Abstract
Purpose
The myofibroblast, a contractile fibroblastic cell expressing α-smooth muscle actin (α-SMA), has been reported to play a role in ligament healing. The aim of this study was to evaluate the feasibility of transplanting culture-derived myofibroblasts in injured rabbit medial collateral ligaments (MCL) and in intact anterior cruciate ligaments (ACL).
Methods
Fibroblasts isolated from the iliotibial band were cultured in the presence of transforming growth factor beta-1 (TGF-β1) for five days and analysed for α-SMA expression. In a concentration of TGF-β1 ≥ 10 ng/ml, the differentiation rate into myofibroblast was 90%. After labelling with PKH26, α-SMA -positive cells were transplanted in intact ACL and in injured MCL of ten rabbits.
Results
Survival of PKH-26+ cells was seen in all intact and damaged ligaments one day after injection. The density of PKH-26+ cells had decreased at seven days postinjection in both ligaments. Double-positive PKH-26+/α-SMA+ cells were only observed in injured MCL at seven days postinjection. Moreover, we found that genetically modified fibroblasts differentiate into myofibroblasts and can be transplanted into ligaments.
Conclusions
Our data demonstrate that culture-born myofibroblasts survive and maintain α-SMA expression up to one week after transplantation. This study provides the first insight into the feasibility of transplanted mechanically active cells for ligament reconstruction.
Introduction
Ligament injuries are encountered frequently in orthopaedics and, if severe, can result in residual laxity, further injury, joint pain and ultimately osteoarthritis. Despite the availability of several therapeutic tools that help repair ligament injuries [1], the healing process results, in most cases—e.g. after anterior cruciate ligament (ACL) injury—in a structure with mechanical properties inferior to those of the native ligament [2–4]. Thus, much effort has been made to improve and optimise the healing environment of injured ligaments [5]. The use of cells as biological vehicles [6–10] or of growth factors [11, 12] has been shown to enhance ligament healing. Recently, the use of a bioactive collagen-based scaffold infiltrated with platelets has yielded a positive effect on the healing of an ACL graft [13]. These data emphasise the importance of cellular and molecular mechanisms in the process of ligament healing and more generally in the field of regenerative orthopaedics [14].
Myofibroblasts, identified as α-smooth muscle actin (α-SMA)-positive fibroblasts [15, 16], have been observed in normal and healing tendons [17, 18] [19] and in the intact and injured human ACL [20–22]. They contribute substantially to the contractile phase of MCL healing, allowing recovery of its original length [23]. We previously demonstrated that myofibroblasts and transforming growth factor beta-R1 (TGF-β-RI) appear in the early phase of the MCL healing process, similarly to that occurring during wound healing [24]. The study reported here investigates whether primary isolated rabbit fibroblasts can differentiate into myofibroblasts in vitro and whether these cells can be transplanted into intact and injured ligaments. Data demonstrate that culture-derived myofibroblasts transplanted in rabbit ligaments, survive and maintain α-SMA expression up to seven days after injection. These results emphasise the potential of such cells to improve ligament healing after injury.
Material and methods
Cell culture
Rabbit fibroblasts were isolated from the iliotibial band (ITB) and amplified on gelatine-coated dishes (NalgeNunc, Rochester, NY, USA) at 37°C, 7% CO2 in Dulbecco’s modified Eagle’s medium (DMEM) (Invitrogen, Basel, Switzerland) containing 10% foetal calf serum (FCS) (Readysystem, Kibbuth Beth Halmek, Israel) and 1% penicillin/streptomycin (Invitrogen). The differentiation into myofibroblasts was induced by incubating primary fibroblasts for five days in DMEM 10% FCS containing 0.1, 1 or 10 ng/ml TGF-β1 (R&D systems Inc., Minneapolis, MN, USA).
GFP lentivirus production
A three-plasmid expression system was used to generate second-generation lentiviral vectors by transient transfection, as previously described [25]. Rabbit fibroblasts were transduced with the lentivirus green fluorescent protein (GFP) at multiplicity of infection (MOI) of 10 before TGF-β1 stimulation.
Induction of MCL injury and myofibroblast transplantation
Animal studies described in this study were approved by the Ethical Committee for Animal Experimentation of the Faculty of Medicine, University of Geneva and Veterinary Authority in accordance with Swiss guidelines. Ten female New Zealand white rabbits (six to eight months old; 3.5 ± 0.5 kg) were purchased from the animal core facility of the University of Geneva. Rabbits were premedicated with xylazine (Rompun®, Bayer HealthCare Pharmaceuticals, NJ, USA), and general anaesthesia was induced and maintained with continuous injection of ketamine (Ketalar®, Parke-Davis) and acepromazine (Vetranquil®, Sanofi-Ceva GmbH, Dusseldorf, Germany). In each animal, the MCL was identified, meticulously undermined and a mop-end tear model created [4, 26]. The ACL was left intact in all experiments. In fact, we observed in a pilot study that complete ACL section resulted in disappearance of the ligament after two weeks (data not shown). Five days after MCL injury, through an anteromedial skin incision, the injured MCL was identified and exposed for cell transplantation. Through an anteromedial arthrotomy, the intact ACL was also identified and exposed for cell transplantation. Rabbit myofibroblasts labelled with PKH-26 (Sigma, St. Louis, MO, USA) (2 × 106 cells per injection in 20 μl DMEM) were injected using a 50-μl syringe with a 27-gauge needle (Hamilton Company, Bonaduz, Switzerland) into injured MCL and into intact ACL. Their contralateral knees served as sham-operated control (20 μl DMEM/no cells). Postoperatively, all animals received daily intramuscular injection of enrofloxacin for three days (Baytril®, Bayer HealthCare Pharmaceuticals) and were allowed free cage activity (cage area: 3 m2). Animals were sacrificed at day one and day seven postinjection (n = 5 per group).
Immunohistochemistry
ACL and MCL were retrieved entirely at days one and seven after surgery, flash frozen, cryosectioned, fixed in phosphate-buffered saline (PBS)/4% formaldehyde and observed with a fluorescent microscope (Axiophot Zeiss) to determine the presence of PKH-26 dyes. Representative sections from each ligament were stained using a mouse monoclonal antibody recognising α-SMA (clone 1A4, [27]). Briefly, nonspecific sites were blocked with 5% bovine serum albumin (BSA) in phosphate-buffered saline for one hour. Sections were then incubated with anti-α-SMA for one hour, followed by 30 min incubation with a goat anti-mouse Alexa 488 (Sigma). All nuclei were stained using a fluorescence-specific 4’-6’-diamidino-2-phenylindole (DAPI) containing mounting medium (Vector Laboratories, Burlingame, CA, USA).
Statistical analysis
Statistical evaluation was performed using Student’s t test, and results were considered significant if P values were < 0.05.
Results
Primary rabbit fibroblasts differentiate into myofibroblasts in vitro
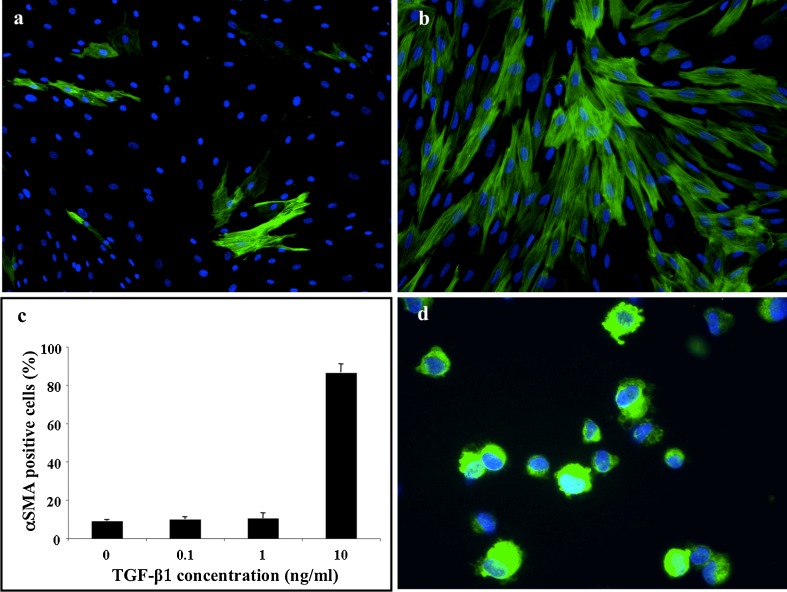
Rabbit fibroblasts isolated from the ITB were incubated for five days with various concentrations of TGF-β1 (0.1, 1 or 10 ng/ml) and analysed by immunofluorescence for α-SMA expression. With a low dose of TGF-β1 (0.1 or 1 ng/ml) in culture medium, no difference in α-SMA expression was observed compared with controls (<10% of α-SMA-positive cells in all conditions, n = 4, Fig. 1a, c). A significant increase in α-SMA expression was observed when fibroblasts were incubated with 10 ng/ml of TGF-β1 for five days (86.4 ± 4.6% of α-SMA-positive cells, n = 4, Fig. 1b, c). Moreover, we observed that culture-derived myofibroblasts maintain their contractile phenotype in vitro up to five days after cessation of TGF-β1 stimulation (data not shown).
Fig. 1.
Transforming growth factor beta-1 (TGF-β1) at 10 ng/ml for 5 days (b, c) for 5 days significantly increases the proportion of α-smooth-muscle actin (SMA)-positive fibroblasts (green staining) compared with untreated cells (a, c). Trypsinisation of rabbit myofibroblasts after TGF-β1 treatment had no effect on α-SMA expression as assessed by cytospin assay (d). These data are representative of at least four different experiments for each condition
Autologous transplantation of culture derived myofibroblast in rabbit ligaments
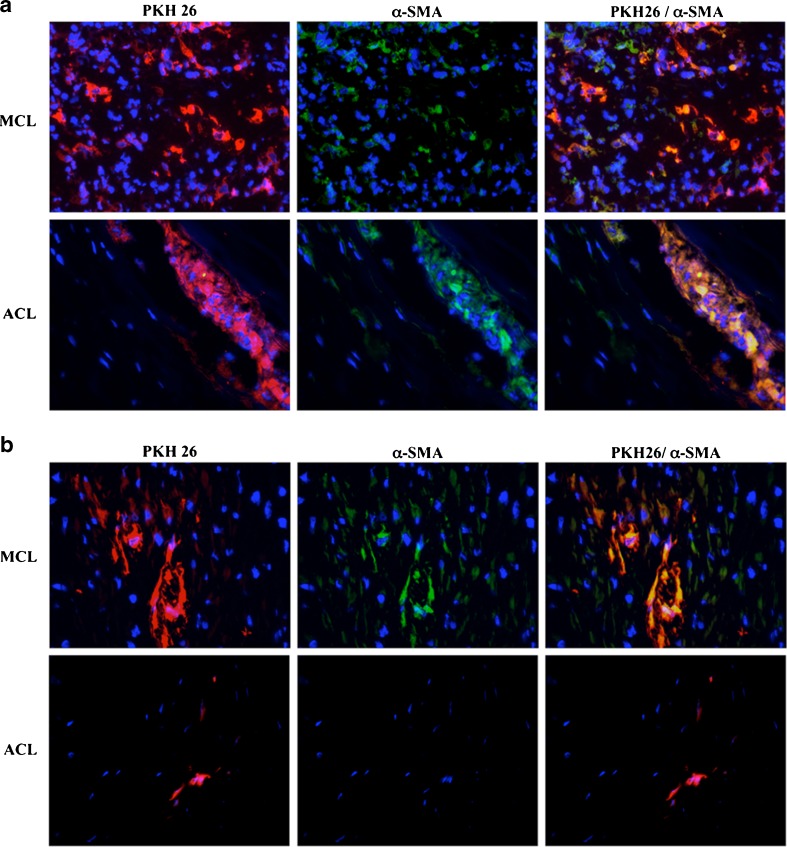
After five days of incubation with 10 ng/ml of TGF-β1, myofibroblasts were trypsinised, marked with PHK-26, a stable lipophilic cell membrane linker, and injected into intact ACL and injured MCL. Prior to transplantation, α-SMA expression was confirmed by immunofluorescence cytospin assay with >80% positive cells (Fig. 1d). At one day postinjection, we observed the presence of PKH-26-positive cells in all injured and intact ligaments. The α-SMA-positive cells were found in the same recipient region in which PKH-26-positive cells were located (Fig. 2a). We also observed a few α-SMA+/PKH-26− cells in the damaged MCL. At seven days postinjection (Fig. 2b), the density of PKH-26-positive cells had decreased in both ligaments, but colocalisation of α-SMA and PKH-26 was still observed in the injured MCL.
Fig. 2.
PKH-26+ cells maintained their myofibroblasts phenotype in vivo up to 7 days after transplantation in rabbit ligament. Culture-derived myofibroblasts labelled with PKH-26 (red) were transplanted in damaged [medial collateral ligaments (MCL)] or intact [anterior cruciate ligament (ACL)] ligaments, and biopsies were performed at 1 (a) or 7 (b) days after cell injection. Tissue sections were analysed using immunofluorescence for α-smooth-muscle actin (α-SMA) (green) and 4’-6’-diamidino-2-phenylindole (DAPI) (blue). The sections shown are representative of five analysed grafts
Myofibroblast as a vehicle for gene therapy
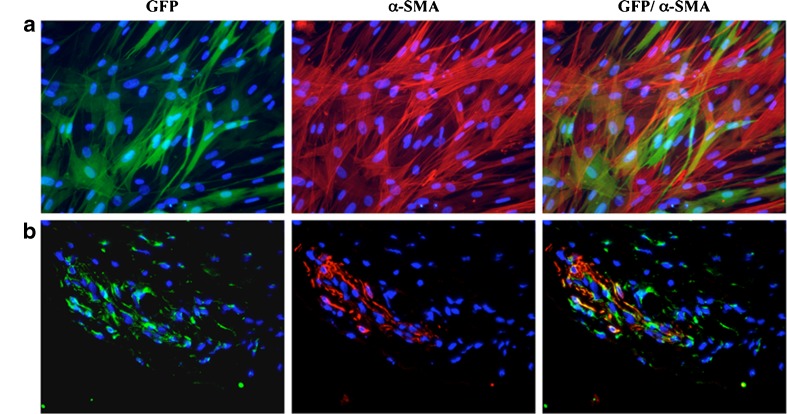
To evaluate the possibility of using myofibroblasts as a vehicle for therapeutic products, rabbit fibroblasts were transduced with a lentivirus encoding the GFP marker protein (>90% of GFP-positive cells) and cultured in the presence of 10 ng/ml TGF-β1 for five days. Alpha-SMA expression was observed in GFP-positive cells, indicating that lentivirus did not alter rabbit fibroblast differentiation capacity in vitro (Fig. 3a). The presence of GFP-positive cells coexpressing α-SMA in the injection region was confirmed by immunofluorescence one day after transplantation (Fig. 3b).
Fig. 3.
Green fluorescent protein (GFP)-transduced fibroblasts maintained their capacity to express α-smooth-muscle actin (SMA) in vitro and in vivo. GFP+ fibroblasts treated with transforming growth factor beta-1 (TGF-β1) (5 days at 10 ng/ml) were analysed for α-SMA expression before transplantation (a) and 1 day after transplantation (b). ACL biopsies were immunostained for α-SMA (red) and the nuclei marked with 4’-6’-diamidino-2-phenylindole (DAPI) (blue). Sections shown are representative of three analysed grafts
Discussion
Cell transplantation for ligament healing relies on the ability of transplanted cells to retain their intrinsic phenotype in the context of a massive endogenous inflammation. This study analysed the fate of rabbit myofibroblasts after autologous transplantation in intact and injured ligaments. In accordance with previous studies [28], we found that under specific conditions, fibroblasts from different origins can be differentiated into myofibroblasts in the presence of TGF-β1. Myofibroblasts were able to maintain their phenotype up to five days in vitro after cessation of TGF-β1 (data not shown). These findings confirm the feasibility of using cultured myofibroblasts for a cell therapy approach.
The second part of the experiment demonstrated that myofibroblasts can be transplanted into damaged ligaments as well as into dense and poorly vascularised tissue, such as an intact ligament, and survive in such environments. Transplanted cells maintain α-SMA expression in the intact and injured ligament at one day after injection. At seven days posttransplantation, double-positive cells (α-SMA+/PKH-26+) were observed in damaged MCL only. The presence of TGF-β1 within the injured ligament, as recently described [24], may create a favourable environment for myofibroblasts in vivo and may explain in part their presence in injured ligament only at seven days postinjection. It is also possible that myofibroblasts remain quiescent after transplantation, and in this state, α-SMA turnover is slow and the cells survive for a long time [29]. Moreover, in addition to promoting myofibroblast differentiation, it is known that combined activation of the adhesion-dependent focal adhesion kinase pathway and the soluble growth-factor-mediated protein kinase B (AKT) pathway confers anoikis/apoptosis resistance to TGF-β1differentiated myofibroblasts [30].
Despite some study limitations (there is no quantitative analysis of cell survival postinjection and no evaluation of the ligament healing process after transplantation), we believe that this study is novel and provides important initial information for the use of mechanically active cells as a source for cell-based therapy. We show evidence that myofibroblasts do not dedifferentiate early after transplantation; therefore, it appears likely that they become fixed to the extracellular matrix, a necessary step for the maintenance of differentiation [31]. Moreover, we show that genetically modified fibroblasts differentiate into myofibroblasts and can be transplanted into ligaments. Injection of genetically modified myofibroblasts expressing growth factors may stimulate and promote formation of new ligament tissue, leading to an accelerated recovery. Growth factors such as epithelial growth factor (EGF), TGF-β1 or platelet-derived growth factor (PDGF) stimulate fibroblasts to proliferate and to reconstitute a collagen-rich extracellular matrix [32, 33].
These findings clearly suggest that cell-therapy approaches using mechanically active cells such as myofibroblasts may represent an interesting strategy for improving the healing of extra- and intra-articular ligaments. Further investigations are required to unveil the full potency of such an approach.
Acknowledgments
This work was supported by the Swiss National Science Foundation (NRP 46 n°4046-058639 and grant n°310030_130700/1), by the Fondation suisse de recherche sur les maladies musculaires, by the Research funds of the Geneva Orthopedic Service and by the Foundation Marcel Levaillant.
Conflict of Interest
None
References
- 1.Woo SL, Abramowitch SD, Kilger R, Liang R. Biomechanics of knee ligaments: injury, healing, and repair. J Biomech. 2006;39(1):1–20. doi: 10.1016/j.jbiomech.2004.10.025. [DOI] [PubMed] [Google Scholar]
- 2.Woo SL, Vogrin TM, Abramowitch SD. Healing and repair of ligament injuries in the knee. J Am Acad Orthop Surg. 2000;8(6):364–372. doi: 10.5435/00124635-200011000-00004. [DOI] [PubMed] [Google Scholar]
- 3.Frank CB. Ligament healing: current knowledge and clinical applications. J Am Acad Orthop Surg. 1996;4(1):74–83. doi: 10.5435/00124635-199603000-00002. [DOI] [PubMed] [Google Scholar]
- 4.Weiss JA, Woo SL, Ohland KJ, Horibe S, Newton PO. Evaluation of a new injury model to study medial collateral ligament healing: primary repair versus nonoperative treatment. J Orthop Res. 1991;9(4):516–528. doi: 10.1002/jor.1100090407. [DOI] [PubMed] [Google Scholar]
- 5.Sanchez M, Anitua E, Lopez-Vidriero E, Andia I. The future: optimizing the healing environment in anterior cruciate ligament reconstruction. Sports Med Arthrosc Rev. 2010;18(1):48–53. doi: 10.1097/JSA.0b013e3181c0ccd5. [DOI] [PubMed] [Google Scholar]
- 6.Day CS, Kasemkijwattana C, Menetrey J, Floyd SS, Jr, Booth D, Moreland MS, Fu FH, Huard J. Myoblast-mediated gene transfer to the joint. J Orthop Res. 1997;15(6):894–903. doi: 10.1002/jor.1100150616. [DOI] [PubMed] [Google Scholar]
- 7.Hildebrand KA, Deie M, Allen CR, Smith DW, Georgescu HI, Evans CH, Robbins PD, Woo SL. Early expression of marker genes in the rabbit medial collateral and anterior cruciate ligaments: the use of different viral vectors and the effects of injury. J Orthop Res. 1999;17(1):37–42. doi: 10.1002/jor.1100170107. [DOI] [PubMed] [Google Scholar]
- 8.Menetrey J, Kasemkijwattana C, Day CS, Bosch P, Fu FH, Moreland MS, Huard J. Direct-, fibroblast- and myoblast-mediated gene transfer to the anterior cruciate ligament. Tissue Eng. 1999;5(5):435–442. doi: 10.1089/ten.1999.5.435. [DOI] [PubMed] [Google Scholar]
- 9.Wang CJ, Weng LH, Hsu SL, Sun YC, Yang YJ, Chan YS, Yang YL. pCMV-BMP-2-transfected cell-mediated gene therapy in anterior cruciate ligament reconstruction in rabbits. Arthroscopy. 2010;26(7):968–976. doi: 10.1016/j.arthro.2009.11.014. [DOI] [PubMed] [Google Scholar]
- 10.Tei K, Matsumoto T, Mifune Y, Ishida K, Sasaki K, Shoji T, Kubo S, Kawamoto A, Asahara T, Kurosaka M, Kuroda R. Administrations of peripheral blood CD34-positive cells contribute to medial collateral ligament healing via vasculogenesis. Stem cells. 2008;26(3):819–830. doi: 10.1634/stemcells.2007-0671. [DOI] [PubMed] [Google Scholar]
- 11.Yasuda K, Tomita F, Yamazaki S, Minami A, Tohyama H. The effect of growth factors on biomechanical properties of the bone-patellar tendon-bone graft after anterior cruciate ligament reconstruction: a canine model study. Am J Sports Med. 2004;32(4):870–880. doi: 10.1177/0363546503261695. [DOI] [PubMed] [Google Scholar]
- 12.Hildebrand KA, Woo SL, Smith DW, Allen CR, Deie M, Taylor BJ, Schmidt CC. The effects of platelet-derived growth factor-BB on healing of the rabbit medial collateral ligament. An in vivo study. Am J Sports Med. 1998;26(4):549–554. doi: 10.1177/03635465980260041401. [DOI] [PubMed] [Google Scholar]
- 13.Joshi SM, Mastrangelo AN, Magarian EM, Fleming BC, Murray MM. Collagen-platelet composite enhances biomechanical and histologic healing of the porcine anterior cruciate ligament. Am J Sports Med. 2009;37(12):2401–2410. doi: 10.1177/0363546509339915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ivkovic A, Marijanovic I, Hudetz D, Porter RM, Pecina M, Evans CH. Regenerative medicine and tissue engineering in orthopaedic surgery. Front Biosci (Elite Ed) 2011;3:923–944. doi: 10.2741/e299. [DOI] [PubMed] [Google Scholar]
- 15.Darby I, Skalli O, Gabbiani G. Alpha-smooth muscle actin is transiently expressed by myofibroblasts during experimental wound healing. Lab Invest. 1990;63(1):21–29. [PubMed] [Google Scholar]
- 16.Schurch W, Seemayer TA, Gabbiani G. The myofibroblast: a quarter century after its discovery. Am J Surg Pathol. 1998;22(2):141–147. doi: 10.1097/00000478-199802000-00001. [DOI] [PubMed] [Google Scholar]
- 17.Ippolito E, Natali PG, Postacchini F, Accinni L, Martino C. Ultrastructural and immunochemical evidence of actin in the tendon cells. Clin Orthop Relat Res. 1977;126:282–284. [PubMed] [Google Scholar]
- 18.Ippolito E, Natali PG, Postacchini F, Accinni L, Martino C. Morphological, immunochemical, and biochemical study of rabbit achilles tendon at various ages. J Bone Joint Surg Am. 1980;62(4):583–598. [PubMed] [Google Scholar]
- 19.Postacchini F, Natali PG, Accinni L, Ippolito E, Martino C. Contractile filaments in cells of regenerating tendon. Experientia. 1977;33(7):957–959. doi: 10.1007/BF01951303. [DOI] [PubMed] [Google Scholar]
- 20.Murray MM, Martin SD, Martin TL, Spector M. Histological changes in the human anterior cruciate ligament after rupture. J Bone Joint Surg Am. 2000;82-A(10):1387–1397. doi: 10.2106/00004623-200010000-00004. [DOI] [PubMed] [Google Scholar]
- 21.Murray MM, Spector M. Fibroblast distribution in the anteromedial bundle of the human anterior cruciate ligament: the presence of alpha-smooth muscle actin-positive cells. J Orthop Res. 1999;17(1):18–27. doi: 10.1002/jor.1100170105. [DOI] [PubMed] [Google Scholar]
- 22.Weiler A, Unterhauser FN, Bail HJ, Huning M, Haas NP. Alpha-smooth muscle actin is expressed by fibroblastic cells of the ovine anterior cruciate ligament and its free tendon graft during remodeling. J Orthop Res. 2002;20(2):310–317. doi: 10.1016/S0736-0266(01)00109-7. [DOI] [PubMed] [Google Scholar]
- 23.Faryniarz DA, Chaponnier C, Gabbiani G, Yannas IV, Spector M. Myofibroblasts in the healing lapine medial collateral ligament: possible mechanisms of contraction. J Orthop Res. 1996;14(2):228–237. doi: 10.1002/jor.1100140210. [DOI] [PubMed] [Google Scholar]
- 24.Menetrey J, Laumonier T, Garavaglia G, Hoffmeyer P, Fritschy D, Gabbiani G, Bochaton-Piallat ML. alpha-Smooth muscle actin and TGF-beta receptor I expression in the healing rabbit medial collateral and anterior cruciate ligaments. Injury. 2011;42(8):735–741. doi: 10.1016/j.injury.2010.07.246. [DOI] [PubMed] [Google Scholar]
- 25.Salmon P, Kindler V, Ducrey O, Chapuis B, Zubler RH, Trono D. High-level transgene expression in human hematopoietic progenitors and differentiated blood lineages after transduction with improved lentiviral vectors. Blood. 2000;96(10):3392–3398. [PubMed] [Google Scholar]
- 26.Murray MM, Spindler KP, Ballard P, Welch TP, Zurakowski D, Nanney LB. Enhanced histologic repair in a central wound in the anterior cruciate ligament with a collagen-platelet-rich plasma scaffold. J Orthop Res. 2007;25(8):1007–1017. doi: 10.1002/jor.20367. [DOI] [PubMed] [Google Scholar]
- 27.Skalli O, Ropraz P, Trzeciak A, Benzonana G, Gillessen D, Gabbiani G. A monoclonal antibody against alpha-smooth muscle actin: a new probe for smooth muscle differentiation. J Cell Biol. 1986;103(6 Pt 2):2787–2796. doi: 10.1083/jcb.103.6.2787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Desmouliere A, Geinoz A, Gabbiani F, Gabbiani G. Transforming growth factor-beta 1 induces alpha-smooth muscle actin expression in granulation tissue myofibroblasts and in quiescent and growing cultured fibroblasts. J Cell Biol. 1993;122(1):103–111. doi: 10.1083/jcb.122.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tomasek JJ, Gabbiani G, Hinz B, Chaponnier C, Brown RA. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat Rev Mol Cell Biol. 2002;3(5):349–363. doi: 10.1038/nrm809. [DOI] [PubMed] [Google Scholar]
- 30.Horowitz JC, Rogers DS, Sharma V, Vittal R, White ES, Cui Z, Thannickal VJ. Combinatorial activation of FAK and AKT by transforming growth factor-beta1 confers an anoikis-resistant phenotype to myofibroblasts. Cell Signal. 2007;19(4):761–771. doi: 10.1016/j.cellsig.2006.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hinz B, Mastrangelo D, Iselin CE, Chaponnier C, Gabbiani G. Mechanical tension controls granulation tissue contractile activity and myofibroblast differentiation. Am J Pathol. 2001;159(3):1009–1020. doi: 10.1016/S0002-9440(10)61776-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.DesRosiers EA, Yahia L, Rivard CH. Proliferative and matrix synthesis response of canine anterior cruciate ligament fibroblasts submitted to combined growth factors. J Orthop Res. 1996;14(2):200–208. doi: 10.1002/jor.1100140206. [DOI] [PubMed] [Google Scholar]
- 33.Marui T, Niyibizi C, Georgescu HI, Cao M, Kavalkovich KW, Levine RE, Woo SL. Effect of growth factors on matrix synthesis by ligament fibroblasts. J Orthop Res. 1997;15(1):18–23. doi: 10.1002/jor.1100150104. [DOI] [PubMed] [Google Scholar]