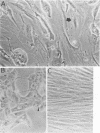
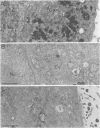
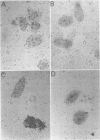
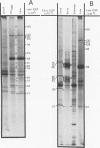
Abstract
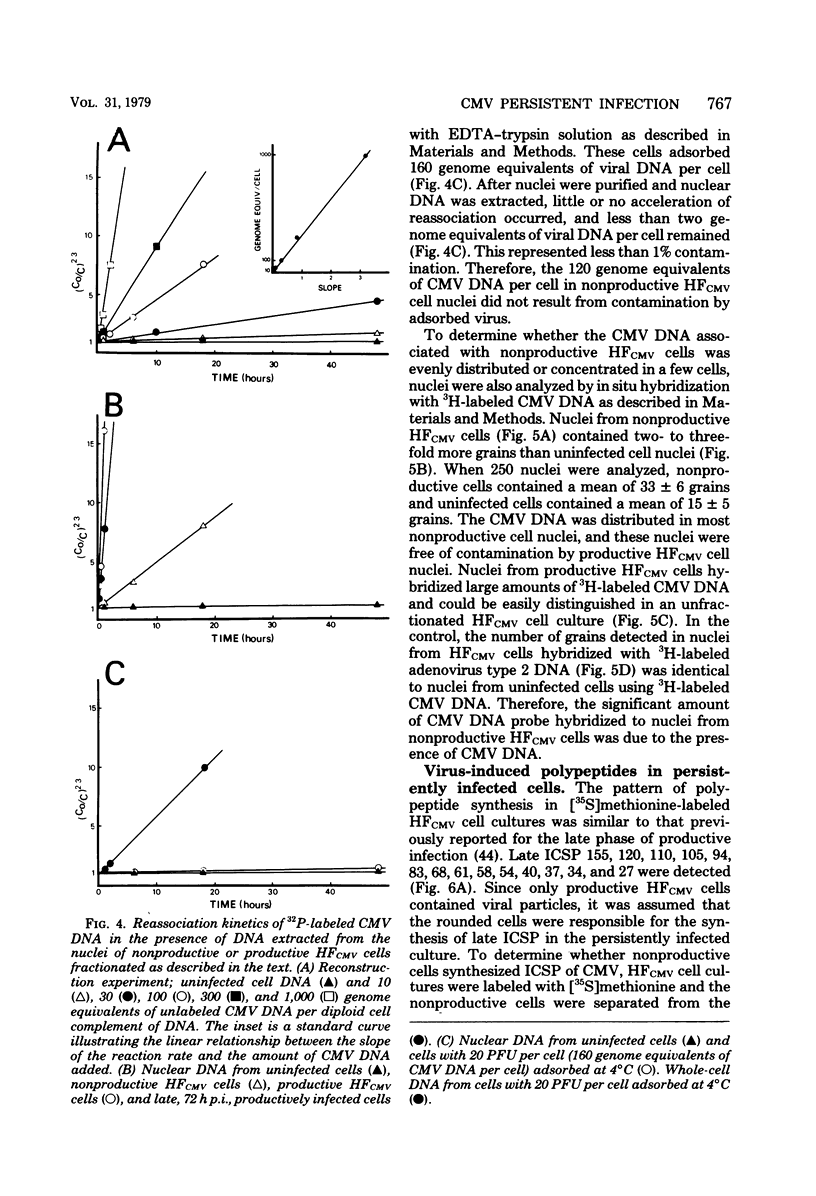
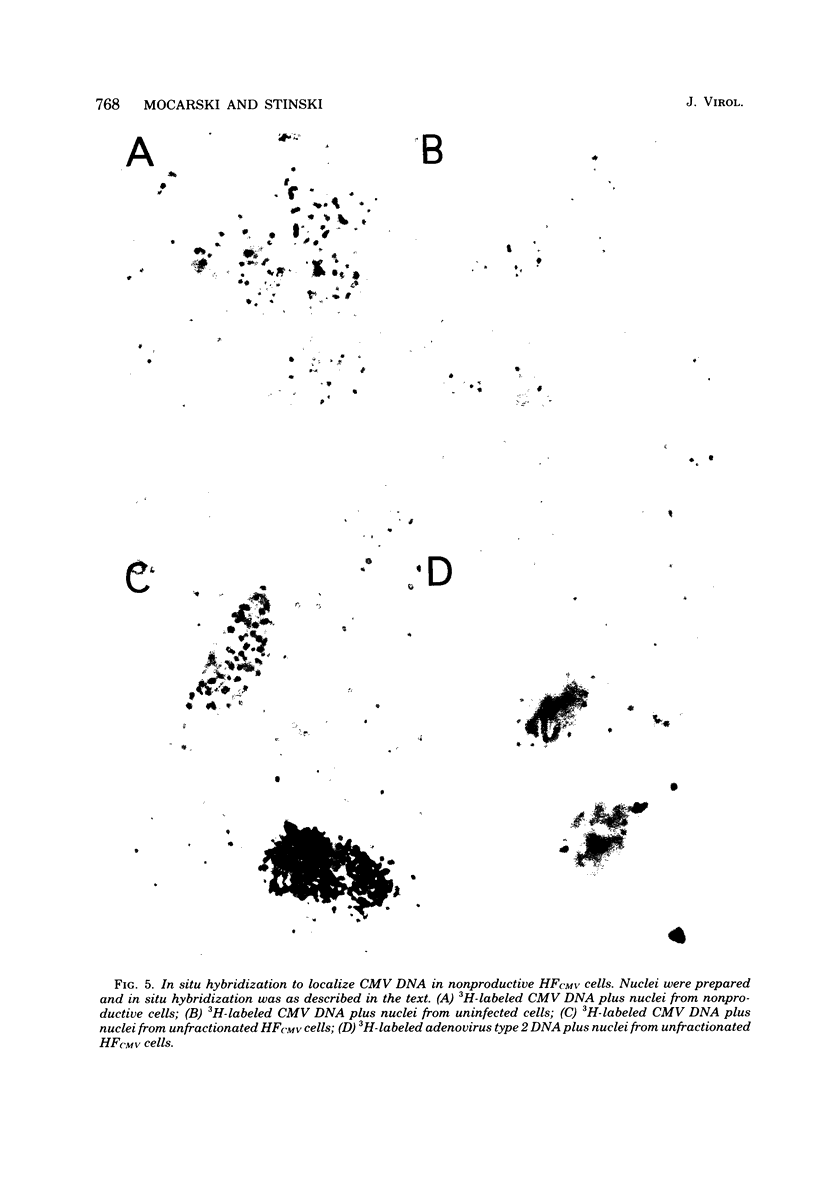
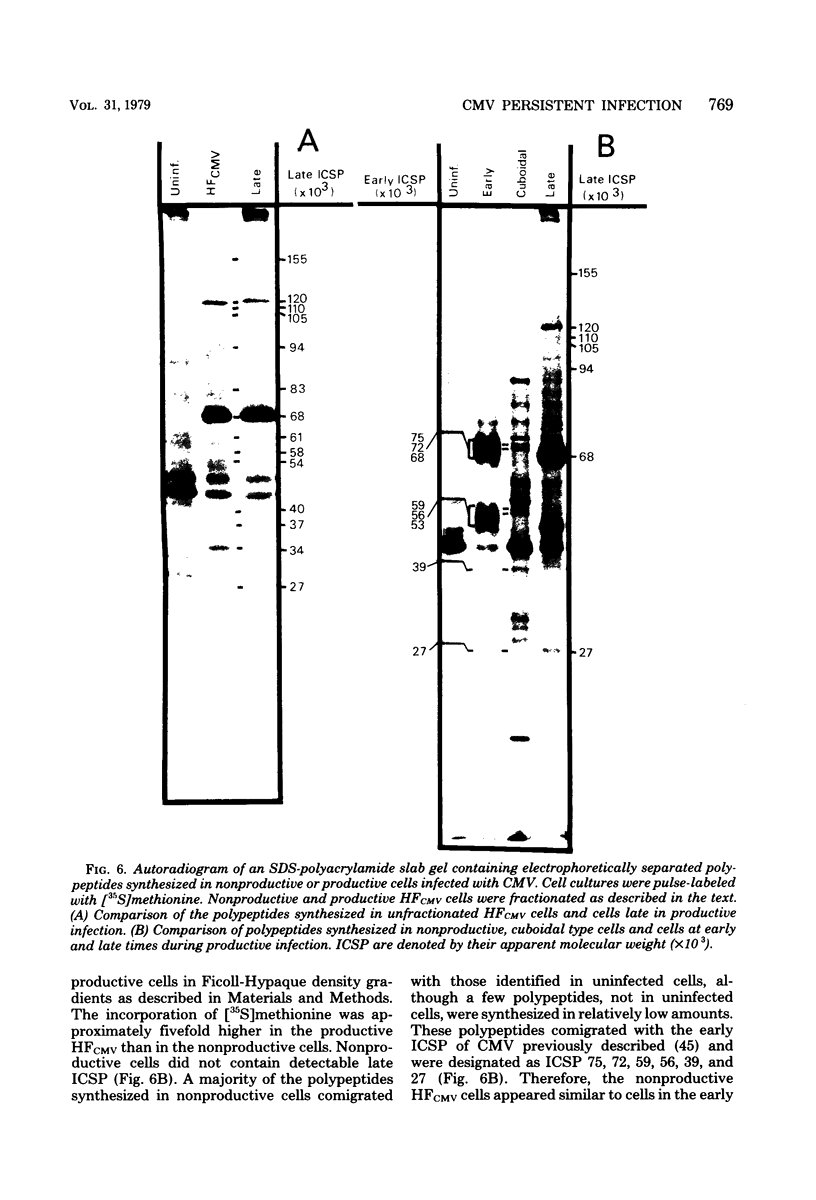
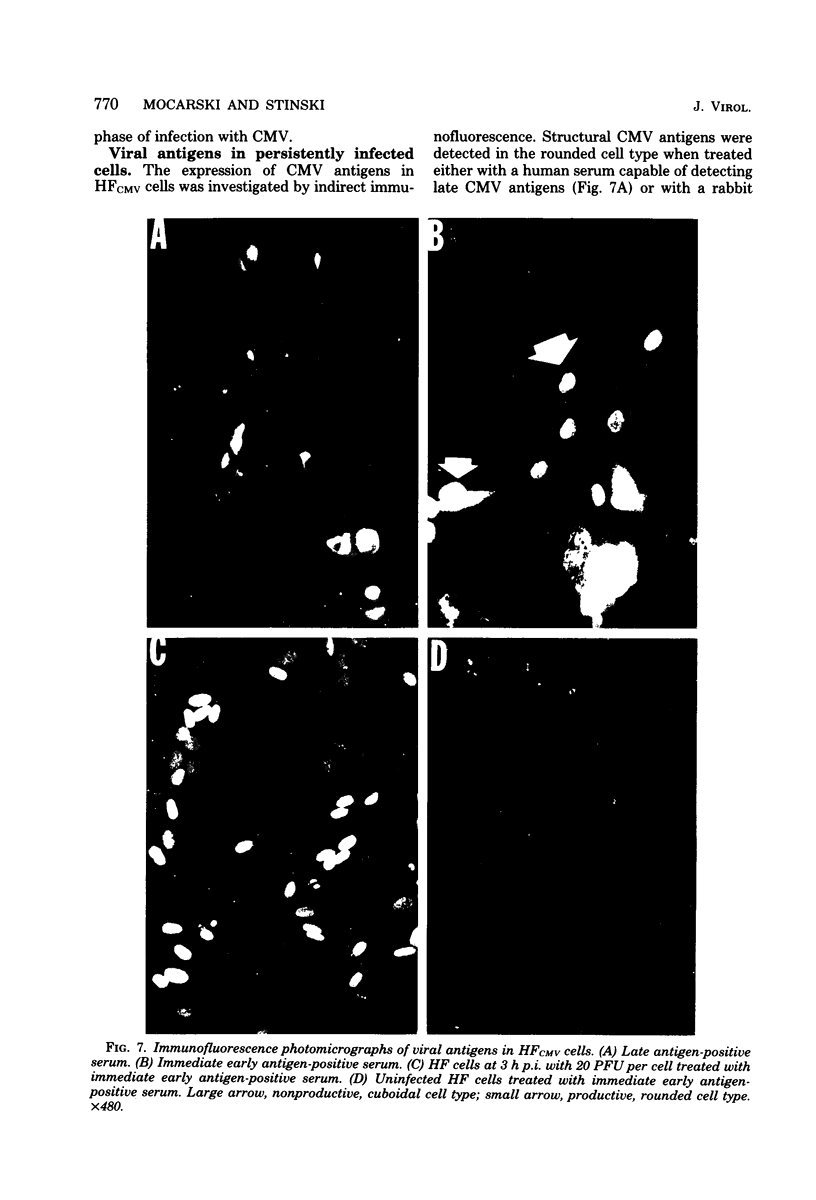
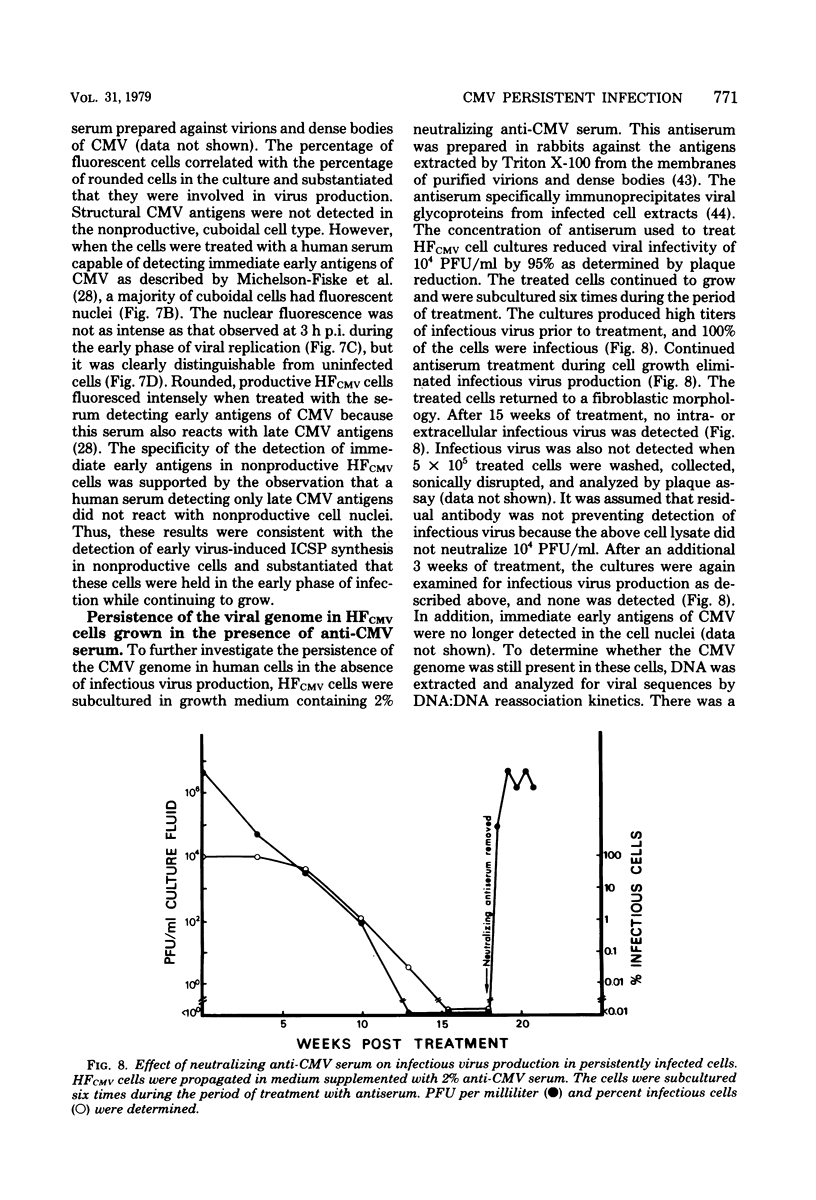
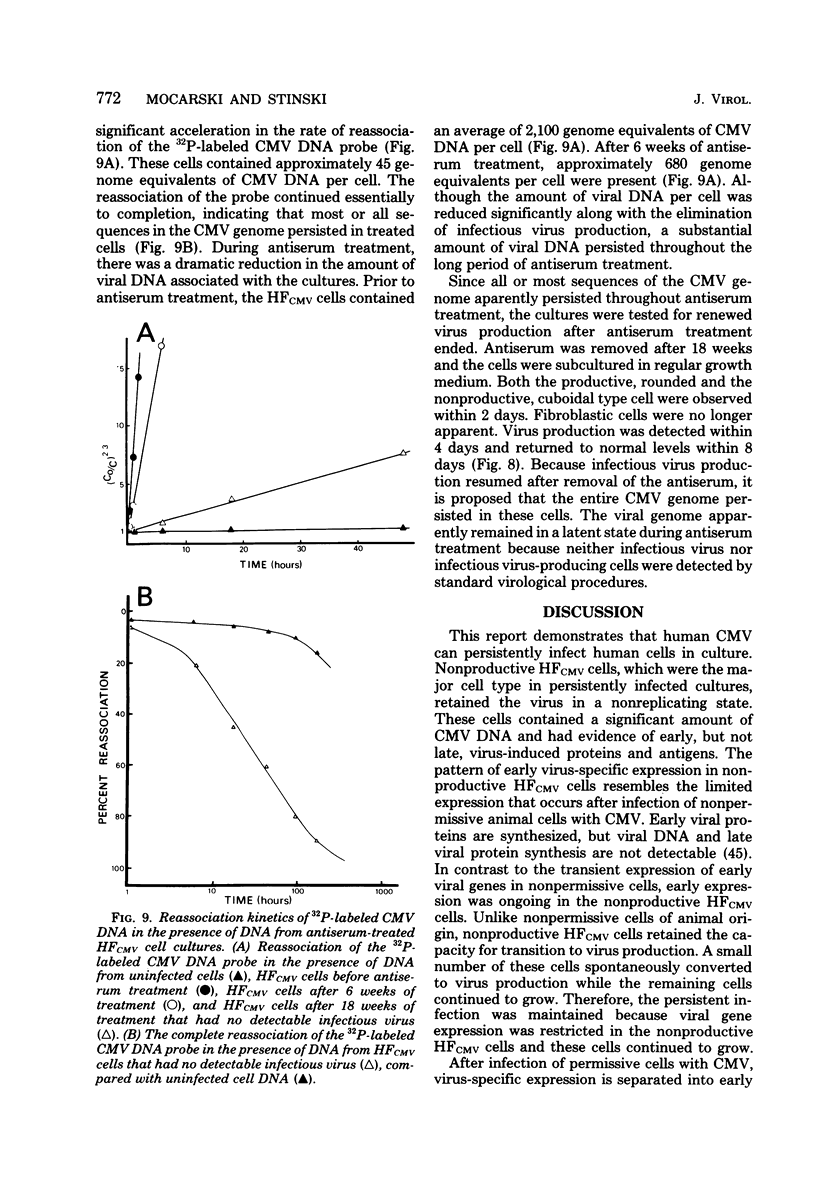
A small percentage of human fibroblast cells survived high-multiplicity infection by cytomegalovirus and were isolated as persistently infected cultures. Approximately 30% of the cells were in the productive phase of infection, since virus-specific structural antigens and virions were associated with these cells. The remaining cells contained neither viral structural antigens nor particles. Nuclear DNA from these nonproductive cells contained approximately 120 genome equivalents of viral DNA per cell as determined by reassociation kinetics. In situ hybridization confirmed that nuclei from nonproductive cells contained a significant amount of viral DNA that was distributed in most of these cells. Early virus-induced proteins and antigens were also detected. Nonproductive cells continued to grow, and there was a slow, spontaneous transition of some of these cells to productive viral replication. The majority of the viral DNA in nonproductive cells persisted with restricted gene expression. When infectious virus production was eliminated by growing the persistently infected cultures in the presence of anticytomegalovirus serum, approximately 45 genome equivalents of the viral DNA persisted per cell. The reassociation reaction approached completion. After removal of the antiserum and subculturing, infectious virus production resumed. Therefore, it was assumed that all sequences of the viral genome remained associated with these cells. Restriction of cytomegalovirus gene expression in persistently infected cell cultures is discussed.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams A., Lindahl T., Klein G. Linear association between cellular DNA and Epstein-Barr virus DNA in a human lymphoblastoid cell line. Proc Natl Acad Sci U S A. 1973 Oct;70(10):2888–2892. doi: 10.1073/pnas.70.10.2888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson-Anvret M., Lindahl T. Integrated viral DNA sequences in Epstein-Barr virus-converted human lymphoma lines. J Virol. 1978 Mar;25(3):710–718. doi: 10.1128/jvi.25.3.710-718.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boldogh I., Gönczöl E., Gärtner L., Váczi G. Expression of the human cytomegalovirus genome in mouse cells and in human-mouse heterokaryons. Arch Virol. 1977;53(1-2):101–108. doi: 10.1007/BF01314851. [DOI] [PubMed] [Google Scholar]
- Brinkley B. R., Murphy P., Richardson L. C. Procedure for embedding in situ selected cells cultured in vitro. J Cell Biol. 1967 Oct;35(1):279–283. doi: 10.1083/jcb.35.1.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chantler J. K., Hudson J. B. Proteins of murine cytomegalovirus: identification of structural and nonstructural antigens in infected cells. Virology. 1978 May 1;86(1):22–36. doi: 10.1016/0042-6822(78)90004-1. [DOI] [PubMed] [Google Scholar]
- Clements J. B., Watson R. J., Wilkie N. M. Temporal regulation of herpes simplex virus type 1 transcription: location of transcripts on the viral genome. Cell. 1977 Sep;12(1):275–285. doi: 10.1016/0092-8674(77)90205-7. [DOI] [PubMed] [Google Scholar]
- Craighead J. E., Kanich R. E., Almeida J. D. Nonviral microbodies with viral antigenicity produced in cytomegalovirus-infected cells. J Virol. 1972 Oct;10(4):766–775. doi: 10.1128/jvi.10.4.766-775.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMarchi J. M., Kaplan A. S. Physiological state of human embryonic lung cells affects their response to human cytomegalovirus. J Virol. 1977 Jul;23(1):126–132. doi: 10.1128/jvi.23.1.126-132.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein M. A., Achong B. G. Recent progress in Epstein-Barr virus research. Annu Rev Microbiol. 1977;31:421–445. doi: 10.1146/annurev.mi.31.100177.002225. [DOI] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Furukawa T., Fioretti A., Plotkin S. Growth characteristics of cytomegalovirus in human fibroblasts with demonstration of protein synthesis early in viral replication. J Virol. 1973 Jun;11(6):991–997. doi: 10.1128/jvi.11.6.991-997.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geder K. M., Lausch R., O'Neill F., Rapp F. Oncogenic transformation of human embryo lung cells by human cytomegalovirus. Science. 1976 Jun 11;192(4244):1134–1137. doi: 10.1126/science.179143. [DOI] [PubMed] [Google Scholar]
- Geelen J. L., Walig C., Wertheim P., van der Noordaa J. Human cytomegalovirus DNA. I. Molecular weight and infectivity. J Virol. 1978 Jun;26(3):813–816. doi: 10.1128/jvi.26.3.813-816.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelb L. D., Kohne D. E., Martin M. A. Quantitation of Simian virus 40 sequences in African green monkey, mouse and virus-transformed cell genomes. J Mol Biol. 1971 Apr 14;57(1):129–145. doi: 10.1016/0022-2836(71)90123-9. [DOI] [PubMed] [Google Scholar]
- Gerber P. Activation of Epstein-Barr virus by 5-bromodeoxyuridine in "virus-free" human cells (complement-fixing antigen-immunofluorescence-leukocytes). Proc Natl Acad Sci U S A. 1972 Jan;69(1):83–85. doi: 10.1073/pnas.69.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green M., Piña M., Kimes R., Wensink P. C., MacHattie L. A., Thomas C. A., Jr Adenovirus DNA. I. Molecular weight and conformation. Proc Natl Acad Sci U S A. 1967 May;57(5):1302–1309. doi: 10.1073/pnas.57.5.1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward S. D., Kieff E. D. Epstein-Barr virus-specific RNA. I. Analysis of viral RNA in cellular extracts and in the polyribosomal fraction of permissive and nonpermissive lymphoblastoid cell lines. J Virol. 1976 May;18(2):518–525. doi: 10.1128/jvi.18.2.518-525.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho M. Virus infections after transplantation in man. Brief review. Arch Virol. 1977;55(1-2):1–24. doi: 10.1007/BF01314475. [DOI] [PubMed] [Google Scholar]
- Honess R. W., Roizman B. Regulation of herpesvirus macromolecular synthesis. I. Cascade regulation of the synthesis of three groups of viral proteins. J Virol. 1974 Jul;14(1):8–19. doi: 10.1128/jvi.14.1.8-19.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeor S. C., Albrecht T. B., Funk F. D., Rapp F. Stimulation of cellular DNA synthesis by human cytomegalovirus. J Virol. 1974 Feb;13(2):353–362. doi: 10.1128/jvi.13.2.353-362.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joncas J. H., Menezes J., Huang E. S. Persistence of CMV genome in lymphoid cells after congenital infection. Nature. 1975 Dec 4;258(5534):432–434. doi: 10.1038/258432a0. [DOI] [PubMed] [Google Scholar]
- Jones P. C., Hayward G. S., Roizman B. Anatomy of herpes simplex virus DNA VII. alpha-RNA is homologous to noncontiguous sites in both the L and S components of viral DNA. J Virol. 1977 Jan;21(1):268–276. doi: 10.1128/jvi.21.1.268-276.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lang D. J. Cytomegalovirus infections in organ transplantation and post transfusion. An hypothesis. Arch Gesamte Virusforsch. 1972;37(4):365–377. doi: 10.1007/BF01241460. [DOI] [PubMed] [Google Scholar]
- Lindahl T., Adams A., Bjursell G., Bornkamm G. W., Kaschka-Dierich C., Jehn U. Covalently closed circular duplex DNA of Epstein-Barr virus in a human lymphoid cell line. J Mol Biol. 1976 Apr 15;102(3):511–530. doi: 10.1016/0022-2836(76)90331-4. [DOI] [PubMed] [Google Scholar]
- MASTER R. W. POSSIBLE SYNTHESIS OF POLYRIBONUCLEOTIDES OF KNOWN BASE-TRIPLET SEQUENCES. Nature. 1965 Apr 3;206:93–93. doi: 10.1038/206093b0. [DOI] [PubMed] [Google Scholar]
- Michelson-Fiske S., Horodniceanu F., Guillon J. C. Immediate early antigens in human cytomegalovirus infected cells. Nature. 1977 Dec 15;270(5638):615–617. doi: 10.1038/270615a0. [DOI] [PubMed] [Google Scholar]
- Misra V., Muller M. T., Chantler J. K., Hudson J. B. Regulation of murine cytomegalovirus gene expression. I. Transcription during productive infection. J Virol. 1978 Aug;27(2):263–268. doi: 10.1128/jvi.27.2.263-268.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller M. T., Hudson J. B. Cell cycle dependency of murine cytomegalovirus replication in synchronized 3T3 cells. J Virol. 1977 May;22(2):267–272. doi: 10.1128/jvi.22.2.267-272.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller M. T., Hudson J. B. Murine cytomegalovirus DNA synthesis in nuclear monolayers. Virology. 1978 Jul 15;88(2):371–378. doi: 10.1016/0042-6822(78)90293-3. [DOI] [PubMed] [Google Scholar]
- Muller M., Misra V., Chantler J. K., Hudson J. B. Murine cytomegalovirus gene expression during nonproductive infection in Go-phase 3T3 cells. Virology. 1978 Oct 15;90(2):279–287. doi: 10.1016/0042-6822(78)90312-4. [DOI] [PubMed] [Google Scholar]
- Nonoyama M., Pagano J. S. Separation of Epstein-Barr virus DNA from large chromosomal DNA in non-virus-producing cells. Nat New Biol. 1972 Aug 9;238(84):169–171. doi: 10.1038/newbio238169a0. [DOI] [PubMed] [Google Scholar]
- Plummer G. Cytomegaloviruses of man and animals. Prog Med Virol. 1973;15:92–125. [PubMed] [Google Scholar]
- Rabinovitch M., DeStefano M. J. Manganese stimulates adhesion and spreading of mouse sarcoma I ascites cells. J Cell Biol. 1973 Oct;59(1):165–176. doi: 10.1083/jcb.59.1.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapp F., Geder L., Murasko D., Lausch R., Ladda R., Huang E. S., Webber M. M. Long-term persistence of cytomegalovirus genome in cultured human cells of prostatic origin. J Virol. 1975 Oct;16(4):982–990. doi: 10.1128/jvi.16.4.982-990.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Roizman B. Herpesviruses, latency and cancer: a biochemical approach. J Reticuloendothel Soc. 1974 Apr;15(4):312–321. [PubMed] [Google Scholar]
- Schachat F. H., Hogness D. S. Repetitive sequences in isolated Thomas circles from Drosophila melanogaster. Cold Spring Harb Symp Quant Biol. 1974;38:371–381. doi: 10.1101/sqb.1974.038.01.040. [DOI] [PubMed] [Google Scholar]
- Smith J. D., De Harven E. Herpes simplex virus and human cytomegalovirus replication in WI-38 cells. I. Sequence of viral replication. J Virol. 1973 Oct;12(4):919–930. doi: 10.1128/jvi.12.4.919-930.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M. J., Britten R. J., Davidson E. H. Studies on nucleic acid reassociation kinetics: reactivity of single-stranded tails in DNA-DNA renaturation. Proc Natl Acad Sci U S A. 1975 Dec;72(12):4805–4809. doi: 10.1073/pnas.72.12.4805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Jeor S., Rapp F. Cytomegalovirus: conversion of nonpermissive cells to a permissive state for virus replication. Science. 1973 Sep 14;181(4104):1060–1061. doi: 10.1126/science.181.4104.1060. [DOI] [PubMed] [Google Scholar]
- Stevens J. G., Cook M. L. Maintenance of latent herpetic infection: an apparent role for anti-viral IgG. J Immunol. 1974 Dec;113(6):1685–1693. [PubMed] [Google Scholar]
- Stinski M. F. Human cytomegalovirus: glycoproteins associated with virions and dense bodies. J Virol. 1976 Aug;19(2):594–609. doi: 10.1128/jvi.19.2.594-609.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stinski M. F., Mocarski E. S., Thomsen D. R. DNA of human cytomegalovirus: size heterogeneity and defectiveness resulting from serial undiluted passage. J Virol. 1979 Jul;31(1):231–239. doi: 10.1128/jvi.31.1.231-239.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stinski M. F., Mocarski E. S., Thomsen D. R., Urbanowski M. L. Membrane glycoproteins and antigens induced by human cytomegalovirus. J Gen Virol. 1979 Apr;43(1):119–129. doi: 10.1099/0022-1317-43-1-119. [DOI] [PubMed] [Google Scholar]
- Stinski M. F. Sequence of protein synthesis in cells infected by human cytomegalovirus: early and late virus-induced polypeptides. J Virol. 1978 Jun;26(3):686–701. doi: 10.1128/jvi.26.3.686-701.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stinski M. F. Synthesis of proteins and glycoproteins in cells infected with human cytomegalovirus. J Virol. 1977 Sep;23(3):751–767. doi: 10.1128/jvi.23.3.751-767.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton W. D. A crude nuclease preparation suitable for use in DNA reassociation experiments. Biochim Biophys Acta. 1971 Jul 29;240(4):522–531. doi: 10.1016/0005-2787(71)90709-x. [DOI] [PubMed] [Google Scholar]
- Takemoto K. K., Linke H., Miyamura T., Fareed G. C. Persistent BK papovavirus infection of transformed human fetal brain cells. I. Episomal viral DNA in cloned lines deficient in T-antigen expression. J Virol. 1979 Mar;29(3):1177–1185. doi: 10.1128/jvi.29.3.1177-1185.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka S., Furukawa T., Plotkin S. A. Human cytomegalovirus stimulates host cell RNA synthesis. J Virol. 1975 Feb;15(2):297–304. doi: 10.1128/jvi.15.2.297-304.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Weller T. H. The cytomegaloviruses: ubiquitous agents with protean clinical manifestations. II. N Engl J Med. 1971 Jul 29;285(5):267–274. doi: 10.1056/NEJM197107292850507. [DOI] [PubMed] [Google Scholar]
- Wentworth B. B., French L. Plaque assay of cytomegalovirus strains of human origin. Proc Soc Exp Biol Med. 1970 Nov;135(2):253–258. doi: 10.3181/00379727-135-35031. [DOI] [PubMed] [Google Scholar]