Abstract
We report an unusual face selective reduction of the exocyclic double bond in the α-methylene-γ-butyrolactone motif of spiro-oxindole systems. The spiro-oxindoles were assembled by an indium metal mediated Barbier-type reaction followed by an acid catalyzed lactonization.
The α-methylene-γ-butyrolactone motif is present in a number of biologically active sesquiterpene lactone natural products, many of which possess useful biological properties (Fig. 1 left side).1 Presence of this lactone moiety with the exocyclic double bond in the natural products is considered to be a major factor in the observed biological activity such as anticancer, antiviral, and anti-inflammatory activities.2 Studies have shown that certain proteins with accessible cysteine functionality tend to bind irreversibly to these natural products.3 Due to its biological relevance, many methods have been reported for the synthesis of α-methylene-γ-butyrolactones.4
Fig. 1.
Biologically relevant α-methylene-γ-butyrolactones and oxindole motifs.
The oxindole framework bearing a spirocyclic quaternary stereocenter at the C3 position represents a privileged structure commonly found in clinical pharmaceuticals and natural products (Fig. 1 right side).5 Oxindole derivatives have been reported to have a range of biological activities such as tyrosine kinase inhibition, cyclin-dependent kinases (CDKs) inhibition, and anti-angiogenic properties.6
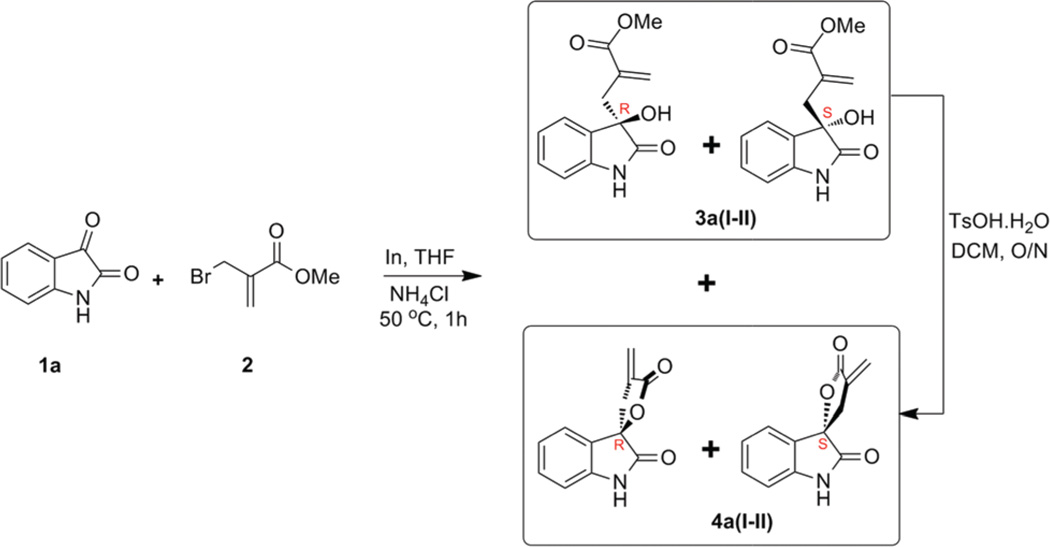
Based on these observations, we postulated that a novel hybrid pharmacophore compound containing both α-methylene-γ-butyrolactones and oxindole presents an interesting scaffold for biological screens. This hybridization concept of conjugating two privileged scaffolds has been successfully applied towards the identification of novel scaffolds/compounds with anticancer, antimalarial and antimicrobial properties.7 To build this novel hybrid pharmacophore, we carried out an indium mediated Barbier-type reaction with isatin (1) and methyl 2-(bromomethyl)acrylate (2).
Previous reports with aldehydes/ketones and methyl 2-(bromomethyl) acrylate (2) yielded cyclic products in a single step.8 However, in our case, reaction of 1a and methyl 2-(bromomethyl) acrylate (2) yielded acyclic and cyclic compounds in a 6 : 1 ratio in 97% yield (Scheme 1).
Scheme 1.
Indium metal mediated Barbier-type reaction between isatin and methyl 2-(bromomethyl)acrylate.
Nevertheless, the acyclic product 3a(I–II) when subjected to p-toluenesulfonic acid in dichloromethane at room temperature was easily converted to the cyclic product 4a(I–II) in excellent yield. When a mixture of enantiomers 4a(I–II) was subjected to hydrogenation, we expected to see two sets of diastereomers 6a(I–IV) (Scheme 2) and thus two sets of doublets for the exocyclic methyl group protons by nuclear magnetic resonance (NMR) spectroscopy. To our surprise, NMR spectra showed only one doublet (~1.5 ppm) that corresponds to the exocyclic methyl group (Fig. 2 top panel). This suggested possible face selective reduction of the double bond.
Scheme 2.
Overall routes to study different configurations.
Fig. 2.
Overlay of 1H-NMR and 1D-NOE difference NMR spectra.
To test if indeed we observe face selective reduction of 4a(I–II), we reversed the sequence of reactions, i.e., reduction followed by cyclization (Scheme 2). Acyclic compound 3a(I–II) was hydrogenated in the presence of 5% Pd/C in THF, which resulted in two sets of inseparable diastereomers 5a(I–IV) in 85% yield (two ester methyl peaks were observed at 3.4 to 3.6 ppm by NMR spectroscopy). Treatment of this inseparable reaction mixture with p-toluenesulfonic acid in dichloromethane gave two sets of inseparable diastereomers 6a(I–IV) in 92% yield, which were clearly resolved by NMR spectroscopy (two doublets at 1.4 and 1.5 ppm respectively, Fig. 2 second panel), thus suggesting a face selective reduction in the former case (4a).
To establish the stereochemistry of the newly created stereo-center alpha to the carbonyl in the lactone ring of compound 6a(I–IV) we conducted Nuclear Overhauser Effect (NOE) experiments. Since the lactone and oxindole rings are perpendicular to each other, we expected to observe crosspeaks between the proton at the C4 position of the oxindole and the protons on the methyl group in only one set of 6a(I–IV) diastereomers.
Unfortunately, NOE experiments with 6a(I–IV) at different mixing times showed no crosspeaks between the proton at the C4 position of the oxindole and the protons on the methyl group. Alternatively, it is known that 1D NOE difference NMR experiments allow long distance transfer of magnetization, therefore a 1D NOE difference NMR experiment was conducted.9 For compound 6a(I–IV), upon saturation of the aromatic region proton (~7.3 ppm), only one set of C–H protons (~3.2 ppm) alpha to the carbonyl was saturated while the other (~3.5 ppm) was not (Fig. 2 bottom panel). This suggests that the proton alpha to the carbonyl on the lactone ring is closer to the aromatic protons in the saturated set of diastereoisomers (RS and SR), while the other set of diastereoisomers (RR and SS) has the methyl group closer to the aromatic C–H. No saturation of signal was observed for either of the exocyclic methyl groups (~1.5 and ~1.4 ppm). At the present time, it is unclear why we did not observe saturation of at least one set of the exocyclic methyl groups.
The same experiment with 6a(I–II) showed saturation of the C–H proton at ~3.2 ppm. Together the data confirm that the reduction of the double bond in the cyclized product 4a(I–II) yields only one set of diastereoisomers 6a(I–II) (RS and SR) in which the methyl group is pointing away from the aromatic ring. We speculated that the source of the facial selectivity during reduction could be due to the orientation of the compound on the catalyst. To investigate this, we synthesized two additional isatin derivatives with methyl groups on the nitrogen atom (1b) and on the C4 and C7 atoms (1c) of the aromatic ring.10 We repeated similar sets of experiments with these substituted isatins (cyclization followed by reduction and reduction followed by cyclization) and carried out 1D NOE difference NMR experiments. The results were similar to what was observed with the unsubstituted isatin compound.
The reaction of 1b and 2 in the presence of indium metal yielded acyclic 3b(I–II) and cyclic 4b(I–II) in a 3 : 1 ratio in 98% overall yield. Acyclic compound 3b(I–II) was easily converted to cyclic compound 4b(I–II) using p-toluenesulfonic acid. When the mixture of enantiomers 4b(I–II) was subjected to hydrogenation, 1H NMR spectra showed only one doublet (~1.5 ppm) that corresponds to the exocyclic methyl group (ESI†). On the other hand, reduction of 3b(I–II) to 5b(1–IV) followed by cyclization gave a mixture of diastereoisomers 6b(I–IV) in 94% yield. Two sets of doublets for exocyclic methyl groups at 1.5 and 1.4 ppm were clearly resolved by NMR spectroscopy (ESI†). Similarly, when 1c was subjected to a reaction with 2, 3c(I–II) was obtained in 92% yield. 3c(I–II) upon reaction with p-toluenesulfonic acid gave cyclic product 4c(I–II) in 96% yield. When the mixture of enantiomers 4c(I–II) was subjected to hydrogenation, 1H NMR spectra of product 6c(I–II) showed only one doublet (~1.59 ppm) that corresponds to the exocyclic methyl group (ESI†). On the other hand, reduction of 3c(I–II) followed by cyclization gave a mixture of inseparable diastereoisomers 6c(I–IV) in 74% yield, which were clearly resolved by NMR spectroscopy (two doublets at 1.6 and 1.4 ppm respectively). This suggested that substitutions on isatin do not contribute to the observed facial selectivity during the reduction of the double bond.
Conclusions
We used isatin analogs and methyl 2-(bromomethyl)acrylate to synthesize the oxindole-α-methylene-γ-butyrolactone compounds (hybrid pharmacophore) by an indium mediated Barbier type reaction. Irrespective of isatin substitutions, a face selective reduction of the exocyclic double bond in the lactone ring was observed. Using 1D NOE difference NMR experiments, we established the stereochemistry of the newly created stereocenter alpha to the lactone carbonyl group. Synthesis and the structural–activity relationship of functionally modified spiro-oxindole compounds are currently under investigation and will be reported in due course.
Supplementary Material
Acknowledgements
This work was supported in part by the Eppley Cancer Center pilot grant, Nebraska Research Initiative and NIH R01CA127239. We would like to thank the Ed Ezell and the Eppley NMR facility and the Natarajan lab members for helpful discussions.
Footnotes
Electronic supplementary information (ESI) available: Experimental procedures, characterisation data, copies of 1H, 13C NMR, COSY, NOESY, 1D NOE difference NMR spectra. See DOI: 10.1039/c2ob27008k
Notes and references
- 1.(a) Kitson RRA, Millemaggi A, Taylor RJK. Angew. Chem., Int. Ed. 2009;48:9426. doi: 10.1002/anie.200903108. [DOI] [PubMed] [Google Scholar]; (b) Elford TG, Hall DG. Synthesis. 2010:893. doi: 10.1021/ja9104478. [DOI] [PubMed] [Google Scholar]
- 2.(a) Hoffmann HMR, Rabe J. Angew. Chem., Int. Ed. 1985;97:96. [Google Scholar]; (b) Picman AK. Biochem. Syst. Ecol. 1986;14:255. [Google Scholar]; (c) Kennedy JWJ, Hall DG. J. Org. Chem. 2004;69:4412. doi: 10.1021/jo049773m. [DOI] [PubMed] [Google Scholar]; (d) Kennedy JWJ. J. Am. Chem. Soc. 2002;124:11586. doi: 10.1021/ja027453j. [DOI] [PubMed] [Google Scholar]; (e) Janecki T, Blaszczyk E, Studzian K, Janecka A, Krajewska U, Rozalski M. J. Med. Chem. 2005;48:3516. doi: 10.1021/jm048970a. [DOI] [PubMed] [Google Scholar]; (f) Ramachandaran PV, Pratihar D, Niar HNG, Walters M, Smith S, Yip-Schneider MT, Wu H, Schmidt CM. Bioorg. Med. Chem. Lett. 2010;20:6620. doi: 10.1016/j.bmcl.2010.09.022. [DOI] [PubMed] [Google Scholar]
- 3.(a) Baeuerle PA, Baltimore D. Cell. 1988;53:211. doi: 10.1016/0092-8674(88)90382-0. [DOI] [PubMed] [Google Scholar]; (b) Ruben SM, Dillon PJ, Schreck R, Henkel T, Chen CH. Science. 1991;251:1490. doi: 10.1126/science.2006423. [DOI] [PubMed] [Google Scholar]; (c) Schmitz ML, Baeuerle PA. EMBO J. 1991;10:3805. doi: 10.1002/j.1460-2075.1991.tb04950.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.(a) Paquette LA, Mendez-Andino J. Tetrahedron Lett. 1999;40:4301. [Google Scholar]; (b) Choudhary PK, Foubelo F, Yus M. Tetrahedron Lett. 1998;39:3581. [Google Scholar]; (c) Loh TP, Lye PL. Tetrahedron Lett. 2001;42:3511. [Google Scholar]; (d) Lee KY, Park DY, Kim JN. Bull. Korean Chem. Soc. 2006;27:1489. [Google Scholar]; (e) Lee KH, Ibuka T, Kim SH, Vestel BR, Hall IH. J. Med. Chem. 1975;18:812. doi: 10.1021/jm00242a010. [DOI] [PubMed] [Google Scholar]; (f) Kabalka GW, Venkataiah B, Chen C. Tetrahedron Lett. 2006;47:4187. [Google Scholar]
- 5.(a) Trost BM, Jiang C. Synthesis. 2006:369. [Google Scholar]; (b) Williams RM, Cox RJ. Acc. Chem. Res. 2003;36:127. doi: 10.1021/ar020229e. [DOI] [PubMed] [Google Scholar]; (c) Galliford CV, Scheidt KA. Angew. Chem., Int. Ed. 2007;46:8748. doi: 10.1002/anie.200701342. [DOI] [PubMed] [Google Scholar]; (d) Marti C, Carreira EM. Eur. J. Org. Chem. 2003:2209. [Google Scholar]; (e) Lin H, Danishefsky SJ. Angew. Chem., Int. Ed. 2003;42:36. doi: 10.1002/anie.200390048. [DOI] [PubMed] [Google Scholar]
- 6.(a) Ma J, Li S, Reed K, Guo P, Gallo JM. J. Pharmacol. Exp. Ther. 2003;305:833. doi: 10.1124/jpet.102.048587. [DOI] [PubMed] [Google Scholar]; (b) Lane ME, Yu B, Rice A, Lipson KE, Liang C, Sun L, Tang C, McMahon G, Pestell RG, Wadler S. Cancer Res. 2001;61:6170. [PubMed] [Google Scholar]
- 7.(a) Romagnoli R, Baraldi PG, Carrion MD, Cruz-Lopez O, Preti D, Tabrizi MA, Fruttarolo F, Heilmann J, Bermejo J, Estevez F. Bioorg. Med. Chem. Lett. 2007;17:2844. doi: 10.1016/j.bmcl.2007.02.048. [DOI] [PubMed] [Google Scholar]; (b) Romagnoli R, Baraldi PG, Carrion MD, Cruz-Lopez O, Cara CL, Balzarini J, Hamel E, Canella A, Fabbri E, Gambari R, Basso G, Viola G. Bioorg. Med. Chem. Lett. 2009;19:2022. doi: 10.1016/j.bmcl.2009.02.038. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Gemma S, Campiani G, Butini S, Joshi BP, Kukreja G. J. Med. Chem. 2009;52:502. doi: 10.1021/jm801352s. [DOI] [PubMed] [Google Scholar]; (d) Rane RA, Telvekar VN. Bioorg. Med. Chem. Lett. 2010;20:5681. doi: 10.1016/j.bmcl.2010.08.026. [DOI] [PubMed] [Google Scholar]; (e) Francis WM, Akira I. Drug Dev. Res. 2010;71:20. [Google Scholar]; (f) Roth BL, Sheffler DJ, Kroeze WK. Nat. Rev. Drug Discovery. 2004;3:353. doi: 10.1038/nrd1346. [DOI] [PubMed] [Google Scholar]
- 8.(a) Choudhary PK, Foubelo F, Yus M. Tetrahedron Lett. 1998;39:3581. [Google Scholar]; (b) Sidduri AR, Knochel P. J. Am. Chem. Soc. 1992;114:7579. [Google Scholar]; (c) Lee KY, Park DY, Kim JN. Bull. Korean Chem. Soc. 2006;27:1489. [Google Scholar]
- 9.Ramirez-Gualito K, Alonso-Rios R, Quiroz-Garcia B, Rojas-Aguilar A, Diaz D, Jimenez-Barbero J, Cuevas G. J. Am. Chem. Soc. 2009;131:18129–18138. doi: 10.1021/ja903950t. [DOI] [PubMed] [Google Scholar]
- 10.(a) Trost BM, Xie J, Seiber JD. J. Am. Chem. Soc. 2011;133:20611–20622. doi: 10.1021/ja209244m. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Wilcox CF, Farley EN. J. Am. Chem. Soc. 1984;106:7195–7200. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.