Abstract
Plant species distributions are expected to shift and diversity is expected to decline as a result of global climate change, particularly in the Arctic where climate warming is amplified. We have recorded the changes in richness and abundance of vascular plants at Abisko, sub-Arctic Sweden, by re-sampling five studies consisting of seven datasets; one in the mountain birch forest and six at open sites. The oldest study was initiated in 1977–1979 and the latest in 1992. Total species number increased at all sites except for the birch forest site where richness decreased. We found no general pattern in how composition of vascular plants has changed over time. Three species, Calamagrostis lapponica, Carex vaginata and Salix reticulata, showed an overall increase in cover/frequency, while two Equisetum taxa decreased. Instead, we showed that the magnitude and direction of changes in species richness and composition differ among sites.
Electronic supplementary material
The online version of this article (doi:10.1007/s13280-012-0312-3) contains supplementary material, which is available to authorized users.
Keywords: Ecosystem structure, Heaths, Meadows, Mountain birch forest, Plant biodiversity, Plant community ecology
Introduction
Plant physiology, biodiversity and distribution in the Arctic and sub-Arctic are constrained by a large number of abiotic factors like temperature, precipitation, snow depth, and length of growing season, as well as biotic factors such as herbivory and competition (e.g. Bliss et al. 1981; ACIA 2005). Several of these factors have changed dramatically over the last century (e.g. Callaghan et al. 2011a; Olsen et al. 2011) and are forecasted to change even further in the future at a rate that is greater than in most other regions on Earth (ACIA 2005). As a consequence, shifts in species distributions and plant community composition in Arctic and sub-Arctic regions are expected, and some changes have already been observed (e.g. ACIA 2005; Tape et al. 2006; Bhatt et al. 2010; Hedenås et al. 2011; Rundqvist et al. 2011; Callaghan et al. 2011a).
The most pronounced vegetation shift supported both by warming experiments (Walker et al. 2006) and observations over the last few decades, is an increased cover, biomass and upward movement of deciduous trees and shrubs (Kullman 2002; Van Bogaert et al. 2010; Hallinger et al. 2010; Hedenås et al. 2011; Rundqvist et al. 2011). Warming experiments also suggest that graminoids will generally increase in abundance (Walker et al. 2006). Many clonal graminoids, and deciduous trees and shrubs may out-compete plants that respond less, or not at all to warming. Earlier studies indicate that low-altitude generalist species may increase in alpine regions while alpine/Arctic species may decrease (Sundqvist et al. 2008; Kullman 2010; Sommer et al. 2010). At particular risk are slow-growing and long-lived specialised alpine/Arctic plants such as Saxifraga oppositifolia (Stenström et al. 1997), some dwarf shrubs such as Cassiope tetragona (Havström et al. 1993; Molau 1997; ACIA 2005), and mosses and lichens (Van Wijk et al. 2003; Walker et al. 2006). The results from warming experiments indicate also that we might expect an overall decrease in local species richness following increased temperatures in the alpine/Arctic regions (Walker et al. 2006). However, while warming experiments, observations and remote sensing have recorded increased plant growth in many areas, several studies have shown relative stability in other areas over recent decades (Prach et al. 2010; Callaghan et al. 2011b; Daniëls and de Molenaar 2011).
Overall, however, unfortunately, there are relatively few long-term biologically focused environmental monitoring programmes in the Arctic (ACIA 2005). Thus, Back to the Future Project (Callaghan et al. 2011c), of which the present study is one part, was established during the International Polar Years (2007 and 2008) to further our understanding of recent decadal-time scale vegetation change by re-sampling and assessing change at sites where historic biotic and abiotic research had been conducted.
We conducted our study in the sub-Arctic near the Abisko Scientific Research Station in northern Sweden where there is a major boundary between Arctic and boreal vegetation. The climate record from the Abisko Station shows substantial change over the past century with mean annual temperature rising by 2.5 °C between 1913 and 2006 (Callaghan et al. 2010). Snow-depth has increased by approximately 10 % per decade, between the 1930–1940s and 2000 (Kohler et al. 2006), but has decreased rapidly since then (Callaghan et al. 2010).
Extensive research has been conducted on the vegetation near Abisko since the first research station opened in 1908 (Andersson et al. 1996). Some of these older studies have been used to determine changes in tree growth (Hedenås et al. 2011), tree and shrub expansion (Rundqvist et al. 2011) and changes in tree species composition and altitudinal range at the tree-line (Van Bogaert et al. 2010, 2011), but in this study, we focus on the ground vegetation.
The aim of this study was to determine if vegetation near Abisko has changed in response to a period of rapid warming between 1970 and 2009. We examined differences in total species richness and species composition between plots established in sub-Arctic vegetation types that were sampled at the beginning of this period and again in 2008 and 2009. We assessed in particular whether there has been a shift in the proportion of various plant life-forms and of low altitude species compared to alpine/Arctic species. We also compared over time the abundance of the most common (i.e. occurs in at least five of the seven datasets) plant species found in these plots.
Materials and Methods
Selection of Study Sites
The two most important criteria used to determine sites for re-sampling were (1) that species level percentage cover or frequency data were recorded, and (2) that it was possible to find either the exact location of the plots or establish new plots close to the old ones in the same type of vegetation as in the initial sampling. In total we re-sampled seven datasets, at seven sites (Fig. 1), from five former studies. One study was initiated in the late 1970s (one dataset) and three others began during the 1980s (five datasets), while one was initiated in the early 1990s (one dataset). The sites associated with these studies and the respective details of their establishment and re-sampling are outlined below. Their locations are depicted in Fig. 1 and they are illustrated in Fig. 2.
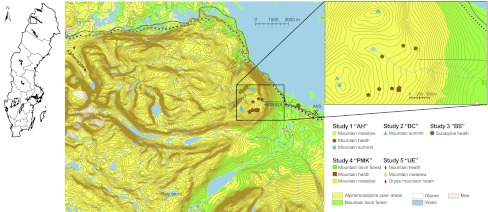
Fig. 1.
Location of the study area. The large map shows the location of the area from which biomass of the 61 clusters (squares) was re-investigated in 2010. The location of the Abisko Scientific Research Station (ANS; cross) where the meteorological station is located is also included. For details of the five different studies, see the key. The “AH”-study consisted of ten plots on Mt. Slåttatjåkka and Mt. Njulla. The “BC” study was situated at a fellfield site at the summit of Slåttatjåkka. The “BS”-study was situated in a dry sub-alpine heath area. The “PMK”-study consisted of three sites (i.e. three datasets): (i) low-herb meadow at Påtjujaure, (ii) Ridonjira mountain birch forest, and (iii) a mountain heath at Mt. Slåttatjåkka. The “UE”-study was situated in three sites at Kärkevagge, one in dry Empetrum heath vegetation, one in alpine low-herb meadow vegetation and one in Dryas heath. ©Lantmäteriet, ärende nr i2012/107
Fig. 2.
Photographs of the sites. a “AH” plot no. 5 located in a dry mountain heath at M. Slåttatjåkka. b The “BC” fellfield site at the summit of Slåttatjåkka: the small picture shows plot no. 3. c B.M. Svenson at the “BS” site situated in a dry sub-alpine heath area. d The low-herb meadow at Påtjujaure, e Mountain birch forest with the arrow pointing at the Ridonjira site, and f the mountain heath at Mt. Slåttatjåkka included in the “PMK”-study. g One of the low-herb meadow plots included in the “UE”-study. Photographs a, b and d–g were taken by H. Hedenås and photograph c was taken by B.Å. Carlsson
Study 1
Headley (1986) established a total of ten, 5 × 5 m plots (“AH”) on Mt. Slåttatjåkka and Mt. Njulla (coordinates N 68°21′24″–89″, E 18°40′30″–43′34″; altitude from 920 to 1170 m a.s.l.) in 1984 in order to study the ecology of Lycopodium sensu lato. The plots could not be accurately relocated in 2009, so ten new plots were chosen in the same area and plant community described in Appendix 1 by Headley (1986). In each plot, a regular spaced grid of 0.5 × 0.5 m cells was lain down over the area and ten, 0.5 × 0.5 m sub-plots were chosen within this space using random number tables. A map was used to record aspect and GPS to record site altitude (Supplementary Table S1). The final decision regarding the new location of the plots was based on the occurrence of Lycopodium spp. and how this resembled notes in Table 2.3 of Headley (1986). Within each plot, the presence of each vascular plant species was noted following Headley (1986).
Study 2
Carlsson and Callaghan (1991) established 20, permanently marked plots (0.25 × 0.25 m) at a 20 × 20 m fellfield site (“BC”) on a south-west facing slope at the summit of Slåttatjåkka (coordinates N 68°21′35″, E 18°40′340″; altitude about 1160 m a.s.l.) in 1984, in order to study the population dynamics of Carex bigelowii. The old vegetation data were recorded in 1986. Nine of the plots were re-sampled in 2009 following the procedure described by Carlsson and Callaghan (1991). Thus the presence/absence data for each vascular plant species were noted in 144 0.02 × 0.02 m quadrats in each of the nine plots.
Study 3
Svensson et al. (1993) established 20 permanently marked plots (0.50 × 0.50 m) (“BS”) in 1992 on vegetated solifluction terraces in a dry sub-alpine heath area (coordinates N 68°21′32″, E 18°48′22″; altitude about 350–355 m a.s.l.) in order to study the population dynamics of Pinguicula alpina and P. vulgaris. Eighteen of the plots were relocated and re-sampled in 2009. The cover of each vascular plant species was estimated in each of the twenty-five 0.1 × 0.1 m quadrats in each plot. The total cover of each species in each of the 18 plots was calculated by totalling the cover recorded in each of the 25 quadrats.
Study 4
The Swedish Environmental Protection Agency established three, permanently marked 40 × 40 m plots in the Abisko area as part of a larger environmental monitoring programme (Program för övervakning av miljökvalitet, PMK; Bernes 1980; PMK 2008). These three sites are detailed below. In all PMK sub-plots, the percentage cover for each plant species was estimated, following the same procedure used by the Swedish Environmental Monitoring Programme (Bernes 1980; PMK 2008).
At Påtjujaure, thirty-one 1 × 1 m permanently marked sub-plots were established in 1983 within the 40 × 40 m plot, in a low-alpine, low-herb meadow with wet areas (N 68°17′29″, E 18°31′49″; altitude 825 m a.s.l.).
In the Ridonjira mountain birch forest (of meadow type with tall herbs), thirty-one 1 × 1 m permanently marked sub-plots were established in 1983 within the 40 × 40 m plot (N 68°21′20″, E 18°45′43″; altitude centre: 388 m a.s.l.).
At Slåttatjåkka, sixteen 1 × 1 m permanently marked sub-plots (N 68°21′18″, E 18°42′38″; altitude centre: 940 m a.s.l.) were established in 1989 within the 40 × 40 m plot at a site consisting mainly of dry Empetrum heath and low-herb meadow with elements of snow-bed vegetation.
Study 5
In 1977–1979, Emanuelsson (1984) initiated a study to assess the trampling impact of hikers on mountain vegetation. Ten circular sectors (“UE”) were established in Kärkevagge, above the tree-line in different types of mountain vegetation. Each circular sector consists of one central trampled zone with two reference non-trampled zones on each side (see Supplementary Information, Figure S1). It was possible to re-locate seven of the circular sectors situated in Kärkevagge in 2009. Three of the circular sectors were situated in dry Empetrum heath vegetation (N 68°24′55″, E 18°18′31″; altitude about 630 m a.s.l.), three were situated in alpine low-herb meadow vegetation (N 68°24′27″, E 18°19′12″; altitude ca. 760 m a.s.l.) and one was situated in Dryas heath vegetation (N 68°23′48″, E 18°19′52″; altitude ca. 830 m a.s.l.). Transects (0.1 × 5 m) were placed in the non-trampled zones and the presence of species was noted at every 0.2 m while cover was estimated every 0.6 m following Emanuelsson (1984). Thus the presence/absence was, in total, noted in fifty 0.1 × 0.1 m sub-plots and cover estimated in a total of eighteen 0.1 × 0.1 m sub-plots, within each circular sector.
Taxonomy and Classification
The taxonomy follows DYNTAXA (2011). We have merged some taxa due to uncertainties in identifications, e.g. in the study “UE” the three Pyrola species were merged to “Pyrola spp.” prior to analysis (Supplementary Information, Tables S2–S8). We also classified the species into plant geographical classes, AA: alpine/Arctic, B: boreal, N: north (northern species, but not boreal or alpine/Arctic) and O: other (cultural, south or southeast origin), based on distribution and origin sensu Hultén (1950). The latter three classes are together called low-altitude species. Further, we classified the species into plant life-forms, G: graminoids, F: forbs, VC: vascular cryptogams, DS: deciduous shrubs, WGS: winter-green shrubs and DT: deciduous trees (Supplementary Information, Tables S2–S8).
Data Analysis
We performed both first- and second-order jackknife analyses, using PC-ORD 4.20 (McCune and Mefford 1999; Walther and Moore 2005), in order to estimate the total species number initially and the present total species number for each study, separately. The number of replicates differed between studies (i.e. sub-plots/plots). For each dataset, we conducted t tests for all species comparing mean differences in frequency or cover between the initial study and the resampling. We applied paired t tests on “AH” “BC” and “BS” datasets and the three “PMK” datasets, and student t tests on “UE” datasets where the relocated plots could not be established to match the original numbers in the “UE” study. The tests were applied in order to assess whether the total number of species, proportion of alpine/Arctic, proportion of low-altitude species, proportion of plant life forms differed overall between the initial and repeated sampling. We also applied Pearson correlation tests to assess whether the difference in species number between the initial and repeated sampling correlated with years between the initial and repeated sampling, and species richness, i.e. both the initial and the present observed number of species. Frequency data were arcsine-transformed and cover data were log-transformed prior to analysis to meet the needs of normality required for the majority of these tests.
Meta-analyses were performed for each of the 17 species that occurred in five or more of the seven datasets. For each dataset, effect size “d” and Hedges “g” (Cooper et al. 2009) based on mean difference of species frequency or cover between the initial and repeated sampling were calculated using the statistical package R 2.11.1 and “MAd version 0.8”. Secondly, we calculated a weighted average effect size of Hedges “g” and lower and upper confidence interval (sensu Cooper et al. 2009). We also performed meta-analyses to assess whether the frequency/cover of plant life-forms differed overall between the initial and repeated sampling. We included only datasets where the plant life forms were represented with at least three species. In each dataset and for each year, we summarised the frequency or cover of the species that was classified as a certain plant life-form. We thereafter calculated weighted average effect size of Hedges “g” and lower and upper confidence intervals as above.
We performed nonparametric Multi-response Permutation Procedures (MRPP) using PC-ORD 4.20 (McCune and Mefford 1999) in order to test whether the species composition differed between years for each dataset separately. We elected to use Sørensens distance measure and used natural weighting (McCune and Grace 2002). T, test statistic, describes the magnitude of separation in species composition between years. The more negative T is, the larger the difference between years. A, i.e. within-group agreement or “effect size”, equals 1 if the species composition in all plots within years is identical, 0 if the species composition equals expectation by chance, and <0 if the species composition within plots differs more than expected by chance. Observed δ, i.e. the observed weighted mean within-group distance, equals 0 if the species composition in all plots within years was identical (McCune and Grace 2002).
Pearson correlation tests were applied to assess whether the Ts and As and observed δs obtained from the MRPP-tests correlated with years between initial and repeated sampling, species richness, i.e. both initial and present observed number of species, first- and second-order jackknife estimates, and the calculated difference in species richness between years. We also assessed whether Ts and As and observed δs were correlated with altitude for five of the datasets (BC, BS, Påtjujaure, Ridonjira and Slåttatjåkka).
Results
Species Richness, Cover and Frequencies
Total species number did not differ generally between the initial and repeated sampling (t6 = 1.868, p = 0.111). Total species numbers were, however, higher in six of the studies in 2008 and 2009 compared to the same studies sampled decades earlier (jackknife estimates; Table 1). The only exception was the Ridonjira study where species richness was higher in 1983 than in 2008 (Table 1). Further, neither proportion of alpine/Arctic species, nor proportion of low-altitude species differed significantly between the initial and repeated sampling (t6 = −0.490, p = 0.641 and t6 = −0.420, p = 0.689, respectively). Moreover, neither proportion of graminoids, forbs, deciduous shrubs, winter-green shrubs nor vascular cryptogams species differed significantly between the initial and repeated sampling (t6 = −0.395, p = 0.707; t6 = 0.312, p = 0.766, t6 = 0.328, p = 0.754, t6 = 0.665, p = 0.531 and t6 = −0.236, p = 0.821, respectively). The difference in total species number between initial sampling and repeated sampling did not correlate with either initial number of species (r = 0.239, p = 0.605), the current number of species (r = 0.684, p = 0.090), or years between sampling time (r = 0.439, p = 0.324; Pearson correlation analyses).
Table 1.
Species richness of vascular plants in the different datasets, divided into old and new datasets
| Study | PMK | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AH | BC | BS | Påtjujaure | Ridonjira | Slåttatjåkka | UE | ||||||||
| 1984 | 2008 | 1986 | 2009 | 1992 | 2009 | 1983 | 2008 | 1983 | 2008 | 1989 | 2008 | 1977–1979 | 2009 | |
| Number of “plots” | 10 | 10 | 9 | 9 | 18 | 18 | 31 | 31 | 31 | 31 | 16 | 16 | 10 | 7 |
| Total sampled area (m2) | 25 | 25 | 0.6 | 0.6 | 4.5 | 4.5 | 31 | 31 | 31 | 31 | 16 | 16 | 5 | 3.5 |
| Observed total number of species | 41 | 53 | 9 | 12 | 30 | 31 | 42 | 58 | 42 | 36 | 35 | 36 | 37 | 49 |
| Number of species per m2 | 1.6 | 2.1 | 16.0 | 21.3 | 6.7 | 6.9 | 1.4 | 1.9 | 1.4 | 1.2 | 2.2 | 2.2 | 7.4 | 14 |
| Prop. of alpine/Arctic species (%)a | 65.9 | 69.8 | 77.8 | 75.0 | 56.7 | 54.8 | 52.4 | 46.5 | 16.7 | 19.4 | 62.9 | 63.9 | 59.5 | 57.1 |
| Prop. of low-altitude species (%)a | 29.2 | 26.4 | 22.2 | 25.0 | 40.0 | 41.9 | 42.9 | 48.3 | 78.6 | 75.0 | 37.1 | 36.1 | 35.1 | 36.7 |
| Prop. of unclassified species (%) | 4.8 | 3.8 | 0 | 0 | 3.3 | 3.2 | 4.8 | 5.1 | 4.8 | 5.6 | 0 | 0 | 5.4 | 6.1 |
| Prop. of graminoids (%) | 22.0 | 20.8 | 33.3 | 23.1 | 10.0 | 16.1 | 23.8 | 25.9 | 28.6 | 25.0 | 11.4 | 16.7 | 16.2 | 20.4 |
| Prop. of forbs (%) | 36.6 | 39.6 | 22.2 | 15.4 | 40.0 | 35.5 | 40.5 | 44.8 | 50.0 | 47.2 | 45.7 | 41.7 | 51.4 | 59.2 |
| Prop. of deciduous shrubs (%) | 14.6 | 15.1 | 11.1 | 23.1 | 13.3 | 16.1 | 19.0 | 15.5 | 2.4 | 0 | 14.3 | 13.9 | 13.5 | 8.2 |
| Prop. of winter-green shrubs (%) | 17.1 | 13.2 | 22.2 | 30.8 | 30.0 | 25.8 | 7.1 | 3.4 | 7.1 | 8.3 | 17.1 | 19.4 | 10.8 | 6.1 |
| Prop. of deciduous trees (%) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 7.1 | 5.6 | 0 | 0 | 0 | 0 |
| Prop. of vascular cryptogams (%) | 9.8 | 11.3 | 11.1 | 7.7 | 6.7 | 6.5 | 9.5 | 10.3 | 4.8 | 13.9 | 11.4 | 8.3 | 8.1 | 6.1 |
| No. of species with ≤2 occurrences | 14 | 28 | 5 | 5 | 4 | 5 | 15 | 15 | 13 | 8 | 6 | 17 | 20 | 23 |
| Jackknife1 estimate of total no. of sp. | 49.1 | 65.6 | 11.7 | 15.6 | 33.8 | 35.7 | 51.7 | 66.7 | 50.7 | 40.8 | 45.3 | 46.3 | 46.9 | 60.1 |
| Jackknife2 estimate of total no. of sp. | 52.7 | 66.8 | 12.6 | 18.0 | 37.3 | 40.2 | 56.5 | 69.7 | 55.5 | 42.8 | 50.9 | 51.0 | 49.3 | 63.5 |
| Years between sampling | 24 | 25 | 17 | 25 | 25 | 19 | 30 | |||||||
| No. of species gained per year | 0.54 | 0.26 | 0.18 | 0.80 | 0.16 | 0.42 | 0.57 | |||||||
| No. of species lost per year | 0.04 | 0.13 | 0.12 | 0.12 | 0.40 | 0.32 | 0.13 | |||||||
| No. of species with a significant increaseb | 2 | 0 | 3 | 11 | 6 | 2 | 4 | |||||||
| No. of species with a significant decreaseb | 3 | 0 | 2 | 7 | 9 | 2 | 0 | |||||||
aLow-altitude species consist of species with boreal, northern or other origin/distribution sensu Hultén (1950)
bAccording to t tests, see Supplementary Information
Relatively few species changed significantly in cover or frequency based on analysis of each species and study separately (Table 1; see Supplementary Information, Tables S2–S8 for details regarding species turnover and results of t tests for each species for each dataset). Three of the 17 species included in the meta-analysis, Calamagrostis lapponica, Carex vaginata and Salix reticulata, increased significantly while two taxa decreased significantly, i.e. Equisetum arvense and the joint taxon E. scirpoides and E. variegatum, between the initial and repeated sampling (Table 2). We revealed that none of the five plant life-forms, included in the meta-analysis, differed significantly in frequency/cover between the initial and repeated sampling (Table S9).
Table 2.
Overall effect size estimates of changes in frequency/cover of individual species occurring in five or more of the datasets (meta-analyses)
| Species | No. of datasets | Weighted average of g | Confidence interval | |
|---|---|---|---|---|
| Lower | Upper | |||
| Anthoxanthum nipponicum syn. A. alpinum | 5 | −0.09 | −0.37 | 0.20 |
| Astragalus alpines & frigidus | 5 | 0.08 | −0.19 | 0.35 |
| Betula nana | 5 | 0.07 | −0.23 | 0.37 |
| Calamagrostis lapponica | 5 | 0.91 | 0.59 | 1.23 |
| Carex bigelowii | 6 | 0.10 | −0.20 | 0.40 |
| Carex vaginata | 5 | 0.32 | 0.05 | 0.59 |
| Empetrum hermaphroditum | 6 | 0.03 | −0.25 | 0.31 |
| Equisetum arvense | 5 | −0.47 | −0.75 | −0.20 |
| Equisetum scirpoides & variegatum | 5 | −0.56 | −0.91 | −0.20 |
| Festuca ovina | 6 | −0.01 | −0.28 | 0.26 |
| Poa alpina | 5 | 0.22 | −0.08 | 0.51 |
| Salix reticulata | 5 | 0.52 | 0.20 | 0.83 |
| Saussurea alpina | 5 | 0.02 | −0.26 | 0.30 |
| Vaccinium myrtillus | 5 | 0.23 | −0.07 | 0.53 |
| Vaccinium uliginosum | 6 | 0.09 | −0.20 | 0.37 |
| Vaccinium vitis-idaea | 7 | 0.10 | −0.14 | 0.35 |
| Viola biflora | 5 | 0.03 | −0.26 | 0.31 |
The change in frequency/cover was significant when zero is not included in the confidence interval (also noted in bold)
Species Composition
Species composition differed between years in three of the seven datasets, namely AH, PMK Påtjujaure and PMK Ridonjira (Table 3; MRPP-tests; Supplementary Information Table S10). T, i.e. magnitude of separation in species composition between years, and A, i.e. homogeneity within groups, did not correlate significantly with either years between initial and repeated sampling, species richness or differences in species richness. Observed δ correlated significantly with both first- and second-order jackknife estimates of total species richness in the new datasets (r = 0.792, p = 0.034 and r = 0.810, p = 0.027, respectively) and nearly significantly with total species richness recorded at the initial sampling (r = 0.695, p = 0.083), total species richness recorded at the re-sampling (r = 0.752, p = 0.051), and first- and second-order jackknife estimates of total species richness in the new datasets (r = 0.732, p = 0.062 and r = 0.704, p = 0.077, respectively).
Table 3.
Overall comparison of vascular plant composition, for each dataset, by the multi-response permutation procedure (MRPP)
| Dataset | δ | T | p | A | |||
|---|---|---|---|---|---|---|---|
| Observed | Expected | Variance | Skewness | ||||
| AH | 0.568 | 0.595 | <0.001 | −0.859 | −3.484 | 0.004 | 0.046 |
| BC | 0.310 | 0.320 | <0.001 | −0.914 | −1.501 | 0.081 | 0.030 |
| BS | 0.472 | 0.470 | <0.001 | −1.918 | 0.463 | 0.588 | −0.005 |
| Påtjujaure | 0.518 | 0.586 | <0.001 | −0.492 | −38.005 | <0.001 | 0.116 |
| Ridonjira | 0.475 | 0.501 | <0.001 | −0.386 | −18.908 | <0.001 | 0.052 |
| Slåttatjåkka | 0.558 | 0.561 | 0.001 | −1.053 | −0.510 | 0.260 | 0.006 |
| UE | 0.717 | 0.707 | 0.007 | −2.109 | 0.349 | 0.515 | -0.013 |
Bold figures denote a significant overall test, i.e. species composition differs between years. Multiple comparisons of vascular plant composition, Påtjujaure and Ridonjira datasets are presented in Table S9 (Supplementary Information)
Discussion
We revealed that the magnitude and direction of changes in species composition and change in species richness differs among sites. We found that the total species number was higher today than earlier at all open and exposed sites whether or not they were located above or below the tree line, while the only site located in the birch forest demonstrated a decrease in species richness. Although we cannot generalise from one forest site, we are not surprised to reveal decreased species richness at the forest site since the tree and shrub cover have increased considerably over the last three decades, changing light regimes and competition which may affect the composition and richness of the ground-vegetation (e.g. Callaghan et al. 2002; Wielgolaski 2005; Walker et al. 2006; Hedenås et al. 2011).
Our result was in contradiction to conclusions from warming experiments, which suggest that we could expect decreased species richness with increasing temperatures (e.g. Walker et al. 2006). Results from earlier surveys in alpine and Arctic areas are, however, not consistent. There are studies conducted in alpine areas that report increased species richness (Grabherr et al. 1994; Klanderud and Birks 2003; see references in Kullman 2010), while others report decreased richness (Moen and Lagerström 2008; Wilson and Nilsson 2009). Some studies on relatively species-poor communities in the Arctic also found little change in species richness (Prach et al. 2010; Callaghan et al. 2011b; Daniëls and de Molenaar 2011). Virtanen et al. (2010) report a more complex pattern with an increase of species in species-poor communities, while species richness remains “relatively constant” in species-rich communities. In contrast, we did not see any correlation between change in species number and either initial species richness or present species richness.
Species composition changed significantly between the initial and repeated sampling at three of the seven sites. However, the result was inconsistent and we found no relationship between magnitude in discrepancy in species composition and either initial species richness or years between initial and repeated sampling. Several of the species that were lost or gained over the years were relatively rare and occurred with low cover/frequencies. Due to their low abundances they will reach statistical significance in t tests, but only have minor influences on the outcome of NMS-ordinations and Multi-response Permutation Procedures.
Our study indicates that low-altitude species have not increased in the area overall, since the proportion of alpine/Arctic species in 2008–2009 was more or less the same as in the initial samplings. Thus, our result contradicts some earlier studies and predictions that amelioration of climatic conditions may lead to low-altitude species colonising higher altitude sites (Sundqvist et al. 2008; Kullman 2010; Sommer et al. 2010), even though our observations span 16–31 years. However, our results agree with other studies which found no increase of low-altitude species (e.g. Wilson and Nilsson 2009).
Overall, we found no general change in cover/frequency of different plant life-forms. Instead, we revealed that the change was highly species-specific. Two graminoid species, Calamagrostis lapponica and Carex vaginata and a small mat-forming willow, Salix reticulata, increased significantly in cover/frequency, while two fern allies’ taxa decreased, Equisetum arvense and the joint taxon E. scirpoides and E. variegatum. The increase in graminoid species cover/frequency was maybe not surprising as warming experiments have shown that several graminoid species are likely to increase in biomass with increasing temperatures (Walker et al. 2006). For example, Calamagrostis lapponica tends to respond to experimentally increased temperatures and/or fertilisation with increased shoot length and biomass (Parsons et al. 1995). It has also been shown that Carex vaginata increases with increasing temperature and nutrient availability, but may decrease due to inter-specific competition (Klanderud 2004). It was somewhat surprising that Salix reticulata was the only shrub that significantly increased overall, since other surveys have shown an extensive increase of shrubs (e.g. Tape et al. 2006; Hallinger et al. 2010; Hedenås et al. 2011; Rundqvist et al. 2011; Myers-Smith et al. 2011). The decrease of the two Equisetum taxa may be related to a change in snow cover, e.g. decreased thickness and related soil moisture, during the last decade (Callaghan et al. 2010), since an earlier study in Canada has shown that Equisetum spp. abundance decreased with decreased snow depth in the Arctic (Schaeffer and Messier 1995).
We found that despite relatively large changes in species composition and species richness at individual sites, the overall changes were relatively moderate. The ecosystems are likely to have high inertia as the open vegetation is dominated by slow-growing clonal species that buffer change (Callaghan et al. 2002; Kullman 2010). However, assuming a lack of change might be too early. We could expect future large-scale sudden shifts in species composition initiated by an outbreak of pathogens (Olofsson et al. 2011), changed herbivory pressure (Tenow et al. 2001; van der Wal 2006; Olofsson et al. 2009), extreme weather conditions such as winter warming (Bokhorst et al. 2009) or competitive displacement by invasive species (Eckstein et al. 2011). The responses of vegetation to such events and disturbance may normally be reversible (van der Wal 2006), but human-induced changes in climate or management may reduce ecosystem resilience and its ability to recover to its former state (ACIA 2005; van der Wal 2006).
Electronic supplementary material
Acknowledgments
We sincerely thank the staff of the Abisko Scientific Research Station for their support, in particular Philpp Theuringer. We also thank Craig E. Tweedie for valuable comments on an earlier version of the manuscript and two anonymous reviewers for valuable comments. The project was financed by a grant from the Swedish Research Council (Vetenskapsrådet) 327-2007-833 as part of the International project “Retrospective and prospective vegetation change in the Polar Regions: Back to the Future Project (BTF; IPY Project number ID No 512). This work was also partially supported by the FORMAS projects “Climate change, impacts and adaptation in the sub Arctic: a case study from the northern Swedish mountains” (214-2008-188) and “Advanced Simulation of Arctic climate change and impact on Northern regions” (214-2009-389).
Biographies
Henrik Hedenås
has a PhD in Ecology from the Department of Ecology and Environmental Science (EMG), Umeå University. He has worked at different positions at EMG, and as researcher at the Abisko Scientific Research Station. He is currently working as an Analyst at the Department of Forest Resource Management, Swedish University of Agricultural Science.
Bengt Å. Carlsson
has a PhD in Plant Ecology from Lund University and is presently working as editor for the Svensk Botanisk Tidskrift.
Urban Emanuelsson
has a PhD from the University of Lund and was Appointed Professor by the Swedish Government in January 2007. He has worked as Director of the Swedish Biodiversity Centre 1995–2008 at The Swedish University of Agriculture and Uppsala University, where he today is Senior Adviser. He is also guest professor at Högskolan Kristianstad, Member of the Board, for Ajtte (Swedish Sáme Museum), and Member of the Board, WWF Sweden, Member of the Royal Academy of Forestry and Agriculture.
Alistair D. Headley
has a PhD from the University of Manchester, UK. He is an independent ecological consultant specialising in assessing herbivore impacts and ecological assessments of upland and peatland habitats, principally in the Scottish highlands.
Christer Jonasson
has a PhD in Physical Geography from Uppsala University and is Associate Professor at Stockholm University. He is Station Manager of the Abisko Scientific Research Station.
Brita M. Svensson
has a PhD in Plant Ecology from Lund University. She is professor in Plant Ecology and Environmental Science at Uppsala University.
Terry V. Callaghan
has a PhD from the University of Birmingham, UK, honorary PhDs from the Universities of Lund, Sweden, and Oulu, Finland, and a DSc from the University of Manchester, UK. He is a Distinguished Research Professor and Member of Royal Swedish Academy of Sciences’, and Professor of Arctic Ecology at the University of Sheffield, UK.
Contributor Information
Henrik Hedenås, Email: henrik.hedenas@slu.se.
Bengt Å. Carlsson, Email: bengt.carlsson@ebc.uu.se
Urban Emanuelsson, Email: urban.emanuelsson@slu.se.
Alistair D. Headley, Email: headleyplantecol@btinternet.com
Christer Jonasson, Email: christer.jonasson@ans.polar.se.
Brita M. Svensson, Email: brita.svensson@ebc.uu.se
Terry V. Callaghan, Email: terry_callaghan@btinternet.com
References
- Arctic Climate Impact Assessment. Cambridge: Cambridge University Press; 2005. [Google Scholar]
- Andersson NÅ, Callaghan TV, Karlsson PS. The Abisko Scientific Research Station. Ecological Bulletins. 1996;45:11–14. [Google Scholar]
- Bernes C. Monitor 1980 en presentation av PMK—Programmet för övervakning av miljökvalitet. Stockholm: Naturvårdsverket; 1980. [Google Scholar]
- Bhatt US, Walker DA, Raynolds MK, Comiso JC, Epstein HE, Jia G, Gens R, Pinzon JE, et al. Circumpolar Arctic tundra vegetation change is linked to sea ice decline. Earth Interactions. 2010;14:8. doi: 10.1175/2010EI315.1. [DOI] [Google Scholar]
- Bliss LC, Heal OW, Moore JJ, editors. Tundra ecosystems: A comparative analysis. Cambridge: Cambridge University Press; 1981. [Google Scholar]
- Bokhorst SF, Bjerke JW, Tømmervik H, Callaghan TV, Phoenix GK. Winter warming events damage sub-Arctic vegetation: Consistent evidence from an experimental manipulation and a natural event. Journal of Ecology. 2009;97:1408–1415. doi: 10.1111/j.1365-2745.2009.01554.x. [DOI] [Google Scholar]
- Callaghan, T.V., B.R. Werkman, and R.M.M. Crawford, eds. 2002. Dynamics of the tundra–taiga interface. AMBIO 12: 3–62. [PubMed]
- Callaghan, T.V., F. Bergholm, T.R. Christensen, C. Jonasson, U. Kokfelt, and M. Johansson. 2010. A new climate era in the sub-Arctic: Accelerating climate changes and multiple impacts. Geophysical Research Letters 37. doi:10.1029/2009GL042064
- Callaghan TV, Christensen TR, Jantze EJ. Plant and vegetation dynamics on Disko Island, West Greenland: Snapshots separated by over 40 years. AMBIO. 2011;40:624–637. doi: 10.1007/s13280-011-0169-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callaghan TV, Tweedie CE, Åkerman J, Andrews C, Bergstedt J, Butler MG, Christensen TR, Cooley D, et al. Multi-decadal changes in tundra Environments and ecosystems: Synthesis of the International Polar Year Back to the Future Project. AMBIO. 2011;40:705–716. doi: 10.1007/s13280-011-0179-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callaghan TV, Tweedie CE, Webber PJ. Multi-decadal changes in tundra environments and ecosystems: The International Polar Year-Back to the Future Project (IPY-BTF) AMBIO. 2011;40:555–557. doi: 10.1007/s13280-011-0162-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsson BÅ, Callaghan TV. Positive plant interactions in tundra vegetation and the importance of shelter. Journal of Ecology. 1991;79:973–983. doi: 10.2307/2261092. [DOI] [Google Scholar]
- Cooper H, Hedges LV, Valentine JC, editors. The handbook of research synthesis and meta analysis. New York: Russell Sage Foundation; 2009. [Google Scholar]
- Daniëls FJA, Molenaar JG. Flora and vegetation of Tasiilaq, Formerly Angmagssalik, Southeast Greenland—A comparison of data from between around 1900 and 2007. AMBIO. 2011;40:650–659. doi: 10.1007/s13280-011-0171-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DYNTAXA. 2011. Taxonomic Database. ArtDatabanken. SLU, Uppsala (in Swedish). http://lampetra2-1.artdata.slu.se:6767/EXEC/0/1akk2jn0er8v9y1aibbr01ve8hw8. Accessed 22 Nov 2011.
- Eckstein RL, Pereira E, Milbau A, Graae BJ. Predicted changes in vegetation structure affect the susceptibility to invasion of bryophyte-dominated subarctic heath. Annals of Botany. 2011;108:177–183. doi: 10.1093/aob/mcr097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emanuelsson, U. 1984. Ecological effects of grazing and trampling on mountain vegetation in northern Sweden. PhD Thesis. Sweden: University of Lund.
- Grabherr G, Gottfried M, Pauli H. Climate effects on mountain plants. Nature. 1994;369:448. doi: 10.1038/369448a0. [DOI] [PubMed] [Google Scholar]
- Hallinger M, Manthey M, Wilmking M. Establishing a missing link: Warm summers and winter snow cover promote shrub expansion into alpine tundra in Scandinavia. New Phytologist. 2010;186:890–899. doi: 10.1111/j.1469-8137.2010.03223.x. [DOI] [PubMed] [Google Scholar]
- Havström M, Callaghan TV, Jonasson S. Differential growth responses of Cassiope tetragona, an arctic dwarf-shrub, to environmental perturbations among three contrasting high- and subarctic sites. Oikos. 1993;66:389–402. doi: 10.2307/3544933. [DOI] [Google Scholar]
- Headley, A.D. 1986. The comparative autecology of some European species of Lycopodium senso lato. PhD Thesis. Manchester: University of Manchester.
- Hedenås H, Olsson H, Jonasson C, Bergstedt J, Dahlberg U, Callaghan TV. Changes in tree growth, biomass and vegetation over a 13-year period in the Swedish sub-Arctic. AMBIO. 2011;40:672–682. doi: 10.1007/s13280-011-0173-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultén, E. 1950. Atlas of the distribution of vascular plants in NW. Europe. AB Kartgeografiska institutet, Esselte AB, Stockholm (in Swedish with English summary).
- Klanderud K. Climatic change effects on species interactions in an alpine plant community. Journal of Ecology. 2004;93:127–137. doi: 10.1111/j.1365-2745.2004.00944.x. [DOI] [Google Scholar]
- Klanderud K, Birks HJB. Recent increase in species richness and shifts in altitudinal distributions of Norwegian mountain plants. The Holocene. 2003;13:1–6. doi: 10.1191/0959683603hl589ft. [DOI] [Google Scholar]
- Kohler J, Brandt O, Johansson M, Callaghan T. A long-term Arctic snow depth record from Abisko, northern Sweden, 1913–2004. Polar Research. 2006;25:91–113. doi: 10.1111/j.1751-8369.2006.tb00026.x. [DOI] [Google Scholar]
- Kullman L. Rapid recent range-margin rise of tree and shrub species in the Swedish Scandes. Journal of Ecology. 2002;90:68–77. doi: 10.1046/j.0022-0477.2001.00630.x. [DOI] [Google Scholar]
- Kullman L. A richer, greener and smaller alpine world: Review and projection of warming-induced plant cover change in the Swedish Scandes. AMBIO. 2010;39:159–169. doi: 10.1007/s13280-010-0021-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCune, M., and M.J. Mefford. 1999. Multivariate analysis of ecological data, version 4.20. Gleneden Beach: MjM Software design.
- McCune M, Grace JB. Analysis of ecological communities. Gleneden Beach: MjM Software design; 2002. [Google Scholar]
- Moen J, Lagerström A. High species turnover and decreasing plant species richness on mountain summits in Sweden: Reindeer grazing overrides climate change. Antarctic, and Alpine Research. 2008;40:382–395. doi: 10.1657/1523-0430(07-031)[MOEN]2.0.CO;2. [DOI] [Google Scholar]
- Molau U. Responses to natural climatic variation and experimental warming in two tundra plant species with contrasting life forms: Cassiope tetragona and Ranunculus nivalis. Global Change Biology. 1997;3:97–107. doi: 10.1111/j.1365-2486.1997.gcb138.x. [DOI] [Google Scholar]
- Myers-Smith IH, Hik DS, Kennedy C, Cooley D, Johnstone JF, Kenney AJ, Krebs CJ. Expansion of canopy-forming willows over the 20th century on Herschel Island, Yukon Territory, Canada. AMBIO. 2011;40:610–623. doi: 10.1007/s13280-011-0168-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olofsson J, Ericson L, Torp M, Stark S, Baxter R. Carbon balance of Arctic tundra under increased snow cover mediated by a plant pathogen. Nature Climate Change. 2011;1:220–223. doi: 10.1038/nclimate1142. [DOI] [Google Scholar]
- Olofsson J, Oksanen L, Callaghan T, Hulme PE, Oksanen T, Suominen O. Herbivores inhibit climate-driven shrub expansion on the tundra. Global Change Biology. 2009;15:2681–2693. doi: 10.1111/j.1365-2486.2009.01935.x. [DOI] [Google Scholar]
- Olsen MS, Callaghan TV, Reist J, Reiersen LO, Ananicheva M, Dahl-Jensen D, Gerland S, Goodison B, et al. The changing Arctic cryosphere and likely consequences: An overview AMBIO 201140Special Report 1111–118. 10.1007/s13280-011-0220-y21446390 [DOI] [Google Scholar]
- Parsons AN, Press MC, Wookey PA, Welker JM, Robinson CH, Callaghan TV, Lee JA. Growth responses of Calamagrostis lapponica to simulated environmental change in the sub-Arctic. Oikos. 1995;72:61–66. doi: 10.2307/3546038. [DOI] [Google Scholar]
- PMK. 2008. The environmental monitoring programme has been funded by the Swedish Environmental Protection Agency and data produced by the Department of Aquatic Science and Assessment at the Swedish University of Agricultural Sciences, SLU. http://www.ma.slu.se/. Accessed 14 Nov 2008.
- Prach K, Kosnar J, Klimesova J, Hais M. High Arctic vegetation after 70 years: A repeated analysis from Svalbard. Polar Biology. 2010;33:635–639. doi: 10.1007/s00300-009-0739-6. [DOI] [Google Scholar]
- Rundqvist S, Hedenås H, Sandström A, Emanuelsson U, Jonasson C, Callaghan TV. Tree and shrub expansion over the past 34 years at the tree-line near Abisko, Sweden. AMBIO. 2011;40:683–692. doi: 10.1007/s13280-011-0174-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaeffer JA, Messier F. Scale-dependent correlations of Arctic vegetation and snow cover. Arctic and Alpine Research. 1995;27:38–43. doi: 10.2307/1552066. [DOI] [Google Scholar]
- Sommer JH, Kreft JH, Jetz W, Mutke J, Barthlott W. Projected impacts of climate change on regional capacities for global plant species richness. Proceedings of the Royal Society B. 2010;277:2271–2280. doi: 10.1098/rspb.2010.0120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenström M, Gugerli F, Henry GHR. Response of Saxifraga oppositifolia L. to simulated climate change at three contrasting latitudes. Global Change Biology. 1997;3:44–54. doi: 10.1111/j.1365-2486.1997.gcb144.x. [DOI] [Google Scholar]
- Sundqvist MK, Björk RG, Molau U. Establishment of boreal forest species in alpine dwarf-shrub heath in subarctic Sweden Mountain birch advance into alpine tundra. Plant Ecology & Diversity. 2008;1:67–75. doi: 10.1080/17550870802273395. [DOI] [Google Scholar]
- Svensson BM, Carlsson BÅ, Karlsson PS, Nordell KO. Comparative long-term demography of three species of Pinguicula. Journal of Ecology. 1993;81:635–645. doi: 10.2307/2261662. [DOI] [Google Scholar]
- Tape K, Sturm M, Racine C. The evidence for shrub expansion in Northern Alaska and the Pan-Arctic. Global Change Biology. 2006;12:686–702. doi: 10.1111/j.1365-2486.2006.01128.x. [DOI] [Google Scholar]
- Tenow, O., H. Bylund, and B. Holmgren. 2001. Impact on mountain birch forests in the past and the future of outbreaks of two geometrid insects. In Nordic Mountain Birch Ecosystems, Man and the Biosphere series, vol. 27, ed. F.E. Wielgolaski, 223–239. New York: The Parthenon Publishing Group.
- Van Bogaert, R., K. Haneca, J. Hoogesteger, C. Jonasson, M. De Dapper, and T.V. Callaghan. 2011. A century of tree line changes in sub-Arctic Sweden show local and regional variability and only a minor role of 20th Century climate warming. Journal of Biogeography. doi:10.1111/j.1365-2699.2010.02453.x.
- Bogaert R, Jonasson C, Dapper M, Callaghan TV. Range expansion of thermophilic Aspen (Populus tremula L.) in the Swedish subarctic. Arctic, Antarctic, and Alpine Research. 2010;42:362–375. doi: 10.1657/1938-4246-42.3.362. [DOI] [Google Scholar]
- Wal R. Do herbivores cause habitat degradation or vegetation state transition? Evidence from the tundra. Oikos. 2006;114:117–186. doi: 10.1111/j.2006.0030-1299.14264.x. [DOI] [Google Scholar]
- Wijk M, Clemmensen KE, Shaver GR, Williams M, Callaghan TV, Chapin FS, III, Cornelissen JHC, Gough L, et al. Long-term ecosystem level experiments at Toolik Lake, Alaska, and at Abisko, Northern Sweden: Generalisations and differences in ecosystem and plant type responses to global change. Global Change Biology. 2003;10:105–123. doi: 10.1111/j.1365-2486.2003.00719.x. [DOI] [Google Scholar]
- Virtanen R, Luoto M, Rämä T, Mikkola K, Hjort J, Grytnes J-A, Birks HJB. Recent vegetation changes at the high-latitude tree line ecotone are controlled by geomorphological disturbance, productivity and diversity. Global Ecology and Biogeography. 2010;19:810–821. doi: 10.1111/j.1466-8238.2010.00570.x. [DOI] [Google Scholar]
- Walker MD, Wahren CH, Hollister RD, Henry GHR, Ahlquist LE, Alatalo JM, Bret-Harte MS, Calef MP, et al. Plant community response to experimental warming across the tundra biome. Proceedings of the National Academy of Sciences. 2006;103:1342–1346. doi: 10.1073/pnas.0503198103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walther BA, Moore JL. The concepts of bias, precision and accuracy, and their use in testing the performance of species richness estimators, with a literature review of estimator performance. Ecography. 2005;28:815–829. doi: 10.1111/j.2005.0906-7590.04112.x. [DOI] [Google Scholar]
- Wielgolaski FE, editor. Plant ecology, herbivory, and human impact in Nordic Mountain Birch Forests. Berlin: Springer; 2005. [Google Scholar]
- Wilson SD, Nilsson C. Arctic and alpine vegetation change over years. Global Change Biology. 2009;15:1676–1684. doi: 10.1111/j.1365-2486.2009.01896.x. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.