Abstract
Winter climate and snow cover are the important drivers of plant community development in polar regions. However, the impacts of changing winter climate and associated changes in snow regime have received much less attention than changes during summer. Here, we synthesize the results from studies on the impacts of extreme winter weather events on polar heathland and lichen communities. Dwarf shrubs, mosses and soil arthropods were negatively impacted by extreme warming events while lichens showed variable responses to changes in extreme winter weather events. Snow mould formation underneath the snow may contribute to spatial heterogeneity in plant growth, arthropod communities and carbon cycling. Winter snow cover and depth will drive the reported impacts of winter climate change and add to spatial patterns in vegetation heterogeneity. The challenges ahead lie in obtaining better predictions on the snow patterns across the landscape and how these will be altered due to winter climate change.
Keywords: Autumn, Empetrum nigrum, Icing, Snow, Snow mould, Winter
Introduction
Historically, there has been a strong research focus on the growing season (spring and summer) for many ecological studies and the same has largely been true for the impacts of climate change on ecosystems. The impact of climate change on plants and animals has been well documented through numerous field observations (Parmesan and Yohe 2003) and manipulation studies (Elmendorf et al. 2012). These achievements have resulted in an in-depth knowledge of how ecosystems may respond to a gradual growing season temperature increase. However, climatic change outside the growing season has received far less attention. Although it is generally acknowledged that winter processes affect organisms with knock-on effects in the following growing season (Inouye 2000; Sturm et al. 2001; Wipf and Rixen 2010; Callaghan et al. 2011), the main biological research focus has more often been on the growing season.
Climate change models also indicate that the frequency of extreme weather events may increase (Christensen et al. 2007). A higher frequency of unseasonal extreme weather increases the risk of mortality for many organisms (Gaines and Denny 1993), which can lead to unexpected knock-on effects throughout an ecosystem. The impact of extreme weather has received considerable research attention during the last decade, but again there has been a major focus on the growing season while autumn and winter have received much less attention (Phoenix and Lee 2004).
In this review, we present an overview of recent studies focussing on winter climate change. Our first goal was to emphasise the importance of climatic changes during the cold season for community development and ecosystem processes. Second, we compared species-specific responses to determine the effect winter climate change has on spatial heterogeneity in community composition. The main focus of this study centres on a field manipulation study on extreme winter warming events conducted at the Abisko Scientific Research Station (ANS) in northern Sweden.
Extreme Winter Warming Simulation
Extreme, short-lived, winter warming events in the Arctic rapidly melt snow and expose ecosystems to unseasonably warm air (2–10 °C) for between 2 and 14 days but upon return to normal winter climate the ecosystem is exposed to much colder temperatures, with potentially devastating impacts for organisms, due to the loss of insulating snow (Phoenix and Lee 2004; Bokhorst et al. 2009). Such events are occurring more frequently in northern Scandinavia (Johansson et al. 2011) and are likely to continue under a warming winter climate. The simulation of week-long extreme winter warming events was performed in a sub-arctic heathland dominated by Empetrum nigrum, Vaccinium myrtillus and V. vitis-idaea. Warming was achieved through infrared heating lamps suspended 50 cm above the snow in twelve plots (1.0 × 2.0 m) during March (in 2007, 2008 and 2009) and in six plots (1.0 × 1.0 m) during January (in 2008 and 2009). The snow pack was slowly melted during 2–3 days. Lamps were thereafter kept ~70 cm above the canopy height to achieve realistic warming with temperatures varying between 1 and 10 °C. Leaf temperatures were checked twice a day and lamp height adjusted where necessary to maintain warming temperatures but avoid overheating. Six of the March plots also had heating cables placed at 5-cm depth in the soil to determine the impact of thawing soils in addition to aboveground warming events (called canopy + soil warming). Soil temperatures fluctuated around 0 °C in the canopy warming treatment but warmed to 7 °C during the canopy and soil warming treatment. Temperatures dropped to well below freezing with much colder temperatures (−15 °C) than in the control plots due to the lack of insulating snow after the heating elements were turned off.
Vascular Plant Responses to Extreme Winter Warming Events
The extreme winter warming events caused considerable damage to many sub-arctic evergreen dwarf shrubs. In particular, the dominant dwarf shrubs E. nigrum, V. myrtillus and V. vitis-idaea were heavily impacted and showed considerable shoot mortality following these simulations. However, only E. nigrum showed a significant decline in live biomass (34 %). The deciduous species V. uliginosum and Deschampsia flexuosa were not affected by these events (Bokhorst et al. 2011a) although this does not mean that deciduous species will not respond to changes in winter snow (Schmidt et al. 2010). Species-specific responses were found during an ecophysiological study into the responsiveness to extreme winter warming events (Bokhorst et al. 2010). Here, E. nigrum was the most physiologically responsive with initiation of photosynthesis, increased (40 %) activity of PSII and bud swelling started (70 % heavier buds) during the winter warming events. V. vitis-idaea and V. myrtillus showed moderate ecophysiological responses. V. vitis-idaea responded stronger to the extreme winter warming events during March compared to a simulation in January, while E. nigrum responded during early and late winter (Bokhorst et al. 2010). These differences may be driven by species-specific climatic requirements for breaking of winter dormancy.
Vaccinium myrtillus was the only deciduous species damaged following the second winter warming events. However, at the same time it showed remarkable response plasticity, which was not present in the evergreen dwarf shrubs. Many damaged V. myrtillus ramets showed complete shoot mortality following the winter warming events, but often developed a single large compensatory shoot with much larger leaves (Fig. 1) later on in the season (Bokhorst et al. 2011a). Length and width measurements (using digital callipers) on five leaves of both large and normal sized leaves from each plot (n = 18) indicated that large leaves were longer (14.8 ± 0.6 mm) and wider (8.3 ± 0.4 mm) than normal ones (8.5 ± 0.3 and 5.0 ± 0.1 mm, ANOVA F1,20 = 76.5, P < 0.001). These larger leaves appear to be a growth strategy whereby the plant invests heavily in a few large leaves due to the extensive loss of its normal leaves (Tolvanen 1997) following the extreme winter warming events. The larger leaves were greener than the normal ones (Bokhorst, personal observation) suggesting chemical changes besides morphological ones. Indeed, the nitrogen content of the large leaves was 18 % higher (P < 0.01) than the normal leaves and the C/N ratio was 18 % lower (P < 0.05) (Fig. 1). These results indicate that this compensatory growth strategy may indeed be a way to effectively utilize remaining stored resources in an attempt to acquire the most carbon with a single shoot with larger leaves of higher nitrogen content (Taub and Lerdau 2000) during the remainder of the growing season. This higher nutrient leaf content will have a subsequent effect on decomposition which is likely to become faster due to the higher N content (Aerts and Chapin 2000). Thus, these species-specific responses could locally promote growth of the microbial community further affecting carbon and nutrient cycling—at least in areas where V. myrtillus makes up a significant proportion of the plant community.
Fig. 1.
Leaf chemistry of compensatory growth by V. myrtillus following extreme winter warming events. a The ratio of ramets with normal ‘small’ leaves to compensatory ‘large’ leaves of V. myrtillus following extreme winter warming events. b Nitrogen content of small and large V. myrtillus leaves. c Carbon to nitrogen ratio of small and large V. myrtillus leaves. Bars Means of n = 6 replicate plots although for the control plots large leaves were n = 3 as these were less available (see Fig. 2a). Error bars SE. *Significant (P < 0.05) differences between treatments in a and leaves in b and c. The data presented in a was previously presented by Bokhorst et al. (2011a)
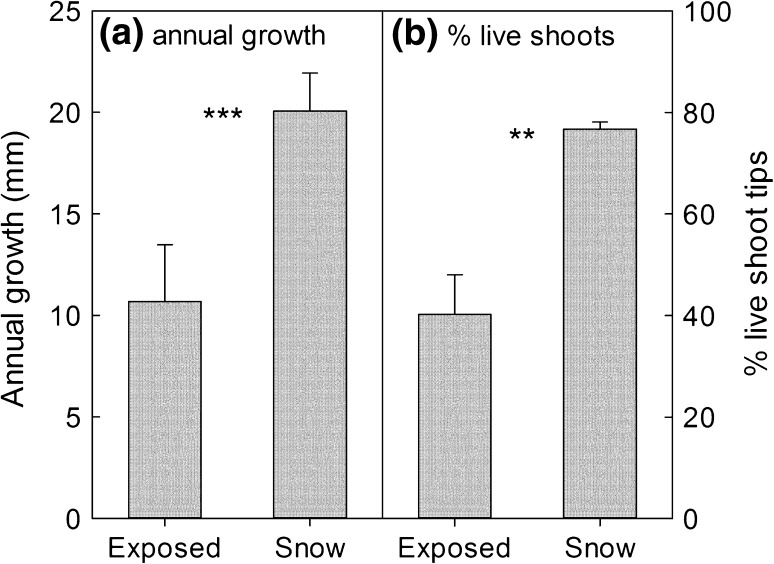
Species-specific responses to winter disturbance may well play a large- and overlooked-role in the heterogeneity of tundra plant communities. Extreme winter warming events cause damage to Arctic vegetation at landscape scales (Bokhorst et al. 2009), but smaller scale patterning in vegetation heterogeneity (e.g. at the “patch” or metre scale) may also be driven by warming in winter. For instance, it is not necessarily an increase in regional temperature that may cause damage, but instead melting of snow in small patches and subsequent warming of the plant canopy by the ambient solar radiation. Of course, melting of snow or its removal by wind at the patch scale is well known to contribute to local vegetation heterogeneity, with greater mortality and reduced productivity of the vegetation arising from desiccation and ice-crystal blast (Callaghan et al. 2011). Such exposed areas may typically contain cushion plants, evergreen dwarf shrubs and tussock graminoids as these growth forms provide protection from wind abrasion and drought stress (Walker et al. 2001). However, damage may be exacerbated where the plant canopy is warmed to well above freezing leading to desiccation or deacclimation of winter freezing tolerance. Evidence for this has been seen at relatively benign sub-arctic birch woodland sites in the vicinity of ANS. Here, non-manipulated patches (<2 m across) of dwarf shrub vegetation, which were exposed due to localised snow melt, showed leaf temperatures approaching 8 °C in March (2007) under full sunlight. Growth (annual stem length increment) and shoot mortality (% of live shoot tips) of the dominant dwarf shrub E. nigrum in eight of these exposed/warmed areas were compared to measurements taken in the immediately adjacent snow-covered patches. Differences between snow covered and exposed/warmed areas were considerable (Fig. 2), with E. nigrum showing nearly twofold greater % live shoots and annual shoot growth in the snow-covered patches (paired t test; % live shoots t = −4.60, P < 0.05; shoot length t = −3.50, P < 0.01). While we cannot attribute damage to the same loss of winter hardiness as occurs in extreme winter warming events, it is apparent that localised loss of snow through warming and melt can induce significant small-scale variation in plant performance.
Fig. 2.
Impacts of localized exposure of E. nigrum shoots during winter. The annual growth (a) and % of alive shoots (b) were reduced in patches where snow had melted during March 2007 near the Abisko Scientific Research Station. Bars are mean of eight open patches and eight adjacent patches which were still snow covered. Error bars are 1 SE
Variation in Ecotype Responses
The vulnerability of species to extreme weather events depends on their physiological and phenotypic characteristics during winter. It is therefore possible that certain ecotypes of a species may be more susceptible than others (Jørgensen et al. 2010). Extensive damage to E. nigrum was observed following a natural winter warming event of December 2007 (Bokhorst et al. 2009) resulting in a 26 % reduction of normalized difference vegetation index (NDVI) across 1400 km2 of northern Scandinavia. The greatest damage to dwarf shrubs following the natural winter warming event occurred in continental mountainous regions—though coastal areas also showed evidence of damage (Bokhorst et al. 2009). The same dwarf shrub species also occur along the Norwegian coast, which have a more variable winter climate and could therefore be expected to respond differently (Graae et al. 2008) to extreme winter warming events. However, similar damage (as reported by Bokhorst et al. 2009) to E. nigrum and other dwarf shrubs was reported along the Norwegian coast during 2006 (Bjerke and Tømmervik 2008). Temperature records from the winter of June 2005 indicated a 2-week period in January with mean weakly air temperatures at 5 °C and snow cover declining to 0. This event lead to dehardening of forage grass, especially ryegrass (Loliumperenne) in agricultural fields and the following frost and ground ice formation caused extensive winterkill (Jørgensen et al. 2010). The same mechanisms were most likely also responsible for the observed damage in coastal dwarf shrubs (Bokhorst et al. 2010). Thus, so far the reports and observations suggest that the different ecotypes (montane vs. coastal) of E. nigrum are susceptible to extreme winter weather events that affect the temporal aspects of snow depth and canopy temperature.
To test whether the observed plant damage in 2006 (Bjerke and Tømmervik 2008) affected the overall vegetation greenness in the landscape, we analysed time series of MODIS scenes, using the methods as described in Bokhorst et al. (2009) to achieve mid-July NDVI (i.e. maximum NDVI for this region). Two areas were selected, one at Kvaløya (1.5 km2) in Hammerfest Municipality, Finnmark County (70°36′N, 23°39′E) and another at Tønsneset-Skarpneset (1.25 km2) in Tromsø Municipality, Troms County (69°44′N, 19°07′E). The photographs in Bjerke and Tømmervik (2008) were from these two locations, and for E.nigrum, they were similar to the illustrations of plant browning in Bokhorst et al. (2009). Our observations of damage at the Hammerfest site were mostly of E.nigrum, which was also the dominant species in this area characterised by dwarf shrub heath. At the Tromsø site, we observed damage on V. myrtillus, E. nigrum and Juniperuscommunis.
The mean NDVI values for Hammerfest and Tromsø were significantly lower in 2006 than in most other years (see Fig. 3). At both the sites the damage affected vegetation performance into the following season (2007). Analyses of NDVI at a larger scale indicated no difference for 2006 in relation to the preceding years (data not shown) suggesting that the damage was local, which is in line with the highly variable spatial distribution of snow along the Norwegian coast; the lowland closest to the sea remained snow-free (snow depth <5 cm) for a much longer period than higher altitudes, as suggested by metrological data from the weather stations in Tromsø and snow water equivalent models for both Tromsø and Hammerfest.1 Thus, the upper part of the 10- to 20-cm-tall dwarf shrubs were exposed to ambient temperatures for most of the winter in the lowlands, but covered by snow at higher altitudes from February until spring thaw in late April with less plant damage as a result. Here, again the importance of snow distribution for the vulnerability of species to extreme winter weather events are exemplified and contribute to our understanding of the spatial heterogeneity of damage and growth.
Fig. 3.
Local NDVI at Hammerfest and Tromsø following a natural winter warming event along the Norwegian coast. a NDVI values near Hammerfest with mean values of 6 pixels and error bars 1 SE. 2006 differed significantly (paired t tests; P values between 0.000 and 0.048) from all other years except for 2002 and 2007, which were near-significantly different from 2006 (P = 0.077 and 0.106, respectively). b NDVI values near Tromsø with mean values of 5 pixels and error bars 1 SE. 2006 differed significantly (paired t tests; P values between 0.007 and 0.067) from all other years except 2005 and 2007 (P = 0.252 and 0.110, respectively)
Response of Fungal Fruiting Bodies to Extreme Winter Warming Events
In the experimental plots near ANS, exposed to the extreme winter warming events we observed a large difference in the number of fungal fruiting bodies between the treatment and control plots at the end of the 2009 growing season (ANOVA F2,15 = 3.7, P < 0.05). During a visual survey count of a 1 × 1 m area in each plot (n = 18) the number of fruiting bodies in the control plots were on average 3.2 (±1.2) m−2, while this was down to 1.0 (±0.6) m−2 and 0.6 (±0.3) m−2 in the canopy and canopy + soil warming plots, respectively. The 2009 growing season was an exceptionally productive year for mushrooms in the Abisko area and the low values in the treatment plots suggest that the community changes associated with the winter warming events—extensive shoot mortality (±50 %), lower (25 %) root growth (Bokhorst et al. 2011a) and a change in the arthropod community (Bokhorst et al. 2012)—also considerably negatively affected the growth of soil fungi and their reproductive output.
Cryptogam Responses to Changes in Snow Cover
Cryptogams play a major functional role in ecosystem carbon and nitrogen sequestration, and preservation of permafrost and changes in their community, due to climate change, may affect these processes. The responsiveness of lichens and mosses to extreme winter warming events in the experimental plots near ANS differed between the two groups. Where the moss Hylocomium splendens was reduced in growth (52 %) and photosynthesis (48 %) there were no impacts on the lichen Peltigera aphthosa (Bjerke et al. 2011). Whereas lichens were not affected by the melting of snow during winter and exposure to much colder temperatures an opposite response was observed when lichens were covered by a deeper snow pack during winter in the Antarctic.
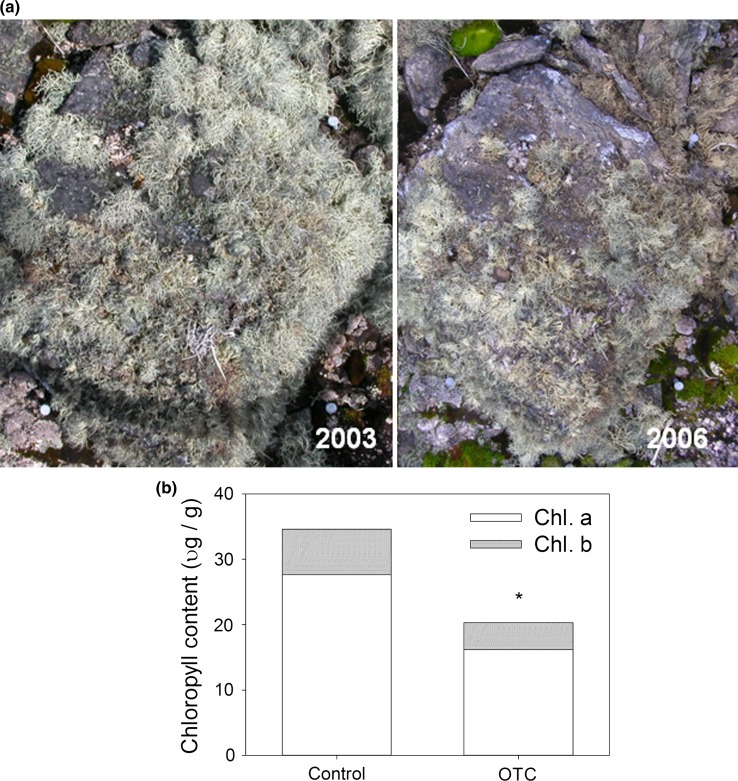
A year-round warming study using open top chambers (OTCs) in a lichen fell-field community at Signy Island (60°71′S 45°59′W) in the Maritime Antarctic is ongoing since the summer of December 2003 (Bokhorst et al. 2007a). Here, the fell-field is dominated by the lichen Usnea antarctica (>50 % of vegetation cover). Following the start of the second year of warming (mean summer warming 0.8 °C) a noticeable decline in lichen cover was observed in the OTCs compared to the control plots (Bokhorst et al. 2007a). Furthermore, this decline was associated with obvious thallus discolouring (Fig. 4). To quantify this apparent ecophysiological deterioration in the lichen thallus, U. antarctica samples were collected from OTCs and control plots during the Austral summer of January 2006 and analysed for total chlorophyll content using acetone extraction. Chlorophyll content of U. antarctica in OTCs was 42 % lower (P < 0.01) than in control plots (Fig. 4).
Fig. 4.
Usnea antarctica deterioration inside OTCs on Signy Island. a The picture of 2003 shows the cover of U. antarctica on a boulder inside an OTC. The picture from 2006 shows the same boulder with an obviously lower lichen cover 3 years later. The distance between the metal pins on the right hand side of each picture is 30 cm. b Chlorophyll content (a and b) of U. antarctica in control plots and OTCs during the summer of 2006. Bars are means of n = 5, *Significant (P < 0.01) difference in chlorophyll (a and b) content between control plots and OTCs
The initial cause behind this decline was sought in the increased temperatures recorded during summer (0.8 °C) and potential water deficiencies due to higher evaporation rates by the OTCs (Bokhorst et al. 2007a). However, considerable snow accumulation had occurred during winter inside the OTCs and the thicker snow cover raised the winter temperature (1 °C) even more than during summer (Bokhorst et al. 2011b). Lichens are metabolically active year-round in the Maritime Antarctic as long as water is available (Kappen 2000) and winter is generally a period of net carbon loss, due to low light conditions under the snow cover. The increased winter temperature recorded in the OTCs may have increased metabolic activity leading to a greater net loss of carbon (Kappen et al. 1995; Schroeter et al. 2000). In addition, a delay in snow melt during spring would also cause a reduction in light levels inside OTCs during a period when these lichens normally acquire the greatest part of their carbon (Schroeter et al. 2000). Even though temperatures were higher inside OTCs during summer this is generally not enough to offset the reduced carbon gain during spring, as has also been shown for this species on Livingston Island (Schroeter et al. 2000). These findings indicate an ecophysiological deterioration in lichen dominated communities, driven by a change in the snow conditions during winter and spring and are consistent with the notion that winter snow cover, and changes therein, have large impacts on lichen growth (Benedict 1991). This lichen decline had a knock-on effect on the soil arthropod community which went into decline (Bokhorst et al. 2008) due to reduced food (Bokhorst et al. 2007b) and shelter opportunities.
Bryophyte responses were negative when winter snow was reduced (Bjerke et al. 2011) but increased productivity has been reported under thicker snow depth (Dorrepaal et al. 2004). Lichen responses showed strong variable responses to changes in snow depth in combination with ambient temperatures. The highly diverse responses by cryptogams provide large uncertainties for the structure of polar ecosystems following winter climate change.
Changes in the Snow Profile
Snowpacks are generally not uniform, and layers of differing density are typically formed through compression, rain-on-snow, melting and refreezing throughout the winter (Colbeck 1991). The formation of ice layers changes the thermal characteristics of the snowpack and can expose underlying plants and animals to much lower temperatures (Callaghan et al. 2004). Ice may cause physical damage as a result of ice forming within plants leading to embolism (Pearce 2001) and create near-anoxic conditions due to decreased gas exchange. Foraging reindeers Rangifer t. tarandus are hampered by the formation of thick and hard ice layers making it hard to reach underlying food (Riseth et al. 2011) and the formation of these ice layers have previously resulted in dramatic population crashes (Putkonen and Roe 2003). The main food source of these reindeer, lichens, may also be affected by the formation of ice especially if they become encased in ice. Bjerke (2009) performed an icing experiment on three lichens species of importance for reindeer feeding: Cladonia rangiferina, C. stellaris and Flavocetraria nivalis and found that ice encapsulation did not affect these lichens. However, at mild subfreezing (−1.5 °C) temperatures, the ice-encased lichens had reduced photosynthetic and chlorophyll fluorescence rates (70 %) suggesting a deterioration of the algal partner. These findings suggest that lichens are sensitive to a warmer and more fluctuating winter climate (Bjerke 2011). The response of lichens to changes in the snowpack (depth, ice formation and timing) are not straightforward and need much more research focus as these organisms play a key role in many polar ecosystems (Cornelissen et al. 2007). The moisture conditions underneath a snowpack are crucial for the activity of many organisms and processes, for example, the formation of snow moulds.
Snow Mould
Keepers of Sami ecological knowledge report that snow mould if accidentally consumed by reindeer may lead to illness. The formation of these snow moulds is primarily driven by the occurrence of wet autumns before formation of the snow pack commences, i.e. when the vegetation, litter and soil layers are still wet (Riseth et al. 2011). These snow moulds or fungi are often observed in tundra and shrub vegetation in northern biomes following snow melt. Snow fungi are abundantly present after prolonged snow cover (Simms 1967; Schmidt et al. 2009) and often disappear within days after snow melt in spring. However, this does mean that they could be present for a significant part of the winter. Indeed, extensive snow mould cover was observed in all of the plots that were exposed by the extreme winter warming simulations during March 2007 but only very few moulds were observed during the simulations of March 2008 and 2009 (Bjerke, personal observations). The meteorological data from ANS indicated rainfall of 90 mm from September up until snowpack formation in November 2006, while this was only 60 and 40 mm for 2007 and 2008, respectively, suggesting that the conditions for snow mould formation during autumn 2006 were better than during the two following years. Olofsson et al. (2011) reported on a plant fungal pathogen infection (Arwidssonia empetri) on E. nigrum as a result of deeper snow during 2008 and 2009 in the same region suggesting that there is large spatial heterogeneity in the formation of such pathogens.
Snow mould presence has been implicated in loss of crops (Matsumoto 2009), damage to shrubs (Olofsson et al. 2011), retarded tree seedling growth (Hessl and Baker 1997; Cunningham et al. 2006) and previously mentioned reindeer illness, even causing mortality, especially for calves and yearlings, after consuming plants covered by mould (Kumpula et al. 2000; Riseth et al. 2011). The effect of these snow fungi on ecosystem processes is receiving more research attention (Schmidt et al. 2009) particularly because they may be involved in the ecosystem carbon balance (Olofsson et al. 2011) and because the visible abundance of hyphae covering all plants (Fig. 5) suggests that these fungi are acquiring large quantities of nutrients from their environment during a large part of winter. At the same time, this high abundance of hyphae could provide a true “smorgasbord” for terrestrial arthropods (Collembola and Acari) that predominantly feed on fungi (Maraun et al. 1998; Hubert et al. 2001; Berg et al. 2004). Therefore, snow fungi could play a direct role in litter decomposition by decomposing organic matter and indirectly by supplying an abundant food source for organisms, such as Collembola, that play a key role in decomposition (Filser 2002; Wall et al. 2008).
Fig. 5.
Snow mould covering vegetation in winter and spring. a Snow mould covering dwarf shrubs exposed during the winter warming event of March 2007 near ANS. b Snow mould completely covering Calluna vulgaris in the spring of 2011 near the Swedish University of Agricultural Sciences in Umeå
During snowmelt of spring 2011, an abundant cover of snow mould was observed in a northern boreal pine forests near the Swedish University of Agricultural Sciences in Umeå (63°49′N 20°15′E). To test the hypothesis that snow fungi would affect Collembola abundance, litter samples with and without visible cover of snow mould (n = 5 for both) were collected (using a 10-cm diameter corer to a depth of 3 cm) and extracted for Collembola using a Tullgren heat extractor (Van Straalen and Rijninks 1982) during 1 week. Total abundance of Collembola was three times higher (P < 0.05) in litter infected by snow mould (2.0 ± 0.6 Collembola per g litter) compared to non-infected litter (0.6 ± 0.2 Collembola g litter). The increased numbers suggest that snow fungi could be a suitable food source for Collembola underneath the snow pack potentially sustaining growth and reproduction for these organisms during a large part of winter. The extensive cover by snow fungi and the feeding on them by Collembola may well affect decomposition and nutrient cycling during winter (Schmidt et al. 2009). As the presence of these snow fungi tends to be highly variable in the landscape (Matsumoto 2009) the impact on the arthropod community and ecosystems processes will be highly spatially distributed.
Summary
Autumn and winter climate change can have major impacts on northern plant communities, with higher plants—particularly shrubs—appearing most prone to damage, while lichens appear tolerant. Beyond this generalisation, however, species-specific differences in sensitivities are apparent due to physiological and phenotypic differences as well as local weather and snow patterns. Belowground, impacts of changing winter climate are less obvious but still apparent, with consequences for ecosystem processes. Changes in the snow profile such as the formation of ice layers through rain-on-snow events is likely to increase. Recent study shows that these may also impact cryptogam communities, though the response of lichens appears to depend on the freezing temperatures following such events (Bjerke 2011).
The potential biological effects of climatic changes during autumn have hardly been studied, but snow mould formation appears to be propagated by a wet autumn climate. The impact of the autumn and winter microbial community on ecosystems processes and trophic cascades may play a large role on the spatial heterogeneity of species distribution and ecosystem development. How climate change will affect these interactions will be hard to predict, but with increases of mild winter weather (Johansson et al. 2011) and changes in the precipitation regime (Ye et al. 2008) predicted for many polar regions, impacts on the growing season are likely. The challenges ahead lie in obtaining better predictions of the snow patterns across the landscape and how and when these will be altered due to warming or rain events. When are species and communities most vulnerable to changes in snow cover and what is the role of the starting conditions during the onset of snow accumulation?
Acknowledgments
We would like to thank Terry V. Callaghan for all the excellent ideas and suggestions during the fieldwork and discussions at Abisko that shaped the work presented here, and his support for the project while director of ANS. This research was supported by a Leverhulme Trust (UK) grant to GKP and TVC, by a grant from the Research Council of Norway awarded to JWB (Contract Nos. 171542/V10 and 216434/E10), by ATANS grants (EU Transnational Access Programme) to JWB, GKP and SB and by the Netherlands Polar Programme (NPP-NWO 851.20.016). This article was improved by the constructive comments of two anonymous reviewers.
Biographies
Stef Bokhorst
is a postdoctoral researcher at the Swedish University of Agricultural Sciences in Umeå. His research interests include the above and belowground response of Polar ecosystems to climate change. Especially, the changes in winter climate and the impacts of extreme weather events for dwarf shrubs, soil arthropods and decomposition are of great interest.
Jarle W. Bjerke
is a senior researcher at the Norwegian Institute for Nature Research. His research interests include the impacts of climate change and land-use on vegetation and terrestrial ecosystems of cold biomes.
Hans Tømmervik
is a senior researcher at the Norwegian Institute for Nature Research. His research is focusing on the application of remote sensing methods and conventional survey methods in mapping and monitoring of boreal and arctic–alpine vegetation changes related to air pollution, climate change, herbivory and reindeer husbandry.
Catherine Preece
is a postdoctoral researcher at the University of Sheffield, UK. Her research has included investigating the impacts of winter icing events on the growth, phenology and physiology of sub-arctic heathland vegetation.
Gareth K. Phoenix
is a senior lecturer (associate professor) at the University of Sheffield, UK. His research interests include the impacts of global change (summer and winter warming, snow regime change, precipitation, UV-B radiation) on arctic ecosystems and the consequences for biogeochemical cycling.
Footnotes
Available at www.senorge.no.
Contributor Information
Stef Bokhorst, Email: stef.bokhorst@slu.se.
Jarle W. Bjerke, Email: jarle.werner.bjerke@nina.no
Hans Tømmervik, Email: hans.tommervik@nina.no.
Catherine Preece, Email: c.preece@sheffield.ac.uk.
Gareth K. Phoenix, Email: g.phoenix@sheffield.ac.uk
References
- Aerts R, Chapin FS. The mineral nutrition of wild plants revisited: A re-evaluation of processes and patterns. Advances in Ecological Research. 2000;30:1–67. doi: 10.1016/S0065-2504(08)60016-1. [DOI] [Google Scholar]
- Benedict JB. Experiments on lichen growth. 2 Effects of a seasonal snow cover. Arctic and Alpine Research. 1991;23:189–199. doi: 10.2307/1551382. [DOI] [Google Scholar]
- Berg MP, Stoffer M, Heuvel HH. Feeding guilds in Collembola based on digestive enzymes. Pedobiologia. 2004;48:589–601. doi: 10.1016/j.pedobi.2004.07.006. [DOI] [Google Scholar]
- Bjerke JW. Ice encapsulation protects rather than disturbs the freezing lichen. Plant Biology. 2009;11:227–235. doi: 10.1111/j.1438-8677.2008.00113.x. [DOI] [PubMed] [Google Scholar]
- Bjerke JW. Winter climate change: Ice encapsulation at mild subfreezing temperatures kills freeze-tolerant lichens. Environmental and Experimental Botany. 2011;72:404–408. doi: 10.1016/j.envexpbot.2010.05.014. [DOI] [Google Scholar]
- Bjerke JW, Tømmervik H. Observerte skader på nordnorske planter i løpet av vår og sommer 2006: omfang og mulige årsaker. Blyttia. 2008;66:90–96. [Google Scholar]
- Bjerke JW, Bokhorst S, Zielke M, Callaghan TV, Bowles FW, Phoenix GK. Contrasting sensitivity to extreme winter warming events of dominant sub-Arctic heathland bryophyte and lichen species. Journal of Ecology. 2011;99:1481–1488. doi: 10.1111/j.1365-2745.2011.01859.x. [DOI] [Google Scholar]
- Bokhorst, S., A. Huiskes, P. Convey, and R. Aerts. 2007a. The effect of environmental change on vascular plant and cryptogam communities from the Falkland Islands and the Maritime Antarctic. BMC Ecology 715. doi:10.1186/1472-6785-1187-1115 [DOI] [PMC free article] [PubMed]
- Bokhorst S, Ronfort C, Huiskes A, Convey P, Aerts R. Food choice of Antarctic soil arthropods clarified by stable isotope signatures. Polar Biology. 2007;30:983–990. doi: 10.1007/s00300-007-0256-4. [DOI] [Google Scholar]
- Bokhorst S, Huiskes AHL, Convey P, Bodegom PMV, Aerts R. Climate change effects on soil arthropod communities from the Falkland Islands and the Maritime Antarctic. Soil Biology and Biochemistry. 2008;40:1547–1556. doi: 10.1016/j.soilbio.2008.01.017. [DOI] [Google Scholar]
- Bokhorst S, Bjerke JW, Tømmervik H, Callaghan TV, Phoenix GK. Winter warming events damage sub-Arctic vegetation: Consistent evidence from an experimental manipulation and a natural event. Journal of Ecology. 2009;97:1408–1415. doi: 10.1111/j.1365-2745.2009.01554.x. [DOI] [Google Scholar]
- Bokhorst S, Bjerke JW, Davey M, Taulavouri K, Taulavuori E, Laine K, Callaghan TV, Phoenix GK. Impacts of extreme winter warming events on plant physiology in a sub-Arctic heath community. Physiologia Plantarum. 2010;140:128–140. doi: 10.1111/j.1399-3054.2010.01386.x. [DOI] [PubMed] [Google Scholar]
- Bokhorst S, Bjerke JW, Street L, Callaghan TV, Phoenix GK. Impacts of multiple extreme winter warming events on sub-Arctic heathland: Phenology, reproduction, growth, and CO2 flux responses. Global Change Biology. 2011;17:2817–2830. doi: 10.1111/j.1365-2486.2011.02424.x. [DOI] [Google Scholar]
- Bokhorst S, Huiskes AHL, Convey P, Sinclair BJ, Lebouvier M, Vijver B, Wall DH. Microclimate impacts of passive warming methods in Antarctica: Implications for climate change studies. Polar Biology. 2011;34:1421–1435. doi: 10.1007/s00300-011-0997-y. [DOI] [Google Scholar]
- Bokhorst S, Phoenix GK, Bjerke JW, Callaghan TV, Huyer-Brugman F, Berg MP. Extreme winter warming events more negatively impact small rather than large soil fauna: Shift in community composition explained by traits not taxa. Global Change Biology. 2012;18:1152–1162. doi: 10.1111/j.1365-2486.2011.02565.x. [DOI] [Google Scholar]
- Callaghan TV, Bjorn LO, Chernov Y, Chapin T, Christensen TR, Huntley B, Ims RA, Johansson M, et al. Responses to projected changes in climate and UV-B at the species level. AMBIO. 2004;33:418–435. doi: 10.1579/0044-7447-33.7.418. [DOI] [PubMed] [Google Scholar]
- Callaghan TV, Johansson M, Brown RD, Groisman PY, Labba N, Radionov V, Bradley RS, Blangy S, et al. Multiple effects of changes in arctic snow cover. AMBIO. 2011;40:32–45. doi: 10.1007/s13280-011-0213-x. [DOI] [Google Scholar]
- Christensen, J.H., B. Hewitson, A. Busuioc, A. Cheng, X. Gao, I. Held, R. Jones, R.K. Kolli, et al. 2007. Regional climate projections. In Climate change 2007: The physical science basis. Contribution of working group I to the fourth assessment report of the intergovernmental panel on climate change, eds. S. Solomon, D. Qin, M. Manning, Z. Chen, M. Marquis, K.B. Averyt, M. Tignor, and H.L. Miller, 847–940. Cambridge: Cambridge University Press.
- Colbeck SC. The layered character of snow covers. Reviews of Geophysics. 1991;29:81–96. doi: 10.1029/90RG02351. [DOI] [Google Scholar]
- Cornelissen JHC, Lang SI, Soudzilovskaia NA, During HJ. Comparative cryptogam ecology: A review of bryophyte and lichen traits that drive biogeochemistry. Annals of Botany. 2007;99:987–1001. doi: 10.1093/aob/mcm030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham C, Zimmermann NE, Stoeckli V, Bugmann H. Growth response of Norway spruce saplings in two forest gaps in the Swiss Alps to artificial browsing, infection with black snow mold, and competition by ground vegetation. Canadian Journal of Forest Research. 2006;36:2782–2793. doi: 10.1139/x06-156. [DOI] [Google Scholar]
- Dorrepaal E, Aerts R, Cornelissen JHC, Callaghan TV, Logtestijn RSP. Summer warming and increased winter snow cover affect Sphagnum fuscum growth, structure and production in a sub-arctic bog. Global Change Biology. 2004;10:93–104. doi: 10.1111/j.1365-2486.2003.00718.x. [DOI] [Google Scholar]
- Elmendorf SC, Henry GHR, Hollister RD, Bjork RG, Bjorkman AD, Callaghan TV, Collier LS, Cooper EJ, et al. Global assessment of experimental climate warming on tundra vegetation: Heterogeneity over space and time. Ecology Letters. 2012;15:164–175. doi: 10.1111/j.1461-0248.2011.01716.x. [DOI] [PubMed] [Google Scholar]
- Filser J. The role of Collembola in carbon and nitrogen cycling in soil. Pedobiologia. 2002;46:234–245. [Google Scholar]
- Gaines SD, Denny MW. The largest, smallest, highest, lowest, longest, and shortest—extremes in ecology. Ecology. 1993;74:1677–1692. doi: 10.2307/1939926. [DOI] [Google Scholar]
- Graae BJ, Alsos IG, Ejrnaes R. The impact of temperature regimes on development, dormancy breaking and germination of dwarf shrub seeds from arctic, alpine and boreal sites. Plant Ecology. 2008;198:275–284. doi: 10.1007/s11258-008-9403-4. [DOI] [Google Scholar]
- Hessl AE, Baker WL. Spruce and fir regeneration and climate in the forest-tundra ecotone of Rocky Mountain National Park, Colorado USA. Arctic and Alpine Research. 1997;29:173–183. doi: 10.2307/1552044. [DOI] [Google Scholar]
- Hubert J, Zilova M, Pekar S. Feeding preferences and gut contents of three panphytophagous oribatid mites Acari: Oribatida. European Journal of Soil Biology. 2001;37:197–208. doi: 10.1016/S1164-5563(01)01083-4. [DOI] [Google Scholar]
- Inouye DW. The ecological and evolutionary significance of frost in the context of climate change. Ecology Letters. 2000;3:457–463. doi: 10.1046/j.1461-0248.2000.00165.x. [DOI] [Google Scholar]
- Johansson C, Pohjola VA, Jonasson C, Callaghan TV. Multi-decadal changes in snow characteristics in sub-Arctic Sweden. AMBIO. 2011;40:566–574. doi: 10.1007/s13280-011-0164-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jørgensen M, Østrem L, Höglind M. De-hardening in contrasting cultivars of timothy and perennial ryegrass during winter and spring. Grass & Forage Science. 2010;65:38–48. doi: 10.1111/j.1365-2494.2009.00718.x. [DOI] [Google Scholar]
- Kappen L. Some aspects of the great success of lichens in Antarctica. Antarctic Science. 2000;12:314–324. doi: 10.1017/S0954102000000377. [DOI] [Google Scholar]
- Kappen L, Sommerkorn M, Schroeter B. Carbon acquisition and water relations of lichens in polar regions—potentials and limitations. Lichenologist. 1995;27:531–545. [Google Scholar]
- Kumpula J, Parikka P, Nieminen M. Occurrence of certain microfungi on reindeer pastures in northern Finland during winter 1996–97. Rangifer. 2000;20:3–8. [Google Scholar]
- Maraun M, Migge S, Schaefer M, Scheu S. Selection of microfungal food by six oribatid mite species Oribatida, Acari from two different beech forests. Pedobiologia. 1998;42:232–240. [Google Scholar]
- Matsumoto N. Snow molds: A group of fungi that prevail under snow. Microbes and Environments. 2009;24:14–20. doi: 10.1264/jsme2.ME09101. [DOI] [PubMed] [Google Scholar]
- Olofsson J, Ericson L, Torp M, Stark S, Baxter R. Carbon balance of Arctic tundra under increased snow cover mediated by a plant pathogen. Nature Climate Change. 2011;1:220–223. doi: 10.1038/nclimate1142. [DOI] [Google Scholar]
- Parmesan C, Yohe G. A globally coherent fingerprint of climate change impacts across natural systems. Nature. 2003;421:37–42. doi: 10.1038/nature01286. [DOI] [PubMed] [Google Scholar]
- Pearce RS. Plant freezing and damage. Annals of Botany. 2001;87:417–424. doi: 10.1006/anbo.2000.1352. [DOI] [Google Scholar]
- Phoenix GK, Lee JA. Predicting impacts of Arctic climate change: Past lessons and future challenges. Ecological Research. 2004;19:65–74. doi: 10.1111/j.1440-1703.2003.00609.x. [DOI] [Google Scholar]
- Putkonen J, Roe G. Rain-on-snow events impact soil temperatures and affect ungulate survival. Geophysical Research Letters. 2003;30:1188. doi: 10.1029/2002GL016326. [DOI] [Google Scholar]
- Riseth JA, Tømmervik H, Helander-Renvall E, Labba N, Johansson C, Malnes E, Bjerke JW, Jonsson C, et al. Sámi traditional ecological knowledge as a guide to science: Snow, ice and reindeer pasture facing climate change. Polar Record. 2011;47:202–217. doi: 10.1017/S0032247410000434. [DOI] [Google Scholar]
- Schroeter B, Kaplan SL, Schulz F, Sancho LG. Seasonal variation in the carbon balance of lichens in the maritime antarctic: Long-term measurements of photosynthetic activity in Usnea aurantiaco-atra. In: Davidson W, Howard-Williams C, Broady P, editors. Antarctic ecosystems: Models for wider ecological understanding. Christchurch: The Caxton Press; 2000. pp. 258–262. [Google Scholar]
- Schmidt SK, Wilson KL, Monson RK, Lipson DA. Exponential growth of snow molds at sub-zero temperatures: an explanation for high beneath-snow respiration rates and Q 10 values. Biogeochemistry. 2009;95:13–21. doi: 10.1007/s10533-008-9247-y. [DOI] [Google Scholar]
- Schmidt NM, Baittinger C, Kollmann J, Forchhammer MC. Consistent dendrochronological response of the Dioecious Salix arctica to variation in local snow precipitation across gender and vegetation types. Arctic, Antarctic, and Alpine Research. 2010;42:471–475. doi: 10.1657/1938-4246-42.4.471. [DOI] [Google Scholar]
- Simms HR. On the ecology of Herpotrichia nigra. Mycologia. 1967;59:902–909. doi: 10.2307/3757200. [DOI] [Google Scholar]
- Sturm M, McFadden JP, Liston GE, Chapin FS, Racinem CH, Holmgren J. Snow-shrub interactions in Arctic tundra: A hypothesis with climatic implications. Journal of Climate. 2001;14:336–344. doi: 10.1175/1520-0442(2001)014<0336:SSIIAT>2.0.CO;2. [DOI] [Google Scholar]
- Taub DR, Lerdau MT. Relationship between leaf nitrogen and photosynthetic rate for three NAD-ME and three NADP-ME C-4 grasses. American Journal of Botany. 2000;87:412–417. doi: 10.2307/2656637. [DOI] [PubMed] [Google Scholar]
- Tolvanen A. Recovery of the bilberry Vaccinium myrtillus L. from artificial spring and summer frost. Plant Ecology. 1997;1301:35–39. doi: 10.1023/A:1009776200866. [DOI] [Google Scholar]
- Straalen NM, Rijninks PC. The efficiency of Tullgren apparatus with respect to interpreting seasonal changes in age structure of soil arthropod populations. Pedobiologia. 1982;24:197–209. [Google Scholar]
- Walker DA, Billings WD, Molenaar JG. Snow-vegetation interactions in tundra environments. In: Jones HG, Pomeroy JW, Walker DA, Hoham RW, editors. Snow ecology: An interdisciplinary examination of snow-covered ecosystems. Cambridge: Cambridge University Press; 2001. pp. 266–324. [Google Scholar]
- Wall DH, Bradford MA, John MGS, Trofymow JA, Behan-Pelletier V, Bignell DDE, Dangerfield JM, Parton WJ, et al. Global decomposition experiment shows soil animal impacts on decomposition are climate-dependent. Global Change Biology. 2008;14:2661–2677. [Google Scholar]
- Wipf S, Rixen C. A review of snow manipulation experiments in Arctic and alpine tundra ecosystems. Polar Research. 2010;29:95–109. doi: 10.1111/j.1751-8369.2010.00153.x. [DOI] [Google Scholar]
- Ye HC, Yang DQ, Robinson D. Winter rain on snow and its association with air temperature in northern Eurasia. Hydrological Processes. 2008;22:2728–2736. doi: 10.1002/hyp.7094. [DOI] [Google Scholar]