Abstract
Purpose
The aim of the study was to verify the ability of nanoparticulate bioactive glass (BAG) to infiltrate into the porous titanium (Ti) layer on Ti-based implants to promote osseointegration.
Methods
The porous titanium layer on Ti-based implants was impregnated with nanoparticulate BAG. The implants without or with BAG were implanted bilaterally in tibial holes of ten New Zealand white rabbits. The rabbits were sacrificed after ten weeks for examinations. Beside histological examination, EDXS analysis of polished cross-sections of explanted implants was also performed with the aim to quantitatively evaluate the bone-to-pore contact and bone-in-pore ratio.
Results
After ten weeks, EDXS analyses of cross-sections of the explanted implants confirmed that bioactive glass was fully resorbed and that the pores throughout the thickness of the porous titanium layer were to a large extent filled with a new bone. In the absence of bioactive glass, only the outer part of the porous layer was filled with bone. The implants without BAG in the porous Ti-layer exhibited similar bone-to-pore contact, while significant improvement of bone ingrowth into the pores was observed for the implants with BAG (38%), as opposed to those without it (22%).
Conclusion
This study confirmed that the nanoparticulate bioactive glass within the porous titanium surface layer on implants promotes osseointegration and stimulates the formation of bone within the pores.
Introduction
Titanium-based implants are the most widely used in orthopaedics and dentistry. Since they are not bioactive, the osseointegration is limited and, therefore, to improve the fixation, different implant designs and surface modifications are used. Implant surfaces are modified mechanically (surface roughening, porous coatings) or chemically (bioactive coatings). Porous metallic coatings with their open and interconnected pores are designed to allow bone to grow into the pores and thus to improve stability of the implant. Porous coatings also reduce the large difference in stiffness between bone and implant [1].
To improve osseointegration, metallic implants are often coated with bioactive coatings such as hydroxyapatite. Hydroxyapatite coatings exhibit strong osteoconductive capacity based on similar chemical composition to natural bone and are being used in clinical practice. They have been also successfully applied to treat bone or articular cartilage defects, and to induce bone ingrowth into and/or onto the soft tissue [2, 3]. Another bioactive material—bioactive glass (BAG) that bonds to living bone trough the apatite layer formed on the surface of the implant—has been confirmed in several studies as highly resorbable and bioactive. It has been shown that BAG stimulates angiogenesis and has an antibacterial effect [4–7]. Their potential to bond to hard, as well as soft tissue, depends on their composition and structure. Despite substantial osteogenic properties, the use of bioactive glass is still limited to non-load bearing applications due to its brittleness. To expand its use to load bearing applications, bioactive glass can be applied as a coating on metals, using one of the various proposed techniques, such as plasma spraying [8], pulsed laser deposition [9], dip-coating in sol [10], etc. These techniques are, however, unable to produce coatings within porous metallic surface layers.
Recent development of nanoparticulate bioactive glass powder in combination with a novel approach [11] enabled preparation of orthopaedic implants with porous metallic surface layer containing bioactive glass within the pores. While commercially available bioactive glass formulations are produced by conventional means, i.e. by melting and milling, the BAG powder used in this study is produced in a direct process by particulate sol–gel synthesis. Consequently, contamination with milling bodies is excluded, and the particles are much smaller, which helps to provide faster bioresorption. With the goal to prove its effectiveness in bone repair, an in-vivo (animal) study was performed. In the present paper, we discuss osseointegration of Ti-alloy implants having a porous titanium surface layer with or without BAG in the pores.
Materials and methods
For the in vivo tests, we used cylindrical Ti6Al4V implants (3 mm x 6 mm) with a ∼ 300-μm thick, porous Ti-layer (vacuum plasma sprayed; Alhenia, Switzerland), with interconnected pores in the size range from 20 to 100 μm. The porous Ti-layers were filled with a bioactive glass powder containing SiO2, CaO, Na2O and P2O5, with particle size from 100 nm to 1 μm, and thermally treated to form a solid, porous BAG structure within the pores. The implants with BAG-impregnated porous Ti-layers were taken from the furnace in a sterile environment, sealed and gamma-sterilized. Implants without bioactive glass in the Ti-layer were tested for comparison.
Surgical procedure
The animal tests were approved by the Veterinary Administration of the Republic of Slovenia. Principles of laboratory animal care (NIH publication No. 85–23, revised 1985) were followed, as well as European and specific national laws (e.g. the current version of the Slovenian Law on the Protection of Animals) where applicable. Ten mature male New Zealand rabbits (mean weight 4.5 ± 1.0 kg) underwent the operative procedure: 12 implants with BAG and eight without BAG were implanted in both rabbit proximal tibias. Anaesthesia was induced by intravenous administration of 0.08 mg/kg acetylpromazine (Vanastress; Vana GmbH, Germany), 30 mg/kg ketamine (Narketan, Vetoquinol) and 3 mg/kg xylazine (Chanazine, Chanelle Pharmaceuticals Manufacturing Ltd) and maintained by intravenous injection of 3 mg/kg propofol (Propofol, Fresenius). A skin incision was made over the medial border of the tibial tuberosity, approximately 10-mm distal to the knee joint, this was followed by dissection to the periosteum and retraction of the tibialis anterior muscle laterally. Then a hole was drilled into the proximal tibial metaphysis at a 90° angle relative to the long axis of the tibia, using a 3.2-mm drill bit. A press-fit implant was pushed into the hole, and the wound was closed in a routine fashion. Ten weeks post-surgery the animals were sacrificed by the administration of the above-mentioned sedation, followed by intravenous administration of T61 euthanasia solution (Intervet/Schering-Plough). The implants were explanted by en-bloc resection of the proximal tibia.
Specimen preparation
Specimens were removed from the formaldehyde, rinsed with 0.2 M cacodylate buffer, dehydrated through a graded series of ethanol, and embedded in Epon (Epoxy Embedding kit, Sigma-Aldrich, Switzerland). The embedded samples were sectioned vertically along the long axes of the implant using a circular water-cooled diamond saw. One cross-section was ground with SiC papers and polished with diamond paste for scanning electron microscopy (SEM) examination, energy-dispersive X-ray analyses (EDXS), and for quantitative analyses of bone ingrowth. The second half was used for further parallel cutting of thin slices for histological examination, which were then additionally thinned to 50–70 μm by grinding, and then finally stained with Van Gieson picrofuchsin red and Stevenel’s blue.
Quantitative analyses of bone ingrowth
The quantitative analysis of bone ingrowth into the pores and bone-to-implant contact was performed on SEM images (JEOL JSM 7600 F) of polished cross-sections of the explanted samples mounted in Epon. To clearly identify the position, amount and composition of the newly formed bone, EDXS analyses (X-ray mapping, point analysis) were performed. For quantification of the volume percentage of bone in pores, we used manual stereological analysis with a standard point-count technique [12]. The relevant area was carefully selected so that only the porous titanium layer was evaluated. The lineal fraction of the bone-to-implant contact was measured using Image Tool software (A Division of Evans Technology, Inc., USA).
Results
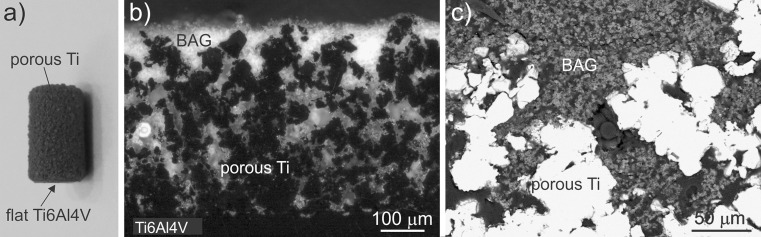
Figure 1a shows the macroscopic view of an implant used in this study. Cross-sections of the implant with BAG-infiltrated porous Ti-layer are presented in Fig. 1b and c (different magnifications). In the optical micrograph (Fig. 1b) the bioactive glass appears as a light phase within the porous titanium layer (dark phase) on the bulk T6Al4V substrate (dark phase in the lower part of the image). It is obvious that the bioactive glass has impregnated the porous titanium layer throughout the whole thickness of layer. Conversely, in the SEM image (Fig. 1c) the metal is white, while the bioactive glass appears as grey porous phase with a few micrometers of large partially-sintered particles. The BAG is also found in very small pores.
Fig. 1.
a Macroscopic image of bone implants used in in vivo test. b Optical micrograph (polarized light) of polished cross-section of the Ti6Al4V implant with porous titanium layer impregnated with bioactive glass. c SEM micrograph at higher magnification
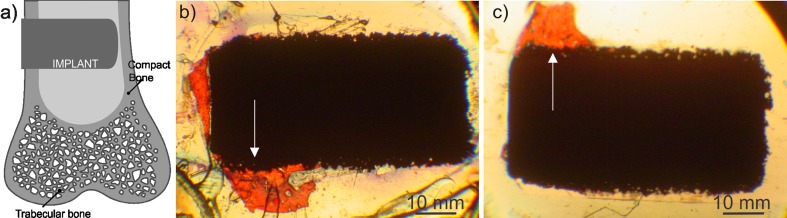
Figure 2a illustrates the position of the implants in the rabbit tibia: only a small part of the 6-mm long cylinder (<2 mm) was fixed in the compact bone, while the rest extended into the bone marrow. Some of the implants were in good contact with bone only on one side. The bone overgrew the outer part of the implant fixed in compact bone, and further expanded on the part positioned in the bone marrow (Fig. 2b). The area of interest was within this 2 mm of the bone-implant interface, as indicated by arrows in Fig. 2b and c.
Fig. 2.
a Implant position in the bone tibia. b Macroscopic images of the histological preparations of samples with bioactive glass (BAG) and without BAG (c). The arrows show the area of interest (the part of the implant in contact with compact bone). Mineralized bone is stained red and the implant is black
Metallographic examination of polished cross-sections
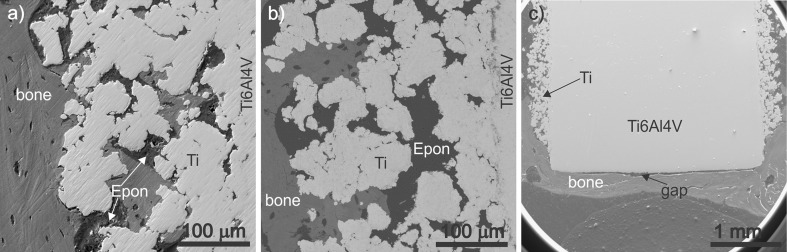
Polished cross-sections of the samples were first observed by scanning electron microscopy (SEM). This technique enables not only morphological examination of metal and bone, but also chemical analyses of the phases by energy-dispersive X-ray spectroscopy (EDXS). Characteristic areas of the polished cross-sections of the samples with or without BAG are presented in Fig. 3a and b, respectively. It is evident that, in both cases, the bone has grown over the metal as well as into the pores of the titanium layer. However, there is a clear difference in the depth of bone growth within the porous titanium layer: the bone overgrew the whole porous area of the samples with BAG within the pores (Fig. 3a), while in the case of the samples without BAG, only the outer part of the porous layer is occupied with the newly formed bone (Fig. 3b). In addition, in both types of samples relatively good bone-to-implant contact can be observed.
Fig. 3.
Scanning electron micrographs of polished cross-section of the sample (a) with bioactive glass (BAG) and without BAG (b). c Lower magnification image of the BAG-containing sample with and without porous titanium layer
A comparison between the porous titanium layer and flat Ti-alloy surface can be seen in Fig. 3c. On the flat side of the implant, a large gap between the implant and the bone was formed that indicates that bone-to-implant contact is much worse for flat than for rough surfaces.
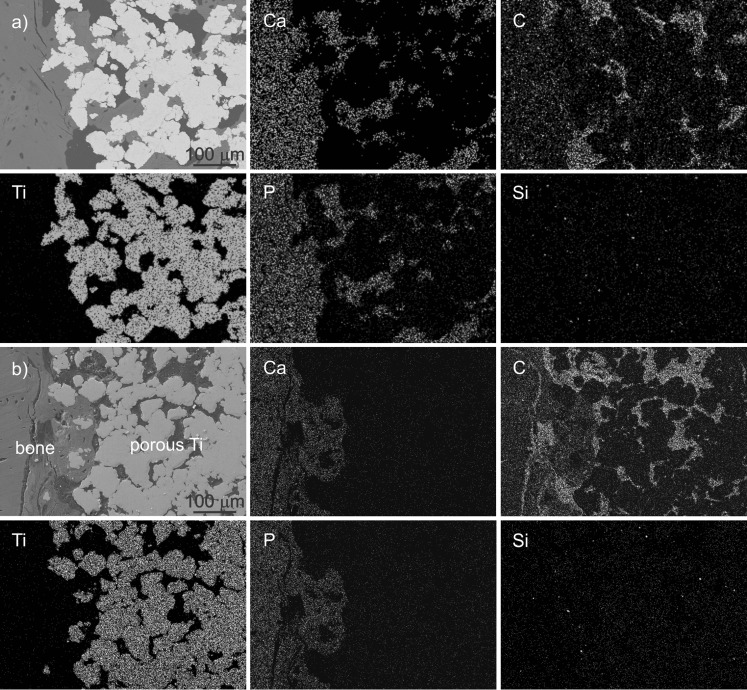
The presence of bone in the porous titanium layer was confirmed by EDXS analysis of the polished cross-sections of the explanted grafts. The atomic ratio of calcium-to-phosphorus content was determined to be close to 1.7, which corresponds to the natural mineral phase of the bone. Distribution of elements within the porous titanium layers with or without the BAG is illustrated in Fig. 4a and b, respectively. The light dots indicate areas with higher concentration of an analysed element. The implant material is identified with high content of Ti, the carbon (C)-rich areas confirm the presence of Epon resin in which samples were mounted, while high calcium (Ca) and phosphorus (P) concentrations in the ratio of ∼1.7 indicate the presence of mineralized bone. Silicon (Si) distribution revealed whether some BAG remained in the pores.
Fig. 4.
a Energy-dispersive X-ray analyses (EDXS) mappings of bone implant cross-sections for the sample with bioactive glass (BAG), showing from left to right on top: SEM image, Ca, C; bottom: Ti, P, Si. b EDXS mappings of the sample without BAG, showing from left to right on top: SEM image, Ca, C; bottom: Ti, P, Si
As can be seen in Fig. 4a and b, Ca and P can be detected much deeper in the porous region of BAG-containing samples compared to samples without BAG. For the latter, the high carbon content and absence of Ca and P in the deeper region of the Ti-layer confirm that the bone did not fill the pores close to the substrate; instead, they were filled with Epon. The minor presence of Si in the sample with BAG indicates that the BAG has fully dissolved during ten weeks of implantation. The detected small amount of Si is approximately the same as in the sample without BAG; therefore we assume that in both samples, this is the consequence of grinding with SiC grinding papers. This proves that, in the early stage, the BAG gradually dissolves providing the necessary concentrations of calcium and silicon ions for promoting the bone formation.
Bone-to-implant contact and bone ingrowth were also evaluated quantitatively. The analyses were performed on the part of implant that was in contact with the compact bone. The part of the implant that was in the bone marrow was excluded, although in the case of samples with BAG, the mineralized bone was found also in the porous region of the implant extending in the bone marrow. The bone-to-implant contact for the samples with and without BAG was equal at around 52%. A much more beneficial effect of BAG was observed in bone occupation of the pores. The pores of the samples with BAG were 38% filled with bone, while only 22% of pores without BAG were filled with bone.
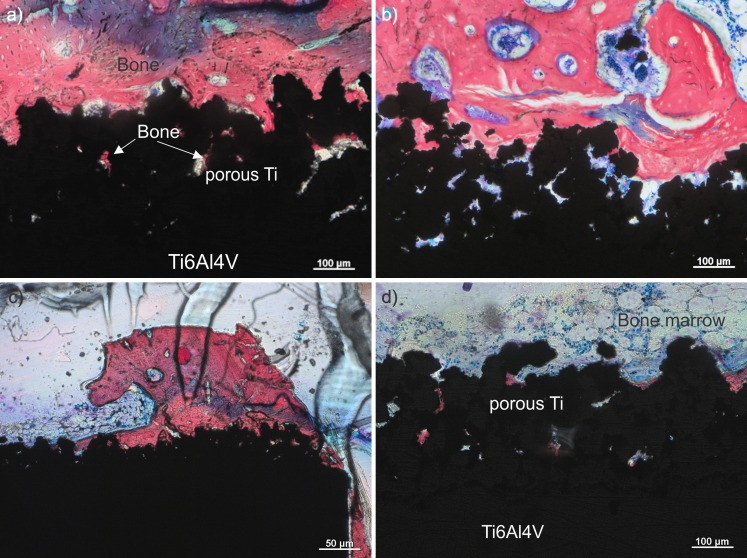
Histological examination
Histological examinations of the stained slices were, in particular, focused in appearance of mineralized newly formed bone in pores and in contact with metal. As under transmitted light only bone that is occupying open pores is visible, much less bone is seen in histological sections (Fig. 5) compared to metallographic samples observed by SEM (Figs. 3 and 4). Ten weeks after implantation, all implants, with and without BAG, showed no sign of inflammation at the interface with the implant (Fig. 5a and b). More mineralized bone (red) was observed in the pores of the samples prepared with BAG, where the bone grew also downwards on the implant surface into the bone marrow region (see Fig. 5c). Newly formed bone in the samples with BAG was found also in the porous regions of the implant that was away from the original compact bone and was actually lying in the medullary cavity of the tibia and surrounded by the bone marrow (Fig. 5d). As the whole porous titanium layer of the implant was initially filled with BAG, bone formation was accelerated not just in the site of the implant facing compact bone, but also on the site of the implant facing bone marrow. In both kinds of implants, i.e. with or without BAG, the contact between the implant and the newly formed compact bone was similar, i.e. good contact with only a few gaps filled with adipose tissue.
Fig. 5.
Characteristic histological sections of the samples with bioactive glass (BAG) (a) and without BAG (b). c Sample with BAG at lower magnification and part of the implant in the bone marrow (d). Mineralized bone is stained red. Osteoblasts and bone marrow cells in b are stained blue. The blue region in the upper part of the image (a) is an artifact caused during the staining with Stevenel’s blue
Discussion
The ability of bioactive glasses to enhance bone formation has been presented in many previous scientific reports [13, 14]. In addition, stimulation effects on angiogenesis as well as antibacterial and inflammatory effects were also reported [4–7]. These effects have been ascribed to the availability of ionic products released from the bioactive glass, especially calcium and silicon ions, which were recently reported to be playing a key role in bone regeneration. The ions released during dissolution of the BAG in the porous metallic structure stimulate several families of genes and activates some growth factors that control osteogenesis [14–16]. Moreover, the surrounding bone is in direct contact with the surface apatite formed during BAG dissolution, without the intervention of any fibrous tissue and consequently a tight chemical bond is formed with the apatite [5, 17]. In spite of all that, due to its poor mechanical properties the application of BAG in orthopaedic surgery has been still limited to non-loaded applications.
On the other hand, porous titanium has been confirmed in clinical practice to significantly improve bone fixation. The advantageous effect of the porous surface layer on implants has also been confirmed in our study: while a gap between the bone and parts of metal with a flat surface was observed in almost all the samples after removal from rabbit tibia, the new bone was well attached to the porous layers with or without BAG.
The above described advantages of bioactive glass have been reported on the basis of numerous in vitro and in vivo studies of BAGs with different chemical compositions and in different forms, such as granules or coatings on metals, while the enhancement of osseointegration of porous metallic structures by impregnation with bioactive glass has not been reported before. This is partly due to technological limitations due to the relatively coarse particles of melt-derived bioactive glasses or their limited resorbability defined by their composition and crystallinity. Also, the bioactive glass used in this study is characterized by small particles size that enables impregnation of the porous titanium. After the thermal treatment, a porous BAG structure with high resorbability is formed within the pores of the titanium layer on the implant. As it has been reported [18], an intermediate layer of Ti-silicide is formed at the BAG-titanium contact. We suppose that the Ti-silicide may, due to silicon presence, contribute to an improved biological response even after bioactive glass has dissolved. It has been also reported that the titanium silicide intermediate layer provides strong adhesion of the bioactive glass to the metallic substrate; however, this is not relevant in our case since the BAG within the pores is not mechanically loaded.
The above-mentioned high resorbability of the bioactive glass is reflected in its absence in the porous titanium layer of the implanted grafts after ten weeks of implantation in rabbit tibia and, instead, there is the presence of newly formed bone. This bone was observed throughout the thickness of the porous layer, which confirms that the dissolution product of the bioactive glass in the pores provided an appropriate and stimulating environment for the bone growth. Conversely, this was not the case for the implants without BAG, where the bone formed only within the outer part of the porous Ti-layer. In addition, the advantageous effect of the porous surface layer on implants has been confirmed: while the new bone was well attached to the porous layers (without or with BAG), a gap between the bone and parts of metal with a flat surface was observed in almost all the samples.
In the case of implants with BAG, the new bone has also formed in the smallest pores, in the deepest parts of the porous titanium layer and also at the site of the implant facing the bone marrow, thus, everywhere where the implant surroundings were saturated with calcium and silicon ions released from the bioactive glass. The porous structure of BAG obviously contributed to fast resorption; in ten weeks, the entire BAG was replaced with a newly formed bone, which indicates favourable dissolution kinetics under physiological conditions.
In conclusion, an in-vivo animal study confirmed that the novel nanoparticulate bioactive glass introduced into the porous titanium surface layer on implants promotes osseointegration. Within ten weeks, the bioactive glass was completely resorbed and substituted with well attached newly formed bone, which overgrew the entire thickness of the porous structure. The observed percentage of pores occupied by bone for the implants contacting BAG (38%) seems not to be sufficiently high, but we believe that the increase from 22% to almost a double value represents a meaningful improvement as it implies faster bone ingrowth during the first weeks after implantation.
Acknowledgements
The work has been performed as a part of the PhD study of Mrs. Nataša Drnovšek, and within the Meddelcoat project (FP6-IP-SME). Financial support of the Slovenian Research Agency is also acknowledged. The authors wish to thank Katja Rade and Gregor Murn for help in samples preparation and analyses and Prof. Rok Romih for his valuable suggestions on preparation for histological observation.
Conflict of interests
The authors declare that they have no conflict of interest.
References
- 1.Ryan G, Pandit A, Apatsidis DP. Fabrication methods of porous metals for use in orthopaedic applications. Biomaterials. 2006;27(13):2651–2670. doi: 10.1016/j.biomaterials.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 2.Daugaard H, Elmengaard B, Bechtold JE, Jensen T, Soballe K. The effect on bone growth enhancement of implant coatings with hydroxyapatite and collagen deposited electrochemically and by plasma spray. J Biomed Mater Res A. 2010;92A(3):913–921. doi: 10.1002/jbm.a.32303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim HW, Koh YH, Li LH, Lee S, Kim HE. Hydroxyapatite coating on titanium substrate with titania buffer layer processed by sol–gel method. Biomaterials. 2004;25(13):2533–2538. doi: 10.1016/j.biomaterials.2003.09.041. [DOI] [PubMed] [Google Scholar]
- 4.Allan I, Newman H, Wilson M. Particulate Bioglass (R) reduces the viability of bacterial biofilms formed on its surface in an in vitro model. Clin Oral Implan Res. 2002;13(1):53–58. doi: 10.1034/j.1600-0501.2002.130106.x. [DOI] [PubMed] [Google Scholar]
- 5.Keshaw H, Forbes A, Day RM. Release of angiogenic growth factors from cells encapsulated in alginate beads with bioactive glass. Biomaterials. 2005;26(19):4171–4179. doi: 10.1016/j.biomaterials.2004.10.021. [DOI] [PubMed] [Google Scholar]
- 6.Lepparanta O, Vaahtio M, Peltola T, Zhang D, Hupa L, Hupa M, Ylanen H, Jukka IS, Matti KV, Eerola E. Antibacterial effect of bioactive glasses on clinically important anaerobic bacteria in vitro. J Mater Sci-Mater M. 2008;19(2):547–551. doi: 10.1007/s10856-007-3018-5. [DOI] [PubMed] [Google Scholar]
- 7.Leu A, Leach JK. Proangiogenic potential of a collagen/bioactive glass substrate. Pharm Res. 2008;25(5):1222–1229. doi: 10.1007/s11095-007-9508-9. [DOI] [PubMed] [Google Scholar]
- 8.Liu X, Tao S, Ding C. Bioactivity of plasma sprayed dicalcium silicate coatings. Biomaterials. 2002;23(3):963–968. doi: 10.1016/S0142-9612(01)00210-1. [DOI] [PubMed] [Google Scholar]
- 9.D'Alessio L, Teghil R, Zaccagnino M, Zaccardo I, Ferro D, Marotta V. Pulsed laser ablation and deposition of bioactive glass as coating material for biomedical applications. Appl Surf Sci. 1999;138–139:527–532. doi: 10.1016/S0169-4332(98)00610-2. [DOI] [Google Scholar]
- 10.Fathi MH, Doostmohammadi A. Bioactive glass nanopowder and bioglass coating for biocompatibility improvement of metallic implant. J Mater Process Technol. 2009;209(3):1385–1391. doi: 10.1016/j.jmatprotec.2008.03.051. [DOI] [Google Scholar]
- 11.Novak S, Drnovšek N, Murn G (2011) Implant having a multilayerd coating and a process for preparing thereof. PCT/SI2011/000020. PCT, Slovenia
- 12.Underwood E (1970) Quantitative stereology. Addison-Wesley, New York
- 13.Hench LL. The story of Bioglass (R) J Mater Sci-Mater M. 2006;17(11):967–978. doi: 10.1007/s10856-006-0432-z. [DOI] [PubMed] [Google Scholar]
- 14.Xynos ID, Edgar AJ, Buttery LDK, Hench LL, Polak JM. Ionic products of bioactive glass dissolution increase proliferation of human osteoblasts and induce insulin-like growth factor II mRNA expression and protein synthesis. Biochem Bioph Res Co. 2000;276(2):461–465. doi: 10.1006/bbrc.2000.3503. [DOI] [PubMed] [Google Scholar]
- 15.Hench LL. Genetic design of bioactive glass. J Eur Ceram Soc. 2009;29(7):1257–1265. doi: 10.1016/j.jeurceramsoc.2008.08.002. [DOI] [Google Scholar]
- 16.Varanasi VG, Owyoung JB, Saiz E, Marshall SJ, Marshall GW, Loomer PM. The ionic products of bioactive glass particle dissolution enhance periodontal ligament fibroblast osteocalcin expression and enhance early mineralized tissue development. J Biomed Mater Res A. 2011;98A(2):177–184. doi: 10.1002/jbm.a.33102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kokubo T. Apatite formation on surfaces of ceramics, metals and polymers in body environment. Acta Mater. 1998;46(7):2519–2527. doi: 10.1016/S1359-6454(98)80036-0. [DOI] [Google Scholar]
- 18.Saiz E, Lopez-Esteban S, Fujino S, Oku T, Suganuma K, Tomsia AP (2003) Characterization of metal/glass interfaces in bioactive glass coatings on Ti-6Al-4 V and Co-Cr alloys. In: Meyers MA, Ritchie RO, Sarikaya M (eds) Nano and microstructural design of advanced materials. Elsevier Science Ltd, Oxford, pp 61–67. doi:10.1016/b978-008044373-7/50034-6