10.1016/s0006-3223(98)00208-x. [DOI] [PubMed] [Google Scholar]
- Salisbury DF, Shenton ME, Sherwood AR, Fischer IA, Yurgelun-Todd DA, Tohen M, McCarley RW. First-episode schizophrenic psychosis differs from first-episode affective psychosis and controls in P300 amplitude over left temporal lobe. Archives of General Psychiatry. 1998;55:173–180. doi: 10.1001/archpsyc.55.2.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulze KK, Hall M-H, McDonald C, Marshall N, Walshe M, Murray RM, Bramon E. P50 Auditory Evoked Potential Suppression in Bipolar Disorder Patients With Psychotic Features and Their Unaffected Relatives. Biological Psychiatry. 2007;62:121–128. doi: 10.1016/j.biopsych.2006.08.006. [DOI] [PubMed] [Google Scholar]
- Shenton ME, Solovay MR, Holzman PS, Coleman M, Gale HJ. Thought disorder in the relatives of psychotic patients. Archives of General Psychiatry. 1989;46:897–901. doi: 10.1001/archpsyc.1989.01810100039007. [DOI] [PubMed] [Google Scholar]
- Solovay MR, Shenton ME, Holzman PS. Comparative studies of thought disordersIMania and schizophrenia. Archives of General Psychiatry. 1987;44:13–20. doi: 10.1001/archpsyc.1987.01800130015003. [DOI] [PubMed] [Google Scholar]
- Spencer KM, Niznikiewicz MA, Shenton ME, McCarley RW. Sensory-evoked gamma oscillations in chronic schizophrenia. Biological Psychiatry. 2008a;63:744–747. doi: 10.1016/j.biopsych.2007.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer KM, Salisbury DF, Shenton ME, McCarley RW. gamma-Band Auditory Steady-State Responses Are Impaired in First Episode Psychosis. Biological Psychiatry. 2008b;64:369–375. doi: 10.1016/j.biopsych.2008.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitzer RL, Endicott J. Schedule for affective disorders and schizophrenia - Lifetime version. New York: New York State Psychiatric Institute; 1978. [Google Scholar]
- Thaker GK. Neurophysiological endophenotypes across bipolar and schizophrenia psychosis. Schizophrenia Bulletin. 2008;34:760–773. doi: 10.1093/schbul/sbn049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiitinen H, May P, Reinikainen K, Naatanen R. Attentive novelty detection in humans is governed by pre-attentive sensory memory. Nature. 1994;372:90–92. doi: 10.1038/372090a0. [DOI] [PubMed] [Google Scholar]
- Turetsky BI, Bilker WB, Siegel SJ, Kohler CG, Gur RE. Profile of auditory information-processing deficits in schizophrenia. Psychiatry Res. 2009;165:27–37. doi: 10.1016/j.psychres.2008.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turetsky BI, Calkins ME, Light GA, Olincy A, Radant AD, Swerdlow NR. Neurophysiological endophenotypes of schizophrenia: the viability of selected candidate measures. Schizophrenia Bulletin. 2007;33:69–94. doi: 10.1093/schbul/sbl060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessman J, Paunio T, Tuulio-Henriksson A, Koivisto M, Partonen T, Suvisaari J, Turunen JA, Wedenoja J, Hennah W, Pietilainen OP, Lonnqvist J, Mannila H, Peltonen L. Mixture model clustering of phenotype features reveals evidence for association of DTNBP1 to a specific subtype of schizophrenia. Biological Psychiatry. 2009;66:990–996. doi: 10.1016/j.biopsych.2009.05.034. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.