Abstract
Novel communities will be formed as species with a variety of dispersal abilities and environmental tolerances respond individually to climate change. Thus, models projecting future species distributions must account for species interactions and differential dispersal abilities. We developed a species distribution model for Arctic char Salvelinus alpinus, a freshwater fish that is sensitive both to warm temperatures and to species interactions. A logistic regression model using lake area, mean annual air temperature (1961–1990), pike Esox lucius and brown trout Salmo trutta occurrence correctly classified 95 % of 467 Swedish lakes. We predicted that Arctic char will lose 73 % of its range in Sweden by 2100. Predicted extinctions could be attributed both to simulated temperature increases and to projected pike invasions. The Swedish mountains will continue to provide refugia for Arctic char in the future and should be the focus of conservation efforts for this highly valued fish.
Keywords: Climate change, Freshwater fish, Species distribution models, Species interactions, Dispersal
Introduction
The global average temperature has increased 0.74 °C since 1906, and the average temperatures in the Northern Hemisphere in 1950–2000 were higher than any other 50-year period in at least the past 1300 years (IPCC 2007). The global surface temperature is predicted to raise 1.8 to 4 °C by 2100 depending on carbon emissions, with the most dramatic temperature increases in polar regions (IPCC 2007). As rising temperatures alter seasonal cycles and render some habitats more or less suitable, species may adapt to the new conditions (either phenotypically or evolutionarily), species may shift their range, or species may go extinct. Phenological, distributional, and genetic changes have already been observed in response to climate change among a wide variety of taxa, including plants, invertebrates, mammals, birds, and fish (Parmesan and Yohe 2003; Bradshaw and Holzapfel 2006). Novel communities will be formed as species with different dispersal abilities, environmental tolerances, and genetics respond individually to climate change (Schweiger et al. 2008; Walther 2010).
Freshwater fishes may be particularly vulnerable to climate change. First, fish are ectothermic poikilotherms, meaning that their body temperatures fluctuate with external temperatures. A variety of physiological rates in poikilotherms scale with body temperature including: metabolism, consumption, excretion, and activity (Fry 1971). Thus, temperature is a critical factor for defining a fish’s ecological niche (Magnuson et al. 1979). In lakes, fish can regulate their metabolism and growth rate by moving between warm, surface water and cold, deep water (Neverman and Wurtsbaugh 1994). However, loss of cold-water microhabitats, such as cold-water pockets in streams, may limit their ability to behaviorally thermoregulate (Torgersen et al. 1999). Second, dispersal barriers may prevent freshwater fishes from colonizing new, thermally suitable habitats (Hein et al. 2011). Freshwater landscapes often exhibit low connectivity due to the presence of barriers (dams, weirs, culverts, waterfalls) and to their dendritic network structure (Fagan 2002; Nilsson et al. 2005). Fish might need to swim long distances downstream and then upstream to colonize an adjacent tributary, and they have almost no ability to colonize isolated lakes (Fagan 2002; Öhman et al. 2006).
Arctic char Salvelinus alpinus is a freshwater fish species whose range might contract substantially under a warmer climate (Reist et al. 2006a). It has a circumpolar distribution with the northernmost extant of all freshwater fishes (Klemetsen et al. 2003). Thus, there is little opportunity for this species to expand its range northward in response to climate change, unless receding glaciers create new habitat (Chu et al. 2005). One model predicts that Arctic char will lose 63 % of its current distribution range in Canada by 2050 given projected temperature and precipitation patterns (Chu et al. 2005). Arctic char’s optimal temperature for growth with unlimited food supply lies between 14.4 and 17.2 °C (Elliott and Elliott 2010). However, under natural conditions the optimum is likely much lower (Elliott 1982). Increased water temperatures could have variable outcomes on Arctic char populations depending on: (1) whether the resultant water temperature is under or above the optimal temperature for growth, (2) how prey production is affected by temperature, (3) which life stage is most affected by climate change (e.g., the upper lethal temperature for eggs is lower than for other life stages, but spring temperatures are also colder), (4) whether cold-water microhabitats are retained, and (5) whether other fish species are present (Elliott 1982; Reist et al. 2006b; Elliott and Elliott 2010; Finstad et al. 2011).
Climate-driven species colonizations may pose a greater threat to Arctic char than temperature increases alone (Hammar 1992; Reist et al. 2006a, b). Arctic char generally occur in species-poor lakes (Hammar 1992; Klemetsen et al. 2003). They are often excluded from environmentally suitable lakes by competitors such as lake trout Salvelinus namaycush, brown trout Salmo trutta, and whitefish Coregonus lavaretus, but are generally superior competitors in cold, unproductive lakes with a long period of ice cover (Hershey et al. 1999; Sandlund et al. 2010; Finstad et al. 2011). In sympatry with other salmonids, the ecological niche of Arctic char is commonly reduced, but the specific habitat used depends on lake morphometry, season, and species composition (Langeland et al. 1991; Sandlund et al. 2010). In addition, Arctic char rarely coexist with pike Esox lucius, which act as both a predator and competitor (Byström et al. 2007; Spens and Ball 2008).
As the only fish species present in many alpine and/or Arctic lakes, Arctic char is an ecologically important species and is also highly valued both for recreational and commercial fishing (Hammar 1992; Eriksson et al. 2006; Reist et al. 2006a). Thus, the aim of this study is to predict the future distribution of Arctic char in Sweden under climate change. We use fish species occurrence and environmental data across 1309 lakes throughout Sweden to develop a distribution model for Arctic char and then apply future climate scenarios to project its occurrence in 9430 lakes. We expect that: (1) warmer temperatures will contract the distribution of Arctic char in Sweden, (2) the climate-driven expansion of pike will cause additional Arctic char extinctions, (3) through competition, brown trout will further limit future Arctic char distributions, and (4) steep terrain in mountain areas will prevent Arctic char from colonizing new lakes at higher elevations. We did not consider interactions with whitefish in this study. The presence/absence of stable coexistence between whitefish and Arctic char was difficult to assess because both species have been introduced to many lakes in Sweden. Lake trout are not native to Sweden and were not considered in this study.
Materials and Methods
Study Area
Sweden spans 55°–69°N and its climate ranges from temperate to sub-arctic. The mean annual air temperature is 8.0 °C in the south and −2.2 °C in the north (means from 1961 to 1990, http://www.smhi.se/klimatdata/). According to climate scenario projections, the mean annual air temperature will be 4 °C warmer by 2100 (SMHI, http://www.smhi.se/klimatdata/). In 2100, maximum mean summer (June–August) air temperatures will be 24 and 14 °C in southern and northern Sweden, respectively. Many lakes are interconnected, and most major rivers flow from the mountains along the western border of the country to the Baltic Sea in the east.
Postglacial Colonization of Fish
After the last glaciation ca. 10 000–15 000 years ago, lakes above the highest postglacial coast line were colonized via rivers entering the Baltic Sea (Ekman 1922), whereas lakes below the highest coast line were colonized directly from the Baltic Sea as lakes were formed by isostatic land uplift. The Baltic Sea is today the most species-rich water body in the region and harbors nearly all freshwater fish species that occur in Swedish lakes and rivers. Arctic char is one of the few species absent from today’s Baltic Sea and from lakes below the highest coastline of the historical Baltic Sea. An initial analysis of our data showed that only one of 675 lakes below the highest coastline had Arctic char. However, during the twentieth century, humans successfully introduced Arctic char to lakes below the highest coastline (Filipsson 1994). Taken together, these observations suggest that the absence of native Arctic char populations below the highest coastline reflects historical conditions. Arctic char either went extinct in the Baltic Sea shortly after the melting of the Wechselian ice sheet and thus was not among the species pool that colonized lakes formed by land uplift, or Arctic char went extinct during some period with unfavorable local conditions. In any case, it is unlikely that present day conditions can explain the absence of Arctic char below the highest coastline. Thus, we excluded all lakes below the highest coastline from further analysis.
Arctic Char Distribution Model
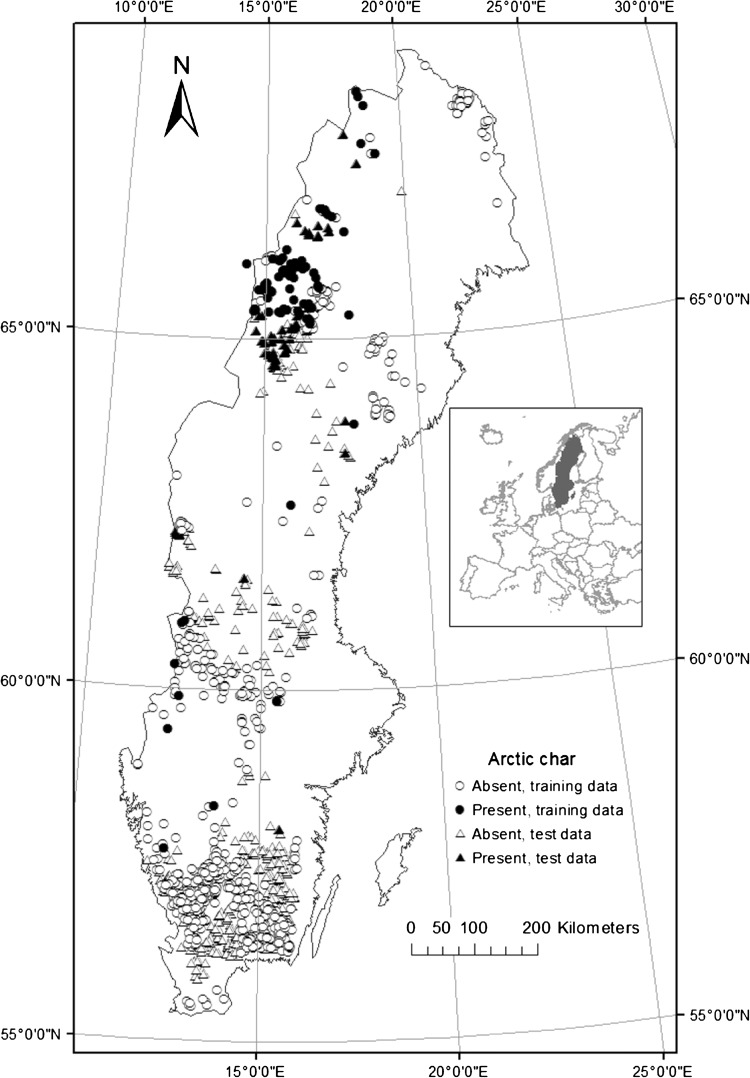
The observed distribution (presence/absence) of Arctic char was modeled with logistic regression, using lake area, average annual temperature (°C), and the presence/absence of brown trout and pike as predictors. Presence/absence data were extracted from a database that includes records of 55 fish species in ca. 18 100 lakes across Sweden. Data sources for the database include governmental records (monitoring reports, stocking programs, interviews and mailed surveys), and interviews with private citizens conducted by Göran Englund and others at Umeå University. For this analysis, we wanted to ensure that lakes with reported presences of Arctic char, brown trout and pike truly represented coexistence lakes. To reduce the incidence of false presences, we only included lakes where gill net surveys had occurred and excluded lakes where any of the three species had: (1) a recorded extinction, (2) a recorded introduction, or (3) an observed presence from another source, but absence from gill net surveys. Table 1 summarizes the co-occurrence patterns of these three species among the 1309 lakes included in the final data set. We also expected negative effects of whitefish on Arctic char distributions (Sandlund et al. 2010), but did not include whitefish in this analysis. An initial analysis showed that whitefish was not a significant predictor of Arctic char distributions and was never included in the best models (Hein et al., unpubl. results). Furthermore, if we included whitefish as a predictor variable, the prevalence of Arctic char lakes in the dataset was substantially reduced (from 10 to 5 %) (Fig. 1).
Table 1.
Co-occurrence patterns of Arctic char, brown trout, and pike in 1309 lakes that compose the gill net data set (e.g., 15 lakes contain Arctic char and none of the other species, whereas 94 lakes contain Arctic char and brown trout)
| Arctic char | Brown trout | Pike | |
|---|---|---|---|
| Arctic char | 15 | 94 | 2 |
| Brown trout | – | 56 | 45 |
| Pike | – | – | 1033 |
All three species occur in 20 lakes, and none of the species occur in 44 lakes
Fig. 1.
Observed Arctic char presence (black symbols) and absence (open symbols) among lakes with gill net data in Sweden. Training data (circles) were used to fit a logistic regression model, and test data (triangles) were used to assess performance of the best model
We used a Geographic Information System (ArcGIS 10.0; ESRI Inc., Redlands, CA, USA) to calculate the areas of all 1309 lakes included in the analysis (mean = 1.3 km2; range = 0.01–67 km2). Average annual air temperatures were on a 50 × 50 km grid and came from the Rossby Centre regional atmospheric climate model (RCA3) (Kjellström et al. 2005), which uses boundary conditions from the global climate model ECHAM4/OPYC3 (Roeckner et al. 1999). The RCA3 model uses observed concentrations of greenhouse gases from 1961 to 1990 and simulated greenhouse gas concentrations from the A2 and B2 emissions scenarios (1991–2100). We averaged simulated temperature data from the B2 emissions scenarios for two time periods: 1961–1990 and 2091–2100. Mean air temperatures for the period 1961–1990 were used to fit the logistic regression models describing the baseline distribution of Arctic char (mean = 1.5 °C, range = −2.1–6.0 °C).
The full dataset was randomly divided by catchment into a training set (81 presences, 761 absences) and test set (50 presences, 417 absences). The training set was used to fit the logistic regression models and the test set was used to evaluate model performance. We used the library for generalized linear models (glm) in R version 2.13.0 (R Development Core Team 2011) and specified a binomial distribution and a logistic link function to fit the models. The full model included all four predictor variables and six interaction terms (Table 2). We built models with various combinations of predictors from the full model and used Akaike’s information criterion (AIC = L + 2m, where L is the likelihood and m is the number of free parameters used in the model) to determine which model performed best (Burnham and Anderson 2002). This method chooses the most parsimonious model (minimum AIC) by calculating the likelihood of the data given each model and penalizing additional parameters with added constants. Models with AIC values within two units of the minimum are considered comparable alternatives. We calculated the following metrics on the test data to assess model performance: percent correctly classified, sensitivity (percent presences correctly classified), specificity (percent absences correctly classified), Kappa, and area under the curve (AUC) (see Fielding and Bell 1997). We used 0.25 as the probability threshold for predicting presence of Arctic char because this value maximized model performance.
Table 2.
Akaike’s Information Criterion (AIC) and difference in AIC (AICi − AICmin) for candidate models predicting the presence/absence of Arctic char
| Model | AIC | ΔAIC |
|---|---|---|
| A + T + B | 275.78 | 40.04 |
| A + P + B | 261.51 | 25.77 |
| A + T + P | 252.67 | 16.93 |
| T + P + B | 244.17 | 8.43 |
| A + T + P + B + A:T + A:P + T:P + A:B + T:B + A:T:P | 237.69 | 1.95 |
| A + T + P + B | 236.58 | 0.84 |
| A + T + P + B + A:T + A:P + T:P + A:B + T:B | 235.75 | 0.01 |
| A + T + P + B + A:T | 235.74 | 0 |
The full model included the following predictor variables: ln lake area (A), average annual air temperature (T), presence/absence of pike (P), presence/absence of brown trout (B), and several interactions. The model in bold was used to predict future distributions of Arctic char
Projected Arctic Char Distribution
One of the regression models was then used to predict the baseline (1961–1990) and the future (2091–2100) distributions of Arctic char for a larger set of lakes (n = 9430). In this data set, we included all lakes with any type of presence record of any fish species, but excluded lakes where brown trout had been introduced or had gone extinct. Because we substituted the observed distribution of brown trout into our model for baseline and future time periods, we did not want presences to only reflect a few stocked individuals. To obtain baseline and future pike presences and absences, we used predictions of pike distributions from a model developed by Hein et al. (2011) that included lake area, average annual air temperature, and locations of natural dispersal barriers as predictors. We evaluated model performance among this larger set of lakes by comparing predictions from 1961 to 1990 with all observed presences and absences of Arctic char. We excluded 185 lakes with Arctic char introductions or extinctions because these lakes were difficult to classify as observed presences or absences.
Results
Arctic Char Distribution Model
A combination of abiotic and biotic variables was critical for accurately predicting the distribution of Arctic char in Sweden. The best generalized linear models included lake area, average annual air temperature, pike, and brown trout as predictors (Table 2). Removal of any single variable caused the AIC value to increase by at least eight units. The most parsimonious model included all four predictors and the interaction between lake area and temperature (Table 2). However, we chose to use a simpler model without the interaction as it was competitive with the best model (Table 2). The model is summarized here:
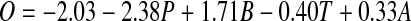 |
1 |
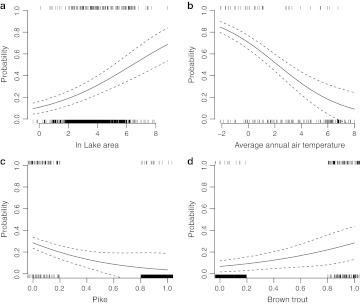
where O is the log odds for Arctic char occurrence, P is the occurrence of pike (0 is absent and 1 is present), B is the occurrence of brown trout, T is the average annual air temperature (°C), and A is the natural logarithm of lake area (ha). All five coefficients were significant at p < 0.01. The probability of Arctic char presence decreased with increasing average annual air temperature and pike presence, but increased with increasing lake area and brown trout presence (Fig. 2). This model performed very well as measured by the test data set; 95 % of lakes were correctly classified as present or absent, sensitivity was 94 %, specificity was 95 %, kappa was 0.78, and AUC was 0.93. Using less restrictive criteria (any type of observed presence, but no introductions or extinctions of Arctic char or brown trout), model performance declined. Eighty-five percent of 9245 lakes were correctly classified as present or absent; sensitivity was 82 %, specificity was 86 %, and kappa was 0.46.
Fig. 2.
Occurrence probability of Arctic char as a function of a ln lake area (ha), b average annual air temperature (°C), c pike occurrence, and d brown trout occurrence. Lake area and average annual air temperature were held constant at the observed mean, pike were kept constant at 0, and brown trout were kept constant at 1 in subplots where they were not plotted directly. Rugs illustrate observed presence (upper) or observed absence (lower) of Arctic char. To improve visibility, presences (1) and absences (0) of pike and brown trout were spread out from 0.8–1.0 and 0.0–0.2, respectively
Projected Arctic Char Distribution
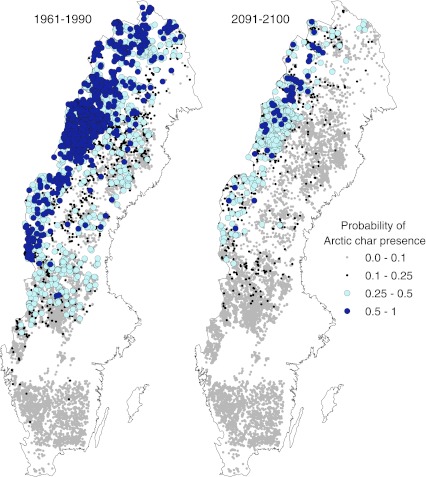
Arctic char distributions will contract by 73 % in the future (Table 3, Fig. 3). Populations that survive into 2091–2100 will occur in the Swedish mountains along the northwestern boarder of the country (Fig. 3). The probability of presence in most Arctic char lakes was >0.5 in 1961–1990, but <0.5 in 2091–2100 (Fig. 3).
Table 3.
Predicted change in the Arctic char distribution across 9430 lakes from the baseline time period (1961–1990) to the future (2091–2100)
| Arctic char occurrence | Number of lakes |
|---|---|
| Remain absent | 7348 |
| Remain present | 549 |
| Colonization | 0 |
| Extinction | 1533 |
Fig. 3.
Probability of Arctic char occurrence in 1961–1990 and in 2091–2100 given the final logistic regression model, which used ln lake area, average annual air temperature, and the occurrence of pike and brown trout as predictor variables. Average annual air temperature was derived from the RCA3 regional atmospheric model using the B2 emissions scenario. Observed brown trout distributions were used for both time periods, but predictions from a separate model (Hein et al. 2011) were used to define pike distributions in both time periods
Predicted Arctic char extinctions were caused by increased temperatures and an expanding pike population (Hypotheses 1 and 2). Most of the 1533 Arctic char extinctions would have been predicted by increased temperature or by pike presence alone. We would have predicted 1292 extinctions had we only increased temperature and assumed pike absence in all lakes. Conversely, we would have predicted 1374 extinctions had we held temperatures constant at the 1961–1990 average and assumed pike to have invaded all lakes. We predicted pike invasions in 388 extinction lakes (Table 3). Arctic char survival was only predicted in six very large pike lakes (29–330 km2). Counter to Hypothesis 3, our model predicted a positive relationship between brown trout and Arctic char presence (Fig. 2d). Thus, brown trout were observed present in 540 of the 549 Arctic char survival lakes. Despite brown trout presence, extinctions were still predicted in small, warm lakes. In summary, our model predicted Arctic char survival if: (1) lake area was >ca. 30 km2, (2) lake area was <ca. 30 km2 and pike was absent, (3) lake area was <ca. 3.5 km2 and brown trout was present, and (4) the lake was sufficiently large and cold (<2.5 °C in 0.02 km2 lakes and <6 °C in 1 km2 lakes).
We did not predict any lakes to become more suitable for Arctic char in the future (Table 3). Therefore, assessing connectivity between observed Arctic char lakes and predicted colonization lakes (Hypothesis 4) was impossible. In Sweden, Arctic char must persist in lakes where they already occur. Connectivity between existing populations might still be important for metapopulation dynamics and evolutionary processes. Most of the lakes predicted to harbor Arctic char in the future had inlet or outlet streams (n = 533), meaning that dispersal between lacustrine populations might at least be possible.
Natural dispersal barriers actually conserved Arctic char populations by preventing pike invasions in mountain lakes. Hein et al. (2011) showed that all Swedish lakes would provide suitable habitat for pike by 2100, but that dispersal barriers would prevent pike invasions in 68.2 % of Swedish lakes. Had we modeled pike invasions in all Swedish lakes, we would have only predicted Arctic char survival in eight very large lakes (29–330 km2). Conversely, had we kept the pike distribution static, 107 Arctic char extinctions attributed to pike invasion would have been predicted survivals.
Discussion
Understanding how the combination of abiotic and biotic processes structures communities at multiple scales is essential for predicting the effects of climate change on biodiversity. A central tenet of ecology is that species interactions prevent species from inhabiting all environmentally suitable habitats; this concept is defined by the realized versus fundamental niche (Hutchinson 1957). Traditionally, it was thought that environmental gradients drive community patterns at large spatial scales and species interactions control distributions at local scales (Pearson and Dawson 2003), but recent research shows that species interactions can also structure communities at large spatial scales (Jackson et al. 2001; Araujo and Luoto 2007; Gotelli et al. 2010). Species distribution models inherently incorporate species interactions because they are fit to species’ realized niches (Pearson and Dawson 2003). However, abiotic variables alone might poorly predict a species’ suitable habitat if they are not tightly linked with the distributions of interacting species (e.g., lakes of the same size could predict presence or absence of Arctic char depending on whether pike is present). Species distribution models of a variety of taxa are substantially improved when the occurrence of interacting species is explicitly included in the model (Heikkinen et al. 2007; Ritchie et al. 2009).
Furthermore, climate-driven changes to a species distribution will be poorly predicted if the interacting species do not respond in unison (Pearson and Dawson 2003; Walther 2010). Spatial mismatch between commensalists or mutualists will limit the future range of one or both species (Araujo and Luoto 2007; Schweiger et al. 2008), whereas mismatch between competitors or predators and prey might provide refugia for vulnerable species (Sharma et al. 2009; Hein et al. 2011). We used a dynamic approach to model future Arctic char distributions, first predicting the climate-driven range expansion of pike in Sweden (Hein et al. 2011) and then substituting future pike distributions into our model for Arctic char. In this case, we would have missed extinctions in 107 lakes if we had not modeled the range expansion of pike. In Scandinavia, brown trout’s distribution will likely expand with climate change (Jonsson and Jonsson 2009), but like Arctic char, they also rarely coexist with pike (Spens and Ball 2008). A species distribution model for brown trout is under development and could be incorporated at a later date.
Future predictions of species distributions can be complex both because multiple predictor variables are used and because dynamic models allow the predictor variables themselves to be affected by climate change. Thus, it is important to investigate how the model functions by, for example, comparing future predictions when only one predictor variable is altered at a time. Using this approach, we learned that most Arctic char extinctions would have been predicted if: (1) temperature increased and pike were absent (84 %) or (2) if temperature was constant and pike invaded all lakes (90 %). Although pike and brown trout were important predictors in our model, they could still be superseded by abiotic predictors: Arctic char survival was predicted in six very large pike lakes and extinction was predicted in 722 small, warm trout-lakes.
The Arctic char distribution model performed very well, but it is a correlative model and might not represent the mechanisms at work. This is fine if spurious correlations remain a good indicator of Arctic char’s distribution in the future, but may lead to false predictions if these “hidden” relationships change. The correlations with pike, temperature, and lake area likely have a mechanistic basis. Introductions of pike often cause extinctions of Arctic char (Filipsson 1994; Byström et al. 2007), and climate scenarios predict temperatures above Arctic char’s optima (Elliott and Elliott 2010). The positive relationship between species diversity and area is a general phenomenon (e.g., Jonsson et al. 2011); species persistence is favored by larger population size and greater habitat heterogeneity in larger lakes (Jackson et al. 2001; Englund et al. 2009). In contrast, it is unlikely that the positive relationship between Arctic char and brown trout has a mechanistic basis because their interactions are known to be competitive rather than mutualistic (Langeland et al. 1991; Jonsson and Jonsson 2009; Finstad et al. 2011).
Thus, we do not expect brown trout presence to aid Arctic char survival in the future. In fact, brown trout have a competitive advantage in more productive lakes with shorter duration of ice cover, and they might competitively exclude Arctic char from small, cold lakes in the future (Jonsson and Jonsson 2009; Finstad et al. 2011). On the other hand, competition between the two species often results in niche partitioning rather than exclusion from an entire lake, with Arctic char shifting their diet to more pelagic resources in summer (Langeland et al. 1991). Environmental factors not included in our model might drive the distributions of both species and thus be responsible for the positive correlation between the two species. Further investigation of Arctic char and brown trout coexistence is needed to fully assess this aspect of our model.
The general trends in future Arctic char distributions predicted by our model are likely robust, but several improvements should be made before model predictions are used to manage individual lakes. First, both climate scenarios and pike invasion pathways need to be downscaled. Climate scenarios for Sweden were available at 50-km resolution, but substantial variation occurs at smaller spatial scales (Yang et al. 2011). A digital elevation model of finer resolution (<50 m) would improve models of stream pathways and predictions of pike invasion (Hein et al. 2011). Second, the link between climate and lake thermodynamics needs to be applied over broader spatial scales (Fang and Stefan 2009). Warmer surface waters will not necessarily exclude char if cold habitat is preserved in deep, stratified lakes (Elliott and Elliott 2010). Third, additional variables are likely important for determining Arctic char distributions, such as historical factors, lake depth, water color, productivity, and the occurrence of other species (Klemetsen et al. 2003; Sandlund et al. 2010; Finstad et al. 2011). The success of Arctic char introductions and the persistence of Arctic char after introductions of other species could be used to investigate Arctic char coexistence patterns further. Finally, when applying predictions to management, one must choose the appropriate probability threshold for predicting Arctic char presence/absence. Liu et al. (2005) advocate using prevalence to define the probability threshold, but a threshold of 0.1 predicted Arctic char in too many low-elevation lakes. A probability threshold of 0.25 resulted in the best model performance, and a threshold of 0.5 greatly reduced performance.
One common criticism of bioclimate envelope models is that they do not incorporate dispersal (Pearson and Dawson 2003; Hein et al. 2011), but this critique may not be as important for species with a contracting distribution. Not a single Swedish lake was predicted to be colonized by Arctic char in the future (Table 3). To avoid climate-driven extinction, Arctic char must therefore survive in lakes where they already occur. Still, high connectivity between existing Arctic char populations might be important for maintaining gene flow and allowing recolonizations after local extinctions (Öhman et al. 2006; Hanski 2011). Conversely, low connectivity between lakes might conserve Arctic char by preventing harmful species invasions. Had pike invaded all future suitable lakes, only eight Arctic char populations were predicted to survive. A more in-depth analysis of metapopulation dynamics in predicted refugia and locations of dispersal barriers may thus be needed for developing a long-term conservation plan for Arctic char.
As the northernmost fish species in the world, Arctic char are important for structuring lake communities (Hershey et al. 1999; Jeppesen et al. 2001) and are deeply woven into human society. They are central to historic subsistence fisheries and to modern recreational and commercial fisheries in circumpolar regions (Eriksson et al. 2006; Reist et al. 2006b). We predicted that Arctic char will lose 73 % of its range in Sweden by 2100 due to climate change, but Arctic char will continue to survive in alpine lakes of sufficient size. Conservation of populations in predicted refugia will be of high priority and will likely require habitat protection, fishery management, and prevention of harmful species invasions.
Acknowledgments
This research was funded by FORMAS (#2007-1149).
Biographies
Catherine L. Hein
is assistant professor at Umeå University. She is interested in understanding the processes that structure freshwater communities, and her work focuses on disturbances induced by climate change, invasive species, and land use change.
Gunnar Öhlund
is a doctoral student at Umeå University. He is interested in processes that structure freshwater communities, and his work focuses on interspecific interactions along environmental gradients.
Göran Englund
is professor in Animal Ecology at Umeå University. He studies the mechanisms controlling distribution patterns in freshwater fish, as well as the role of space for predator–prey dynamics.
Contributor Information
Catherine L. Hein, Email: clhein@gmail.com
Gunnar Öhlund, Email: gunnar.ohlund@emg.umu.se.
Göran Englund, Email: goran.englund@emg.umu.se.
References
- Araujo MB, Luoto M. The importance of biotic interactions for modelling species distributions under climate change. Global Ecology and Biogeography. 2007;16:743–753. doi: 10.1111/j.1466-8238.2007.00359.x. [DOI] [Google Scholar]
- Bradshaw WE, Holzapfel CM. Climate change—Evolutionary response to rapid climate change. Science. 2006;312:1477–1478. doi: 10.1126/science.1127000. [DOI] [PubMed] [Google Scholar]
- Burnham KP, Anderson DR. Model selection and multimodel inference: A practical information-theoretic approach. New York: Springer; 2002. [Google Scholar]
- Byström P, Karlsson J, Nilsson P, Kooten T, Ask J, Olofsson F. Substitution of top predators: Effects of pike invasion in a subarctic lake. Freshwater Biology. 2007;52:1271–1280. doi: 10.1111/j.1365-2427.2007.01763.x. [DOI] [Google Scholar]
- Chu C, Mandrak NE, Minns CK. Potential impacts of climate change on the distributions of several common and rare freshwater fishes in Canada. Diversity and Distributions. 2005;11:299–310. doi: 10.1111/j.1366-9516.2005.00153.x. [DOI] [Google Scholar]
- Ekman, S. 1922. The history of animal distributions on the Scandinavian Peninsula. Stockholm: Albert Bonniers Förlag (in Swedish).
- Elliott JM. The effects of temperature and ration size on the growth and energetics of salmonid fish in captivity. Comparative Biochemistry and Physiology. 1982;73:81–92. doi: 10.1016/0300-9629(82)90096-2. [DOI] [Google Scholar]
- Elliott JM, Elliott JA. Temperature requirements of Atlantic salmon Salmo salar, brown trout Salmo trutta and Arctic charr Salvelinus alpinus: predicting the effects of climate change. Journal of Fish Biology. 2010;77:1793–1817. doi: 10.1111/j.1095-8649.2010.02762.x. [DOI] [PubMed] [Google Scholar]
- Englund G, Johansson F, Olofsson P, Salonsaari J, Öhman J. Predation leads to assembly rules in fragmented fish communities. Ecology Letters. 2009;12:663–671. doi: 10.1111/j.1461-0248.2009.01322.x. [DOI] [PubMed] [Google Scholar]
- Eriksson T, Andersson J, Byström P, Hörnell-Willebrand M, Laitila T, Sandström C, Willebrand T. Fish and wildlife in the Swedish mountain area—Resources, use and management. International Journal of Biodiversity and Management. 2006;2:334–342. doi: 10.1080/17451590609618154. [DOI] [Google Scholar]
- Fagan WF. Connectivity, fragmentation, and extinction risk in dendritic metapopulations. Ecology. 2002;83:3243–3249. doi: 10.1890/0012-9658(2002)083[3243:CFAERI]2.0.CO;2. [DOI] [Google Scholar]
- Fang X, Stefan HG. Simulations of climate effects on water temperature, dissolved oxygen, and ice and snow covers in lakes of the contiguous United States under past and future climate scenarios. Limnology and Oceanography. 2009;54:2359–2370. doi: 10.4319/lo.2009.54.6_part_2.2359. [DOI] [Google Scholar]
- Fielding AH, Bell JF. A review of methods for the assessment of prediction errors in conservation presence/absence models. Environmental Conservation. 1997;24:38–49. doi: 10.1017/S0376892997000088. [DOI] [Google Scholar]
- Filipsson O. New fish populations as a result of stocking or spreading of fish. Information från Sötvattenslaboratoriet. 1994;2:1–65. [Google Scholar]
- Finstad AG, Forseth T, Jonsson B, Bellier E, Hesthagen T, Jensen AJ, Hessen DO, Foldvik A. Competitive exclusion along climate gradients: Energy efficiency influences the distribution of two salmonid fishes. Global Change Biology. 2011;17:1703–1711. doi: 10.1111/j.1365-2486.2010.02335.x. [DOI] [Google Scholar]
- Fry FEJ. The effect of environmental factors on the physiology of fish. In: Hoar WS, Randall DJ, editors. Fish physiology. New York: Academic Press; 1971. [Google Scholar]
- Gotelli NJ, Graves GR, Rahbek C. Macroecological signals of species interactions in the Danish avifauna. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:5030–5035. doi: 10.1073/pnas.0914089107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammar J. The significance of the Arctic char (Salvelinus alpinus) species complex in Sweden: Distribution, biology and status of an ice-age reminiscence. In: Maitland PS, editor. Proceedings of the seventh ISACF workshop on Arctic char. Drottningholm: International Society of Arctic Char Fanatics; 1992. pp. 47–63. [Google Scholar]
- Hanski I. Habitat loss, the dynamics of biodiversity, and a perspective on conservation. AMBIO. 2011;40:248–255. doi: 10.1007/s13280-011-0147-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heikkinen RK, Luoto M, Virkkala R, Pearson RG, Korber JH. Biotic interactions improve prediction of boreal bird distributions at macro-scales. Global Ecology and Biogeography. 2007;16:754–763. doi: 10.1111/j.1466-8238.2007.00345.x. [DOI] [Google Scholar]
- Hein CL, Öhlund G, Englund G. Dispersal through stream networks: Modelling climate-driven range expansions of fishes. Diversity and Distributions. 2011;17:641–651. doi: 10.1111/j.1472-4642.2011.00776.x. [DOI] [Google Scholar]
- Hershey AE, Gettel GA, McDonald ME, Miller MC, Mooers H, O’Brien WJ, Pastor J, Richards C, et al. A geomorphic-trophic model for landscape control of Arctic lake food webs. BioScience. 1999;49:887–897. doi: 10.2307/1313648. [DOI] [Google Scholar]
- Hutchinson GE. Concluding remarks. Cold Spring Harbor Symposium on Quantitative Biology. 1957;22:415–457. doi: 10.1101/SQB.1957.022.01.039. [DOI] [Google Scholar]
- IPCC (Intergovernmental Panel on Climate Change). 2007. Climate change 2007, Synthesis report. In Contribution of working groups I, II, and III to the fourth assessment report of the Intergovernmental Panel on Climate Change, ed. Core Writing Team, R.K. Pachauri, and A. Reisinger. Geneva, Switzerland: IPCC.
- Jackson DA, Peres-Neto PR, Olden JD. What controls who is where in freshwater fish communities—The roles of biotic, abiotic, and spatial factors. Canadian Journal of Fisheries and Aquatic Sciences. 2001;58:157–170. [Google Scholar]
- Jeppesen E, Christoffersen K, Landkildehus F, Lauridsen T, Amsinck SL, Riget F, Sondergaard M. Fish and crustaceans in northeast Greenland lakes with special emphasis on interactions between Arctic charr (Salvelinus alpinus), Lepidurus arcticus and benthic chydorids. Hydrobiologia. 2001;442:329–337. doi: 10.1023/A:1017508211819. [DOI] [Google Scholar]
- Jonsson M, Englund G, Wardle DA. Direct and indirect effects of area, energy and habitat heterogeneity on breeding bird communities. Journal of Biogeography. 2011;38:1186–1196. doi: 10.1111/j.1365-2699.2010.02470.x. [DOI] [Google Scholar]
- Jonsson B, Jonsson N. A review of the likely effects of climate change on anadromous Atlantic salmon Salmo salar and brown trout Salmo trutta, with particular reference to water temperature and flow. Journal of Fish Biology. 2009;75:2381–2447. doi: 10.1111/j.1095-8649.2009.02380.x. [DOI] [PubMed] [Google Scholar]
- Kjellström, E., L. Barring, U. Hansson, C. Jones, P. Samuelsson, M. Rummukainen, A. Ullerstig, U. Willen, et al. 2005. A 140-year simulation of European climate with the new version of the Rossby Center regional atmospheric climate model (RCA3). Swedish Meteorological and Hydrological Institute, Report 108, Norrköping, Sweden.
- Klemetsen A, Amundsen PA, Dempson JB, Jonsson B, Jonsson N, O’Connell MF, Mortensen E. Atlantic salmon Salmo salar L., brown trout Salmo trutta L. and Arctic charr Salvelinus alpinus (L.): A review of aspects of their life histories. Ecology of Freshwater Fish. 2003;12:1–59. doi: 10.1034/j.1600-0633.2003.00010.x. [DOI] [Google Scholar]
- Langeland A, Abeelund JH, Jonsson B, Jonsson N. Resource partitioning and niche shift in Arctic charr Salvelinus alpinus and brown trout Salmo trutta. Journal of Animal Ecology. 1991;60:895–912. doi: 10.2307/5420. [DOI] [Google Scholar]
- Liu CR, Berry PM, Dawson TP, Pearson RG. Selecting thresholds of occurrence in the prediction of species distributions. Ecography. 2005;28:385–393. doi: 10.1111/j.0906-7590.2005.03957.x. [DOI] [Google Scholar]
- Magnuson JJ, Crowder LB, Medwick PA. Temperature as an ecological resource. American Zoologist. 1979;19:331–343. [Google Scholar]
- Neverman D, Wurtsbaugh WA. The thermoregulatory function of diel vertical migration for a juvenile fish, Cottus extensus. Oecologia. 1994;98:247–256. doi: 10.1007/BF00324211. [DOI] [PubMed] [Google Scholar]
- Nilsson C, Reidy CA, Dynesius M, Revenga C. Fragmentation and flow regulation of the world’s large river systems. Science. 2005;308:405–408. doi: 10.1126/science.1107887. [DOI] [PubMed] [Google Scholar]
- Öhman J, Buffam I, Englund G, Blom A, Lindgren E, Laudon H. Associations between water chemistry and fish community composition: A comparison between isolated and connected lakes in northern Sweden. Freshwater Biology. 2006;51:510–522. doi: 10.1111/j.1365-2427.2006.01514.x. [DOI] [Google Scholar]
- Parmesan C, Yohe G. A globally coherent fingerprint of climate change impacts across natural systems. Nature. 2003;421:37–42. doi: 10.1038/nature01286. [DOI] [PubMed] [Google Scholar]
- Pearson RG, Dawson TP. Predicting the impacts of climate change on the distribution of species: Are bioclimate envelope models useful? Global Ecology and Biogeography. 2003;12:361–371. doi: 10.1046/j.1466-822X.2003.00042.x. [DOI] [Google Scholar]
- R Development Core Team. 2011. R: A language and environment for statistical computing, R Foundation for Statistical Computing. Vienna: R Development Core Team. http://www.R-project.org.
- Reist JD, Wrona FJ, Prowse TD, Power M, Dempson JB, Beamish RJ, King JR, Carmichael TJ, et al. General effects of climate change on Arctic fishes and fish populations. AMBIO. 2006;35:370–380. doi: 10.1579/0044-7447(2006)35[370:GEOCCO]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Reist JD, Wrona FJ, Prowse TD, Power M, Dempson JB, King JR, Beamish RJ. An overview of effects of climate change on selected Arctic freshwater and anadromous fishes. AMBIO. 2006;35:381–387. doi: 10.1579/0044-7447(2006)35[381:AOOEOC]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Ritchie EG, Martin JK, Johnson CN, Fox BJ. Separating the influences of environment and species interactions on patterns of distribution and abundance: Competition between large herbivores. Journal of Animal Ecology. 2009;78:724–731. doi: 10.1111/j.1365-2656.2008.01520.x. [DOI] [PubMed] [Google Scholar]
- Roeckner E, Bengtsson L, Feichten J, Lelieveld J, Rodhe H. Transient climate change simulations with a coupled atmosphere-ocean GCM including the Tropospheric sulfur cycle. Journal of Climate. 1999;12:3004–3032. doi: 10.1175/1520-0442(1999)012<3004:TCCSWA>2.0.CO;2. [DOI] [Google Scholar]
- Sandlund OT, Museth J, Naesje TF, Rognerud S, Saksgard R, Hesthagen T, Borgstrom R. Habitat use and diet of sympatric Arctic charr (Salvelinus alpinus) and whitefish (Coregonus lavaretus) in five lakes in southern Norway: Not only interspecific population dominance? Hydrobiologia. 2010;650:27–41. doi: 10.1007/s10750-009-0075-4. [DOI] [Google Scholar]
- Schweiger O, Settele J, Kudrna O, Klotz S, Kuhn I. Climate change can cause spatial mismatch of trophically interacting species. Ecology. 2008;89:3472–3479. doi: 10.1890/07-1748.1. [DOI] [PubMed] [Google Scholar]
- Sharma S, Jackson DA, Minns CK. Quantifying the potential effects of climate change and the invasion of smallmouth bass on native lake trout populations across Canadian lakes. Ecography. 2009;32:517–525. doi: 10.1111/j.1600-0587.2008.05544.x. [DOI] [Google Scholar]
- Spens J, Ball JP. Salmonid or nonsalmonid lakes: Predicting the fate of northern boreal fish communities with hierarchical filters relating to a keystone piscivore. Canadian Journal of Fisheries and Aquatic Sciences. 2008;65:1945–1955. doi: 10.1139/F08-103. [DOI] [Google Scholar]
- Torgersen CE, Price DM, Li HW, McIntosh BA. Multiscale thermal refugia and stream habitat associations of Chinook salmon in northeastern Oregon. Ecological Applications. 1999;9:301–319. doi: 10.1890/1051-0761(1999)009[0301:MTRASH]2.0.CO;2. [DOI] [Google Scholar]
- Walther GR. Community and ecosystem responses to recent climate change. Philosophical Transactions of the Royal Society B-Biological Sciences. 2010;365:2019–2024. doi: 10.1098/rstb.2010.0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang ZL, Hanna E, Callaghan TV. Modelling surface-air-temperature variation over complex terrain around Abisko, Swedish Lapland: Uncertainties of measurements and models at different scales. Geografiska Annaler Series A-Physical Geography. 2011;93A:89–112. doi: 10.1111/j.1468-0459.2011.00005.x. [DOI] [Google Scholar]