Abstract
This study investigated the effects of long-term-enhanced UV-B, and combined UV-B with elevated CO2 on dwarf shrub berry characteristics in a sub-arctic heath community. Germination of Vaccinium myrtillus was enhanced in seeds produced at elevated UV-B, but seed numbers and berry size were unaffected. Elevated UV-B and CO2 stimulated the abundance of V. myrtillus berries, whilst UV-B alone stimulated the berry abundance of V. vitis-idaea and Empetrum hermaphroditum. Enhanced UV-B reduced concentrations of several polyphenolics in V. myrtillus berries, whilst elevated CO2 increased quercetin glycosides in V. myrtillus, and syringetin glycosides and anthocyanins in E. hermaphroditum berries. UV-B × CO2 interactions were found for total anthocyanins, delphinidin-3-hexoside and peonidin-3-pentosidein in V. myrtillus berries but not E. hermaphroditum. Results suggest positive impacts of UV-B on the germination of V. myrtillus and species-specific impacts of UV-B × elevated CO2 on berry abundance and quality. The findings have relevance and implications for human and animal consumers plus seed dispersal and seedling establishment.
Electronic supplementary material
The online version of this article (doi:10.1007/s13280-012-0311-4) contains supplementary material, which is available to authorized users.
Keywords: CO2, Elevated, UV-B, Enhanced, Arctic, Reproduction, Berry, Abundance, Secondary metabolites
Introduction
UV-B radiation is a biologically important component of the solar spectrum and is known to increase when the ozone layer thins (Allen et al. 1998). Ozone depletion events will continue into the future at high latitudes, regardless of the success of the 1987 Montreal Protocol (Manney et al. 2011). In addition to variations in UV-B due to ozone depletion, UV-B fluxes are affected by cloud cover, vegetation canopies, topography and aspect (Paul and Gwynn-Jones 2003). It has also been suggested that future changes in UV-B will occur due to the influence of climate change on cloud cover (McKenzie et al. 2011).
Concern about the ecological consequences of ozone depletion in the late 1980s generated a large number of publications on the effects of enhanced UV-B on terrestrial ecosystems. Over the past two decades these ranged from simple growth room studies of individual plant effects, to outdoor manipulations where UV-B was either screened (Ballaré et al. 2001; Day et al. 2001; Phoenix et al. 2002; Robson et al. 2003; Albert et al. 2011) or artificially enhanced via fluorescent light frames. Outdoor UV-B enhancement experiments were initiated across the globe and utilised a range of lighting systems from simple square wave (on/off) step-wise control to sophisticated temporal and seasonally modulated systems (Björn et al. 1997; Markham et al. 1998; Phoenix et al. 2001; Musil et al. 2002; Caldwell et al. 2003; Rozema et al. 2006 etc.). UV-B enhancement experiments were most relevant to high latitude regions in relation to ozone depletion impacts. However, there has been growing interest in the biological role of UV-B in both the ecological and the agricultural contexts (e.g. Jansen et al. 2008), and continued reporting from ozone depletion and other studies have provided valuable ecological insight into the role of UV-B as a biological factor.
During the early 1990s, it was also appreciated that atmospheric CO2 concentrations were rising and more recently it has been suggested that concentrations will increase from the 2007 level of 379 ppm to between 445 and 710 ppm by 2050 (IPCC 2007). The study described herein was established in this context at Abisko in Northern Sweden and responded to concerns by looking at the interactive effects of enhanced UV-B in combination with elevated CO2. Early UV-B × CO2 studies focussed mainly on short-term plant growth responses in glasshouses (Teramura et al. 1990; Wand et al. 1996; Tosserams et al. 2001; Qaderi and Reid 2005). Recent studies have also investigated food crops and their reproductive physiology in response to UV-B and CO2 (Koti et al. 2005; Qaderi et al. 2007; Singh et al. 2010). In this study, the reproductive characteristics of the dominant dwarf shrubs in a sub-arctic heath were considered in relation to enhanced UV-B and elevated CO2 treatment.
UV-B alone has been studied in the context of seed production and viability (van de Staaij et al. 1997; Wang et al. 2008; Lake et al. 2009) and also as a potential tool to manipulate or beneficially enhance the secondary metabolite chemistry of fruit and vegetables (see review by Jansen et al. 2008). For example, research has been conducted on UV-B impacts on grape berries due to commercial interest in fruit characteristics and resultant wine quality. Solar radiation increased the quercetin glycoside (flavonol) concentration in their skin (Price et al. 1995). Berli et al. (2008) further showed that polyphenolics, anthocyanins and resveratrol were stimulated in grapes when ripened in the presence of solar UV-B, suggesting a significant role for UV-B as a regulator of fruit phenolic chemistry during reproduction.
Studies on the effects of elevated CO2 on plant reproduction are also numerous, and mainly focus on crops or the seeds of forage plants (e.g. Jablonski et al. 2002; Hikosaka et al. 2011). Elevated CO2 has the potential to increase the size of berries, because of increased photosynthesis and more effective carbohydrate accumulation, as described by Wang et al. (2003) in field-grown strawberries. In addition, elevated CO2 in strawberries also stimulated the anthocyanin, flavonoid and phenolic contents of the fruit. This suggests a potential beneficial role for elevated CO2 on fruit production, although Gonçalves et al. (2009) found no effects of elevated CO2 on grape anthocyanin and tannin content in red wine vines.
Where elevated UV-B and CO2 are studied together they have the capacity to interact affecting reproductive characteristics. For example, Wand et al. (1996) studied the short-term effects of UV-B and elevated CO2 on the reproduction of the winter ephemeral forb, Dimorphotheca pluvialis. Reproductive phenology was delayed and reproductive biomass reduced at elevated CO2. However, UV-B stimulated reproduction significantly at ambient CO2 but not at elevated CO2. Qaderi et al. (2007) suggested that UV-B was detrimental to siliquas and seeds, but that some of these negative effects were ameliorated by elevated CO2. Schultz (2000) reviewed the effects of enhanced UV-B radiation (in conjunction with separate elevated CO2 experiments) on grape varieties. He found that UV-B increased levels of flavonoids, amino acids and carotenoid pigments in fruit skins. Singh et al. (2010) completed further study on how multiple stresses (including enhanced UV-B) affected the tropical legume Vigna unguiculata (cowpea), showing that UV-B (in combination with elevated temperatures) caused the greatest negative impact on the reproductive capacity of this species, and that genotype dependent responses interacted with elevated CO2.
The only study that has looked at the simultaneous effects of elevated CO2 and enhanced UV-B on fruit in natural communities was conducted at the same experimental site used in the current study (Gwynn-Jones et al. 1997). Three years’ exposure to elevated CO2 had no effects on berry production. However, enhanced UV-B stimulated Vaccinium myrtillus berry abundance (expressed per m−2). Phoenix et al. (2001) also observed a stimulation of flower abundance, berry abundance and total berry fresh weight (expressed per m−2) in the dwarf shrub V. myrtillus with UV-B. They also highlighted large inter-annual variation and found significant responses to UV-B in particular years. For the competing species within this community, Empetrum hermaphroditum, no significant impacts of UV-B (Gwynn-Jones et al. 1997; Phoenix et al. 2001) and CO2 (Gwynn-Jones et al. 1997) were apparent on fruit abundance.
Dwarf shrub berries are particularly valued by the human populations at Northern latitudes as an autumn harvest, but are also consumed by a wide range of animals (Anderson 1985). From a human perspective, the fruit contain high concentrations of flavonoids and anthocyanins (Heinonen et al. 1998; Faria et al. 2005; Heinonen 2007)—which can scavenge cancer-causing free-radicals (Martin-Aragón et al. 1998; Taruscio et al. 2004) and reduce the oxidative stress caused by these compounds in animals (Johnson and Felton 2001). There is therefore commercial interest in the chemistry of such berries, particularly if they have potential health benefits to both humans and animals via polyphenolic antioxidants (Meyer et al. 1997; Jansen et al. 2008). For example, there is already laboratory evidence suggesting that the consumption of V. myrtillus berry flavonoids by small mammals can increase the antioxidant capacity of their blood plasma which could promote their fitness (Talavera et al. 2006).
This study investigated the long-term effects (15 years treatment) of enhanced UV-B on seed germination of V. myrtillus L. (bilberry) and E. hermaphroditum L. (mountain crowberry) in seeds collected in 2006. This was followed by an investigation of long-term (17 years)-enhanced UV-B combined with elevated CO2 treatment on berry size, quality and abundance (expressed per m−2) of the dominant dwarf shrub species in 2009 [V. myrtillus, E. hermaphroditum, V. vitis-idaea L. (lingonberry)]. Data collection for some elements was practicable only in specific years when there were sufficient berries across the two replicated experiments to permit valid analyses. Based on previous study (Gwynn-Jones et al. 1997), it was hypothesised that elevated CO2 would not affect berry size and abundance. However, UV-B was expected to stimulate berry abundance but not berry size. Enhanced UV-B exposure of plants was also expected to produce seeds that have lower germination success. Both the UV-B and the elevated CO2 were hypothesised to stimulate berry tissue polyphenolic compounds. The ecological implications of the results are considered in relation to fruit consumption and seed dispersal by animals.
Materials and Methods
Experimental Site and Treatments
Experiments simulating enhanced UV-B and elevated CO2 were conducted at Abisko Scientific Research Station in Northern Sweden (68.35°N, 18.82°E). The vegetation studied was sub-arctic Empetrum-Vaccinium myrtillus forest heath community as described by Sonesson and Lundberg (1974). For this study, data collection was dictated by the availability of replicable samples across the two experiments, we employed: (1) an experiment with an enhanced UV-B treatment only and (2) an experiment with combined UV-B and elevated CO2 treatments.
Enhanced UV-B Treatment (Experiment 1)
Vacciniummyrtillus and E. hermaphroditum berries were collected in 2006 to investigate seed germination characteristics from an experiment following 15 years of enhanced UV-B treatment (1991–2006). Vegetation was exposed to enhanced UV-B treatment simulating ~15 % ozone depletion conditions under cloudless skies (see Johanson et al. 1995a, b). Six fluorescent tubes (Q-PANEL UVB-313, Cleveland, OH, USA) housed in bespoke lamp holders were suspended above vegetation by aluminium frames (2.5 × 1.3 × 1.5-m high). Four of the frames represented a control and maintained vegetation under ambient UV-B conditions using window glass to cover the UV-B lamps and absorb all radiation <320 nm. Four treatment frames exposed vegetation to enhanced UV-B conditions with lamps filtered with UV transparent Perspex and cellulose diacetate filters to absorb radiation <280 nm but allow UV-B exposure (see Johanson et al. 1995a, b for full experimental details). Treatments started during snowmelt (late April 1991) and ran until mid-September of every growing season. Lamps were connected to timers providing square wave enhancement of UV-B, which was seasonally adjusted (every 2 weeks). For a summary of site climatic data (temperature, precipitation and photosynthetically active radiation) from 1991 to 2009, see supplementary data provided (Tables S1, S2 and S3).
Enhanced UV-B and Elevated CO2 Treatment (Experiment 2)
We separately assessed berry numbers, size and quality from a further experiment investigating looking at enhanced UV-B effects in combination with elevated CO2 (1993–2009; i.e. Ambient conditions; +UV-B; +CO2; +UV-B +CO2, n = 4). In combination with UV-B treatments (see above for details), 16 open-top chambers (OTCs) made of UV transmitting Perspex (0.85-m diameter, 0.5-m height and vegetation area 0.73 m2) were placed on ambient and enhanced UV-B plots. The OTCs used for this study also elevated air temperature by 1 °C as described by Gwynn-Jones et al. (1997) (details of local climatological conditions are provided as supplementary data, Tables S1, S2 and S3).
The vegetation within these OTCs was exposed to either ambient air or air enriched with CO2 to elevated atmospheric levels of 600 ppm, against an ambient level of 360–386 ppm (Gwynn-Jones et al. 1997). Examples of CO2 concentration measurements within the elevated CO2 plots are provided as supplementary data (see Fig. S1).
Assessing Seed Germination Responses to UV-B Treatment (from Experiment 1)
A representative and spatially randomised field sample (~25 g) of mature E. hermaphroditum and V. myrtillus berries were harvested from the ambient and enhanced UV-B treatment plots (n = 4) from the 1st to the 11th of August 2006. The berry samples were immediately air dried at room temperature for 72 h and transferred to air-tight sealed containers for transport to the UK. On arrival, the berries were re-hydrated in distilled water for 36 h. Seeds were extracted from berry mesocarp using fine tweezers and transferred to a Petri dish which contained a moistened disc of filter paper, maintained at saturation with distilled water. Fifty seeds obtained from several berries were distributed evenly on each filter paper disc. The procedure was repeated so that six sub-replicate Petri dish germination vessels were generated per experimental treatment plot. The germination vessels were then sealed in zip-lock bags to prevent moisture loss and transferred to a controlled temperature facility to facilitate seed stratification.
During the period of warm seed stratification required for E. hermaphroditum germination (Baskin et al. 2002) fungal growth problems arose, so this part of the study was discontinued. The germination of V. myrtillus required only a 20-week period of cold stratification (~0 °C) (Baskin and Baskin 1998) and this species remained unaffected by fungus. Following stratification, the germination vessels were removed from their zip-lock bags, which allowed air to circulate within them, and transferred to a controlled environment chamber for a germination trial that ran until >25 % of seeds had germinated in all treatments (54 days). The controlled environment chamber provided a diurnal cycle of 14 h of 190 μmol m−2 s−1 light at 20 °C and 10 h in darkness at 10 °C.
The filter papers were kept moist throughout with periodic additions of distilled water. Every 4 days during the trial, seeds were examined under magnification to observe germination incidence and to check for any potential fungal development.
Assessing Berry Abundance and Size in Response to UV-B and CO2 (from Experiment 2)
Total berry number for each species (including V. vitis-idaea) was counted within each plot (ripe and unripe). Percentage species cover of the dominant dwarf shrubs (V. myrtillus, E. hermaphroditum, V. vitis-idaea) was measured in order to correct berry abundance data for species abundance; abundance data were presented as number of berries per m2 cover of the species assessed. Cover was determined using a quadrat frame (0.5 × 0.5 m) placed within the chambers and the individual % cover from 100 squares within the quadrat was determined by eye. Total % cover was determined as the sum of all values from the 100 squares assessed.
Vacciniummyrtillus and E. hermaphroditum ripe berries were also collected (27th July–9th Aug 2009) to determine berry size. Six berries per plot were collected and measured for diameter by passing them through a Perspex ruler with precision drilled holes (4.5–10.5 mm at 0.5-mm interval).
Further ripe berries were also freeze dried at −50 °C for 48 h (MODULYOD 230, Thermo Electron Corporation, Holbrook, USA) for tissue quality analyses. For this element experimental samples were collected over a 2-week timescale to reduce temporal variation in fruit quality. We selected a short harvesting period as the stage of berry development is important for consistency in chemical analyses, as has been shown in other studies on red berries (e.g. Macheix et al. 1990). Phenolic compounds, for example, usually accumulate in berries as they mature (Heinonen et al. 1998). In our study, we only sampled ripe berries, determined by colour, at the same developmental stage (when berry colour was fully established) for chemical analysis. Using colour reduced sampling variation but it should be emphasised that there were no visible differences in berry appearance or size according to treatments. We acknowledge that some berries will have ripened after the sampling period used. For our third species (V. vitis-idaea) this 2-week time period was too short to collect sufficient berries of similar ripeness (based on colour) from across the experimental site, hence this species was not studied in detail.
Freeze-dried berries were next dissected and their seeds were extracted and counted, the remaining exocarp (skin) and mesocarp together (hereafter collectively described as ‘berry tissue’) were subsequently assessed for methanol soluble polyphenolic content. For all species studied, protocols were developed from previous study at the same site (Gwynn-Jones et al. 1997; Phoenix et al. 2001). In agreement with this previous study the experimental treatments did not appear to affect plant phenology, berry development or ripening.
Berry Tissue Polyphenols
A broad range of polyphenols were studied to provide an insight into secondary metabolite responses to the treatments. The extraction of phenolics from berry tissue of V. myrtillus and E. hermaphroditum was conducted using the same berry samples as the seed number and berry size measurements. A bulked (plot basis) 100–200 mg sample of de-seeded berry tissue (exocarp plus mesocarp) was weighed, then extracted for phenolic compounds by grinding in 2 ml 70 % aqueous methanol for 2 min per sample and stored at 5 °C. Extract was centrifuged for 5 min at 14.5 k rpm and the supernatant collected with sediment pellets rinsed with 0.5 ml of 70 % aqueous methanol, centrifuged at 14.5 k rpm and supernatant pooled. This was heated at 80 °C for 10 min to denature oxidising enzymes. Methanol was removed by vacuum centrifugation with application of heat.
Evaporated solute was made up to 1 ml in water and centrifuged for 5 min at 14.5 k rpm and samples (500 μl) were partially purified with Waters Sep-PAK (500 mg) C18 reverse-phase cartridges, dried down under air and re-suspended in 500 μl 50 % aqueous methanol. Hydroxycinnamic acids, anthocyanins and flavonols were analysed by reverse-phase HPLC with an on-line photodiode array detector (PDA). The HPLC system used consisted of a WATERS 717 plus Autosampler, WATERS 515 HPLC pump, WATERS 996 Photodiode Array Detector and a PC with Empower software. Phenols were separated on a Waters C18 reversed-phase Nova-Pak cartridge (4.0 mm, 8.0 × 100 mm). Mobile phase A consisted of purified water–acetic acid (95:5, v/v) and mobile phase B of HPLC grade methanol. Initial conditions were A:B (100:0, v/v) and the percentage of mobile phase B was increased linearly to 100 % over 50 min with a flow rate of 2 ml min−1. The sample injection volume was 60 μl. UV and light absorption was monitored over the wavelength range of 240–600 nm, to enable identification of anthocyanins with characteristic absorption profiles in the 500–550 nm range. Compounds were quantified by integrating the areas underneath the curve for absorbance at 340 nm for hydroxycinnamates and flavonols and 280 nm for anthocyanins. Peaks associated with individual compounds were distinguished according to retention times (Rt) and peak maxima (nm), and were quantified against standards.
The area under the curve of peaks for hydroxycinnamates, flavonols and anthocyanins were converted to chlorogenic/coumaric acid, quercetin and peonidin equivalents (μg per peak), respectively, using appropriate response factors (2.65 × 10−6 for chlorogenic at 340 nm, 7 × 10−7 for coumaric acid at 340 nm, 2.09 × 10−6 for quercetin at 340 nm and 1.8 × 10−6 cyanidin at 280 nm).
Compounds were identified by HPLC/MSn with a LC–MS system (Thermo Electron Corporation, Waltham, USA) comprising a Finnigan Surveyor PDA Plus detector, a Finnigan LTQ linear ion trap with ESI source and a Waters C18 reversed-phase Nova-Pak column (4 μm, 3.9 × 100 mm). The auto-sampler tray temperature was kept at 5 °C and the column temperature was maintained at 30 °C. Sample injection volume was 10 μl, the detection wavelength was set at 240–600 nm and the flow rate was 1 ml min−1, with 100 μl min−1 going to the mass spectrometer. The mobile phase consisted of purified water–formic acid (A; 100:0.1, v/v) and HPLC grade MeOH–formic acid (B; 100:0.1, v/v). The initial condition was A: B (5:95, v/v), and the percentage of B increased linearly to 50 % over 45 min.
Mass spectra were acquired in negative ionisation mode. Ionisation parameters were optimised by infusion of chlorogenic acid standard at a constant rate into the LC flow. Interface and MSD parameters were as follows: sheath gas 30 au, auxiliary gas 15 U, spray voltage 4 kV, capillary temperature 320 °C, capillary voltage −1 V and tube lens offset −68 V. Initial MS/MS fragmentation was carried out at normalised collision energy 35 % and isolation width 2.0 (m/z). Compounds were identified by comparison with their molecular mass and with fragmentation patterns of commercial standards for phenolic acids, flavonol aglycones and anthocyanins obtained from Sigma-Aldrich, UK and PhytoLab, Vestenbergsgreuth, Germany.
Statistical Analyses
Seed germination responses were derived from Petri dish germination vessels, which were sub-replicated six times for each of the eight experimental plots. Data were averaged to obtain a single mean value for each plot at each observation time point and were recorded at 4-day intervals over 36 days from the initial point of germination giving 11 time points overall. This yielded separate data for (1) germination incidence (as a running total for each time point during the trial) and (2) germination rate over time (expressed as the number of new seedlings germinating per day). Daily germination rates were obtained from counts of newly germinated seedlings at each observation point, divided according to the period between observations. All data were tested for experimental treatment effects by repeated measures analysis in a generalised linear mixed effects model (GLMM) using the statistical programme R v2.12.2 (R Development Core Team 2011). The model was Poisson corrected to account for the distribution of the data. Sampling time point and plot were nested, respectively, as mixed (random) effects within the model.
Berry abundance analyses of V. myrtillus, E. hermaphroditum and V. vitis-idaea were conducted using the statistical programme R v2.12.2 (R Development Core Team 2011). Main factor effects and interaction (UV-B × CO2) were determined using a generalised linear model (GLM) or GLMM, with species percentage cover defined as a random (uncontrolled) experimental variable within the model for berry abundances. Data were logarithmically transformed where necessary to gain homogeneity of variances and the model then tested for the treatment effects of UV-B or CO2 on berry number. Probability plots using the Kolmogorov–Smirnov normality test of each vegetation type were conducted and the area (% cover) of the species considered to ensure that they were not affected by treatment. Differences at P < 0.05 level were considered significant.
Berry tissue polyphenolic content was analysed using a nested ANOVA to partition variability both within and between the replicate plots, using Minitab version 14 (Minitab Ltd., Coventry, UK). However, seed number and HPLC polyphenolic data for statistical analyses were assessed using a mean value basis per plot. Data were first assessed using the Kolmogorov–Smirnov normality test to test for deviation from normal distribution and tests for equality of variances were conducted before using balanced ANOVAs (Minitab Ltd., Coventry, UK).
Results
V. Myrtillus Seed Germination in Response to UV-B
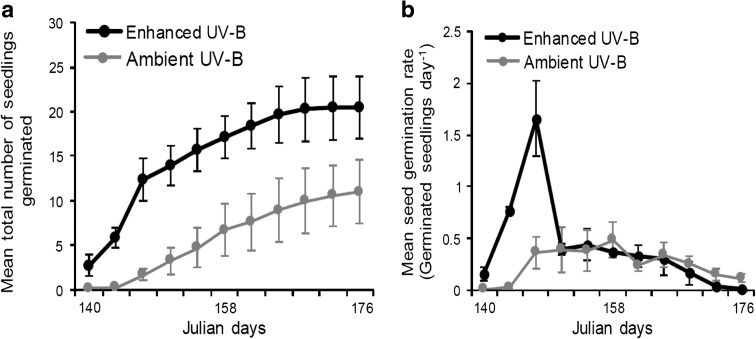
Enhanced UV-B had a significant positive effect on V. myrtillus seed germination. Seeds from the UV-B treatment germinated earlier and more rapidly, with twice the abundance overall than those from the ambient UV-B treatment (Fig. 1). The repeated measures analysis of these data by GLMM determined that over the trial period the enhanced UV-B treatment exerted a highly significant effect on the running total for germination success (P < 0.001) and a significant effect on germination rate (P = 0.02) (Table 1), as calculated at 4-day intervals when observations were made of newly emerging seedlings. The reduced significance for germination rate is likely to derive from the high initial rate of germination in seeds from the UV-B treatments (Fig. 1). Owing to high initial germination rates, proportionally fewer un-germinated seeds from the UV-B treatment remained during the rest of the trial period, such that germination rates inevitably declined to levels similar to those of the ambient UV-B treatment.
Fig. 1.
Time-series line plots showing V. myrtillus germination responses over 36 days, according to experimental UV-B treatment (ambient UV-B versus elevated UV-B): a mean total number of germinated seedlings per treatment, b mean daily seed germination rate per treatment
Table 1.
Summary of GLMM repeated measures analyses on time-series analyses for V. myrtillus germination at 11 time points during 54 days of experimentation
| Model | AIC | f | P | dfd | dfn |
|---|---|---|---|---|---|
| V. myrtillus total germination success | |||||
| UV-B treatment | 558.2 | 71.6 | *** | 1 | 75 |
| V. myrtillus daily germination rate | |||||
| UV-B treatment | 90.2 | 5.4 | * | 1 | 75 |
Experimental responses were tested according to UV-B treatment (ambient UV-B versus elevated UV-B). Akaike information criterion (AIC), degrees of freedom for numerator and denominator (dfn, dfd), f and P values for significance given: *** <0.001, * <0.05
Enhanced UV-B and Elevated CO2 Impacts on Berry Number, Diameter and Seed Size
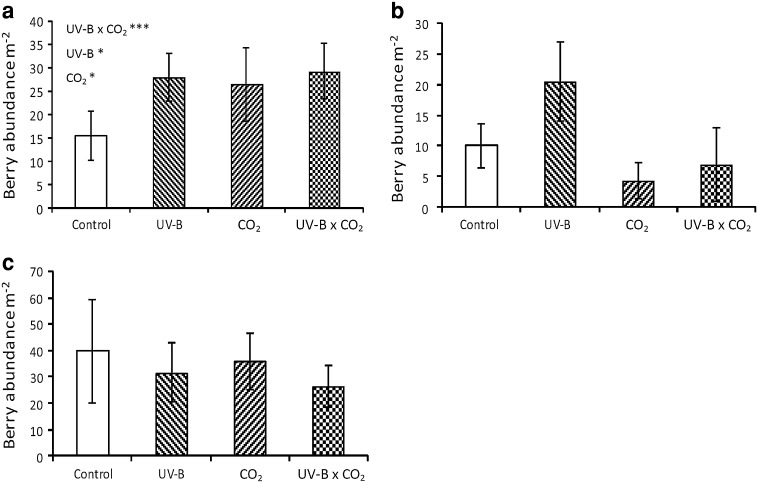
Vacciniummyrtillus berry abundance was significantly increased by both the elevated CO2 and the enhanced UV-B; however, the combined treatments had no greater effect than either treatment in isolation (Fig. 2a; Table 2). UV-B alone promoted berry abundance in the related species V. vitis-idaea and in E. hermaphroditum, but no effects of elevated CO2 nor interactions were observed (Fig. 2b, c; Table 2). No significant treatment effects were observed for berry diameter or seed number in any of the species studied (Table 3).
Fig. 2.
Berry abundance responses during August 2009 to community experimental treatment [enhanced UV-B, elevated CO2, UV-B and CO2 against ambient (control) conditions] for 17 field seasons. Species shown are: aV. myrtillus, bV. vitis-idaea and cE. hermaphroditum. Mean and standard error given according to experimental treatment. Statistical significance: * P < 0.05, *** P < 0.001. See Table 2 for further statistical information
Table 2.
Responses of species (V. myrtillus, E. hermaphroditum and V. vitis-idaea) 2009 berry abundance to community experimental treatment [enhanced UV-B, elevated CO2, UV-B and CO2 against ambient (control) conditions] over 17 field seasons
| Model | Log-likelihood | z | AIC | P | dfd | dfn |
|---|---|---|---|---|---|---|
| V. myrtillus berry abundance | ||||||
| UV-B × CO2 | −54.8 | −4.9 | 119.6 | *** | 12 | 15 |
| UV-B | −70.2 | 2.2 | 146.5 | * | 14 | 15 |
| CO2 | −70.0 | −2.4 | 146.0 | * | 12 | 15 |
| E. hermaphroditum berry abundance | ||||||
| UV-B × CO2 | −63.1 | 0.9 | 136.2 | n/s | 12 | 15 |
| UV-B | −63.5 | −2.4 | 133.1 | n/s | 14 | 15 |
| CO2 | −64.7 | 1.9 | 135.4 | n/s | 12 | 15 |
| V. vitis-idaea berry abundance | ||||||
| UV-B × CO2 | −32.5 | 1.0 | 75.0 | n/s | 8 | 15 |
| UV-B | −36.4 | 3.12 | 78.7 | ** | 12 | 15 |
| CO2 | −41.5 | −0.1 | 89.1 | n/s | 14 | 15 |
Statistical significance of response variable according to Poisson-corrected GLMM, with individual species percentage cover defined as a random experimental variable. Log-likelihood, Akaike information criterion (AIC), degrees of freedom for numerator and denominator (dfn, dfd), z and P values given. Significant P values in the table are highlighted in bold, according to * P < 0.05, ** P < 0.01, *** P < 0.001 levels of statistical significance
Table 3.
Diameters and seed numbers of V. myrtillus and E. hermaphroditum berries collected from plants grown in a community at enhanced UV-B, elevated CO2, UV-B and CO2 plus ambient (control) conditions following 17 field seasons of exposure
| Growth measures | Control | UV-B | CO2 | UV-B/CO2 | UV-B effect | CO2 effect | UV-B × CO2 interaction | Plot | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F | P | F | P | F | P | F | P | |||||
| V. myrtillus | ||||||||||||
| Berry diameter (mm) | 7.9 ± 0.2 | 8.6 ± 0.2 | 7.8 ± 0.1 | 8.1 ± 0.2 | 3.03 | 0.107 | 1.42 | 0.256 | 0.41 | 0.533 | 3.49 | *** |
| Seed number | 53.9 ± 9.9 | 63.3 ± 6.2 | 56.4 ± 12.4 | 52.6 ± 10.9 | 0.08 | 0.787 | 0.16 | 0.692 | 0.42 | 0.531 | ||
| E. hermaphroditum | ||||||||||||
| Berry diameter (mm) | 7.2 ± 0.3 | 7.5 ± 0.2 | 7.7 ± 0.2 | 7.4 ± 0.2 | 0.00 | 0.989 | 0.16 | 0.696 | 0.84 | 0.378 | 3.33 | *** |
| Seed number | 6.7 ± 0.3 | 7.1 ± 0.3 | 6.9 ± 0.2 | 6.9 ± 0.6 | 0.24 | 0.634 | 0.00 | 0.968 | 0.17 | 0.688 | ||
Samples were collected in August 2009 and show mean ± standard error of the mean (SEM) showing variation within the data set. Significant P values in the table are highlighted in bold, according to *** P < 0.001 level of statistical significance. Significant plot effects are evident for both the species
Berry Tissue Polyphenolic Content
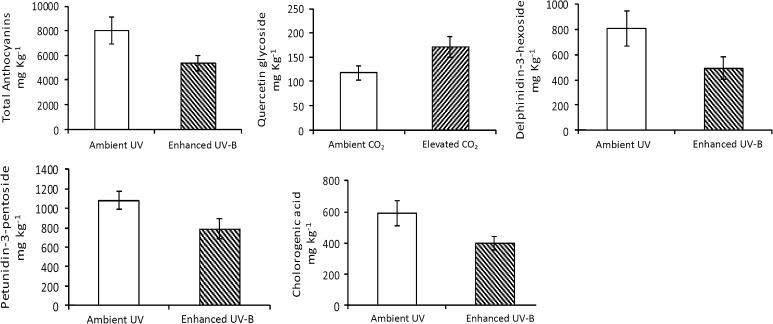
There was a consistent and significant negative effect of UV-B on the quantity of polyphenolics in the berry tissue of V. myrtillus (Fig. 3; Table 4). Total anthocyanin, chlorogenic acid, delphinidin-3-hexoside and petunidin-3-pentoside were all reduced at enhanced UV-B. The only effect of elevated CO2 for this species was an increase in quercetin glycosides. There were also UV × CO2 interactive effects apparent for the anthocyanins delphinidin-3-hexoside and petunidin-3-pentoside (Table 4).
Fig. 3.
UV-B or CO2 treatment effects on flavonoid concentrations for V. myrtillus berries collected in August 2009 following community experimental treatment [enhanced UV-B, elevated CO2, UV-B and CO2 against an ambient (control) conditions] following 17 field seasons. Mean and standard error given according to the experimental treatment. See Table 4 for further statistical information
Table 4.
Responses of V. myrtillus berry tissue flavonoid concentration to plant community experimental treatment [enhanced UV-B, elevated CO2 conditions, UV-B and CO2 against ambient (control) conditions] following 17 field seasons
| Effect | dfd | Total Anthocyanins | Chlorogenic acid | Quercetin glycosides | Delphinidin-3-hexoside | Cyanidin-3-hexoside | Petunidin-3-pentoside | Peonidin-3-pentosidein | Malvidin-3 hexoside/pentoside | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F | P | F | P | F | P | F | P | F | P | F | P | F | P | F | P | ||
| UV-B | 14 | 4.91 | 0.04* | 5.22 | 0.04* | 1.27 | 0.28 | 5.25 | 0.04* | 0.13 | 0.72 | 6.01 | 0.03* | 2.49 | 0.13 | 0.51 | 0.49 |
| CO2 | 14 | 1.47 | 0.25 | 1.24 | 0.28 | 4.95 | 0.04* | 4.05 | 0.07 | 0.62 | 0.44 | 0.55 | 0.47 | 0.10 | 0.76 | 0.36 | 0.56 |
| UV-B × CO2 | 12 | 2.31 | 0.15 | 0.06 | 0.80 | 1.09 | 0.32 | 6.42 | 0.03* | 4.05 | 0.06 | 3.43 | 0.08 | 5.62 | 0.04* | 3.27 | 0.09 |
Statistical significance of log-transformed response variable data according to GLM, with denominator degrees of freedom (dfd), F and P values given. Significant P values in the table are highlighted in bold, according to * P < 0.05 level of statistical significance
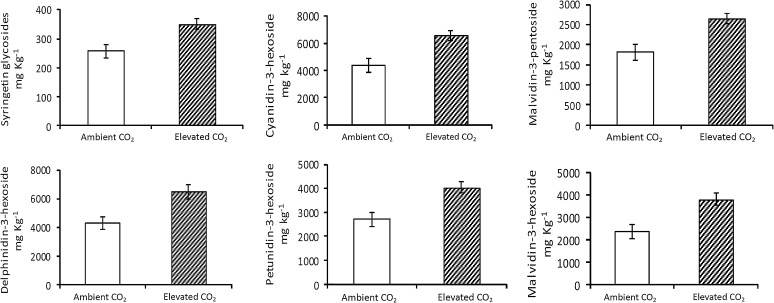
In E. hermaphroditum, there was a significant positive effect of elevated CO2 on syringetin glycosides and the five measured anthocyanins (delphinidin -3-hexoside, cyanidin-3-hexoside, petunidin-3-hexoside, malvidin-3-pentoside, malvidin-3-hexoside, Fig. 4; Table 5). However, when all data were combined there was no significant (P > 0.05) effect on total anthocyanin content (Table 5). There were also no further significant treatment effects (UV-B) or interactions (UV-B × CO2) on the berry tissue of E. hermaphroditum.
Fig. 4.
Significant CO2 treatment effects on flavonoid concentrations for E. hermaphroditum berries in August 2009 following community experimental treatment [enhanced UV-B, elevated CO2, UV-B and CO2 against an ambient (control) conditions] following 17 field seasons. Mean and standard error given according to the experimental treatment. No data are present for UV-B effects as no significant impacts (P > 0.05) were observed. See Table 5 for further statistical information
Table 5.
Responses of E. hermaphroditum berry tissue flavonoid concentration to community experimental treatment [enhanced UV-B, elevated CO2 conditions, UV-B and CO2 against an ambient (control) conditions] following 17 field seasons
| Effect | dfd | Total anthocyanin | P-Coumaroyl quinicacid | Syringetin glycosides | Delphinidin-3-hexoside | Cyanidin-3-hexoside | Petunidin-3-hexoside | Malvidin-3-pentoside | Malvidin-3-hexoside | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F | P | F | P | F | P | F | P | F | P | F | P | F | P | F | P | ||
| UV-B | 14 | 0.09 | 0.77 | 0.84 | 0.78 | 2.57 | 0.13 | 0.55 | 0.47 | 0.98 | 0.34 | 1.87 | 0.19 | 2.34 | 0.15 | 1.41 | 0.25 |
| CO2 | 14 | 0.24 | 0.63 | 1.67 | 0.21 | 9.09 | <0.01** | 10.6 | <0.01** | 11.5 | <0.01** | 11.3 | <0.01** | 10.4 | <0.01** | 9.53 | <0.01** |
| UV-B × CO2 | 12 | 1.48 | 0.25 | 0.00 | 0.99 | 1.67 | 0.22 | 0.40 | 0.53 | 1.74 | 0.22 | 0.60 | 0.45 | 0.77 | 0.40 | 0.38 | 0.55 |
Statistical significance of log-transformed response variable data according to GLM, with denominator degrees of freedom (dfd), F and P values given. Significant P values in the table are highlighted in bold, according to ** P < 0.01 level of statistical significance
Discussion
The berries of the dwarf shrub species studied are widely distributed at high latitudes in the Northern hemisphere and valued from a human perspective (Anderson 1985; Heinonen 2007). They also represent an important food source for animals and birds, and are a very rich source of phenolic antioxidants (Prior et al. 1998), especially anthocyanins (Heinonen 2007), which provide nutritional benefit to both humans and animals. Although mainly clonal in growth, these species can effectively reproduce via seed (Szmidt et al. 2002) and dispersal is achieved via consumption by bears, foxes, small mammals and bird consumers. Ptarmigan and grouse in particular facilitate seedling recruitment into disturbed areas (Ritchie 1956; Tybirk et al. 2000).
Seed Germination Responses to UV-B
This study initially examined the effects of enhanced UV-B on seed germination of the dominant dwarf shrub species V. myrtillus. The results indicated that UV-B had a strongly positive effect on germination, in terms of both the rate and the overall success (Fig. 1; Table 1). Graae et al. (2011) suggest that V. myrtillus seeds already germinate effectively under natural conditions and data herein suggest that UV-B exposure of the parent could further promote germination success. Positive effects of UV-B on reproductive output have already been reported in the literature including increased berry production of sub-arctic heath vegetation (Gwynn-Jones et al. 1997; Phoenix et al. 2001); positive effects on seed production in a Mediterranean shrub species (Stephanou and Manetas 1998); promotion of reproductive development with ambient UV in two Antarctic vascular plant species (Day et al. 1999, 2001); positive effects of UV on seed production and germination of an European alpine forb (van de Staaij et al. 1997). The mechanisms responsible for this wide range of positive reproductive responses to UV-B are unclear. However, increased germination success in this study may derive from investment in reproductive resources, which may facilitate the survival of a species under stressful conditions (Harper and Ogden 1970).
Although reproductive output has been considered in response to UV-B, there has been limited work to date examining the effects of enhanced parental UV-B exposure on seed germination and seedling viability, particularly in species from natural ecosystems. A study on the Mediterranean shrub Cistus creticus, found seed production was increased by enhanced UV-B (Stephanou and Manetas 1998) but there was no effect on germination. Germination rates were reduced by UV-B during a similar study on the perennial South African forb D. sinuata (Musil 1996). Oilseed rape was investigated by Demchik and Day (1996) who found that parental UV-B exposure detrimentally affected seed germination, but only under varying degrees of UV-B exposure during the germination period, which may have directly inhibited germination.
Although our experiments did not directly expose the seeds to UV-B during germination (as is likely to happen under field conditions), the findings nevertheless demonstrate investment by V. myrtillus to seed fertility, which is attributable to UV-B exposure. This apparently positive effect could potentially be counteracted under field conditions, because direct UV-B exposure may inhibit germination (Sullivan and Teramura 1988).
The results shown represent the first evidence of a change in the normal reproductive functioning of V. myrtillus at the seed level due to UV-B exposure (Fig. 1; Table 1). This deciduous dwarf shrub species appears better adapted than evergreen competitors to exploit seedling dispersal as an adaptive reproductive strategy (Tolvalen and Laine 1997). Therefore, this as an adaptive response mechanism in V. myrtillus to UV-B underlines the ecological significance of these findings for competitive dynamics within the sub-arctic heath community. However, further ecological factors including the availability of suitable disturbed patches (see Graae et al. 2011) may dictate the success of seedling establishment.
Enhanced UV-B and Elevated CO2 Impacts on Berry Abundance, Size and Tissue Quality
The long-term treatment effects of enhanced UV-B and elevated CO2 on the berry reproductive characteristics and associated polyphenolics were also studied. Data collection focussed on berries collected from a single season (2009) when berry numbers were consistently high. Large inter-annual variation of berry production is recognised in this system (Phoenix et al. 2001). The berry abundances of the dwarf shrub species in this study were also lower than the values presented by Phoenix et al. (2001) for previous years at the same point. Furthermore, data could not be collected in the subsequent seasons 2010 and 2011 as there were insufficient berries within all plots for fully replicated analyses.
In line with previous study on the same species (Gwynn-Jones et al. 1997; Phoenix et al. 2001) and other species in the Mediterranean (Stephanou and Manetas 1998), flower/berry abundance was stimulated by enhanced UV-B in both of the Vaccinium species studied (Fig. 2; Table 2). This response also occurred with E. hermaphroditum, a finding not reported in prior studies. Such dwarf shrub species predominantly propagate by clonal growth (Eriksson 1989). However, under stressful conditions resource allocation prioritises species/genotype survival by promoting sexual reproduction (Zvereva and Kozlov 2005). Hence, long-term UV-B stress impacts on Vaccinium species and E. hermaphroditum may be inducing berry production in a similar way but the underlying mechanism is unknown.
Vacciniummyrtillus berry abundance was also higher at elevated CO2 (Fig. 2; Table 2), which agrees with previous study on strawberries (Wang et al. 2003). However, in this study, the response is considered to be via the effects of elevated CO2 on resource availability rather than a stimulation of flower production. Berries represent a local sink for carbohydrates, and berry abortion, at least in grapes, is determined by photoassimilate availability (Caspari et al. 1998). Previous elevated CO2 research has suggested that plants can maintain higher rates of photosynthesis in the longer term at elevated CO2 but the additional photoassimilates do not often manifest in increased vegetative growth (Körner 2006). Reproductive sinks therefore represent an obvious destination for any additional photoassimilates produced at elevated CO2.
No effects of elevated CO2 were observed on the berry abundance of the competing species V. vitis-idaea and E. hermaphroditum (Fig. 2; Table 2), which is in line with previous research (Gwynn-Jones et al. 1997). Both these species are evergreen and this may explain why they respond differently to elevated CO2 compared to the deciduous species V. myrtillus. Evergreen species such as E. hermaphroditum are slow growing but very competitive species (Tybirk et al. 2000). They favour asexual vegetative layering for expansion but can produce large numbers of berries (Szmidt et al. 2002). Shevtsova et al. (1995) further noted that only the deciduous species V. myrtillus was sensitive to climate in terms of sexual reproduction.
Changes in dwarf shrub berry abundance in response to UV-B or CO2 were not reflected by changes in size. Berry diameter and mean number of seeds per berry for V. myrtillus and E. hermaphroditum were unaffected by treatment (Table 3). These findings differ from those of laboratory based studies on seeds where UV-B reduced seed size and numbers (e.g. Lake et al. 2009; Singh et al. 2010).
UV-B is known to induce photoprotective responses in plants, with increases in leaf flavonoids being the commonest effect reported in meta-analyses (Searles et al. 2001; Newsham and Robinson 2009). However, past study on sub-arctic dwarf shrub vegetation suggests that enhanced UV-B did not stimulate the antioxidant capacity of the leaves (Taulavuori et al. 1998). This study was the first to investigate berry polyphenolic responses to enhanced UV-B and elevated CO2. For V. myrtillus only, UV-B significantly reduced berry concentrations of total anthocyanins, delphinidin-3-hexoside and petunidin-3-pentoside and chlorogenic acid at enhanced UV-B, whilst there were no significant effects (P > 0.05) on all other compounds studied (Fig. 3; Table 4). This contrasts with previous study that has shown a stimulation of tomato polyphenolics in response to UV-B (Giuntini et al. 2008). Our study therefore represents the first study to date that highlights negative UV-B impacts on fruit tissue polyphenolics. We emphasize that these findings were not the consequence of any sampling bias as berry collection was randomised across treatments, and berries were also not visibly different in size or colour.
Higher levels of polyphenolics in dwarf shrub berry tissue may be expected at elevated CO2, based on previous study on field-grown strawberries showing higher phenolic acids, flavonoids, anthocyanins and antioxidant capacity (Wang et al. 2003). Increased photoassimilate supply could stimulate more essential polyphenolics (Peñuelas and Estiarte 1998; Gonçalves et al. 2009) Results for E. hermaphroditum agree with this hypothesis, showing higher levels of syringetin glycosides and the five characterised anthocyanins (Fig. 4; Table 5; delphinidin-3-hexoside, cyanidin-3-hexoside, petunidin-3-hexoside, malvidin-3-pentoside, malvidin-3-hexoside). By contrast, effects of elevated CO2 on V. myrtillus were limited with only an increase in quercetin glycoside observed but this particular response was in agreement with Wang et al. (2003) working on strawberries. Hence overall, consumers of E. hermaphroditum may gain higher antioxidant intake at elevated CO2, whilst UV-B reduces the polyphenolic content of V. myrtillus.
Conclusion
This study has shown important species-specific responses to UV-B and CO2 in the quality and abundance of berries in dwarf shrub species with a wide geographical distribution. However, the mechanisms explaining the responses are unclear and merit further investigation. Our data confirm previous observations on berry abundance (Gwynn-Jones et al. 1997; Phoenix et al. 2001) but provide significant added value by further investigating seed germination and berry polyphenolics. The findings suggest possible implications for the feeding behaviour of Arctic frugivorous birds and animals with potentially important consequences for the dispersal and relative recruitment capacity of the competing species that make up this plant community. It is already known that some European bird species show preferential feeding towards berries with higher antioxidant contents (Catoni et al. 2008), which could have important implications for the palatability and, therefore seed dispersal, of these species. Specific effects of UV-B on the germination of V. myrtillus in particular years could have implications for plant community dynamics, biodiversity and ecosystem functioning. Furthermore, our findings have cultural and nutritional relevance for the people of the Northern regions as berries from the species studied are widely recognised and exploited as a valuable food resource for humans.
Electronic supplementary material
Acknowledgments
The authors are grateful to the UK Natural Environmental Research Council for supporting this research (grant NE/H023690/1). We also thank the UV4Growth cost network for supporting the study. Finally, we thank all staff at Abisko Naturvetenskpliga station and the Swedish Royal Academy of Sciences for making this study possible via their unswerving support over the past two decades.
Biographies
Dylan Gwynn-Jones
is a Senior Lecturer in Ecology at IBERS, Aberystwyth University. His research interests include the response of arctic plants and communities to climate change factors.
Alan G. Jones
is a postdoctoral researcher at IBERS, Aberystwyth University and researches plant and soil responses to environmental change.
Alice Waterhouse
is an Environmental Biology graduate from IBERS, Aberystwyth University and contributed to this work during her undergraduate dissertation.
Ana Winters
is a research scientist at IBERS, Aberystwyth University and has research interests in secondary metabolites.
David Comont
is an Environmental Biology graduate of IBERS, Aberystwyth University and is currently studying Ph.D. on environmental impacts and interactions on plants and communities.
John Scullion
is a Senior Lecturer in Soil Biology at IBERS, Aberystwyth University. His research interests include the response plants and soil to environmental changes.
Rosie Gardias
is a Countryside Conservation graduate of IBERS, Aberystwyth University and is currently studying Ph.D. on modelling and understanding Arabidopsis thaliana.
Bente Graee
is an Assistant Professor at the Norwegian University of Science and Technology. She has research interests in seed ecology and climate change.
John A. Lee
is an Emeritus Professor in Plant Ecology at Sheffield University. He has continued research interests in how various environmental perturbations (Nitrogen, climate change, UV-B) affect plants and communities.
Terry V. Callaghan
is a Professor in Arctic Ecology based at the Swedish Royal Academy of Sciences. He is a world leading authority in his field of research.
References
- Albert KR, Mikkelsen TN, Ro-Poulsen H, Michelsen A, Arndal MF, Bredahl L, Hakansson KB, Boesgaard NM, et al. Improved UV-B screening capacity does not prevent negative effects of ambient UV irradiance on PSII performance in high arctic plants. Results from a six year UV exclusion study. Journal of Plant Physiology. 2011;167:1542–1549. doi: 10.1016/j.jplph.2010.05.023. [DOI] [PubMed] [Google Scholar]
- Allen DJ, Nogues S, Baker NR. Ozone depletion and increased UV-B radiation: Is there a real threat to photosynthesis? Journal of Experimental Botany. 1998;49:1775–1788. [Google Scholar]
- Anderson M. The Saami reindeer-breeders of Norwegian Lapland. American Scientist. 1985;73:524–532. [Google Scholar]
- Ballaré CL, Rousseaux CM, Searles PS, Zaller JG, Giordano CV, Robson MT, Caldwell MM, Sala OE, et al. Impacts of solar ultraviolet-B radiation on terrestrial ecosystems of Tierra del Fuego (southern Argentina). An overview of recent progress. Journal of Photochemical Photobiology B. 2001;62:67–77. doi: 10.1016/S1011-1344(01)00152-X. [DOI] [PubMed] [Google Scholar]
- Baskin, C.C., and J.M. Baskin. 1998. Seeds; Ecology, biogeography, evolution of dormancy and germination. San Diego: Academic Press.
- Baskin CC, Zackrisson O, Baskin JM. Role of warm stratification in promoting germination of seeds of Empetrum hermaphroditum (Empetraceae), a circumboreal species with a stony endocarp. American Journal of Botany. 2002;89:486–493. doi: 10.3732/ajb.89.3.486. [DOI] [PubMed] [Google Scholar]
- Berli F, D’Angelo J, Cavagnaro B, Bottini R, Wuilloud R, Silva MF. Phenolic composition in grape (Vitis vinifera L. cv. Malbec) ripened with different solar UV-B radiation levels by capillary zone electrophoresis. Journal of Agricultural and Food Chemistry. 2008;56:2892–2898. doi: 10.1021/jf073421+. [DOI] [PubMed] [Google Scholar]
- Björn LO, Callaghan TV, Johnsen I, Lee JA, Manetas Y, Paul ND, Sonesson M, Wellburn A, Coops D, et al. The effects of UV-B radiation on European heathland species. Plant Ecology. 1997;128:253–264. doi: 10.1023/A:1009782207376. [DOI] [Google Scholar]
- Caldwell MM, Ballaré CL, Bornman JF, Flint SD, Björn LO, Teramura AH, Kulandaivelu G, Tevini M. Terrestrial ecosystems, increased solar ultraviolet radiation and interactions with other climatic change factors. Photochemical and Photobiological Science. 2003;2:29–38. doi: 10.1039/b211159b. [DOI] [PubMed] [Google Scholar]
- Caspari HW, Lang A, Alspach P. Effects of girdling and leaf removal on fruit set and vegetative growth in grape. American Journal of Ecology and Viticulture. 1998;49:359–366. [Google Scholar]
- Catoni C, Schaefer HM, Peters A. Fruit for health: The effect of flavonoids on humoral immune response and food selection in a frugivorous bird. Functional Ecology. 2008;22:649–654. doi: 10.1111/j.1365-2435.2008.01400.x. [DOI] [Google Scholar]
- Day TA, Ruhlan CT, Grobe CW, Xiong F. Growth and reproduction of Antarctic vascular plants in response to warming and UV radiation reductions in the field. Oecologia. 1999;119:24–35. doi: 10.1007/s004420050757. [DOI] [PubMed] [Google Scholar]
- Day TA, Ruhland CT, Xiong FS. Influence of solar ultraviolet-B radiation on Antarctic terrestrial plants: Results from a 4-year field study. Journal of Photochemical Photobiology B. 2001;62:78–87. doi: 10.1016/S1011-1344(01)00161-0. [DOI] [PubMed] [Google Scholar]
- Demchik SM, Day T. Effect of enhanced UV-B radiation on pollen quantity, quality and seed yield in Brassica rapa (Brassicaceae) American Journal of Botany. 1996;85:573–579. doi: 10.2307/2445915. [DOI] [Google Scholar]
- Eriksson O. Seedling dynamics and life histories in clonal plants. Oikos. 1989;55:231–238. doi: 10.2307/3565427. [DOI] [Google Scholar]
- Faria A, Oliveira J, Neves P, Gameiro P, Santos-Buelga C, Freitas V, Mateus N. Anti-oxidant properties of prepared blueberry. Journal of Agricultural and Food Chemistry. 2005;53:6896–6902. doi: 10.1021/jf0511300. [DOI] [PubMed] [Google Scholar]
- Giuntini D, Lazzeri V, Calvenzani V, Dall’Asta C, Galaverna G, Tonelli C, Petroni K, Ranieri A. Flavonoid profiling and biosynthetic gene expression in mesocarp and peel of two tomato genotypes grown under UV-B-depleted conditions during ripening. Journal of Agricultural and Food Chemistry. 2008;56:5905–5915. doi: 10.1021/jf8003338. [DOI] [PubMed] [Google Scholar]
- Gonçalves B, Falco V, Moutinho-Pereira J, Bacelar E, Peixoto F, Correia C. Effects of elevated CO2 on grapevine (Vitis vinifera L.): Volatile composition, phenolic content, and in vitro antioxidant activity of red wine. Journal of Food Chemistry. 2009;57:265–273. doi: 10.1021/jf8020199. [DOI] [PubMed] [Google Scholar]
- Graae B, Ejrnæs R, Lang SI, Meineri E, Ibarra PT, Bruun HH. Strong microsite control of seedling recruitment in tundra. Oecologia. 2011;166:565–576. doi: 10.1007/s00442-010-1878-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gwynn-Jones D, Lee JA, Callaghan TV. Effects of enhanced UV-B radiation and elevated carbon dioxide concentrations on a sub-arctic forest heath ecosystem. Plant Ecology. 1997;128:243–249. doi: 10.1023/A:1009771125992. [DOI] [Google Scholar]
- Harper JL, Ogden J. The reproductive strategy of higher plants. I. The concept of strategy with special reference to Senecio vulgaris L. Journal of Ecology. 1970;58:681–698. doi: 10.2307/2258529. [DOI] [Google Scholar]
- Heinonen M. Antioxidant activity and antimicrobial effect of berry phenolics—a Finnish perspective. Molecular Nutrition & Food Research. 2007;51:684–691. doi: 10.1002/mnfr.200700006. [DOI] [PubMed] [Google Scholar]
- Heinonen IM, Meyer AS, Frankel EN. Antioxidant activity of berry phenolics on human low-density lipoprotein and liposome oxidation. Journal of Agricultural and Food Chemistry. 1998;46:4107–4112. doi: 10.1021/jf980181c. [DOI] [Google Scholar]
- Hikosaka K, Kinugasa T, Oikawa S, Onoda Y, Hirose T. Effects of elevated CO2 concentration on seed production in C3 annual plants. Journal of Experimental Botany. 2011;62:1523–1530. doi: 10.1093/jxb/erq401. [DOI] [PubMed] [Google Scholar]
- IPCC. 2007. Climate change 2007: Synthesis report. Contribution of Working groups I, II and III to the fourth assessment report of the intergovernmental panel on climate change. R.K. Pachauri, and Reisinger, A., eds. Geneva: IPCC.
- Jablonski LM, Wang X, Curtis PS. Plant reproduction under elevated CO2 conditions: A meta-analysis of reports on 79 crop and wild species. New Phytologist. 2002;156:9–26. doi: 10.1046/j.1469-8137.2002.00494.x. [DOI] [Google Scholar]
- Jansen MAK, Hectors K, O’brien NM, Guisez Y, Potters G. Plant stress and human health: Do human consumers benefit from UV-B acclimated crops? Plant Science. 2008;175:449–458. doi: 10.1016/j.plantsci.2008.04.010. [DOI] [Google Scholar]
- Johanson U, Gehrke C, Björn LO, Callaghan TV, Sonesson M. The effects of enhanced U radiation on a subarctic heath ecosystem. AMBIO. 1995;24:106–111. [Google Scholar]
- Johanson U, Gehrke C, Björn LO, Callaghan TV. The effects of enhanced UV-B on the growth of dwarf shrubs in a sub-arctic heathland. Functional Ecology. 1995;9:713–719. doi: 10.2307/2390243. [DOI] [Google Scholar]
- Johnson KS, Felton GW. Plant phenolics as dietary antioxidants for herbivorous insects: A test with genetically modified tobacco. Journal of Chemical Ecology. 2001;27:2579–2597. doi: 10.1023/A:1013691802028. [DOI] [PubMed] [Google Scholar]
- Körner C. Plant CO2 responses: An issue of definition, time and resource supply. New Phytologist. 2006;172:393–411. doi: 10.1111/j.1469-8137.2006.01886.x. [DOI] [PubMed] [Google Scholar]
- Koti S, Reddy KR, Reddy VR, Kakani VG, Zhao DL. Interactive effects of carbon dioxide, temperature, and ultraviolet-B radiation on soybean (Glycine max L.) flower and pollen morphology, pollen production, germination, and tube lengths. Journal of Experimental Botany. 2005;56:725–736. doi: 10.1093/jxb/eri044. [DOI] [PubMed] [Google Scholar]
- Lake JA, Field KJ, Davey MP, Beerling DJ, Lomax BH. Metabolomic and physiological responses reveal multi-phasic acclimation of Arabidopsis thaliana to chronic UV radiation. Plant, Cell and Environment. 2009;32:1377–1389. doi: 10.1111/j.1365-3040.2009.02005.x. [DOI] [PubMed] [Google Scholar]
- Macheix J-J, Fleuriet A, Billot J. Fruit phenolics. Boca Raton: CRC Press; 1990. [Google Scholar]
- Manney GL, Santee ML, Rex M, Livesey NJ, Pitts MC, Veefkind P, Nash ER, Wohltmann I, et al. Unprecedented arctic ozone loss in 2011. Nature. 2011;478:469–475. doi: 10.1038/nature10556. [DOI] [PubMed] [Google Scholar]
- Markham KR, Ryan KG, Bloor SJ, Mitchell KA. An increase in the luteolin: apigenin ratio in Marchatia polymorpha on UV-B enhancement. Ecological biochemistry. 1998;48:791–794. [Google Scholar]
- Martin-Aragón S, Basabe B, Benedi JM, Villar AM. Anti-oxidant action of Vaccinium myrtillus L. Phytotherapy Research. 1998;12:104–106. doi: 10.1002/(SICI)1099-1573(1998)12:1+<S104::AID-PTR265>3.0.CO;2-O. [DOI] [Google Scholar]
- McKenzie RL, Aucamp PJ, Bais AF, Björn LO, Ilyas M, Madronich S. Ozone depletion and climate change: Impacts on UV radiation. Photochemical & Photobiological Sciences. 2011;10:182–198. doi: 10.1039/c0pp90034f. [DOI] [PubMed] [Google Scholar]
- Meyer AS, Yi O, Pearson DA, Waterhouse AL, Frankel EN. Inhibition of human low-density lipoprotein oxidation in relation to composition of phenolic antioxidants in grapes (Vitis vinifera) Journal of Agricultural and Food Chemistry. 1997;45:1638–1643. doi: 10.1021/jf960721a. [DOI] [Google Scholar]
- Musil CF. Accumulated effect of elevated ultraviolet-B radiation over multiple generations of the arid-environment annual Dimorphotheca sinuata DC. (Asteraceae) Plant, Cell and Environment. 1996;9:1017–1027. doi: 10.1111/j.1365-3040.1996.tb00208.x. [DOI] [Google Scholar]
- Musil CF, Chimphango SBM, Dakora FD. Effects of elevated ultraviolet-B radiation on native and cultivated plants of southern Africa. Annals of Botany. 2002;90:127–137. doi: 10.1093/aob/mcf156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newsham KK, Robinson SA. Responses of plants in polar regions to UVB exposure: A meta-analysis. Global Change Biology. 2009;15:2574–2589. doi: 10.1111/j.1365-2486.2009.01944.x. [DOI] [Google Scholar]
- Paul ND, Gwynn-Jones D. Ecological roles of solar UV radiation: Towards an integrated approach. Trends in Ecology & Evolution. 2003;18:48–55. doi: 10.1016/S0169-5347(02)00014-9. [DOI] [Google Scholar]
- Peñuelas J, Estiarte M. Can elevated CO2 affect secondary metabolism and ecosystem function? Trends in Ecology & Evolution. 1998;13:20–24. doi: 10.1016/S0169-5347(97)01235-4. [DOI] [PubMed] [Google Scholar]
- Phoenix GK, Gwynn-Jones D, Callaghan TV, Sleep D, Lee JA. Effects of global change on a sub-arctic heath: effects of enhanced UV-B radiation and increased summer precipitation. Journal of Ecology. 2001;89:256–267. doi: 10.1046/j.1365-2745.2001.00531.x. [DOI] [Google Scholar]
- Phoenix GK, Gwynn-Jones D, Callaghan TV. Ecological importance of ambient solar ultraviolet radiation to a sub-arctic heath community. Plant Ecology. 2002;165:263–273. doi: 10.1023/A:1022276831900. [DOI] [Google Scholar]
- Price SF, Breen PJ, Valladao M, Watson BT. Cluster sun exposure and quercetin in pinot noir grapes and wine. American Society for Enology and Viticulture. 1995;46:187–194. [Google Scholar]
- Prior RL, Cao G, Martin A, Sofic E, McEwen J, O’Brien C, Lischner N, Ehlenfeldt M, et al. Antioxidant capacity as influenced by total phenolic and anthocyanin content, maturity, and variety of Vaccinium species. Journal of Agricultural and Food Chemistry. 1998;46:2686–2693. doi: 10.1021/jf980145d. [DOI] [Google Scholar]
- Qaderi MM, Reid DM. Growth and physiological responses of canola (Brassica napus) to UV-B and CO2 under controlled environment conditions. Physiologia Plantarum. 2005;125:247–259. doi: 10.1111/j.1399-3054.2005.00566.x. [DOI] [Google Scholar]
- Qaderi MM, Reid DM, Yeung EC. Morphological and physiological responses of canola (Brassica napus) siliquas and seeds to UVB and CO2 under controlled environment conditions. Environmental and Experimental Botany. 2007;60:428–437. doi: 10.1016/j.envexpbot.2006.12.019. [DOI] [Google Scholar]
- R Development Core Team. 2011. R: A language and environment for statistical computing, reference index version 2.12.2. R Foundation for Statistical Computing, Vienna. http://www.R-project.org. Accessed 11 Feb 2011.
- Ritchie JC. Vaccinium myrtillus L. Journal of Ecology. 1956;44:291–299. doi: 10.2307/2257181. [DOI] [Google Scholar]
- Robson TM, Pancotto VA, Flint SD, Ballare CL, Sala OE, Scopel AL, Caldwell MM. Six years of solar UV-B manipulations affect growth of Sphagnum and vascular plants in a Tierra del Fuego peatland. New Phytologist. 2003;160:379–389. doi: 10.1046/j.1469-8137.2003.00898.x. [DOI] [PubMed] [Google Scholar]
- Rozema J, Boele P, Solheim B, Zielke M, Buskens A, Doorenbosch M, Fjin R, Herder J, et al. Stratospheric ozone depletion: High arctic tundra plant growth on Svalbard is not affected by enhanced UV-B after 7 years of UV-B supplementation in the field. Plants and Climate Change: Tasks for vegetation science. 2006;34:121–136. doi: 10.1007/978-1-4020-4443-4_9. [DOI] [Google Scholar]
- Schultz HR. Climate change and viticulture: A European perspective on climatology, carbon dioxide and UV-B effects. Australian Journal of Grape and Wine Research. 2000;6:2–12. doi: 10.1111/j.1755-0238.2000.tb00156.x. [DOI] [Google Scholar]
- Searles PS, Flint SD, Caldwell MM. A meta-analysis of plant field studies simulating stratospheric ozone-depletion. Oecologia. 2001;127:1–10. doi: 10.1007/s004420000592. [DOI] [PubMed] [Google Scholar]
- Shevtsova A, Ojala A, Neuvonen S, Vieno M, Haukioja E. Growth and reproduction of dwarf shrubs in a subarctic plant community: Annual variation and above-ground interactions with neighbors. Journal of Ecology. 1995;2:263–275. [Google Scholar]
- Singh SK, Kakani VG, Surabhi GK, Reddy KR. Cowpea (Vigna unguiculata [L.] Walp) genotypes response to multiple abiotic stresses. Journal of Photochemistry and Photobiology. 2010;100:135–146. doi: 10.1016/j.jphotobiol.2010.05.013. [DOI] [PubMed] [Google Scholar]
- Sonesson M, Lundberg B. Late quaternary forest development of the Torneträsk area, North Sweden. Oikos. 1974;25:121–133. doi: 10.2307/3543633. [DOI] [Google Scholar]
- Stephanou M, Manetas Y. Enhanced UV-B radiation increases the reproductive effort in the Mediterranean shrub Cistus creticus under field conditions. Plant Ecology. 1998;134:91–96. doi: 10.1023/A:1009773105854. [DOI] [Google Scholar]
- Sullivan JH, Teramura AH. Effects of ultraviolet-B radiation on seedling growth in the Pinaceae. American Journal of Botany. 1988;75:225–230. doi: 10.2307/2443888. [DOI] [Google Scholar]
- Szmidt AE, Nilsson MC, Briceno E, Zackrisson O, Wang XR. Establishment and genetic structure of Empetrum hermaphroditum populations in northern Sweden. Journal of Vegetation Science. 2002;13:627–634. [Google Scholar]
- Talavera S, Felgines C, Texier O, Besson C, Mazur A, Lamaison JL, Remesy C. Bioavailability of a bilberry anthocyanin extract and its impact on plasma antioxidant capacity in rats. Journal of the Science of Food and Agriculture. 2006;86:90–97. doi: 10.1002/jsfa.2327. [DOI] [Google Scholar]
- Taruscio TG, Barney DL, Exon J. Content and profile of flavanoid and phenolic acid compounds in conjunction with the antioxidant capacity for a variety of northwest Vaccinium berries. Journal of agricultural and food chemistry. 2004;52:3169–3176. doi: 10.1021/jf0307595. [DOI] [PubMed] [Google Scholar]
- Taulavuori E, Bäckman M, Taulavuori K, Gwynn-Jones D, Johanson U, Laine K, Callaghan TV, Sonesson M, et al. Long-term exposure to ultraviolet-B radiation in the sub-arctic does not cause oxidative stress in Vaccinium myrtillus. New Phytologist. 1998;140:691–697. doi: 10.1046/j.1469-8137.1998.00302.x. [DOI] [PubMed] [Google Scholar]
- Teramura AH, Sullivan JH, Ziska LH. Interaction of elevated ultraviolet-B radiation and CO2 on productivity and photosynthetic characteristics in wheat, rice, and soybean. Plant Physiology. 1990;94:470–475. doi: 10.1104/pp.94.2.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolvalen A, Laine K. Effects of reproduction and artificial herbivory on vegetative growth and resource levels in deciduous and evergreen dwarf shrubs. Canadian Journal of Botany. 1997;75:656–666. doi: 10.1139/b97-073. [DOI] [Google Scholar]
- Tosserams M, Visser A, Groen M, Kalis G, Magendas E, Rozema J. Combined factors of CO2 concentration and enhanced UV-B radiation on faba bean. Plant Ecology. 2001;154:195–210. doi: 10.1023/A:1012907118290. [DOI] [Google Scholar]
- Tybirk K, Nilsson MC, Michelson A, Kristensen HL, Shevtsova A, Strandberg MT, Johansson M, Nielsen KE, et al. Nordic Empetrum dominated ecosystems: Function and susceptibility to environmental changes. AMBIO. 2000;29:90–97. [Google Scholar]
- Staaij JWM, Bolink E, Rozema J, Ernst WHO. The impact of elevated UV-B (280–320 nm) radiation levels on the reproduction biology of a highland and lowland population of Silene vulgaris. Plant Ecology. 1997;128:173–179. doi: 10.1023/A:1009710907336. [DOI] [Google Scholar]
- Wand SJE, Midgley GF, Musil CF. Growth, phenology and reproduction of an arid-environment winter ephemeral Dimorphotheca pluvialis in response to combined increases in CO2 and UV-B radiation. Environmental Pollution. 1996;94(247):254. doi: 10.1016/s0269-7491(96)00116-9. [DOI] [PubMed] [Google Scholar]
- Wang SY, Bunce JA, Maas JL. Elevated carbon dioxide increases contents of antioxidant compounds in field-grown strawberries. Journal of Agricultural and Food Chemistry. 2003;51:4315–4320. doi: 10.1021/jf021172d. [DOI] [PubMed] [Google Scholar]
- Wang SY, Qiu N, Wang X, Ma Z, Du G. Effects of enhanced UV-B radiation on fitness of an alpine species Cerastium glomeratum Thuill. Journal of Plant Ecology. 2008;1:197–202. doi: 10.1093/jpe/rtn018. [DOI] [Google Scholar]
- Zvereva EL, Kozlov MV. Growth and reproduction of dwarf shrubs, Vaccinium myrtillus and V. vitis-idaea, in a severely polluted area. Basic and Applied Ecology. 2005;6:261–274. doi: 10.1016/j.baae.2004.11.003. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.