Abstract
Trees are taller than shrubs, grasses, and herbs. What is the disadvantage of being tall so that trees are restricted to warmer regions than low stature life forms? This article offers a brief review of the current state of biological treeline theory, and then explores the significance of tallness from a carbon balance, freezing resistance, and microclimatological perspective. It will be argued that having of a woody stem is neither a burden to the carbon balance nor does it add to the risk of freezing damage. The physiological means of trees to thrive in cold climates are similar to small stature plants, but due to their size, and, thus, closer aerodynamic coupling to air circulation, trees experience critically low temperatures at lower elevation and latitude than smaller plants. Hence, trees reach a limit at treeline for physical reasons related to their stature.
Keywords: Climate, Forest limit, Growth, Stress, Temperature, Timberline
Biological Theory of Treeline Information
The title of this article addresses the central issue of treeline ecology. What makes trees more susceptible to the high elevation or high latitude climate than shrubs, grasses, or herbs? While trees are obviously taller, what are the processes that cause size to become decisive when it gets cold? The article explores this field by addressing three questions: (1) Is the trunk a burden in a carbon balance perspective? (2) Are perennially exposed tissues at greater risk of freezing damage? (3) Does size physically enhance the action of adverse climate? Before entering these themes, I will briefly summarize current knowledge about the biological aspects of tree life at treeline in order to justify the focus on these three themes. The following summary is based on a more exhaustive assessment by Körner (2012) and will consider the biological drivers of large-scale biogeographic patterns rather than local effects and peculiarities, such as disturbances that are not specific to treelines in a global comparison.
Treeline Climate
Across the world, and irrespective of the local tree species, forests find a rather abrupt end at latitude- and region-specific high elevations, yielding terrain to a high diversity of taxa, belonging to other, low stature life forms, such as shrubs, graminoids, or herbs. The past treeline debate was too strongly focused on northern cool temperate, mostly conifer-dominated treelines, with winter phenomena often considered to play a central role, although treelines are found at similar thermal isolines in non-seasonal, tropical climates (Körner and Paulsen 2004; Körner 2007). As had been discussed in many previous accounts, the treeline most often is not represented by a sharp line, but forms a highly dynamic ecotone (Kullman 1990; Stöcklin and Körner 1999; Callaghan et al. 2002; Sveinbjörnsson et al. 2002; Holtmeier 2009), commonly fragmented by features of the land surface (e.g., lack of soil, water logging), physical disturbance (storms, rockfall, avalanches, fire), biological disturbances (insect outbreaks, browsing, pathogens), or human interferences (logging, pastoralism). Since these disturbances can prevent trees from growing anywhere, they need to be clearly separated from biological causes of tree absence above the high elevation treeline, which must be related to tree development (reproduction, life history), metabolic constraints (physiology), or the action of environmental extremes (stress). In this article, I will consider such biological causes only. It should be these causes that help understanding the treeline phenomenon globally, given all other, non-biological causes for tree absence, vary from place to place and could never lead to the worldwide treeline pattern as it was already depicted by von Humboldt and Bonpland (1807; see also Troll 1973).
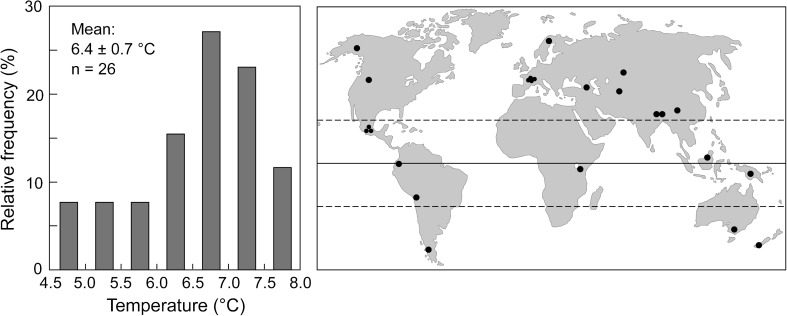
Based on data collected right at the treeline, the current natural high elevation tree limit is associated with a growing season that is at least 90 days long (constrained by temperatures passing through a 0 °C weekly mean threshold) and during which the mean air temperature is 6.4 ± 0.7 °C (±SD for 5 biomes, i.e., tropical, subtropical, warm temperate, cool temperate, subarctic-boreal; Körner 2012; Fig. 1). Using an algorithm that applies the above climatic criteria, the global pattern of natural, climate-driven treeline positions can be modeled with great confidence (Körner 2007, 2012; Körner et al. 2011). There is one problem associated with such temperature data, collected over the past two decades: the climate has become significantly warmer than it used to be and the current position of treeline in many parts of the world is lagging behind regional climate (Harsch et al. 2009). One simple reason is that trees take a long time to grow in size (e.g., meeting a 3-m-size criterion as a convention to separate a tree from a shrub). Hence, depending on regional climatic warming, temperatures collected at treeline may deviate by up to 1 K from thermal equilibrium. The reason why the above 6.4 °C mean temperature of the treeline isotherm is slightly lower then originally calculated by Körner and Paulsen (2004; 6.7 ± 0.8 °C) is the inclusion of new data (tropical and one arctic site) that may have seen less climatic warming (and thus, supposedly are closer to equilibrium), while previously published means had more stations from the Alps, which saw a 1.1–2 K warming over the past century. Accounting for such mismatch of regional treeline position with regional climate, a mean below 6 °C but above 5 °C would possibly be closer to the steady state isotherm associated with treeline, in the long run. Surprisingly, the measured mean temperatures do not significantly change with increasing season length; hence, they represent close approximations for arctic, temperate, subtropical as well as tropical treelines. Mean air temperatures measured at treeline turned out to match with mean soil temperatures measured in complete shade under trees, hence, both serve equally well as treeline proxies (see the above references).
Fig. 1.
Frequency of mean on-site temperatures at treeline for the growing season (as constrained by a weekly mean temperature >0 °C) from across the globe, obtained by data loggers buried at −10 cm soil depth in complete shade (from Körner and Paulsen 2004 and newer data in Körner 2012). These rooting zone temperatures are matching weekly means of ambient air temperature. In order to avoid over-representing regions better covered by data, multiple sites’ data for a given region (e.g., the Alps) have been aggregated, hence, the total number of seasonal means is reduced to 26 out of >40 locations monitored (the remaining regional means were aggregated into five biomes before averaging; see the text). The two coldest sites (leftmost bar) are from a wet site in Ecuador and a site from Alaska (over permafrost)
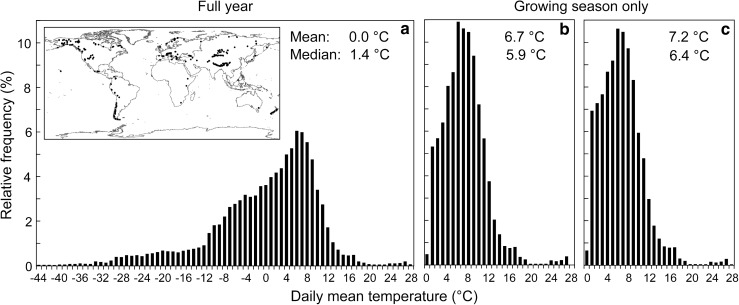
Given the close correlation between temperature and treeline position, it was tempting to explore this relatedness globally, using geographical information systems, such as the climate data base Worldclim (Hijmans et al. 2005) and treeline positions obtained from Google Earth (unpublished results by Jens Paulsen). Data for 370 treeline locations across the globe (Fig. 2) had been explored statistically for best fit, in a three-step procedure by (a) obtaining the daily mean temperature for treeline elevation for all days of the year (fitted from monthly means by cubic splines), (b) as before, but for all days with a daily mean temperature at or above 0.9 °C (found to statistically best define season length at treeline), and (c) additionally accounting for moisture constraints of season length (drought). Quite surprisingly, this purely statistical procedure based on satellite images without any site visits, yielded a global mean temperature for the growing season at treeline of 6.4 °C, similar to the results of the data logging campain, but the best fit was achieved using a 94-day minimum season length with a 0.9 °C threshold for days to be considered belonging to the growing season. Hence, the two completely independent approaches resulted in similar thermal treeline proxies. The remaining differences may reflect the inclusion of different sampling sites, but more likely, some mismatch of the climate data base with on-site climate and hence, treeline position.
Fig. 2.
Frequency of daily mean temperatures at treeline for 370 treeline sites across the globe obtained by combining temperatures from the Woldclim climate data base and treeline positions assessed with Google Earth (a unconstrained season, i.e., all days of the year; b restricted to days with a daily mean >0.9 °C, c as b, but disregarding days too dry for tree growth (solving the local water balance equation with a bucket model). Note, data in b and c are spanning such a wide range, because they include daily mean temperatures for all individual days that exceed 0.9 °C from all climatic zones (J. Paulsen and C. Körner, unpublished)
These numbers offer a good basis for testing hypotheses, although they are not per se referring directly to a biological mechanism. They simply reflect a highly repeated coincidence that makes it hard to believe that there is no relationship with a common set of biological causes. The temperatures obtained are more consistent than one could have expected, given the wide range of regional climates and floras in the mountains of the world. The variance of ±0.7 K across biomes corresponds to a mean uncertainty of ±100–150 m of elevation, sufficiently robust at a global scale (and much more precise regionally), to encourage a discussion of the likely causes of such a global treeline isotherm.
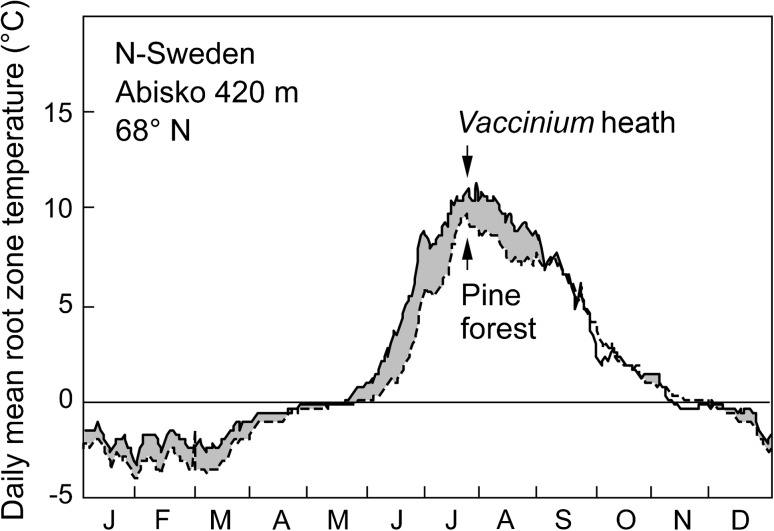
Central to this debate is the distinction between trees (e.g., >3 m) and tree seedlings or krummholz (small stunted individuals or shrub). Tree seedlings may be found at several hundred meters above treeline, nested among alpine shrub or in sheltering microtopography. Near the ground, the climate is warmer during the day, and tree seedlings share those benefits with the low stature alpine flora (Fig. 3), except for disturbed areas with open ground and lack of shelter.
Fig. 3.
Thermal benefits of being a shrub when it becomes cold. Root zone temperatures (−10 cm) measured in a subarctic climate, at the upper limit of Pinus sylvestris at 420 m near Abisko, N-Sweden (68°N), and beneath adjacent dwarf shrubs just outside the fragmenting forest
As one approaches the elevational or latitudinal tree limit, trees get smaller and grow slower, but the decline is not gradual, but accelerates over the last few tens of meters of elevation (Paulsen et al. 2000). Diameter growth declines slower than does height growth, presumably because cambial growth is less constrained by the prevailing temperatures at treeline than apical growth. This may be related to the exposed, and thus, ‘colder’ position of apical meristems compared to the cambial tissue of stems. Crowding of trees has rather ambiguous effects. In the earliest life stage, cluster-recruitment may exert some facilitative microclimatic benefits (as suggested by Smith et al. 2003), but the available data, rather point at benefits in terms of mechanics and browsing rather than in physiology (Schönenberger 2001; Körner 2012). At later stages, trees profit from isolation, both at high elevation and at arctic treelines, given the small diurnal heating of their canopy by direct sun exposure, but a warmer rooting zone beneath unshaded ground. Hence, open stands are generally considered warmer and more favorable for tree growth at treeline (Matyssek et al. 2009).
Tree Recruitment at Treeline
For a tree to grow at treeline, there must be a successful seedling in the first place, and there must be more birth than older tree death for a population to be sustained, because of the high mortality in the earliest life stages. Hence, recruitment limitation is one of the possible causes for treeline formation, but it is difficult to assess. A single demographic census cannot provide an answer, because both, recruitment and death show irregular temporal patterns, possibly reflecting exceptionally positive or negative weather situations or disturbances (Kullman 2007). Age distributions of individuals near treeline commonly point at waves (episodes) of successful recruitment followed by significant intervals with none (e.g., Gervais and MacDonald 2000). It needs only a few exceptionally good summers in a century to maintain a tree population with life expectancies exceeding 150 years.
Based on tree demography, there is no evidence of a systematic and general (long-term) recruitment limitation at treeline. Quite often, the number of seedlings is greater above the treeline, than within the uppermost forest. These seedlings initially profit from shelter among alpine vegetation and microtopography, but fully sky exposed ones, without any shelter, may experience stress during their earliest life stage, like other low stature plants would (Germino and Smith 1999). There are many examples of successful seedling establishment above treeline that ended in crippled shrub rather than upright tree stature. Seedbed conditions at and above treeline differ so widely locally as well as across the globe that it is impossible to arrive at a consistent global treeline position tied to a common isotherm based on seedling success. Seed limitation (a shortage of viable seeds) does not seem to be a major problem, because of the very short distances between the montane forest and treeline, should trees right at treeline not produce sufficient viable seeds (Körner 2012). While there is no question that there must be sufficient seedling survival, the critical step is the transition from the ‘shrub’ stage of young trees to the upright sapling and tree stage. This is when apical meristems get exposed to the direct action of low air temperature.
Growth Restrictions at Treeline
There is now broad evidence that plant tissue cannot be built at temperatures close to 0 °C, and there is very slow (if any) growth activity up to +5 °C, both in cambial and apical meristems, above and below the ground, matching a long known threshold for growth in winter crops as well as trees (for reviews, see Rossi et al. 2007; Körner 2008). This means, most nights during the growing season (which may last 12 months in the tropics) are too cold for any length growth of shoots or thickness growth of stems, and also many hours during the day will permit only marginal growth. Roots may profit from higher temperatures in open stands (Fig. 3) because of solar ground warming, one of the possible reasons why isolated trees are often found above/beyond the forests limit. As soon as the forest canopy closes, the rooting zone gets as cool as the free atmosphere, and root growth will come close to a halt at around +5 °C as well (Alvarez-Uria and Körner 2007). While growth becomes negligible below +5 °C, leaf photosynthesis still reaches 50–70 % of its full capacity at this temperature in cold-adapted taxa (Tranquillini 1979; Wieser and Tausz 2007). From this it can be expected that growth processes are far more temperature limited than processes associated with photoassimilate production, when it gets cold.
Among the physiological causes of declining tree growth as one approaches the treeline, in addition to those direct temperature effects on meristems discussed above, limitations by water, nutrients, and carbon may come into play. However, whether a treeline forms is not a matter of a certain rate of growth, but rather of sustained growth at whatever rate and thus, tree presence. Hence, should any of these resources reduce the rate of growth, this would not imply a causal relationship with the current position of the treeline. During the cold, late nineteenth century, treeline trees in the Alps hardly grew for several decades (ring width of <0.1 mm), but the position of the treeline was not affected (Paulsen et al. 2000). Hence, resource limitation must not be seen in the context of an agronomic (yield-oriented) limitation concept, but in an ecological context related to persistence, fitness, and survival. Robustness is not commonly related to fast growth when environmental conditions get demanding.
Exept for mountain deserts or semi-deserts, water is not a resource known to become increasingly scarce at high elevation or high latitude. The so-called winter desiccation in temperate zone mountains (Larcher 1985; Mayr 2007) has never been shown to affect adult trees or trees at or below treeline, but rather affects isolated seedlings and young saplings above treeline in some of these (more continental) regions. Since (up to a certain limit) treeline elevation is negatively correlated with precipitation (the drier the climate, the higher the treeline, with record elevations close to 5000 m a.s.l. in Tibet and Bolivia), there is no reason to consider water shortage as a common treeline determinant. Heavy snowpack or a wet (and thus cloudy) climate are reducing treeline elevation in some maritime or humid tropical regions. Water logging is a key factor in arctic lowland treelines but not on mountain slopes. Obviously, such water-related effects could never explain the uniformity of treeline elevation relative to temperature across the globe.
In other than horticultural conditions, soil nutrients are hardly ever available in growth saturating amounts (provided nothing else is limiting tree growth). Hence, in the long run, addition of growth limiting nutrients will commonly stimulate plant growth anywhere, including trees at treeline. However, a growth stimulation by fertilizer addition does not permit any inferences with regard to nutrient limitation as a treeline determinant. Such an interpretation would need to account for long-term ecological implications of stimulated growth (should it occur) with regard to tree robustness against stress and pathogens. As far as known, nutrient concentrations in treeline trees are not indicating deficits compared to the adjacent, lower montane forest. In many cases foliage nutrient concentrations rather tend to increase with elevation (Körner 2012).
As mentioned above, carbon acquisition by photosynthesis is far less sensitive to low temperature than growth. Despite some adjustment of metabolism to higher specific rates at low temperature, respiratory carbon losses are reduced at treeline because of the predominant cold temperatures, particularly at night. This field has been explored quite exhaustively for temperate zone treelines (Wieser and Tausz 2007), where carbon balance issues are often considered more critical than in lower latitude treelines, because of the long dormant season. However, all evidence reviewed in Wieser and Tausz (2007) stands against this presumption, rendering carbon constraints even less likely in non-seasonal, tropical treelines. In addition to perfect thermal adjustments of photosynthesis to low temperatures, stable carbon isotope research confirmed a general higher CO2 uptake efficiency of leaves at high elevation, presumably, a response to reduced partial pressure of CO2 (Zhu et al. 2010 and further references therein). A global survey of carbon reserve formation in trees at treeline points at improved rather than diminished supplies (Hoch and Körner 2011). This is explained by the greater thermal limitation of growth processes (meristems, sink activity) than assimilation (source activity).
In summary, low temperatures are the dominant factor for treeline formation worldwide and the abruptness of the termination of upright tree growth must be intrinsically related to the nature (stature) of trees, because other, low stature life forms, including many woody species, cope well with life conditions at much higher elevations. Since all physiological parameters point at perfect low temperature adaptation of trees at treeline, not different to other cold-adapted plants, stature itself remains as the most critical factor, and perhaps, tied to stature, the ‘need’ for substantial longevity (sustained intactness of stature). In the following, I will thus, explore the biological ‘costs’ of a big stem, the effects of crown exposure to stressful conditions, and consequences of stature itself for the impacts of cold climate.
Are There Any Extra Costs Associated with Having a big stem?
The most obvious feature of a tree is its stem. It has to be built and maintained. The total amount of biomass in tree stems, major branches and roots is in the order of 95 % of its total biomass, with leaves representing on average 1–2 % in deciduous and 3–4 % in evergreen trees, and ca. 1.5 % of the biomass is fine roots <3 mm in diameter (Table 1). So, the leaf to fine root biomass ratio is ca. 2.5 in evergreen conifers and 0.8 in deciduous trees, which reflects the difference in specific leaf area and leaf longevity. If one considers foliage and <3 mm fine roots alone, thus converting a tree into a rosette herb with no stem, the leaf mass fraction of such a stemless plant would be 74 % in conifers and 52 % in deciduous trees. Hence, without accounting for shoot and coarse root mass, trees are as ‘leafy’ as herbs. It is important, that all these biomass data, including the leaf and fine root fractions, were obtained and calculated per m2 of closed forest. Once the tree canopy is closed and trees matured, the leaf and fine root mass per unit of land area stays fairly constant, while stems get heavier as trees become older. Another limitation of such compartmentation considerations is the functional duration of investments (leaf and fine root duration). Commonly, the specific ‘cost’ (volume density) of tissues is balanced by their longevity in terms of carbon and nutrient amortization.
Table 1.
Dry matter allocation in mature forest trees from 73 literature references
| Biomass fraction | Evergreen conifers | Deciduous tree species |
|---|---|---|
| Shoot fraction (%) | 80 | 83 |
| Coarse root fraction (%) | 17 | 14 |
| Leaf fraction (%) | 4.2 | 1.3 |
| <3 mm root fraction (%) | 1.7 | 1.4 |
Note, because not all authors offer all biomass fractions, the means do not add up to 100 % (data from Körner 1994)
While leaves turn over once every year in deciduous trees, the mean leaf duration is 4 to 12 years (e.g., PinusversusPicea) in evergreen conifers at treeline, with only the last 3–5 years significantly contributing to carbon gain (Matyssek 1985). Evergreen conifers have 3–4 times more foliage biomass, of commonly half or less the photosynthetic capacity per unit projected leaf area and half the protein concentration than in deciduous trees. Since the carbon gain scales with leaf mass per area (Oren et al. 1986) the annual gain becomes fairly similar for evergreen and deciduous taxa (Matyssek 1985) and so is the daily water loss (Schulze et al. 1985). In the case of evergreen conifers, carbon uptake continues during mild weather in the dormant period, so that carbon reserves reach a maximum before bud break in spring (Hoch and Körner 2003). These relationships can be assumed not to change significantly with elevation (Körner 2012), but young trees have a larger fraction of leaf mass and smaller stem and coarse root fractions, and the leaf mass fraction was found to increase with elevation in two evergreen taxa (Table 2; no change in Larix; Bernoulli and Körner 1999). Disregarding the 1–2 % of dry matter of fine roots thinner than 3 mm, the bulk coarse root plus shoot mass approximates 91 % of total biomass in Larix, and 80–84 % in the two pine species. Because of the greater foliage longevity at high elevation (balancing the shorter growing season) the foliage to sap wood ratio increases with elevation (Matyssek et al. 2009). So there is no evidence that trees at treeline operate at reduced foliage mass fraction, rather the reverse, the foliage mass fraction tends to be higher, and neither photosynthetic capacity nor foliage nutrients decline with elevation, as discussed above.
Table 2.
Dry matter allocation (% of total) in 27-year-old trees (1–2 m height) at the treeline near Davos (Switzerland; n = 20–24 individuals per species)
| Biomass | Evergreen | Deciduous |
|---|---|---|
| Leaf mass fraction | 15–16 | 7 |
| Stem + branch + rootstock | 69–73 | 79 |
| Root mass fraction | 11–12 | 14 |
Evergreen species: Pinus uncinata and P. cembra, deciduous species: Larix decidua (from Bernoulli and Körner 1999)
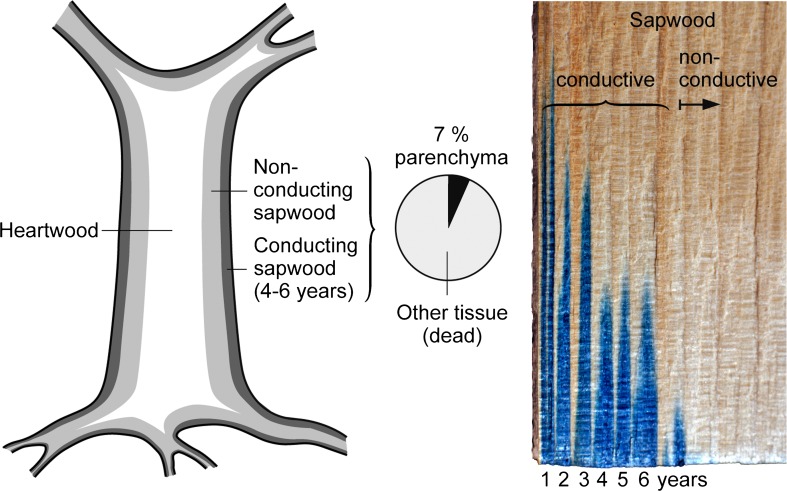
In a seasonal climate, trees add a single growth layer to the stem year by year, which represents between 0.5 and 2 % (mostly around 1 %) of the existing mass, depending on tree age. Hence, the total amount of new shoot mass added per year is similar to the annual leaf turnover. These grows layers of stems (hereafter addressed as tree rings) are commonly active for 5–20 years, with most (>80 %) of the water conductivity confined to the last 2–6 years (Fig. 4). Hence, a ring serves/amortizes itself over several years, different from herbaceous plants and grasses, that recycle such tissue annually. While every year, a new ring is added, one ring is (statistically) transferred from the active to the inactive state (sapwood to heartwood transition). Once transferred, this ring is disposed for delayed recycling after the tree has died. Heartwood has little static and no metabolic function, as long as pathogens stay away. This trivial fact is important, because, unlike low stature vegetation, most of the stem consists of dead tissue that, for reasons of plant anatomy, will enter recycling only after the tree has died. That tissue is ‘trapped’ inside the trunk and is not creating any additional ‘cost’.
Fig. 4.
Tree stems are composed of active and dead parts, commonly addressed as sapwood and heartwood. Sapwood is commonly defined by color or moisture, and includes conductive and non-conductive parts, the latter commonly bigger than the first. Depending on species, about 7 % of all wood tissue volume is initially made of parenchyma, only a fraction of which is active. The remaining wood volume is composed of trachea, tracheids, and fibers, all metabolically inactive, once built. The radial variation of sap flow velocity is illustrated by methylene blue dye for a stem of a transpiring pine tree cut while submersed
The critical issue is the amount and quality of sapwood, the fraction of which (per cross-sectional stem area) depends on species, tree age, increases with insertion height, and varies with growth conditions, including water supply. Pines from a dry location where found to have less sapwood than trees from a moist location (Sterck et al. 2008). What is considered sapwood is a matter of definition (Bosshard 1984). Often sapwood is defined by color compared to the heartwood (e.g., Knapic and Pereira 2005), by pH sensitive staining (e.g., Gould and Harrington 2008), and most often by moisture (e.g., Münster-Swendsen 1987; Sellin 1994; Björklund 1999), generally, and misleadingly, treated as conductive sapwood, although water content does not imply that there is conductivity. Color based definitions tend to overestimate sapwood, and moisture based ones certainly overestimate the conductive part. The transitions between heartwood, non-conductive and conductive sapwood are gradual, but most of the sapwood is not or hardly conductive, but serves for water, nutrient and mobile carbon compound storage, with the volume of active tissue (parenchyma, ray tissue) in conifer sapwood commonly between 5 and 10 percent (Huber and Prütz 1938; Fig. 4). Peripheral tissue (recent tree rings) is more active than central tissue. According to Bosshard (1984) the most active (and presumably most conductive) part of sapwood is between four and six years old. Active sap wood can also be defined by the presence of sugars and starch reserves, which are most commonly confined to the outermost (youngest) part of sap wood. In individuals of 16 tropical tree species of 30 to 160 cm diameter, most of the reserves were found in the outer 5 cm (in some species up to 10 cm; Hoch et al. 2003; Würth et al. 2005) with a sharp inward decline (see the review by Sala et al. 2011).
Hence, >90 % of sapwood is non-parenchymal tissue (tracheids, trachea), with no metabolic activity after formation. Assuming a physiologically active sap wood area of 30 % of the cross-sectional area (and thus stem mass) and an upper limit of the active parenchyma volume-fraction of 10 %, 3 % of stem volume at most, is active parenchyma cells, and 27 % is the remaining ‘active’ sapwood that would have no metabolic activity, irrespective of whether it contains actually conductive elements or not. Over all tree rings in such a sapwood example, the innermost tissue would be close to dead and the outermost most active. Assuming a linear centripedal decline in metabolism, an average activity applied to the complete sapwood, would correspond to full activity of half of the tissue, i.e. 1.5 % of stem volume (instead of 3 %, in this upper limit estimate of parenchyma fraction; 1 % would perhaps be more realistically). This comes close to the mean annual volume increment of an average stem. Assuming the same tissue dry matter density, this roughly yields a 1:1:1 ratio of foliage, active stem parenchyma cells and fine roots in deciduous trees (e.g., Larix) and a greater foliage fraction (with less metabolic activity) in evergreen species (see above).
Despite all the uncertainties regarding specific tissue volumes and their functional duration, the important point is that the stem does not represent a metabolic burden any different from herbaceous or grass plants, with the same general relationships presumably applying to woody parts in shrubs. The annual production of a tree ring, >90 % of which is dead, corresponds to the annually produced, but also annually recycled above-ground biomass in herbaceous plants. By definition, dead heartwood and the >90 % dead fraction of sapwood cells, cause stems to largely represent non-recycled, annually accumulated dead plant material that exerts no ongoing metabolic cost. In comparison to herbaceous plants, an annual C budget of tree stems (including branches and coarse roots) has to account for the formation of one new growth layer (a dry mass similar to new foliage or the <3 mm fine root pool), and ca. 1 % stem volume equivalent of fully active parenchyma cells in sapwood (even less % when expressed per total trunk). The remaining >99 % of the total xylem dry matter does not contribute to metabolic costs. Cambial and phloem tissue will perhaps double the active cell fraction per complete stem, leading to a 1:2:1 active biomass ratio (leaf : life shoot : fine root) in deciduous trees, very similar to many perennial herbs and grasses (Körner 2003).
Given that the leaf mass fraction (expressed as the fraction of all active tissue) often correlates with growth rate, these allocation patterns explain, why the annual productivity of non-water-limited grassland and forests per unit land area is not systematically different across the globe (ca. 200 g dry biomass m−2 month−1 of growing season, Körner 1999). Not surprisingly, the rates of stem respiration measured as CO2 efflux are quite low (Wieser and Bahn 2004), so low that the total stem respiration of treeline trees during the long temperate zone winter corresponds to the carbon uptake during 1-2 bright days in spring or summer (Wieser 1997). Tree stems are unlikely to add a particular burden to the tree’s carbon balance in comparison to axial tissue in non-tree plants.
Tissue Exposure to Extreme Temperatures
A common assumption is that tall plant stature causes perennial tissues to remain exposed to free atmosphere conditions year round, and thus, causing tissues to be at higher risk of freezing damage, whereas shrubs, grasses and forbs experience mutual shelter and, at higher latitudes, are protected by snow during the coldest part of the year. This assumption is not fully correct. First, tall stature is in fact preventing significant radiational cooling of the leaf canopy below air temperature during clear nights, because of faster convective heat transfer, whereas low stature vegetation does cool below atmospheric temperatures (well known ground frost; Squeo et al. 1991). Second, since treelines occur worldwide, including the tropics, treeline formation cannot be related to snow effects, and even at high latitude, snow cover is unreliable, often missing in early or late winter, and showing significant slope exposure effects, not mirrored in treeline position.
Since freezing stress is likely at any treeline, it represents a potential globally important factor, but the climate and stress tolerance data for different climatic regions illustrate substantial leeway between the freezing resistance of treeline trees and the actual long-term temperature minima observed at treeline (Sakai and Okada 1971; Sakai and Larcher 1987; see review by Körner 2012). Hence, freezing damage may affect young, unhardened shoots early in the season, and canopy damages supposedly related to freezing had been observed in the tropics, but these partial tissue losses would not cause a systematic placement of treeline at a common growing season isotherm, because the seasonal means and annual extremes are not closely related. Trees at humid tropical treelines are much less resistant (ca. −5 to −15 °C, often around -8 °C), than trees at temperate and boreal treelines in winter (around −35 °C), but during the growing season, the freezing resistance of these temperate/boreal trees is similar to that in year-round active trees at tropical treelines. All these stress related effects may regionally add modulative constraints, but they cannot explain the general decline of tree vigor as one approaches the thermal tree limit worldwide, while shrubs and herbaceous vegetation is doing well upslope.
Besides tissue damage, freezing could affect trees in a specific way related to their stature, namely by affecting the xylem conductivity in their exposed stems through either blockade (ice formation) or by inducing embolism (cavitation) by freeze–thaw cycles (Mayr 2007). However, to date, no negative effects of such freezing induced cavitation in the subsequent growing season had been shown for treeline trees, and the desiccation of evergreen foliage, as a consequence of inhibited water supply (winter desiccation) remains restricted to exposed individuals above treeline, mostly small saplings, in continental regions. These phenomena have been shown to induce some limited damage locally, but their geographic range and abundance is restricted, and thus, these specific actions of low temperature cannot explain the global treeline phenomenon. These local constraints may, however, have led to local tree mortality. At a global scale, there is no evidence that tall stature makes trees more susceptible to freezing damage.
The Disadvantage of Being Tall When the Growing Season Gets Cool
While protecting trees from experiencing significant radiative cooling and thus enhanced freezing stress, a high degree of aerodynamic coupling to the free atmosphere enforces convective heat exchange also during favorable periods of the growing season, causing trees to experience climatic conditions similar to those recorded by weather stations. Trees are thus, not taking significant (or enough) thermal advantage from solar heating, as is the case in all smaller stature vegetation (Fig. 3). A life form that evolved in response to competition for light, ground fires and browsing, is selected against, when the mean air temperature approaches the general thermal limit of plant growth, and when plant architecture does not facilitate significant departures from those adverse ambient temperatures. In contrast, all smaller stature plants are periodically experiencing a warmer microenvironment because of reduced heat exchange near the ground (high aerodynamic exchange resistance; Grace 1988; Grace et al. 1989; Körner 2007). This is also the simple explanation, why the bioclimatic treeline (a line connecting the uppermost patches of upright tree individuals) follows mountain topography like the shore lines of a lake (with only moderate variation), reflecting the average thermal layering of the atmosphere. The main reason why we have a treeline below the shrub limit or the general limit of higher plant life in mountains, is associated with the physical (aerodynamic) consequences of being tall.
There is a second, though minor drawback of tree stature when it gets cold. Successful tree life, requires the build-up of perennial structures that need a minimum period of time to mature and thus, reach a robustness that permits survival during less favorable periods. This is to some extent true for all latitudes, but particularly so at higher latitudes, when the growing season gets short. In addition to the minimum mean growing season temperature of ca. 6 °C, the global survey of treeline conditions also revealed that the mean duration of the growing season must be at least 94 days. A shorter season does not permit to complete xylogenesis and/or mature evergreen foliage. Shrubs, and particularly herbaceous plants are far more flexible, some can even survive a full year without being freed from snow pack, and seasons as short as 45 days suffice to complete a seasonal life cycle. Low stature life forms thus, take advantage from short lived foliage and developmental flexibility (variable number of leaf cohorts and rates of leaf turnover) compared to trees. Since treelines are commonly built at elevations (isotherms) where the season length significantly exeeds 94 days (the mean for the Alps is 135 days), season length constraints do not come into play very often.
Conclusions
The low temperature tree limit is reflecting rather basic constraints of plant tissue formation common to all cold-adapted plants, including, for instance, winter crops. Trees are affected by low temperature in such a way that their elevational limit follows a common isotherm across mountain terrain. The reason for this tight association between treeline and temperature is the intimate linkage between tree canopy temperature and the altitudinal gradient of temperature of the free atmosphere (contrasting low stature vegetation that is able to build its own, periodically warmer microenvironment). The fact that this boundary is often distorted or interrupted by a suite of disturbances and adverse local conditions, not associated with treeline in general, should not disguise from the more fundamental drivers set by the biology of trees in interaction with the free atmosphere. The identification and functional explanation of large-scale biogeographic patterns such as the treeline phenomenon, require a comparative approach, a clear separation of common from particulate drivers, and a distinction between physiological phenomena and disturbance regimes, with only the first permitting generalization. Since tree life at the natural treeline is primarily temperature controlled, and since tree canopies are closely coupled to ambient air conditions, undisturbed treeline position will inevitably track (with some delay) any sustained change in temperature, as it did in the geological past.
In this article I have shown that neither the burden of a massive stem, nor the year-round exposure to low temperature extremes of the atmosphere (because of tall stature), are placing trees at a disadvantage over shrubs or herbs. The tree architecture itself makes the main difference between trees and low stature vegetation, through its closer physical (aerodynamic) linkage to the conditions of the free atmosphere.
Acknowledgments
With great pleasure I dedicate this article to Professor Terry Callaghan as part of the festschrift at the occasion of his retirement from directorship at the Abisko Research Station. Much of the treeline theory presented here developed during my sabbatical stays at the station. I thank Jens Paulsen for providing his unpublished statistics on global treeline climatology and Susanna Riedl for the artwork. This paper developed while funded by the European Research Council, advanced Grant 233399, TREELIM.
Christian Körner
is a professor in Botany. His main research interests are the ecology of plants in cold environments, treeline biology, and the consequences of elevated atmospheric CO2 for plants and ecosystems.
References
- Alvarez-Uria P, Körner C. Low temperature limits of root growth in deciduous and evergreen temperate tree species. Functional Ecology. 2007;21:211–218. doi: 10.1111/j.1365-2435.2007.01231.x. [DOI] [Google Scholar]
- Bernoulli M, Körner C. Dry matter allocation in treeline trees. Phyton. 1999;39:7–12. [Google Scholar]
- Björklund L. Identifying heartwood-rich stands or stems of Pinus sylvestris by using inventory data. Silva Fennica. 1999;33:119–129. [Google Scholar]
- Bosshard HH. Holzkunde. Basel: Birkhäuser; 1984. [Google Scholar]
- Callaghan TV, Crawford RMM, Eronen M, Hofgaard A, Payette S, Rees WG, Skre O, Sveinbjörnsson B, et al. The dynamics of the tundra-taiga boundary: An overview and suggested coordinated and integrated approach to research. AMBIO. 2002;12:3–5. [PubMed] [Google Scholar]
- Germino MJ, Smith WK. Sky exposure, crown architecture, and low-temperature photoinhibition in conifer seedlings at alpine treeline. Plant, Cell and Environment. 1999;22:407–415. doi: 10.1046/j.1365-3040.1999.00426.x. [DOI] [Google Scholar]
- Gervais BR, MacDonald GM. A 403-year record of July temperatures and treeline dynamics of Pinus sylvestris from the Kola Peninsula, northwest Russia. Arctic, Antarctic, and Alpine Research. 2000;32:295–302. doi: 10.2307/1552528. [DOI] [Google Scholar]
- Gould, P.J., and C.A. Harrington. 2008. Extending sapwood—Leaf area relationships from stems to roots in Coast Douglas-fir. Annals of Forest Science. doi:10.1051/forest:2008067.
- Grace J. The functional significance of short stature in montane vegetation. In: Werger MJA, Aart PJM, During HJ, Verhoeven JTA, editors. Plant form and vegetation structure. The Hague: SPB Academic Publishing; 1988. pp. 201–209. [Google Scholar]
- Grace J, Allen SJ, Wilson C. Climate and the meristem temperatures of plant communities near the tree-lines. Oecologia. 1989;79:198–204. doi: 10.1007/BF00388479. [DOI] [PubMed] [Google Scholar]
- Harsch MA, Hulme PE, McGlone MS, Duncan RP. Are treelines advancing? A global meta-analysis of treeline response to climate warming. Ecology Letters. 2009;12:1040–1049. doi: 10.1111/j.1461-0248.2009.01355.x. [DOI] [PubMed] [Google Scholar]
- Hijmans RJ, Cameron SE, Parra JL, Jones PG, Jarvis A. Very high resolution interpolated climate surfaces for global land areas. International Journal of Climatology. 2005;25:1965–1978. doi: 10.1002/joc.1276. [DOI] [Google Scholar]
- Hoch G, Körner C. The carbon charging of pines at the climatic treeline: A global comparison. Oecologia. 2003;135:10–21. doi: 10.1007/s00442-002-1154-7. [DOI] [PubMed] [Google Scholar]
- Hoch, G., and C. Körner. 2011. Global patterns of mobile carbon stores in trees at high elevation treeline. Global Ecology and Biogeography. doi:10.1111/j.1466-8238.2011.00731.x.
- Hoch, G., A. Richter, and C. Körner. 2003. Non-structural carbohydrates in temperate forest trees. Plant Cell Environ 26: 1067–1081.
- Holtmeier F-K. Mountain timberlines. Ecology, patchiness, and dynamics. Berlin: Springer; 2009. [Google Scholar]
- Huber B, Prütz G. Über den Anteil von Fasern, Gefässen und Parenchym am Aufbau verschiedener Hölzer. Holz als Roh- und Werkstoff. 1938;1:377–381. doi: 10.1007/BF02613180. [DOI] [Google Scholar]
- Knapic S, Pereira H. Within-tree variation of heartwood and ring width in maritime pine (Pinus pinaster Ait.) Forest Ecology and Management. 2005;210:81–89. doi: 10.1016/j.foreco.2005.02.017. [DOI] [Google Scholar]
- Körner C. Biomass fractionation in plants: A reconsideration of definitions based on plant functions. In: Roy J, Garnier E, editors. A whole plant perspective on carbon–nitrogen interactions. The Hague: SPB Academic Publishing; 1994. pp. 173–185. [Google Scholar]
- Körner C. Alpine plants: Stressed or adapted? In: Press MC, Scholes JD, Barker MG, editors. Physiological plant ecology. Oxford: Blackwell; 1999. pp. 297–311. [Google Scholar]
- Körner C. Alpine plant life. Berlin: Springer; 2003. [Google Scholar]
- Körner C. Climatic treelines: Conventions, global patterns, causes. Erdkunde. 2007;61:315–324. doi: 10.3112/erdkunde.2007.04.02. [DOI] [Google Scholar]
- Körner C. Winter crop growth at low temperature may hold the answer for alpine treeline formation. Plant Ecology and Diversity. 2008;1:3–11. doi: 10.1080/17550870802273411. [DOI] [Google Scholar]
- Körner C. Alpine treelines. Basel: Springer; 2012. [Google Scholar]
- Körner C, Paulsen J. A world-wide study of high altitude treeline temperatures. Journal of Biogeography. 2004;31:713–732. doi: 10.1111/j.1365-2699.2003.01043.x. [DOI] [Google Scholar]
- Körner, C., J. Paulsen, and E.M. Spehn. 2011. A definition of mountains and their bioclimatic belts for global comparison of biodiversity data. Alpine Botany 121: 73–78.
- Kullman L. Dynamics of altitudinal tree-limits in Sweden: A review. Norsk Geologisk Tidsskrift. 1990;44:103–116. doi: 10.1080/00291959008552248. [DOI] [Google Scholar]
- Kullman L. Tree line population monitoring of Pinus sylvestris in the Swedish Scandes, 1973–2005: Implications for tree line theory and climate change ecology. Journal of Ecology. 2007;95:41–52. doi: 10.1111/j.1365-2745.2006.01190.x. [DOI] [Google Scholar]
- Larcher, W. 1985. Winter stress in high mountains. In Establishment and tending of subalpine forest: Research and management, ed. H. Turner, and W. Tranquillini. Berichte der Eidgenössischen Anstalt für das forstliche Versuchswesen 270: 11–20.
- Matyssek, R. 1985. Der Kohlenstoff-, Wasser-, und Nahrstoffhaushalt der wechselgrünen und immergrünen Koniferen Lärche, Fichte, Kiefer. PhD thesis. Bayreuth, Germany: University of Bayreuth.
- Matyssek R, Wieser G, Patzner K, Blaschke H, Haberle KH. Transpiration of forest trees and stands at different altitude: Consistencies rather than contrasts? European Journal of Forest Research. 2009;128:579–596. doi: 10.1007/s10342-008-0243-5. [DOI] [Google Scholar]
- Mayr S. Limits in water relations. In: Wieser G, Tausz M, editors. Trees at their upper limit. Berlin: Springer; 2007. pp. 145–162. [Google Scholar]
- Münster-Swendsen M. Index of vigour in Norway spruce (Picea abies Karst.) Journal of Applied Ecology. 1987;24:551–561. doi: 10.2307/2403892. [DOI] [Google Scholar]
- Oren R, Schulze E-D, Matyssek R, Zimmermann R. Estimating photosynthetic rate and annual carbon gain in conifers from specific leaf weight and leaf biomass. Oecologia. 1986;70:187–193. doi: 10.1007/BF00379238. [DOI] [PubMed] [Google Scholar]
- Paulsen J, Weber UM, Körner C. Tree growth near treeline: Abrupt or gradual reduction with altitude? Arctic, Antarctic, and Alpine Research. 2000;32:14–20. doi: 10.2307/1552405. [DOI] [Google Scholar]
- Rossi S, Desauriers A, Anfodillo T, Carraro V. Evidence of threshold temperatures for xylogenesis in conifers at high altitudes. Oecologia. 2007;152:1–12. doi: 10.1007/s00442-006-0625-7. [DOI] [PubMed] [Google Scholar]
- Sakai, A., and W. Larcher. 1987. Frost survival of plants. Responses and adaptation. Ecological Studies 62. Berlin: Springer.
- Sakai A, Okada S. Freezing resistance of conifers. Silvae Genetica. 1971;20:91–97. [Google Scholar]
- Sala, A., W. Fouts, and G. Hoch. 2011. Carbon storage in trees: Does relative carbon supply decrease with tree size? In Size- and age-related changes in tree structure and function (Tree Physiology 4), ed. F. C. Meinzer, B. Lachenbruch and T. E. Dawson, 287–306. Berlin: Springer. doi:10.1007/978-94-007-1242_11.
- Schönenberger W. Cluster afforestation for creating diverse mountain forest structures—A review. Forest Ecology and Management. 2001;145:121–128. doi: 10.1016/S0378-1127(00)00579-X. [DOI] [Google Scholar]
- Schulze E-D, Cermak J, Matyssek R, Penka M, Zimmermann R, Vasicek F, Gries W, Kucera J. Canopy transpiration and water fluxes in the xylem of the trunk of Larix and Picea trees—A comparison of xylem flow, porometer and cuvette measurements. Oecologia. 1985;66:475–483. doi: 10.1007/BF00379337. [DOI] [PubMed] [Google Scholar]
- Sellin A. Sapwood-heartwood proportion related to tree diameter, age and growth rate in Picea abies. Canadian Journal of Forest Research. 1994;24:1022–1028. doi: 10.1139/x94-133. [DOI] [Google Scholar]
- Smith WK, Germino MJ, Hancock TE, Johnson DM. Another perspective on altitudinal limits of alpine timberlines. Tree Physiology. 2003;23:1101–1112. doi: 10.1093/treephys/23.16.1101. [DOI] [PubMed] [Google Scholar]
- Squeo A, Rada F, Azocar A, Goldstein G. Freezing tolerance and avoidance in high tropical Andean plants: Is it equally represented in species with different plant height? Oecologia. 1991;86:378–382. doi: 10.1007/BF00317604. [DOI] [PubMed] [Google Scholar]
- Sterck FJ, Zweifel R, Sass-Klaassen U, Chowdhury Q. Persisting soil drought reduces leaf specific conductivity in Scots pine (Pinus sylvestris) and pubescent oak (Quercus pubescent) Tree Physiology. 2008;28:529–536. doi: 10.1093/treephys/28.4.529. [DOI] [PubMed] [Google Scholar]
- Stöcklin J, Körner C. Recruitment and mortality of Pinus sylvestris near the nordic treeline: The role of climatic change and herbivory. Ecological Bulletins. 1999;47:168–177. [Google Scholar]
- Sveinbjörnsson B, Hofgaard A, Lloyd A. Natural causes of the tundra-taiga boundary. AMBIO. 2002;12:23–29. [PubMed] [Google Scholar]
- Tranquillini, W. 1979. Physiological ecology of the Alpine Timberline. Tree existence at high altitudes with special references to the European Alps. Ecological Studies 31. Berlin: Springer.
- Troll C. The upper timberlines in different climatic zones. Arctic and Alpine Research. 1973;5:A3–A18. [Google Scholar]
- von Humboldt, A., and A. Bonpland. 1807. Ideen zu einer Geographie der Pflanzen nebst einem Naturgemälde der Tropenländer. Tübingen: F.G. Cotta; Paris: F. Schoell.
- Wieser G. Carbon dioxide gas exchange of cembran pine (Pinus cembra) at the alpine timberline during winter. Tree Physiology. 1997;17:473–477. doi: 10.1093/treephys/17.7.473. [DOI] [PubMed] [Google Scholar]
- Wieser G, Bahn M. Seasonal and spatial variation of woody tissue respiration in a Pinus cembra tree at the alpine timberline in the central Austrian Alps. Trees - Structure and Function. 2004;18:576–580. [Google Scholar]
- Wieser G, Tausz M. Trees at their upper limit—Treelife limitation at the Alpine Timberline. Dordrecht: Springer; 2007. [Google Scholar]
- Würth MKR, Pelaez-Riedl S, Wright SJ, Körner C. Non-structural carbohydrate pools in a tropical forest. Oecologia. 2005;143:11–24. doi: 10.1007/s00442-004-1773-2. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Siegwolf RTW, Durka W, Körner C. Phylogenetically balanced evidence for structural and carbon isotope responses in plants along elevational gradients. Oecologia. 2010;162:853–863. doi: 10.1007/s00442-009-1515-6. [DOI] [PubMed] [Google Scholar]