Abstract
Purpose
The purpose of our study is to describe the rationale, the surgical technique and the early clinical and radiographic results of the treatment of patients with early stage osteonecrosis of the femoral head (ONFH) by performing: core decompression, injection of autologous bone marrow concentrate and the use of a new composite injectable bone substitute (PRO-DENSE®), as a mechanical supplementation associated with decompression.
Methods
The study included 37 hips (31 patients, 14 females, 17 males; mean age 43.9 years, range 24–56 years) with stages IC–IIIA ONFH. The outcome was determined by the changes in the Harris hip score (HHS), by progression in radiographic stages and by the need for hip replacement. The mean follow-up was 20.6 months (range 12–32 months).
Results
At final follow-up the mean HHS increased from 68 points pre-operatively to 86 points post-operatively. The radiological results showed that 29 hips (78.4 %) improved or had no further collapse. The overall clinical success rate of the procedure was 86.5 %, with three conversions to THA, and a failure rate of only 3.3 % in the pre-collapse group.
Conclusions
We are encouraged by these early results using core decompression, injection of the autologous bone marrow concentrate and backfilling the defect with an injectable bioceramic for the treatment of early stages of ONFH; as far as a conclusion can be drawn from the current data, this treatment seems to relieve hip pain and prevent the progression of ONFH in the majority of the cases.
Introduction
The natural history of symptomatic osteonecrosis of the femoral head (ONFH) is a relentless progression to collapse and eventually incongruity of the joint. Several forms of intervention have been advocated for the treatment of early stages of ONFH [1, 2].
Core decompression, one of the most widely accepted hip preservative treatment modalities for ONFH, has been extensively reported during the last two decades. However its efficacy in preventing disease progression has been questioned and has stimulated the application of alternative techniques to augment the core decompression [3, 4].
Multiple adjuncts to core decompression, such as vascularised and non vascularised fibular and other structural bone grafts, non structural grafts, demineralised bone matrix, bone marrow concentrate, bone morphogenetic proteins, and porous tantalum implants, have been developed to accelerate the healing process in addition to providing, in some cases, a structural support [5–13].
However, in recent years considerable research and development activity have been expended to find new and more satisfactory bone substitutes with osteconductive and osteoinductive properties for use in the treatment of surgically created defects [14–16].
On the basis of this new research the aim of this paper was to describe and evaluate a new technique to augment core decompression in ONFH with a new calcium sulphate (CaSO4)/calcium phosphate (CaPO4) composite graft (PRO-DENSE® Wright Medical Technology, Arlington TN USA), enhanced with autologous bone marrow concentrate. PRO-DENSE® is a fully synthetic composite material that is designed to be mixed intraoperatively and injected to cure in vivo and to form a strong composite like a bone cement. Pre-clinical studies have shown stronger bone regenerate than both autograft and CaSO4 alone. Furthermore, it has been engineered so that the triphasic resorption pattern exhibited by this material in vivo would enhance the bone forming properties [17].
The rationale of this technique is that the mechanical properties exhibited by this material could provide a stronger osteoinductive scaffold, and the resorption pattern a faster new bone incorporation for the core tract. Furthermore the injection of the autologous bone marrow concentrate is intended to provide osteoinductive properties for bone regeneration in the necrotic area.
The purpose of this study is to describe the rationale, the surgical technique and the early clinical and radiographic results of this procedure in 37 hips which have been prospectively followed up for an average of 20 months.
Material and methods
We prospectively evaluated the results of a series of 37 hips in 31 patients with ONFH treated at the author’s institution from March 2009 to December 2010 by core decompression, injection of bone marrow concentrate in the necrotic area and backfilling of the core tract with PRO-DENSE®.
The study protocol was approved by the institutional review board and all patients gave informed consent for participation in the study.
The indication for the procedure was ONFH without collapse, Steinberg I or II, or with early segmental collapse, Steinberg IIIa, in patients who were less than 65 years of age. Contraindications included a femoral head with advanced segmental collapse (a stage of IIIb or higher according to Steinberg), or who had previous surgery to the affected hip, or were aged 65 years or more. There were 14 women (16 hips) and 17 men (21 hips). The mean age at the time of the index procedure was 43.9 years (range 24–56 years).
We identified the following risk factors and associated conditions with ONFH: corticosteroid use (defined as a dose greater than 2 g prednisone or its equivalent per month for three months minimum) (14 patients, 17 hips); further pharmacological treatment for bone marrow transplantation (four patients, six hips); alcohol abuse (defined as alcohol consumption of more than 400 mL per week) (ten patients, 13 hips); systemic lupus erythematosus (one patients, one hip). The remaining six cases were classified as having idiopathic ONFH because no specific risk factors could be identified.
The diagnosis of ONFH was made using anteroposterior (AP) and frog lateral radiographs and MRI scans. The patients were classified according to the University of Pennsylvania system for staging the necrotic lesion which include an assessment of the size and the location of the necrotic lesion. This staging system has gained increasing acceptance in the orthopaedic community, since it is concise and delineates clearly the progression and extent of ONFH [18].
According to Steinberg Classification we had three cases (8.1 %) of stage 1c, seven cases (18.9 %) of stage 2a, 11 cases (29.7 %) of stage 2b, nine cases (24.3 %) of stage 2c, and seven cases of stage 3a (18.9 %).
Surgical technique and rehabilitation
The patients were placed supine on a fracture table with the hip internally rotated to neutralise the normal anteversion of the neck.
The bone-marrow harvesting was performed during the same operative session of the core decompression. About 40 ml of bone marrow was obtained from the anterior iliac crest with a 10-mL syringe, each time changing the site or direction of aspiration, and then separated with an automatic blood cell processor at 4,000 rpm for two minutes, followed by a second centrifugation at 3,500 rpm for eight minutes.
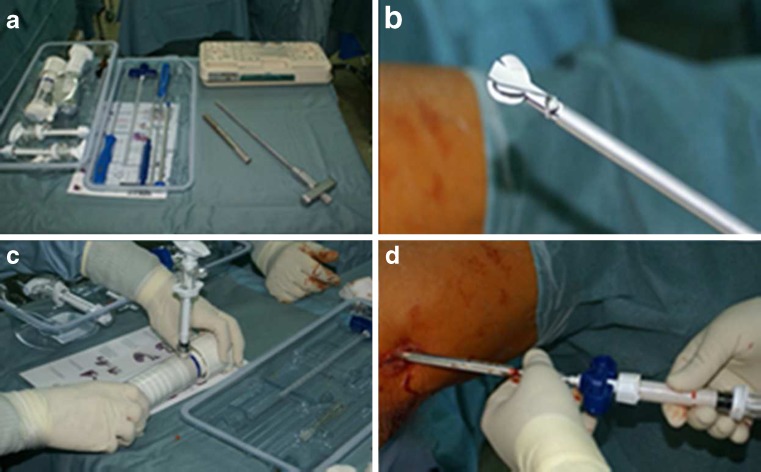
A small lateral incision was performed centred over the flare of the greater trochanter and a 3.2-mm guide pin, provided in a self-contained core decompression kit (Fig.1a), was inserted in the lateral cortex at the level of the upper margin of the lesser trochanter. The pin was guided into the centre of the necrotic lesion using a biplanar fluoroscopic view; this identifies the core tract. A tissue protector was inserted and, with a specific cannulated reamer of the same kit, we reamed the core to a 9-mm diameter. A biopsy of the necrotic area was taken for pathological analysis to confirm the diagnosis.
Fig. 1.
a Components from a self-contained disposable core decompression kit. b X-REAM™ Percutaneous Expandable Reamer. c Vacuum bone cement mixing apparatus for mixing the liquid and powder components of the CaSO4/CaPO4 composite. d The resulting composite is then injected into the core tract through a cannula. The graft is injectable for approximately five minutes and sets up in 20–30 minutes
After conducting standard debridement, a specialised reamer with an expanding tip (Fig. 1b) (X-REAM™ Percutaneous Expandable Reamer, Wright Medical Technology, Inc, Arlington, TN) was inserted to remove a greater volume of the necrotic lesion, and once most of the necrotic area was removed, we injected about 15–20 cc of bone marrow concentrate into the head with a dedicated syringe.
After that, the liquid and powder components of the CaSO4/CaPO4 composite were combined in a vacuum bone cement mixing apparatus (Fig.1c). The whole cavity, starting retrograde from the necrotic area, was filled with about ten millilitres of the CaSO4/CaPO4 injectable composite (Fig.1d), waiting for the composite to harden in vivo. The composite hardens without exothermic reaction, is injectable for approximately five minutes, and sets up in 20–30 minutes. Fluoroscopy was used to confirm that the entire cavity was filled.
After operation patients were mobilised with protected weight bearing for at least six weeks. After that period patients progressed to full weight bearing as tolerated and were encouraged to avoid impact loading of the hip.
Patient evaluation
All patients were followed-up prospectively both clinically and radiographically. The clinical follow-up consisted of the determination of preoperative and postoperative serial Harris hip scores (HHS) and serial AP, frog lateral were used for the radiographic follow-up.
Outcome was graded as excellent when the HHS was greater than 90 points, good when it was between 80 and 89 points, fair when it was between 70 and 79 points, and poor when it was less than 70 points. Radiographic evolution was evaluated for progression of the necrotic lesion, changes in the contour of the femoral head, or the presence of secondary osteoarthritis.
Clinical failure was defined as an HHS below 75 points or if the patient needed a further hip procedure such as THA or osteotomy for any reason. A radiographic failure was defined as a new area of collapse or an increased collapse of greater than 2 mm on plain radiographs during follow-up, or the development of progressive osteoarthritis. In these cases an MRI scan was performed to define the stage of necrotic progression.
Wilcoxon signed-rank test was used to compare preoperative versus postoperative values in non-parametric values as pain score and HHS score. A p-value of less than or equal to 0.05 was taken to indicate statistically significant difference.
Results
No complications related to the procedure were seen during or after the operation. We were able to review all the patients with a mean follow-up of 20 months (range 10–36 months). The results are summarised in Table 1.
Table 1.
Results
| Stage | Mean Harris hip score | Radiographic progression | Stage of necrotic progression | Clinical failure | Conversion to THA | |
|---|---|---|---|---|---|---|
| Pre-op | Post-op | |||||
| I C (n = 3) | 74 | 94* | – | – | – | |
| II A (n = 7) | 71 | 91* | 1 (14.2 %) → | 1 hip IIIA | – | – |
| II B (n = 11) | 70 | 90* | 2 (18.2 %) → | 1hip IIIA | 1 (9.0 %) | – |
| 1 hip IIIB | ||||||
| II C (n = 9) | 67 | 89* | 2 (22.2 %) → | 1 hip IIIA | 2 (22.2 %) | 1 (11.1 %) |
| 1 hip IIIC | ||||||
| III A (n = 7) | 62 | 79* | 3 (42.8 %) → | 1 hip IVA | 2 (28.5 %) | 2 (28.5 %) |
| 2 hips IVC | ||||||
| Total (n = 37) | 68 | 86* | 8 (21.6 %) | 5 (13.5 %) | 3 (8.1 %) | |
* p < 0.05
The average preoperative HHS was 68 points (range 41–93 points), and the average score at the latest follow-up was 86 points (range 38–100 points) indicating a significant difference between before and after operation (p < 0.05). The HHS was determined for all hips at the final follow-up examination. The scores of three hips which had undergone total hip replacement were determined just before surgery.
Results were rated as excellent in 23 (62.2 %) hips, good in nine (24.3 %), fair in three (8.1 %), bad in two (5.4 %). The HHS improved from 74 to 94 in stage 1c, from 71 to 91 in stage 2a, from 70 to 90 in stage 2b, from 67 to 89 in stage 2c, and from 62 to 79 in stage 3a.
The radiological results showed that 29 hips (78.4 %) improved or had no further collapse (Fig. 2). We noted a radiographic progression rate of 14.2 % in stage IIA and 18.2 % in stage IIB (patients with minor lesion size), while progression rate rose in stage IIC (22.2 %) and stage IIIA patients (42.8 %). The mean onset of this progression was at 13 months postoperatively (range seven to 20 months).
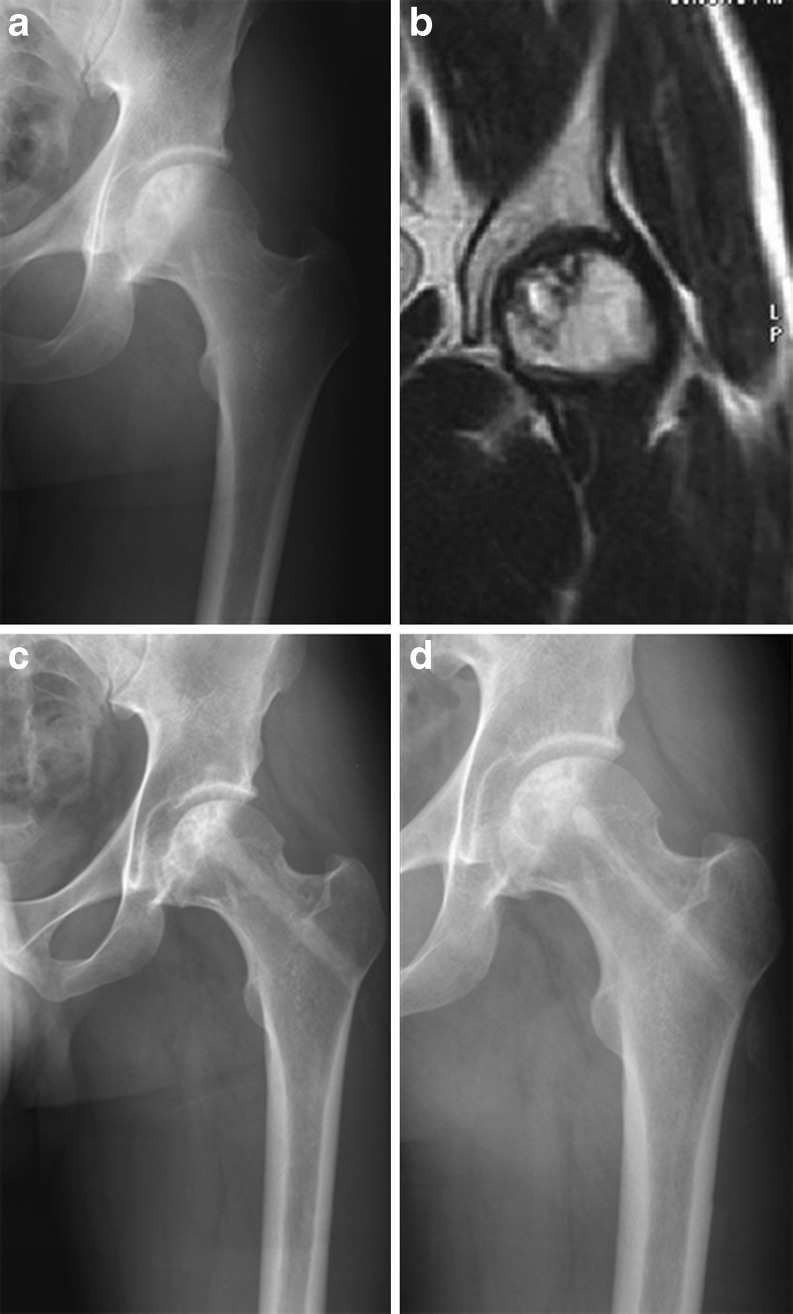
Fig. 2.
a Preoperative anteroposterior (AP) radiograph of the left hip in a symptomatic 42-year-old man with osteonecrosis of the femoral head (ONFH). b Preoperative MRI of the femoral heads confirms the presence of a Steinberg 2c osteonecrotic lesion. c Six months follow-up AP radiograph of the same hip after core decompression, injection of autologous bone marrow concentration and backfilling of core tract with PRO-DENSE. d An AP radiograph taken 30 months after the index procedure shows no collapse of the femoral head and degradation of the ceramic with bone ingrowth and bony remodelling
According to the MRI results of these cases one hip of stage IIA, one hip of stage IIB, and one of stage IIC progressed to IIIA stage; one hip of stage IIB progressed to stage IIIB; one hip of stage IIC progressed to IIIC; one hip of stage IIIA progressed to stage IVA and two hips of stage IIIA progressed to stage IVC.
However, a clinical failure, as previously defined, was present in only five (13.5 %) patients, three of which had to be converted to a total hip arthroplasty after eight, 12 and 14 months from the procedure, due to persistent pain as well as radiographic progression of the disease.
The overall rate of conversion to arthroplasty was 8.1 % in the whole group and only 3.3 % in the pre-collapse group (Steinberg I to II).
Discussion
The rationale of core decompression in the treatment of early stages of ONFH is to reduce or decompress the intraosseous pressure in the femoral head resulting from venous congestion and other pathways, promote vascular invasion and facilitate regeneration of the necrotic tissue [6, 19].
The disadvantage of core decompression alone is the lack of structural support to the subchondral plate. It has been demonstrated that the loss of mechanical integrity of the necrotic zone is a major structural determinant leading to subcondral fracture and loss of sphericity and that core decompression alone may even compromise the structural behaviour of the necrotic head. Finite-element models have demonstrated that core decompression that penetrates the body of the necrotic lesion puts the head at a greater risk of structural collapse compared to the untreated situation, while appropriately placed bone grafts afford considerable relief of stress in the necrotic cancellous bone [20, 21].
Summarising these results, evidence suggests that subchondral mechanical support in the weight-bearing segment of the femoral head is essential during the period of revascularisation and reconstitution of the femoral bone if segmental collapse is to be prevented. A meta-analysis that evaluated core decompression alone found that further surgical intervention was necessary in 16 %, 37 %, and 71 % of at Steinberg stages I, II, and III, respectively [22].
Thus, many grafting techniques have been used in the treatment of ONFH, and these grafts have been of two types: structural or osteoinductive. Structural grafts are usually fibular grafts and are either vascularised or nonvascularised; non-structural grafts are morcelised bone chip alone or augmented with demineralised bone matrix [5–8] .
In all cases the primary function of the bone or bone substitute is to provide a mechanical and structural support and reinforcement of the necrotic segment until the lesion is restored biologically. The results in the literature are satisfactory and these procedures have demonstrated that they provide decompression of the femoral head, removal of necrotic bone, and structural support and scaffolding to allow repair and remodelling of subchondral bone [4, 23].
However, with some of the newer bone graft substitutes and biological bone stimulation, the success rate of these procedures may be further enhanced, and our hypothesis was that a new CaSO4/CaPO4 composite graft, the PRO-DENSE®, could have several theoretical advantages in the mechanical support of core decompression.
When tested in pre-clinical studies, in a canine critical-sized bone defect, at 13 weeks compressive strength and stiffness of restored bone was greater when bone defects were treated with the CaSO4/CaPO4 synthetic graft than when treated with conventional CaSO4 pellets. Furthermore, the results seen in this canine critical defect model and aqueous dissolution testing indicate a positive multiphase bone resorption pattern: in the early post-operative period, the CaSO4 resorbs first through simple dissolution leaving behind an open-pore structure that allows for vascular infiltration and new bone deposition on the remaining CaPO4 scaffold [17].
The staged resorption and dense bone formation evidenced in canine studies would be desirable in core decompression techniques where healthy bony ingrowth is the goal. However it must be stressed that animal studies of bone substitute degradation cannot be transferred directly to humans [24].
The early clinical data for use with this CaSO4-CaPO4 composite has been encouraging. This composite has been used for benign bone tumour series and patients treated with this material reported high functional scores with low complication rates [25].
As a further step of this technique, since the bioceramic mainly addresses the mechanical properties of the femoral head, to enhance the biological environment of the necrotic area we completed the procedure with an injection of autologous bone marrow concentrate, that was intended to provide osteoinductive properties for bone healing and bone regeneration [11, 15, 26].
Besides the preclinical studies and some promising anecdotal reports [27], to the best of our knowledge, this is one of the first studies of this novel biomaterial in the treatment of ONFH after core decompression of the femoral head.
The results of our study compare favourably with these premises. At an average follow-up of about two years, the overall clinical success rate of the procedure was 86.5 %, with three conversions to THA, and a failure rate of only 3.3 % in the pre-collapse group (Steinberg I to II).
The results of core decompression were best for early-stage lesions and were worse for patients with postcollapse disease, i.e. radiological success was noted in 100 % of patients in stage I, 76.67 % of patients in stage IIB and 50.96 % of patients in stage IIC and IIIA cases.
There was a difference between the percentage of clinical success and radiological success in this study. Some patients demonstrated radiological failure but no clinical failure with a HHS above 75 points.
In conclusion, although a short period of follow-up and a small number of patients are the principal limitations of this study we are encouraged by these early results using core decompression, injection of the autologous bone marrow concentration and backfilling the defect with an injectable, synthetic, bioceramic (PRO-DENSE®) for the treatment of early stage, particularly Steinberg I and II, ONFH. However, it should be noted that the results of this technique were best for early-stage lesions and worse for patients with postcollapse disease; furthermore, no conclusions can yet be drawn on the amount and rate of bone formation and the rate of bone substitute dissolution.
Compared to bone grafts this technique combines many clinical advantages: it is a minimally invasive and short procedure, it eliminates the morbidity and complication of graft harvesting, the use of the percutaneous expanding reamer allows for increased debridement of the necrotic zone, and finally it burns no bridges regard to future treatment options for patients with progression of disease.
Further studies with longer follow-up are needed to better establish the kinetics of resorption and new bone formation of this bioceramic.
Acknowledgments
Conflict of interest
The authors declared that they have no conflict of interest.
References
- 1.Hungerford DS, Mont MA. The natural history of untreated asymptomatic hips in patients who have non-traumatic osteonecrosis. J Bone Joint Surg Am. 1998;80:765–6. [PubMed] [Google Scholar]
- 2.Nam KW, Kim YL, Yoo JJ, Koo KH, Yoon KS, Kim HJ. Fate of untreated asymptomatic osteonecrosis of the femoral head. J Bone Joint Surg Am. 2008;90:477–484. doi: 10.2106/JBJS.F.01582. [DOI] [PubMed] [Google Scholar]
- 3.Lieberman JR. Core decompression for osteonecrosis of the hip. Clin Orthop Relat Res. 2004;418:29–33. doi: 10.1097/00003086-200401000-00006. [DOI] [PubMed] [Google Scholar]
- 4.Marker DR, Seyler TM, Ulrich SD, Srivastava S, Mont MA. Do modern techniques improve core decompression outcomes for hip osteonecrosis. Clin Orthop Relat Res. 2008;466:1093–1103. doi: 10.1007/s11999-008-0184-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Korompilias AV, Lykissas MG, Beris AE, Urbaniak JR, Soucacos PN. Vascularised fibular graft in the management of femoral head osteonecrosis: twenty years alter. J Bone Joint Surg Br. 2009;91:287–93. doi: 10.1302/0301-620X.91B3.21846. [DOI] [PubMed] [Google Scholar]
- 6.Steinberg ME, Larcom PG, Strafford B, Hosick WB, Corces A, Bands RE, Hartman KE. Core decompression with bone grafting for osteonecrosis of the femoral head. Clin Orthop Relat Res. 2001;386:71–78. doi: 10.1097/00003086-200105000-00009. [DOI] [PubMed] [Google Scholar]
- 7.Wang BL, Sun W, Shi ZC, Zhang NF, Yue DB, Guo WS, Shi SH, Li ZR. Treatment of nontraumatic osteonecrosis of the femoral head using bone impaction grafting through a femoral neck window. Int Orthop. 2009;34(5):635–639. doi: 10.1007/s00264-009-0822-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lieberman JR, Conduah A, Urist MR. Treatment of osteonecrosis of the femoral head with core decompression and human bone morphogenetic protein. Clin Orthop Relat Res. 2004;429:139–44. doi: 10.1097/01.blo.0000150312.53937.6f. [DOI] [PubMed] [Google Scholar]
- 9.Hernigou P, Beaujean F. Treatment of osteonecrosis with autologous bone marrow grafting. Clin Orthop Relat Res. 2002;405:14–23. doi: 10.1097/00003086-200212000-00003. [DOI] [PubMed] [Google Scholar]
- 10.Yoshioka T, Mishima H, Akaogi H, Sakai S, Li M, Ochiai N. Concentrated autologous bone marrow aspirate transplantation treatment for corticosteroid-induced osteonecrosis of the femoral head in systemic lupus erythematosus. Int Orthop. 2011;35(6):823–829. doi: 10.1007/s00264-010-1048-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gao YS, Zhang CQ. Cytotherapy of osteonecrosis of the femoral head: a mini review. Int Orthop. 2010;34:779–782. doi: 10.1007/s00264-010-1009-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shuler MS, Rooks MD, Roberson JR. Porous tantalum implant in early osteonecrosis of the hip: preliminary report on operative, survival, and outcomes results. J Arthroplast. 2007;22:26–31. doi: 10.1016/j.arth.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 13.Floerkemeier T, Lutz A, Nackenhorst U, Thorey F, Waizy H, Windhagen H, Lewinski G. Int Orthop. 2011;35(10):1461–6. doi: 10.1007/s00264-010-1138-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mont MA, Jones LC, Einhorn TA, Hungerford DS, Reddi AH. Osteonecrosis of the femoral head. Potential treatment with growth and differentiation factors. Clin Orthop Relat Res. 1998;355:314–335. doi: 10.1097/00003086-199810001-00032. [DOI] [PubMed] [Google Scholar]
- 15.Cui Q, Botchwey EA. Emerging ideas. Treatment of pre-collapse osteonecrosis using stem cells and growth factors. Clin Orthop Relat Res. 2011;469:2665–2669. doi: 10.1007/s11999-010-1738-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li Y, Han R, Geng C, Wang Y, Wei L. A new osteonecrosis animal model of the femoral head induced by microwave heating and repaired with tissue engineered bone. Int Orthop. 2009;33(2):573–580. doi: 10.1007/s00264-008-0672-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Urban RM, Turner TM, Hall DJ, Inoue N, Gitelis S. Increased bone formation using calcium sulfate-calcium phosphate composite graft. Clin Orthop Relat Res. 2007;457:110–117. doi: 10.1097/BLO.0b013e318059b902. [DOI] [PubMed] [Google Scholar]
- 18.Steinberg ME, Steinberg DR. Classification systems for osteonecrosis: an overview. Orthop Clin North Am. 2004;35:273–283. doi: 10.1016/j.ocl.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 19.Petrigliano FA, Lieberman JR. Osteonecrosis of the hip: novel approaches to evaluation and treatment. Clin Orthop Relat Res. 2007;465:53–62. doi: 10.1097/BLO.0b013e3181591c92. [DOI] [PubMed] [Google Scholar]
- 20.Penix AR, Cook SD, Skinner HB, Weinstein AM, Haddad RJ., Jr Femoral head stresses following cortical bone grafting for aseptic necrosis. A finite element study. Clin Orthop. 1983;173:159–165. [PubMed] [Google Scholar]
- 21.Brown TD, Pedersen DR, Baker KJ, Brand RA. Mechanical consequences of core drilling and bone-grafting on osteonecrosis of the femoral head. J Bone Joint Surg Am. 1993;75:1358–1367. doi: 10.2106/00004623-199309000-00011. [DOI] [PubMed] [Google Scholar]
- 22.Castro FP, Barrack RL. Core decompression and conservative treatment for avascular necrosis of the femoral head: a meta-analysis. Am J Orthop. 2000;29:187–194. [PubMed] [Google Scholar]
- 23.Mont MA, Jones LC, Hungerford DS. Nontraumatic osteonecrosis of the femoral head: ten years later. J Bone Joint Surg Am. 2006;88:1117–1132. doi: 10.2106/JBJS.E.01041. [DOI] [PubMed] [Google Scholar]
- 24.Meyer S, Floerkemeier T, Windhagen H. Histological osseointegration of a calciumphosphate bone substitute material in patients. Biomed Mater Eng. 2007;17:347–356. [PubMed] [Google Scholar]
- 25.Fillingham YA, Lenart BA, Gitelis S (2012) Function after injection of benign bone lesions with a bioceramic. Clin Orthop Relat Res. doi:10.1007/s11999-012-2251-5 [DOI] [PMC free article] [PubMed]
- 26.Gangji V, Hauzeur JP. Treatment of osteonecrosis of the femoral head with implantation of autologous bone-marrow cells. J Bone Joint Surg Am. 2005;87:106–112. doi: 10.2106/JBJS.D.02662. [DOI] [PubMed] [Google Scholar]
- 27.Hungerford DS. The use of an injectable calcium sulfate/calcium phosphate composite in the treatment of osteonecrosis of the femoral head. J Bone Joint Surg Br. 2009;91:331. [Google Scholar]