Abstract
Purpose
The aim of this study was to evaluate the safety and efficiency, as well as the incorporation characteristics of a specific type of xenograft used for iliac crest defects post-harvesting tri-cortical iliac crest bone graft.
Methods
Sixteen patients diagnosed with chronic anterior pelvic pain were operated for pubic symphysis fusion. The tri-cortical graft harvested from the iliac crest was inserted into the pubic symphysis and compressed with a reconstruction plate. The defect in the iliac crest was filled with a block of cancellous bovine substitute (Tutobone®). The length of iliac crest defect, time to fusion of pubic symphysis, time to incorporation of the graft and complications were recorded. The postoperative pain and patients’ satisfaction were evaluated.
Results
The median age of patients was 36.5 years (range 27–75). Fusion was obtained in 15 patients (94 %). The median time to fusion was four months (range three to seven). The length of the iliac crest bone defect ranged from 40 to 70 mm. Integration of the bovine substitute was achieved in 15 patients (94 %) over a median period of three months (range two to six). The postoperative median pain score was 2 (range 1–5). Twelve patients (75 %) reported good satisfaction. No major complications or allergic reactions were observed.
Conclusions
The xenograft used in this study provided a safe and effective method of reconstruction of iliac crest donor site defects. It has satisfactory incorporation, high biocompatibility and no signs of inflammatory reactions. This new technique is simple and easily reproducible in routine clinical practice.
Introduction
Iliac crest bone grafts are considered to be the gold standard method in bone grafting procedures. Due to their osteogenic, osteoinductive and osteoconductive properties, they have been widely used in a variety of orthopaedic, neurosurgical, and maxillofacial procedures to promote bone healing and restore bone defects [1, 2].
Despite their many advantages, harvesting of iliac bone grafts can be associated with significant morbidity [3]. The overall complication rate ranges from 9 to 49 % [4] including infections, hematomas, seromas, hernias, persistent pain, fracture of the ilium and poor cosmetic outcome [5]. To decrease the risk of these potential complications, various harvesting methods have been described using trephines, specific reamer systems and special osteotomes or applying minimally invasive approaches [6]. Moreover, authors have proposed different protocols in order to control pain, which remains one of the major complications after iliac crest bone graft harvesting [1].
In cases where a graft possessing structural properties is needed, tri-cortical bone from the iliac crest can be harvested. This type of graft is usually large, its harvesting requires a bigger approach and results in the creation of considerable defects at the donor site of the iliac wing.
Reconstruction of the resulting iliac crest defect has been reported in several in vivo and clinical studies, with promising results. Different types of materials have been used, including degradable hydroxyapatite-bioactive glass composites, bioactive ceramic prosthesis and spacers, autogenous ribs, metallic plates and polyurethane bone graft substitutes [7–12].
Xenografts (tissue from different species) have also been utilised for a variety of clinical conditions in the form of deproteinized and defatted bovine bone, which has a chemical composition and architectural geometry that is almost identical to that of human bone [13–15].
All of the above graft materials have been used in order to minimize donor-site pain, to promote bone healing [10, 11, 16, 17], and to improve cosmesis [7–9, 18, 19] and functional outcome [12, 20–22].
The purpose of the present study was to determine the safety and efficiency, as well as the incorporation characteristics of a specific type of xenograft (cancellous bovine bone, Tutobone®, Tutogen Medical GmbH, Neunkirchen a. Brand, Germany) used for iliac crest defects post-harvesting tri-cortical iliac crest grafts.
Patients and methods
Between January 2005 and January 2010 patients diagnosed with chronic anterior pelvic pain secondary to osteoarthrosis, post-partum pelvic instability or pubis symphysis dysfunction and underwent pelvic reconstruction in the form of fusion requiring a tri-cortical graft harvested from the iliac crest were eligible to participate in this study. Exclusion criteria were patients that underwent fusion but a tricortical graft was not harvested as it was not deemed to be necessary and other type of graft material was used. All the patients were operated by the same orthopaedic surgeon.
Surgical technique
Under general anaesthesia and with the patient supine on a radiolucent table, a Phannesteal incision was used to expose the pubis symphysis. Using osteotomes, the cartilage was removed and a vascular bed was created in such way as to accept the fitting of the appropriate tricortical graft harvested based on the patient’s wish from either the left or the right iliac crest. For the harvesting of the tricortical graft, the first lateral window of the ilioinguinal approach was used. Using an electric saw the appropriate size was harvested based on the length of the bed prepared at the pubis symphysis. Fusion was facilitated by insertion and compression with a reconstruction plate of the tri-cortical graft into the pubic symphysis. The defect in the iliac crest was filled with a block of cancellous bovine substitute (Tutobone®; Fig. 1). The appropriate size of the substitute was measured and a press-fit technique was applied. Where appropriate, more than one Tutobone® xenograft cancellous block was inserted and compressed into the defect. Depending on the stability of the inserted graft as assessed intra-operatively and at the surgeon’s discretion, where it was felt to be necessary, a 3.5-mm screw was inserted fixing the xenograft block to the iliac blade for optimum stability.
Fig. 1.
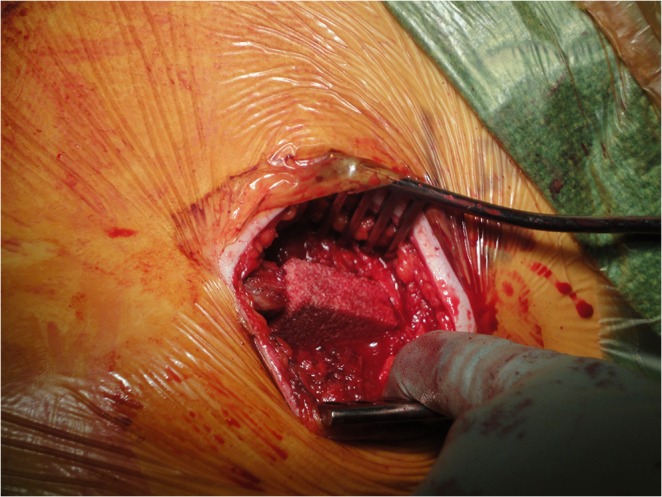
Placement of Tutobone graft in the iliac crest defect
The patients’ demographics, length of iliac crest defect, time to fusion of pubic symphysis, and time to incorporation of the Tutobone® were recorded. Radiological examination consisted of anteroposterior, inlet and outlet pelvic radiographs. Radiographic incorporation of the bone graft was reported by an independent musculoskeletal radiologist assessing the images taken at follow-up. Incorporation was defined as bridging of the interface between the graft and the native bone by bone, callus, or trabeculae and obliteration of the graft interface [23, 24].
The degree of pain and tenderness in deep palpation over the donor site was evaluated with the use of a visual analogue scale at the final follow-up appointment. All donor-site-related complaints or complications were recorded. An assessment tool for patients’ subjective satisfaction at the final follow-up visit was used consisting of a 3-category scale (patient comfortable with the donor site, A; patient with some problems at the donor site, B; patient not satisfied at all with the donor site, C). The minimum follow-up was 18 months (range 18–61).
Results
Out of 43 patients, 16 met the inclusion criteria (Table 1). There were 15 females (94 %) and one male (6 %), with a median age of 36.5 years (range 27–75). The indication for surgery in most of the cases (68.7 %) was pregnancy related pelvic girdle pain syndrome (PPGP) according to the algorithm of management as previously described [25]. The rest had their pubic symphysis fused as a result of a pelvic fracture or pubis osteitis [26].
Table 1.
Basic characteristics of the cohort of patients where the Tutobone–Bovine xenograft was used to cover the created defect at the iliac crest of patients post tricortical autograft harvest
| Number of patients | Gender | Age (years) | Indication for tricortical bone harvest | Time to successful symphysis pubis fusion (months) | Complications, secondary procedures | Time to successful radiological incorporation of the Xenograft (months) | Last follow-up (months) | VAS pain/tenderness at iliac crest grafted defect | Pain description | Subjective satisfaction |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 31 | PSD – PPGP | 5 | Local hematoma | 2.5 | 12 | 0/10 | None | A |
| 2 | F | 58 | PSD – PPGP | 5.5 | Prominence of plate at PS | 2 | 21 | 1–2/10 | Occasional | A |
| 3 | F | 31 | PSD – PPGP | 8 | Bilateral SIJ fusions | 3 | 39 | 1/10 | Swelling | A |
| 4 | F | 27 | PSD – PPGP | 4 | - | 2 | 26 | 1–2/10 | Occasional | A |
| 5 | F | 31 | PSD – PPGP | 7 | Bilateral SIJ fusions | no incorporation | 34 | 1–2/10 | Occasional | A |
| 6 | F | 58 | PSD – PPGP | 8 | - | 4 | 35 | 5/10 | Skin sensitivity | B |
| 7 | F | 33 | PSD – PPGP | 5 | - | 2 | 36 | 9/10 | In pressing | C |
| 8 | F | 35 | PSD – PPGP | 11 | - | 6 | 31 | 1–2/10 | Feels itchy | A |
| 9 | F | 59 | Non-union PFX | 8 | Bilateral SIJ fusions | 3 | 34 | 2/10 | Occasional - when lying on side | A |
| 10 | F | 48 | PSD – PPGP | 12 | Inguinal hernia | 3 | 39 | 0/10 | None | A |
| 11 | F | 75 | PFX | no fusion | Infection at PS fusion - removal of metalwork | 2 | 16 | 0/10 | None | A |
| 12 | F | 36 | Non-union PFX | 6 | Prominence at the iliac crest | 6 | 24 | 3/10 | In cold weather | A |
| 13 | M | 46 | Non-union PFX | 9 | - | 4 | 16 | 2/10 | Persistent | A |
| 14 | F | 27 | PSD – PPGP | 10 | - | 2 | 56 | 1–2/10 | Occasional | A |
| 15 | F | 37 | PSD - unsuccessful fusion | 8 | Prominence at the iliac crest | 4 | 22 | 4/10 | In cold weather &skin sensitivity | A |
| 16 | F | 62 | PFX | 8 | - | 3 | 61 | 0/10 | None | A |
| Median | 36.5 | 8 | 3 | 32.5 | 1–2/10 | |||||
| Range | 25–75 | 4–12 | (2–5) | (12–61) | (0/10–9/10) | |||||
A patient comfortable with the donor site, B patient with some problems at the donor site, C patient not satisfied at all with the donor site, f female, m male, PFX pelvic fracture, PPGP pregnancy-related pelvic girdle pain syndrome, PS pubis symphysis, PSD pubis sumphysis dysfunction, SIJ sacroiliac joint/s, VAS visual analogue scale
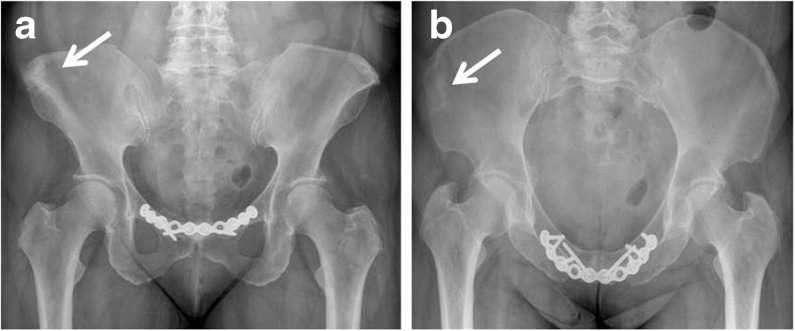
Fusion of pubic symphysis was obtained in all but one patient (94 %; Fig. 2). The median time to fusion was four months (range three to seven months). There was one failed fusion of the symphysis secondary to infection requiring removal of the plate and administration of antibiotics with no further consequences. In another patient a palpable mass over the pubis symphysis was detected, due to the prominence of the reconstruction plate. The length of the iliac crest bone defect post-harvest ranged from 40 to 70 mm. Integration of the bovine substitute was achieved in 15 patients (94 %) over a median period of three months (range two to six).
Fig. 2.
a Antero-posterior pelvic view showing pubis symphysis fusion and complete incorporation of the xenograft within 3 months post-surgery (patient no. 2). b Inlet pelvic view showing complete incorporation of the xenograft within 3 months post-surgery (patient no. 2). White arrow indicates incorporation site of xenograft
At the last follow-up visit (median 32.5 months, range 18–61 months) the postoperative median visual analogue scale pain/tenderness score was 2 (range 1–5). Of the 16 patients, 12 (75 %) stated no or minimal (1–2/10) pain at the donor site. One patient had sharp pain on direct pressure at the harvesting site and two patients had moderate pain during cold weather. One patient had moderate pain with light touch at the skin incision. Overall, three patients complained of cosmetic irregularity and scar hypersensitivity. According to the subjective 3-scale assessment, 14 patients (87.5 %) were at state A, one at state B and one at state C (Table 1).
Regarding the postoperative complications, there was one patient with wound hematoma at the donor site that was resolved without any intervention. No allergic reactions were recorded with this bovine graft. In one patient, the substitute did not show incorporation, but no further symptoms were reported. All patients reported the substitute felt as part of their body, and they did not have any feelings of movement or instability at the grafted site. In two of them the incorporated xenograft was palpable as a deformity to the congruency of the iliac crest. There were no muscle hernias or neuropathies at the harvesting site of the iliac crest.
Discussion
Minimally invasive techniques for harvesting iliac crest bone graft have evolved in an attempt to reduce donor site morbidity and to increase the harvested volume [27]. Despite their reported advantages such as a reduced risk of infection, less chance of fracture of the ilium, reduced blood loss and operating time, these approaches are still problematic. In the case of tri-cortical autologous iliac crest bone harvesting, local donor site morbidity is significant since the created bone defect is large, palpable, increases the risk of abdominal contents’ hernias and exposes bleeding surfaces of cancellous bone predisposing to hematomas, seromas and infections.
This cohort study describes the safety and efficacy of a specific surgical technique of filling the corticocancellous defect at the donor site. Certain limitations have to be considered in the herein report including the small number of cases evaluated, the retrospective nature of the study and the lack of a control group. However, this is the first report in the literature of the use of this specific xenograft and technique. Strengths of this study include the long follow-up time and the consistency of the surgical technique as one surgeon performed all the procedures. Assessment of graft’s incorporation was based on the report of a senior radiologist being independent from the surgical team and as such any potential bias from the analysis of the radiographs by the surgical team was eliminated. A strength of the study can also be considered the fact that the radiological evidence obtained was supplemented by the clinical findings and the subjective patient-reported outcome.
In general terms, reconstruction of iliac crest bone defects using this cancellous bovine substitution material was associated with a satisfactory radiological and clinical outcome. In clinical practice it offered an easy-to-reproduce technique of addressing sizable donor-site defects after harvesting of tri-cortical iliac bone graft. Graft incorporation is usually related to the vitality of the host bed and the amplitude of local pre-osteogenic or osteogenic cell populations. We believe that the iliac host bed was not only well vascularised but also rich in osteo-progenitor cells [34]. Moreover, the custom-made sizing of the inserted graft and the press fit insertion, supplemented when needed by screw fixation, offered the required local stability, maximising graft incorporation.
The xenograft serves primarily as void filler that allows osteo-conduction of the host cells into its mass, resulting in progressive incorporation of the graft into the host bone. Incorporation is a series of events (creeping substitution) including gradual replacement of grafted bone by host bone through a mechanism of osteoclastic resorption followed by deposition of new bone [28]. Experimentally, about half of a massive cortical bone autograft will be replaced by living bone after six months of implantation [29]. However, in human allografts or xenografts substitution of less than 10 % has been observed at the same period of time [30].
The bovine cancellous xenograft used in this study offers many advantages. Its biomechanical characteristics have been well tested indicating no structural deficits after its chemical processing [31]. The main indications are areas where trabecular bone is required, filling in of bone defects and plastic reconstruction of damaged bone regions. Moreover, these indications can be extended to iliac crest defects of any size and shape, since it can be fashioned without difficulty.
In a recent retrospective study, the clinical and radiological outcomes of subtalar fusion with and without bovine cancellous bone grafting were evaluated [32]. High rates of failure were found and the authors advised an extreme caution when considering the use of this material in subtalar joint fusions. However, they recognized that vascular insufficiency, which usually characterizes the patients undergoing subtalar fusion, could have contributed to the poor graft integration.
In contrast, Lakdawala et al. [33] reported satisfactory outcomes with good osseointegration with the use of Tutobone® in patients who underwent revision knee arthroplasty. Furthermore, Meyer et al. [34] evaluated the histological osseointegration of this substitute used in high tibial osteotomy and revision hip arthroplasty in humans. Analysis of the biopsies showed high biocompatibility of the material without any fibrous tissue filled interface. Similarly, the clinical and radiological outcome of the patients in the present study indicated a good incorporation of the xenograft, no loss of alignment and no loss of bone load or infection. The majority of patients were subjectively satisfied having minimal or no pain and no major complications.
Similar case series exist reporting on other methods of addressing the same problem and offer the comparative groups of cases for the present cohort (Table 2) [7–12, 16, 19–22].
Table 2.
Comparison of different materials used in reconstruction of the iliac crest defects
| Authors, Year | No. of cases; Material used to cover the iliac crest defect |
Defect length | Graft incorporation | Pain after surgery | Patients’ satisfaction | Allergic reaction |
|---|---|---|---|---|---|---|
| Asano et al. (1994) [10] | 60 | 15–70 mm | 98.3 % | 93 % no pain | 95 % excellent | 0 |
| Bioactive ceramic (apatite wollastonite-containing glass ceramic) | ||||||
| Ito et al. (2005) [11] | 31 | 15–70 mm | 84 % | 84 % no pain | 78 % excellent | 0 |
| Bioactive ceramic (apatite wollastonite-containing glass ceramic) | ||||||
| Acharya et al. (2010) [9] | 26 | n/a | 80.7 % | 96 % no pain | n/a | 0 |
| Bioactive ceramic (Chitra hydroxyapatite-bioactive glass ceramic) | ||||||
| Bojescul et al. (2005) [19] | 10 | n/a | 100 % | Significantly lower | n/a | 0 |
| ProOsteonTM-Coralline hydroxy-apatite | ||||||
| Harris et al. (1994) [22] | 28 | n/a | 100 % | 100 % “acceptable levels” | n/a | 0 |
| Autologous rib | ||||||
| Defino and Rodriguez-Fuentes (1999) [12] | 15 | n/a | 86.7 % | 93 % no pain | 100 % satisfied | 0 |
| Autologous rib | ||||||
| Bapat et al. (2008) [20] | 20 | >25 mm | 95 % | Significantly lower | n/a | 0 |
| Autologous rib | ||||||
| Dave et al. (2007) [21] | 26 | 3.5–5 mm | 100 % | 100 % no pain | n/a | 0 |
| Autologous rib | ||||||
| Halsnad et al. (2004) [7] | 4 | n/a | n/a | 100 % less pain | 100 % satisfied | 0 |
| Metal plate | ||||||
| Huemer et al. (2004) [8] | 2 | n/a | n/a | 100 % less pain | 100 % satisfied | 0 |
| Metal plate | ||||||
| Gil-Albarova and Gil-Albarova (2011) [16] | 9 | n/a | 100 % | 100 % no pain | 8.2 (median, scale 0–10) | 0 |
| Autologous graft | ||||||
| Present study (2011) | 16 | 40–70 mm | 94 % | Median VAS of 3 (0–9) | 87.5 % “very satisfied” | 0 |
| Tutobone, Xenograft |
n/a not applicable or not available, VAS visual analogue scale
Bapat et al. [20] and Dave et al. [21] used rib autograft for reconstruction of the iliac crest during surgery of thoracic spine. The rib, which was removed at the time of thoracotomy, was used and fixed to the iliac crest with a press-fit technique after fashioning it from both ends. No rib graft complication was recorded, the graft was well incorporated and the patients had significantly lower pain. Rightfully, the authors stated that this technique is limited to patients undergoing thoracotomy or thoraco-phreno-lumbotomy for spinal reconstruction.
Bojescul et al. [19] reported a prospective randomized study of coralline hydroxyapatite used to backfill iliac crest donor sites versus no void filler in five and seven cases respectively. Seventy-five percent of patients showed bone ingrowth on plain radiographs and CT scan, and 100 % showed biological activity on technetium bone scans. All patients reported mild pain to no pain postoperatively. However, the sample of patients was small and follow-up was limited to 12 months.
Japanese authors [10, 11] have investigated the long-term results of bioactive ceramic spacers and their efficacy in reconstruction of the iliac donor site defect. They reported satisfactory outcome in high percentages, concluding that ceramic materials can be beneficial for reconstruction of the iliac crest defects.
Recently, a cost-effective technique proposed by Gil-Albarova [16] included the use of bone graft obtained from the bone defect itself. A transverse fence of appropriate thin tri-cortical chips taken from the posterior lateral wall of the donor site was used to reconstruct the iliac crest. No hardware was placed and bone defect healing was satisfactory. However, further studies are needed to prove the efficacy of this technique in reconstruction of larger defects.
Conclusion
Cancellous bovine xenograft used in this study provides a safe and effective method of reconstruction of iliac crest donor site defects. It has satisfactory incorporation, high biocompatibility and no signs of inflammatory reactions. This new technique is simple, relatively fast and easily reproducible in routine clinical practice. A larger series of patients and prospective control studies are required to expand its use and verify the promising results.
Acknowledgments
Conflict of interest
No benefits have been received in any form by any of the authors with regard to the preparation of this manuscript. All authors declare that there is no conflict of interest.
References
- 1.Mazock JB, Schow SR, Triplett RG. Posterior iliac crest bone harvest: review of technique, complications, and use of an epidural catheter for postoperative pain control. J Oral Maxillofac Surg: Off J Am Assoc Oral Maxillofac Surg. 2003;61:1497–1503. doi: 10.1016/j.joms.2003.03.001. [DOI] [PubMed] [Google Scholar]
- 2.Guerado E, Fuerstenberg CH. What bone graft substitutes should we use in post-traumatic spinal fusion? Injury. 2011;42(Suppl 2):S64–S71. doi: 10.1016/j.injury.2011.06.200. [DOI] [PubMed] [Google Scholar]
- 3.Dimitriou R, Mataliotakis GI, Angoules AG, Kanakaris NK, Giannoudis PV. Complications following autologous bone graft harvesting from the iliac crest and using the RIA: a systematic review. Injury. 2011;42(Suppl 2):S3–S15. doi: 10.1016/j.injury.2011.06.015. [DOI] [PubMed] [Google Scholar]
- 4.Banwart JC, Asher MA, Hassanein RS. Iliac crest bone graft harvest donor site morbidity. A statistical evaluation. Spine. 1995;20:1055–1060. doi: 10.1097/00007632-199505000-00012. [DOI] [PubMed] [Google Scholar]
- 5.Younger EM, Chapman MW. Morbidity at bone graft donor sites. J Orthop Trauma. 1989;3:192–195. doi: 10.1097/00005131-198909000-00002. [DOI] [PubMed] [Google Scholar]
- 6.Westrich GH, Geller DS, O'Malley MJ, Deland JT, Helfet DL. Anterior iliac crest bone graft harvesting using the corticocancellous reamer system. J Orthop Trauma. 2001;15:500–506. doi: 10.1097/00005131-200109000-00007. [DOI] [PubMed] [Google Scholar]
- 7.Halsnad SM, Dhariwal DK, Bocca AP, Evans PL, Hodder SC. Titanium plate reconstruction of the osseous defect after harvest of a composite free flap using the deep circumflex iliac artery. Br J Oral Maxillofac Surg. 2004;42:254–256. doi: 10.1016/j.bjoms.2004.01.015. [DOI] [PubMed] [Google Scholar]
- 8.Huemer GM, Puelacher W, Schoeller T. Improving the iliac crest donor site by plate insertion after harvesting vascularized bone. J Craniomaxillofac Surg. 2004;32:387–390. doi: 10.1016/j.jcms.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 9.Acharya NK, Mahajan CV, Kumar RJ, Varma HK, Menon VK. Can iliac crest reconstruction reduce donor site morbidity?: a study using degradable hydroxyapatite-bioactive glass ceramic composite. J Spinal Disord Tech. 2010;23:266–271. doi: 10.1097/BSD.0b013e3181a990fc. [DOI] [PubMed] [Google Scholar]
- 10.Asano S, Kaneda K, Satoh S, Abumi K, Hashimoto T, Fujiya M. Reconstruction of an iliac crest defect with a bioactive ceramic prosthesis. Eur Spine J. 1994;3:39–44. doi: 10.1007/BF02428315. [DOI] [PubMed] [Google Scholar]
- 11.Ito M, Abumi K, Moridaira H, Shono Y, Kotani Y, Minami A, Kaneda K. Iliac crest reconstruction with a bioactive ceramic spacer. Eur Spine J. 2005;14:99–102. doi: 10.1007/s00586-004-0765-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Defino HL, Rodriguez-Fuentes AE. Reconstruction of anterior iliac crest bone graft donor sites: presentation of a surgical technique. Eur Spine J. 1999;8:491–494. doi: 10.1007/s005860050211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Scotti C, Wirz D, Wolf F, Schaefer DJ, Burgin V, Daniels AU, Valderrabano V, Candrian C, Jakob M, Martin I, Barbero A. Engineering human cell-based, functionally integrated osteochondral grafts by biological bonding of engineered cartilage tissues to bony scaffolds. Biomaterials. 2010;31:2252–2259. doi: 10.1016/j.biomaterials.2009.11.110. [DOI] [PubMed] [Google Scholar]
- 14.Kubosch D, Milz S, Sprecher CM, Sudkamp NP, Muller CA, Strohm PC. Effect of graft size on graft fracture rate after anterior lumbar spinal fusion in a sheep model. Injury. 2010;41:768–771. doi: 10.1016/j.injury.2009.08.014. [DOI] [PubMed] [Google Scholar]
- 15.Candrian C, Barbero A, Bonacina E, Francioli S, Hirschmann MT, Milz S, Valderrabano V, Heberer M, Martin I, Jakob M. A novel implantation technique for engineered osteo-chondral grafts. Knee Surg Sports Traumatol Arthrosc. 2009;17:1377–1383. doi: 10.1007/s00167-009-0766-4. [DOI] [PubMed] [Google Scholar]
- 16.Gil-Albarova J, Gil-Albarova R (2011) Donor site reconstruction in iliac crest tricortical bone graft: surgical technique. Injury. 11 Oct 2011 [Epub ahead of print] [DOI] [PubMed]
- 17.Gogolewski S, Gorna K, Turner AS. Regeneration of bicortical defects in the iliac crest of estrogen-deficient sheep, using new biodegradable polyurethane bone graft substitutes. J Biomed Mater Res A. 2006;77:802–810. doi: 10.1002/jbm.a.30669. [DOI] [PubMed] [Google Scholar]
- 18.Resnick DK. Reconstruction of anterior iliac crest after bone graft harvest decreases pain: a randomized, controlled clinical trial. Neurosurgery. 2005;57:526–529. doi: 10.1227/01.NEU.0000170558.70876.E3. [DOI] [PubMed] [Google Scholar]
- 19.Bojescul JA, Polly DW, Jr, Kuklo TR, Allen TW, Wieand KE. Backfill for iliac-crest donor sites: a prospective, randomized study of coralline hydroxyapatite. Am J Orthop. 2005;34:377–382. [PubMed] [Google Scholar]
- 20.Bapat MR, Chaudhary K, Garg H, Laheri V. Reconstruction of large iliac crest defects after graft harvest using autogenous rib graft: a prospective controlled study. Spine. 2008;33:2570–2575. doi: 10.1097/BRS.0b013e318185287d. [DOI] [PubMed] [Google Scholar]
- 21.Dave B, Modi H, Gupta A, Nanda A. Reconstruction of iliac crest with rib to prevent donor site complications: A prospective study of 26 cases. Indian J Orthop. 2007;41:180–182. doi: 10.4103/0019-5413.33678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harris MB, Davis J, Gertzbein SD. Iliac crest reconstruction after tricortical graft harvesting. J Spinal Disord. 1994;7:216–221. doi: 10.1097/00002517-199407030-00003. [DOI] [PubMed] [Google Scholar]
- 23.Corrales LA, Morshed S, Bhandari M, Miclau T., 3rd Variability in the assessment of fracture-healing in orthopaedic trauma studies. J Bone Joint Surg Am. 2008;90:1862–1868. doi: 10.2106/JBJS.G.01580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shibuya N, Jupiter DC, Clawson LD, La Fontaine J (2012) Incorporation of bovine-based structural bone grafts used in reconstructive foot surgery. J Foot Ankle Surg 51(1):30–33 [DOI] [PubMed]
- 25.Kanakaris NK, Roberts CS, Giannoudis PV. Pregnancy-related pelvic girdle pain: an update. BMC Med. 2011;9:15. doi: 10.1186/1741-7015-9-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kanakaris NK, Angoules AG, Nikolaou VS, Kontakis G, Giannoudis PV. Treatment and outcomes of pelvic malunions and nonunions: a systematic review. Clin Orthop Relat Res. 2009;467:2112–2124. doi: 10.1007/s11999-009-0712-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sharma S, Schneider LF, Barr J, Aarabi S, Chibbaro P, Grayson B, Cutting CB. Comparison of minimally invasive versus conventional open harvesting techniques for iliac bone graft in secondary alveolar cleft patients. Plast Reconstr Surg. 2011;128:485–491. doi: 10.1097/PRS.0b013e31821b6336. [DOI] [PubMed] [Google Scholar]
- 28.Delloye C, Cornu O, Druez V, Barbier O. Bone allografts: What they can offer and what they cannot. J Bone Joint Surg Br. 2007;89:574–579. doi: 10.2106/JBJS.E.00943. [DOI] [PubMed] [Google Scholar]
- 29.Enneking WF, Burchardt H, Puhl JJ, Piotrowski G. Physical and biological aspects of repair in dog cortical-bone transplants. J Bone Joint Surg Am. 1975;57:237–252. [PubMed] [Google Scholar]
- 30.Enneking WF, Mindell ER. Observations on massive retrieved human allografts. J Bone Joint Surg Am. 1991;73:1123–1142. [PubMed] [Google Scholar]
- 31.Thull R, Sturm A, Pesch H-J. Mechanische Eigenschaften nativer und praparierter Spongiosa. In: Pesch H-J, Stob H, Kummer B, editors. Osteologie aktuell VII. Heideberg: Springer; 1993. pp. 157–163. [Google Scholar]
- 32.Patil S, Auyeung J, Gower A. Outcome of subtalar fusion using bovine cancellous bone graft: a retrospective case series. J Foot Ankle Surg. 2011;50:388–390. doi: 10.1053/j.jfas.2011.04.019. [DOI] [PubMed] [Google Scholar]
- 33.Lakdawala A, Todo S, Scott G. The significance of surface changes on retrieved femoral components after total knee replacement. J Bone Joint Surg Br. 2005;87:796–799. doi: 10.1302/0301-620X.87B6.15776. [DOI] [PubMed] [Google Scholar]
- 34.Meyer S, Floerkemeier T, Windhagen H. Histological osseointegration of Tutobone: first results in human. Arch Orthop Trauma Surg. 2008;128:539–544. doi: 10.1007/s00402-007-0402-z. [DOI] [PubMed] [Google Scholar]