Abstract
Current atmospheric warming due to increase of greenhouse gases will have severe consequences for the structure and functioning of arctic ecosystems with changes that, in turn, may feed back on the global-scale composition of the atmosphere. During more than two decades, environmental controls on biological and biogeochemical processes and possible atmospheric feedbacks have been intensely investigated at Abisko, Sweden, by long-term ecosystem manipulations. The research has addressed questions like environmental regulation of plant and microbial community structure and biomass, carbon and nutrient pools and element cycling, including exchange of greenhouse gases and volatile organic compounds, with focus on fundamental processes in the interface between plants, soil and root-associated and free-living soil microorganisms. The ultimate goal has been to infer from these multi-decadal experiments how subarctic and arctic ecosystems will respond to likely environmental changes in the future. Here we give an overview of some of the experiments and main results.
Keywords: Tundra, Warming, Field experiments, Plant–microbe interactions, Carbon and nitrogen cycling
Long-Term Ecosystem Manipulation Studies of Plant–Microbe–Soil Interactions
During the previous two decades, a large number of plot-scale field manipulations of the environment have been established and maintained across the subarctic and arctic, many of which include soil and air warming with various types of passive greenhouses (Callaghan et al. 2004; van Wijk et al. 2004; Elmendorf et al. 2012). In some of the experimental sites, the scientific focus has been on plant–microbe–soil interactions and biogeochemistry. One of the earliest experiments, involving factorial manipulation of light, temperature, and nutrient availability has been maintained during more than two decades at a mesic treeline heath near Abisko, northern Sweden (Jonasson et al. 1999; Rinnan et al. 2007a) (Fig. 1a). This represents one of the longest ongoing ecosystem manipulation experiments in northern latitudes. The experiment consists of summer warming by open top, passive 1.2 × 1.2 m tent-like greenhouses, addition of fertilizer (simulating enhanced nutrient availability as an indirect effect of warming), and attenuation of 50 % of the light (simulating enhanced cloud cover) (Michelsen et al. 1996). Similar greenhouses and open-top chambers (OTCs) have also been used in warming experiments near Abisko in mountain birch (Betula pubescens ssp. czerepanovii) forest understory (Press et al. 1998; Sjögersten and Wookey 2009; Olsrud et al. 2010), alpine fellfield heath (Jonasson et al. 1999), wet heath (Rinnan et al. 2008) (Fig. 1b), higher altitude dry heat, meadow and tussock tundra (Björk et al. 2007; Molau 2010) and a peat bog (Dorrepaal et al. 2009) and typically lead to 1–2 °C soil warming, and to 3–4 °C air warming above ambient summer temperature. By placing heating cables in the soil, additional soil warming in forest understory has been achieved (Hartley et al. 1999), also in an experiment which included combined treatment with elevated CO2 (Olsrud et al. 2010). OTCs have furthermore been erected in late autumn and early spring to increase the frequency of freeze–thaw cycles in order to examine how these cycles affect microbes, soil arthropods, and nutrient availability (Konestabo et al. 2007). In winter, OTCs and snow addition have been applied on a peat bog in order to cause erratic snowmelt and to alter snow accumulation patterns (Aerts et al. 2006). In winter, warming has also been applied with infra-red heaters and soil cables to study the consequences of extreme winter warming events on heath ecosystem biota and processes (Bjerke et al. 2011; Bokhorst et al. 2011).
Fig. 1.

Ecosystem manipulations near Abisko, Northern Sweden. a Mesic Cassiope tetragona–Empetrum hermaphroditum–Betula nana heath near the treeline, with manipulations of light (dome-shaped greenhouses with shading cloth), temperature (open-top plastic greenhouses), and nutrient availability (NPK fertilizer). b Mixed, wet dwarf shrub heath with open-top greenhouses and mountain birch litter addition plots in a fully factorial design. c Measurement of greenhouse gas exchange in species removal experiments in the understory of a mountain birch birch (Betula pubescens ssp. czerepanovii) forest. Photos by Anders Michelsen
In addition to warming treatments, nutrients have frequently been added to experimental plots (Press et al. 1998; Jonasson et al. 1999). In high doses, responses to such additions (Jonasson and Michelsen 1996) increase our understanding of how nutrients act as drivers in these, often nutrient-poor ecosystems. In low doses, the additions are more similar to the natural pulse-increase in nutrient availability, which may take place periodically during, e.g., snow melt in spring or freeze–thaw cycles in autumn (Konestabo et al. 2007; Larsen et al. 2007a), with likely effects on microbial immobilization of nutrients (Andresen et al. 2008).
As an alternative to addition of inorganic nutrients, birch leaf litter has been added annually since 1999 in a long-term litter addition and warming experiment at a wet heath near Abisko (Sjursen et al. 2005; Rinnan et al. 2007b, 2008; Sorensen and Michelsen 2011) (Fig. 1b). The amount added each autumn is comparable to the litterfall in the mountain birch forest, now undergoing warming-induced expansion in Northern Scandinavia (Kullman and Öberg 2009; Rundqvist et al. 2011). The nutrients are gradually released from the litter by leaching and litter decomposition, and the experiment probably better simulates the stimulation of mineralization, as one of the probable effects of enhanced nutrient availability by warming (Rinnan et al. 2008).
Other experiments in the Abisko region have included changes of substrate quality, nutrient availability, and microbial biomass by addition of labile carbon (sugar) to stimulate microbial growth, fungicide (benomyl) and bactericides (penicillin and streptomycin) to control specific microorganisms, and plant removals (Fig. 1c) to examine plant–plant and plant–microbe interactions (Christensen et al. 1999; Michelsen et al. 1999; Haugwitz and Michelsen 2011; Haugwitz et al. 2011; Faubert et al. 2012). In addition, effects of enhanced UV-B on vascular plant, lichen, and moss performance and on nitrogen-fixing potential of cyanobacteria associated with mosses have been evaluated in experiments conducted in the area (Solheim et al. 2002; Arroniz-Crespo et al. 2011).
Plant Functional Types, Nutrient Availability, and Responsiveness to Changes
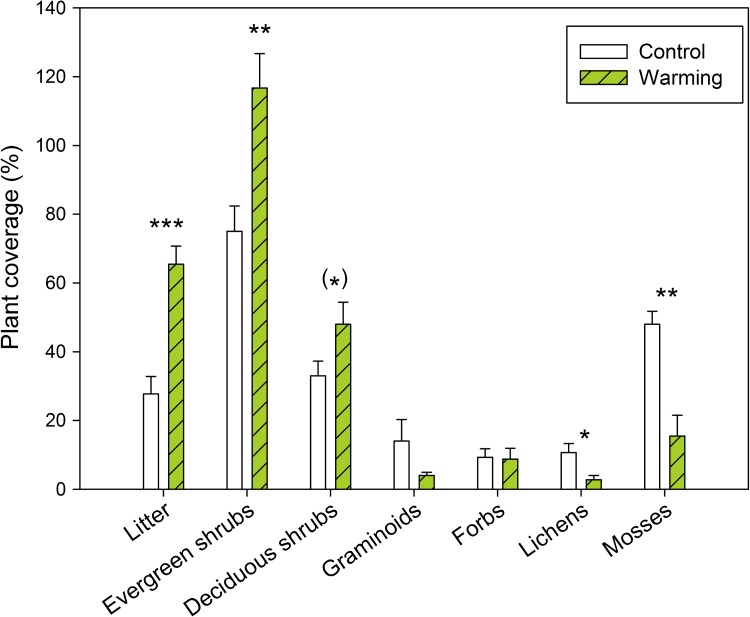
The experiments in the forest understory (Press et al. 1998), the wet (Sorensen and Michelsen 2011) and the mesic (Graglia et al. 2001) heaths and the high altitude fellfield (Michelsen et al. 1996) have shown that both warming and, in particular, enhanced nutrient availability promote a higher biomass of dwarf shrubs, but that sub-canopy mosses and lichens decline strongly as the plant canopy closes. Hence, plant species diversity is expected to be reduced in subarctic heaths following warming because of loss of cryptogams, while plant C accumulation is expected to increase as woody plants with large stem masses increase their cover. However, if extreme events of winter warming occur, this may counteract shrub expansion because vascular plants are more sensitive to damage than lichens (Bjerke et al. 2011). At the mesic heath, effects of warming have progressed during the two decades of study (Table 1). Warming already led to significant increases in evergreen shrubs during the first 5 years of treatment (Jonasson et al. 1999), while the effect became significant for deciduous shrubs first after a decade (Table 1). After two decades, these effects were maintained, with about 50 % increase in shrub cover, and a reduction of moss and lichen cover to one-third of that in unperturbed plots (Fig. 2). As in other subarctic heaths, grasses, and forbs had low cover and did not respond significantly, even after two decades of warming (Sorensen et al. 2012). These responses are consistent with a global assessment of experimental climate warming on tundra vegetation, which has shown that shrubs increase with warming only where ambient temperature is highest, i.e., in the Subarctic and Low Arctic, whereas graminoids increase primarily in high arctic sites (Elmendorf et al. 2012).
Table 1.
Medium and long-term plant and soil responses to experimental warming with open-top tents at mesic Cassiope tetragona heath at the treeline near Abisko, Northern Sweden since 1989
| Response variable/Time span | 5-9 yrs | 10-14 yrs | 15-19 yrs | 20-23 yrs |
|---|---|---|---|---|
| Plant | ||||
| Deciduous shrub cover or biomass | – | ↑ | n.d. | (↑) |
| Evergreen shrub cover or biomass | ↑ | – | n.d. | ↑ |
| Herb cover or biomass | – | – | n.d. | – |
| Moss cover or biomass | – | ↓ | n.d. | ↓ |
| Lichen cover or biomass | – | ↓ | n.d. | ↓ |
| Fine root biomass | ↑ | ↑ | – | n.d. |
| Deciduous leaf N concentration | – | ↑ | – | – |
| Evergreen leaf N concentration | (↓) | – | – | – |
| Soil | ||||
| Soil inorganic N concentration | – | – | ↑ | n.d. |
| Soil inorganic P concentration | ↑ | – | – | n.d. |
| Soil fungal biomass | – | – | – | n.d. |
| Microbial biomass C concentration | – | – | ↓ | n.d. |
| Soil C stock | – | – | n.d. | n.d. |
Data are from measurements at peak plant biomass in late July or early August. Soil data are 0–10 cm depth. Direction of arrows indicates significant (P < 0.05) responses, evaluated with one-way ANOVA comparing warmed versus control plots (n = 6); “–” signifies no change; arrows in brackets indicate a tendency towards significance (P < 0.05 < P < 0.10); n.d. not determined
Data sources: Responses after 5–9 years: Jonasson et al. (1999), Ruess et al. (1999), Graglia et al. (2001); 10–14 years: Graglia et al. (2001); Clemmensen et al. (2006), van Wijk et al. (2004), Sorensen et al. (2008a, b), Michelsen and Jonasson (unpubl.); 15–19 years: Rinnan et al. (2007a, 2011b); 20–23 years: van Driel and Michelsen (unpubl.), Sorensen et al. (2012)
Fig. 2.
Plant coverage at mesic treeline heath near Abisko, N. Sweden after 20 years of summer warming with open-top plastic greenhouses. Mean coverage (%) of different plant functional types (± SE, n = 6) in the control and warming treatment in analysis of 100 pin-points in July 2008. Results of one-way ANOVA’s for different plant functional groups are indicated; (*) P < 0.10, * P < 0.05, ** P < 0.01, *** P < 0.001. Modified from Sorensen et al. (2012)
Warming has resulted in either unchanged or occasionally increased nutrient availability (Table 1). For instance, inorganic phosphorus (P) availability increased after 5 years of warming at the mesic heath (Jonasson et al. 1999) and soil ammonium concentration increased after 15 years of treatment at the same site (Rinnan et al. 2007a), while midsummer responses were insignificant in other years (e.g., Sorensen et al. 2008b). Increased concentrations are consistent with data from several other sites showing stimulated mineralization rates (Robinson et al. 1995; Schmidt et al. 2002), which is a prerequisite for enhancement of plant nutrient pool sizes (Sorensen et al. 2008a, b).
In initial phases of warming, the expanding evergreen shrubs at the mesic heath responded by a tendency towards reduced leaf N concentration (Table 1; Michelsen et al. 1996) due to accelerated growth but intensified N limitation. By contrast, deciduous plants showed increased leaf N concentration but first after more than a decade (Sorensen et al. 2008b), suggesting that N mineralization now was sufficient to meet plant demand. After two decades, the N concentrations in warmed plots were similar to controls (Table 1). However, these responses differ not only between different time-spans of the warming experiments but also between ecosystem types. In evergreens from warmed fellfield plots, leaf N was reduced still after a decade and the tannin to N ratio was increased (Hansen et al. 2006). Consequently, if these responses persist in the fellfield, the changes could lead to lower tissue quality for herbivores and reduced decomposition, because of higher recalcitrance of litter.
Nutrient addition led to increased vascular plant biomass in several subarctic ecosystem types (Press et al. 1998; Jonasson et al. 1999), but plant biomass changes may be dampened due to immobilization of nutrients in microbial biomass (Jonasson et al. 1999; Haugwitz et al. 2011). These results suggest that the strong microbial nutrient sink in subarctic and alpine heaths may mitigate changes in the plant biomass even if warming increases the mineralization rate.
Belowground C Pools and Environmental Changes
In many subarctic heaths, the major part of the total ecosystem carbon (C) pool is found in the soil, due to temperature constraints on decomposition (Jonasson et al. 1999). Hence, although the total plant C pool may increase by warming (Fig. 2; Table 1), the changes in soil pools may be decisive for overall ecosystem C balance. For instance, at the mesic heath (Fig. 1a) soil C was unchanged following a decade of warming (but increased by fertilizer addition) (Clemmensen et al. 2006; Table 1). This contrasts with expectations of increased warming-induced losses of C to the atmosphere from tundra ecosystems (Mack et al. 2004). While more CO2 is emitted from the system in warmed plots (Illeris et al. 2004), the soil C stock at least to 15 cm depth is unchanged (Clemmensen et al. 2006). This suggests that a major part of the respired C is derived from recently fixed labile C from litter and root exudates (Grogan et al. 2001), and another part possibly from deeper soil layers, emphasizing that gains in plant C pools must be compared to potential losses in soil pools in the entire soil horizon in order to estimate the long-term fate of C storage and release (Mack et al. 2004).
Warming significantly increased the biomass of fine roots at the mesic heath, both after 5 and 10 years of treatment (Table 1), and in higher altitude dry heath warming increased the specific root length in the fine root fraction (Björk et al. 2007). Likewise, at the wet heath (Fig. 1b), the root biomass and the dissolved organic C (DOC) concentration in the rooting zone also increased, by about 30 and 17 %, respectively (Rinnan et al. 2008), possibly due to a higher supply of root exudates (Rinnan et al. 2008). However, warming led to 30 % higher losses of DOC below the rooting zone at the mesic heath (Michelsen et al., unpublished data), suggesting that warming will increase CO2 emission from subarctic streams and water bodies through DOC derived from terrestrial ecosystems (Christensen et al. 2007) and reduce the quality of drinking water.
Mycorrhizal Fungi: Function, Interactions with Host Plants, and Responses to Environmental Changes
Mycorrhizal fungi form an important part of the belowground microbial community at subarctic and arctic heaths (Michelsen et al. 1998). Responses to environmental changes in these microbial groups are important, because mycorrhizal fungi supply the host plants with nutrients from the soil in exchange for labile C from the plant, and because fungal composition and diversity influences plant productivity, stability and diversity. In the Subarctic and Low Arctic, most studies of fungal responses to warming and nutrient addition (Clemmensen and Michelsen 2006; Clemmensen et al. 2006) or plant species interactions (Urcelay et al. 2003) have focused on ectomycorrhizal fungi, because many of these fungi easily can be distinguished morphologically and because they colonize roots of widespread arctic shrubs species as dwarf birch (Betula nana) and willows (Salix spp). For instance, the ectomycorrhizal community on roots of the prostrate willow (Salix herbacea x polaris) at an arctic-alpine fellfield consists of at least seven fungal species, of which some may also be found in boreal forests. Despite a short growing season of only 2.5 months or less, the fungal colonization of short root tips is high, and gradually increasing from 60 % at snowmelt (late June) to 85 % in early autumn (late August) (Clemmensen and Michelsen 2006).
Eleven years of warming of the fellfield generally had modest effects on the fungal community, with a balanced increase in the number of colonized root tips of all fungal morphotypes and no change in the total percentage of colonized root tips (Clemmensen and Michelsen 2006). However, one dominant fungal species (Cenococcum geophilum) decreased by warming and fertilization, which may indicate that a shift from drought stress tolerant fungi towards a dominance of fungi more tolerant to high level of mineral nutrients may take place if nutrient availability increases substantially due to anthropogenic disturbances (Clemmensen and Michelsen 2006). At the mesic heath (Fig. 1a), fungal biomass in fine roots increased in warmed plots due to higher root mass (Clemmensen et al. 2006) but did not increase in the soil (Table 1). Increased abundance of ectomycorrhizal fungi in a warmer climate may contribute to increased belowground C sink in tundra ecosystems due to the role of fungal biomass in humus formation, while C turnover may be accelerated due to enhanced activity by heterotrophic fungi and bacteria.
Although ectomycorrhizal shrubs dominate many arctic shrub tundra types and roots of most boreal and subarctic tree species, the ericoid mycorrhizal fungi, which form symbiosis with plants in the heather family, are probably more important for biogeochemical cycling of C and N in many tundra types. This is, firstly, because wide areas of both arctic and subarctic tundra and subarctic forest understory are dominated by such plants, and secondly because these fungi to a larger extent than ectomycorrhizal fungi produce enzymes that break down soil organic matter. In addition to ericoid mycorrhiza, ericaceous roots also harbor the so-called dark septate endophytes (DSE), fungi with unknown ecological function (Olsrud et al. 2007). Both fungal groups consist of many individual species, and molecular analysis of the fungal community of ericaceous roots from a mire near Abisko showed that the fungal community consisted of more than 30 operational taxonomic units of ericoid mycorrhizal fungi and DSE fungi (Kjøller et al.2010). Furthermore, while four co-existing ericaceous plants at local scale shared fungal symbionts, plants a few meters away hosted a different fungal community (Kjøller et al. 2010). It is uncertain whether this spatial distribution of fungal species is due to random founder effects, plant biomass or environmental conditions, as nutrient availability or temperature.
In order to couple fungal community characterization to ecosystem function, effects of enhanced CO2 and warming on overall fungal colonization of ericaceous roots by ericoid mycorrhizal and DSE fungi was recently studied in a mountain birch forest understory with ericoid shrubs and a grass near Abisko (Olsrud et al. 2010). The ericoid mycorrhizal fungi increased under elevated atmospheric CO2 concentrations, but did not respond to warming following 6 years of treatment. This suggests that the higher ericoid mycorrhizal colonization under elevated CO2 might be due to increased transport of C belowground to acquire limiting resources such as N, which was diluted in the plant leaves under enhanced CO2. By contrast, arbuscular-mycorrhizal fungi showed reduced colonization of grass roots in elevated CO2, possibly because they could not match the enhanced root growth. The DSE fungi in grass roots increased by warming of the birch forest understory but did not respond to enhanced CO2 (Olsrud et al. 2010). However, the implications for plant N uptake and soil C storage remain unclear as the ecological function of this fungal group is unknown, and more research on this fungal group is warranted.
Nitrogen Cycling in Strongly N-Limited Ecosystems
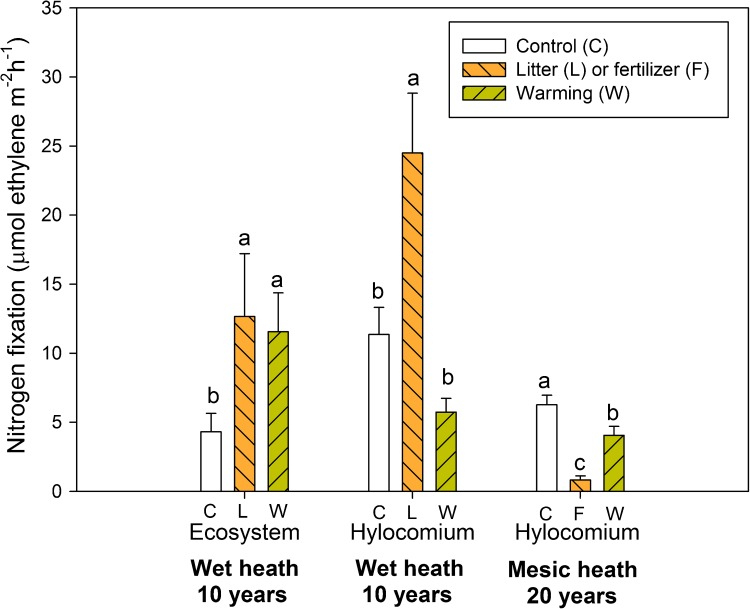
Characterization of the controls on nitrogen (N) cycling is critical to understanding the functioning of high latitude ecosystems, and to predicting responses to perturbations. Plant C accumulation is limited by the lack of N in many tundra ecosystems because N input with deposition is low (e.g., 0.23 g N m−2 year−1 in the Abisko region in N. Sweden; Karlsson et al. 2009; Sorensen and Michelsen 2011), partly due to long distance to N sources such as industry, agriculture, and traffic. Nitrogen fixation in lichens with cyanobacteria as symbionts, and in cyanobacteria associated with mosses, contribute with “new” N of the same order of magnitude as N deposition, with high fixation in surface crusts with cyanobacteria, intermediate fixation in moss and lower fixation in dense heaths (Sorensen et al. 2006). Nitrogen fixation associated with mosses is promoted by enhanced precipitation, while lichen-associated N2 fixation is sensitive to enhanced UV-B (Solheim et al. 2002). A decade of warming led to a doubling of N2 fixation in the wet heath at ecosystem level (Fig. 3) (Sorensen and Michelsen 2011). However, N2 fixation associated with specific moss species tended to be reduced in warmed plots, partly because of drying, and partly because the denser canopy formed by the shrubs in the longer term led to reduced moss and lichen cover (Fig. 2). Although litter addition stimulated N2 fixation after 10 years of treatment of the wet heath (Fig. 3), probably due to the relatively high P content in birch litter (Sorensen and Michelsen 2011), the very high nutrient input with NPK fertilizer strongly reduced N2 fixation at the mesic heath (Fig. 3) (Sorensen et al. 2012). If N input increases due to deposition, N2 fixation is likely to decrease. Similarly, both dryer conditions and shading from an increased vegetation cover in the long run leads to a negative feedback on N2 fixation.
Fig. 3.
Long-term effects of warming, litter addition and fertilizer addition on nitrogen fixation measured with the acetylene reduction method at ecosystem level (20 × 20 cm heath squares), and in plugs with the moss Hylocomium splendens. Data are from the wet heath after 10 years of treatment, and from the mesic heath after 20 years of treatment. Data are means across growing season, adapted from Sorensen and Michelsen (2011) and Sorensen et al. (2012). Significant differences between treatments investigated with repeated measures mixed model ANOVA with Tukey adjusted means comparison. Treatments influenced means in all cases, P < 0.05. Within site or type, bars with the same letters are not significantly different (± SE, n = 6)
As for the N input, also the N export from many subarctic ecosystems is presently very low. One reason may be that plants partition the dissolved N, so that the most productive plants take up the most abundant form of N, while less productive species tap less abundant forms (McKane et al. 2002). The availability of N in different forms (NO3−, NH4+, amino acids), and the cycling between plants, microbes, fungal mycelium and soil has been intensively investigated at Abisko, both in experiments designed to address fundamental questions on plant N acquisition and mycorrhizal function (Andresen et al. 2008; Clemmensen et al. 2008; Krab et al. 2008), and in climate change related experiments (Sorensen et al. 2008a, b; Olsrud and Michelsen 2009).
The 15N natural abundance has been used as a tool to investigate plant and microbial N acquisition at subarctic and arctic heaths (Michelsen et al. 1998). Ericoid mycorrhizal plants are clearly the most depleted in 15N, ectomycorrhizal plants less depleted and non- and arbuscular-mycorrhizal plants relatively more enriched in 15N (Michelsen et al. 1998), a pattern which has been confirmed as global phenomenon, although much less pronounced than in subarctic and arctic settings (Craine et al. 2009). The pattern is probably both due to differences in N forms acquired by plants with different mycorrhizal symbionts, and due to fractionation during transfer of N from fungi to host plant (Michelsen et al. 1998; Craine et al. 2009; Yano et al. 2010). Hence, as multiple reasons may explain 15N natural abundance patterns, studies with 15N enrichment may often provide clearer information on N transfers within ecosystem compartments and exchange with the environment. For instance, by injection of 15N-enriched compounds separately in soil and in root-free soil compartments with mycorrhizal fungal access solely, it has been shown that ectomycorrhizal dwarf birch (Betula nana) roots showed highest preference for ammonium while the fungal component showed low nitrate uptake (Clemmensen et al. 2008). Furthermore, ecto- and ericoid mycorrhizal plants show higher N uptake than non-mycorrhizal plants in long-term studies, with uptake of N in both inorganic and organic form, as amino acids (Andresen et al. 2008).
Microbes generally immobilize more than half and even up to 90 % of pulse-added 15N after a few days, while plants typically access less than 5 % (Andresen et al. 2008; Sorensen et al. 2008a, b). Low plant species diversity in some ecosystem types may imply that the plant community potentially could be less efficient in retaining N, which, consequently, may be leached from the ecosystem, for instance, during freeze–thaw in autumn and spring when plant N uptake still may take place but lysis of soil microbes is high (Larsen et al. 2007a). However, in most subarctic heath and forest understory systems, plants seem efficient in taking up N released from dying microbes. For instance, when adding low amounts of 15N to a species-rich subarctic heath, and to a more species-poor birch forest understory, the N cycling in both ecosystems was dominated by a substantial turnover of 15N recently acquired by microbes that coincided with a significant increase in plant 15N uptake (Grogan and Jonasson 2003; Andresen et al. 2008).
Strong demand for N led to high plant retention of 15N in the species-rich mesic heath (Fig. 1b) even 1 year after 15N pulse-addition (Sorensen et al. 2008a, b), but 15N export could potentially be higher in the species-poor ecosystem type with less diversified niches of plant functional types, which remain to be investigated. At the mesic heath, microbes harbored 90 % of the added 15N one day after addition, but less than 30 % after 1 year, when more than 40 % of the total 15N was strongly bound in recalcitrant non-microbial soil organic matter fractions, to which plants without ericoid mycorrhiza have limited or no access.
Interestingly, 14 years of summer climate manipulations at the mesic heath had little or no effect on the partitioning of added 15N between plant, microbial and soil pools (Sorensen et al. 2008a, b). However, in the short term, environmental factors affect plant nutrient and C acquisition. For instance, light attenuation similar to an enhanced cloud cover led to reduced photosynthesis and allocation to mycorrhizal fungi, but mycorrhizal uptake of 15N13C-labeled amino acid was still high, demonstrating that plants prioritize N uptake in nutrient deficient subarctic tundra types (Olsrud and Michelsen 2009).
Soil Microbial Community Responses to Manipulations
Because microbial activity governs the processing of soil C and because the microbial pool of N and P equals or even exceeds the amount stored in vegetation (Jonasson et al. 1999; Schmidt et al. 2002), it is vital to know how environmental factors affect the microbial biomass. The fumigation-extraction technique has been used to follow effects of experimental manipulations on microbial biomass over 15 years (Fig. 4).
Fig. 4.
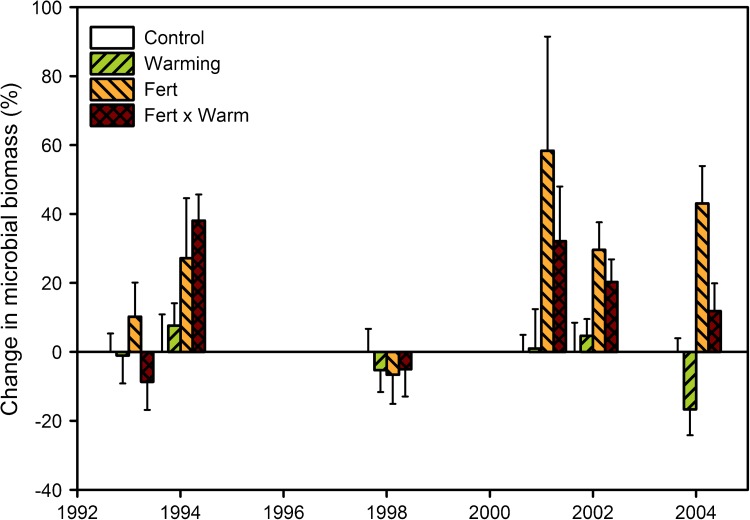
Microbial biomass C in mesic shrub heath with open-top chambers and fertilizer addition plots in a fully factorial design. Treatments were initiated in 1989. The figure shows the relative change in microbial biomass C compared to control plots (± SE, n = 6). Data modified from Jonasson et al. (1999), Ruess et al. (1999), S. Jonasson and A. Michelsen (unpublished), Rinnan et al. (2007a) and Sorensen et al. (2008a, b). Microbial biomass C differed between years (P < 0.001; repeated measures two-way ANOVA) and was generally significantly higher in fertilized plots (P < 0.01) but with a tendency towards interaction between year and fertilizer treatment (P < 0.1)
The warming had not significantly affected microbial biomass C, N, or P in the mesic heath soils until after 15 manipulation years (Jonasson et al. 1999; Ruess et al. 1999; Rinnan et al. 2008), when the microbial biomass C concentration was 17 % lower in the warmed than in the control soil of the mesic heath (Rinnan et al. 2007a; Fig. 4). The reduction was not expected because the same treatment led to increased shrub biomass (Table 1) (Sorensen et al. 2008a, b, 2012). One of the reasons for the unchanged or even reduced microbial biomass could be top-down regulation caused by grazing by fungal and bacterial feeding nematodes, which doubled in abundance after 8 treatment years (Ruess et al. 1999). More specific analyses of the microbial community support the finding of reduced microbial biomass. Bacterial community growth rate was significantly decreased by warming, by as much as 73 % after 17 treatment years (Rinnan et al. 2011a). As microbial community composition had been little affected by warming (Rinnan et al. 2007b, 2008), the reduced growth rate appears to be due to lower substrate quality in the warmed soil. Changes were more distinct at the mesic heath with 15 manipulation years (Rinnan et al. 2007a) than in the wet heath site with 7 manipulation years (Rinnan et al. 2008). While the fungal biomass was unchanged (Table 1), it is likely that bacterial growth was limited by substrate availability, since the mainly N-limited bacterial communities had shifted towards limitation by C and P in response to 7 years of warming (Rinnan et al. 2007b).
In the long term, warming is expected to lead to higher soil nutrient availability following stimulated nutrient mineralization in soil (Callaghan et al. 2004). However, in situ buried bag studies suggest that the soil temperature increase by 1–2 °C as in the passive warming (Michelsen et al. 1996; Ruess et al. 1999) does not significantly increase the microbial net N mineralization (Robinson et al. 1995; Schmidt et al. 2002; Jonasson et al. 2006; Rinnan et al. 2007b). When soil warming by 5 °C was accomplished by heating cables buried 5 cm below the soil surface, seasonal net mineralization was transiently doubled after 2 years, but the difference disappeared after 5 years (Hartley et al. 1999). Hence, fertilization treatments at high doses, intended to mimic the expected higher soil nutrient availability in response to warming, do not therefore reflect long-term effects of climate warming but rather they serve as a means to study effects of alleviated nutrient limitation.
Effects of fertilization, a much more dramatic treatment than warming, have developed over long time: in the mesic heath, microbial biomass was marginally affected by fertilization after 5 (Jonasson et al. 1999) and 6 (Ruess et al. 1999) years but not after 10 treatment years (S. Jonasson and A. Michelsen, unpublished data). After 12 treatment years, there was 58 % more microbial biomass C in the fertilized soil as compared to unfertilized soil (Sorensen et al. 2008a), and this increase remained at 43 % with 3 more years of manipulation treatment (Rinnan et al. 2007a) (Fig. 4). At the alpine fellfield, which had been fertilized for 10 years, however, the microbial biomass was unchanged 6 years after the treatment ceased, except if labile C in the form of sugar also had been added, in which case microbial biomass C, N, and P was still higher than in control plots (Haugwitz et al. 2011). An analysis of the microbial community composition at the lower altitude heaths using lipid biomarkers has shown that the fertilization-induced increase in microbial biomass is mainly due to an increase in the amount of soil fungi (Rinnan et al. 2007a). Tracking incorporation of isotopic signature from different C substrates to microbial biomarker lipids has further revealed that fertilization stimulates fungal uptake of simple organic substrates and the breakdown of more complex substrates, as, e.g., vanillin (Rinnan et al., unpublished data).
The treatment with mountain birch litter addition, used to mimic the natural fertilization effect of the increasing leaf litter deposition following the ongoing deciduous shrub expansion, has not altered the N concentrations or pools in soil or microbial biomass, but it has increased the concentrations of inorganic and microbial biomass P (Rinnan et al. 2008). While the total microbial biomass was unaffected by this treatment, the relative proportion of Gram-positive bacteria in the microbial community increased (Rinnan et al. 2008). When combined with warming, litter addition significantly increased the bacterial growth rate and altered the chemical quality of the soil (Rinnan et al. 2007b).
Most manipulation treatments suffer from unwanted side effects. For soil microbial communities, the most critical of these is probably drying of soils in warming treatments, as water availability is one of the most important factors affecting microbial activity. It has been suggested that litter decomposition is limited by water availability in plots warmed by OTCs (Aerts 2006). The warming treatment of the mesic heath reduced the soil water content in the uppermost 5 cm by 37 % (Rinnan et al. 2007a) despite the access of rainwater to the plots both from the open top of the greenhouses and from surface runoff on the sloping terrain. Here and in the combined warming and litter addition treatment (Rinnan et al. 2007b), higher evaporation and transpiration from a higher plant biomass may partly explain the drying. Hence, drying of the soil surface may accompany warming, also under natural field conditions.
The warming-induced changes in microbial activity may influence the soil C stores, because microbial activity is one of the most important drivers determining the C balance of arctic soils under climate change. The responses in warming experiments described above have all been measured during the growing season, when the microbial biomass in these types of soils is largely constant (Jonasson et al. 1999). However, major fluctuations take place during winter and early spring, when the snowmelt water and repeated freeze–thaw cycles perturb the soil environment (Larsen et al. 2007a).
Exchange of Greenhouse Gases in Subarctic Heath Ecosystems
Arctic soils harbor such a large amount of C so that its release to the atmosphere would seriously alter the atmospheric C concentrations. Carbon dioxide is taken up from the atmosphere by photosynthetic activity and released by respiration of plants, animals and microorganisms. The balance between uptake and release determines whether an ecosystem is a sink or source of CO2, and this C balance can be altered by environmental perturbations and climate change. In general, it is expected that climate change stimulates plant biomass production and photosynthetic uptake of CO2, but increases loss of C from soils (Mack et al. 2004).
Most research into effects of climate change on C balance of subarctic ecosystems has focused on wet peaty ecosystems with CH4 emission comprising a significant part of the ecosystem gas exchange. Here, we concentrate on heaths, which are considered a small sink of CO2 and CH4 from the atmosphere. In the Torneträsk catchment area in Northern Sweden, the average CO2 uptake by heaths is estimated to be 3 g C m−2 year−1 (Christensen et al. 2007). However, spatial variation in CO2 exchange is high (Fox et al. 2008), and the same site can be a sink in some years and a source of CO2 in other years (Christensen et al. 2007).
After a decade of experimental manipulations at the mesic heath in Abisko (Fig. 1a), a chamber technique was used for in situ measurements of ecosystem CO2 fluxes over the growing season (Illeris et al. 2004). Fertilization increased both ecosystem respiration and gross ecosystem production, while warming had no significant effects (Illeris et al. 2004). In contrast, summer warming by OTCs in tundra in Alaska and Canada, and in other tundra types in Scandinavia has been shown to increase annual CO2 efflux (Welker et al. 2000, 2004; Sjögersten and Wookey 2009). The contrasting responses to warming may be due to differences in soil moisture conditions, which strongly control ecosystem respiration, especially in mesic-dry heath systems.
The wet heath site (Fig. 1b) was a net sink for CO2 during the growing season, but both warming and litter addition treatments reduced net ecosystem CO2 exchange so that the system shifted into a net source of CO2 when the treatments were combined (Tiiva et al. 2008). The elevated temperature increased the decomposition of the added leaf litter and possibly also enhanced the release of previously accumulated C from the peaty soil, leading to higher CO2 release than the photosynthetic CO2 uptake by the increased plant biomass (Rinnan et al. 2008; Tiiva et al. 2008). Although respiration derived from recently photosynthesized C is more temperature-sensitive than respiration from soil organic matter C, climate warming can also accelerate respiration of deeper soil C (Dorrepaal et al. 2009).
Wintertime C losses can account to a significant part of the annual C budget (Fahnestock et al. 1999), and the C efflux is likely to increase if the snow depth increases (Welker et al. 2000; Larsen et al. 2007a). In the mesic heath, cold-season (October–May) respiration accounted for 22 % of the annual respiratory CO2 release, and rather surprisingly, photosynthetic activity outside the growing season accounted for 19 % of the annual gross CO2 uptake, partly compensating for the respiratory C losses (Larsen et al. 2007b). Freeze–thaw cycles appear to have few if any effects on CO2 release (Larsen et al. 2007b).
The heath ecosystems are in general small net sinks for the greenhouse gas methane, which is consumed by methanotrophic bacteria in the aerobic soil. Inorganic N additions have been observed both to stimulate (Christensen et al. 1997) and inhibit (Christensen et al. 1999) methane consumption in subarctic heaths. Emission of another strong greenhouse gas, N2O, from subarctic heath soils is minimal, and is stimulated by inorganic N additions (Christensen et al. 1999).
Emission of Biogenic Volatile Organic Compounds (BVOCs)
The subarctic heath and forest ecosystems are sources for a plethora of reactive trace gases called biogenic volatile organic compounds (BVOCs), which are emitted from plants both constitutively and in response to biotic and abiotic stresses. As an example of the emitted compounds, willow (Salix) species, mosses and sedges emit isoprene (Ekberg et al. 2009, 2011; Rinnan et al. 2011b), the crowberry (Empetrum hermaphroditum) sesquiterpenes (Faubert et al. 2012), Vaccinium species monoterpenes (Faubert et al. 2012), and the dwarf birch (Betula nana L.) and the mountain birch (Betula pubescens ssp. czerepanovii) emit many monoterpenes and sesquiterpenes (Haapanala et al. 2009; Rinnan et al. 2011b). Although the BVOCs are mainly released from vegetation, soils and microbial processes also contribute to the total ecosystem BVOC emissions (Faubert et al. 2012).
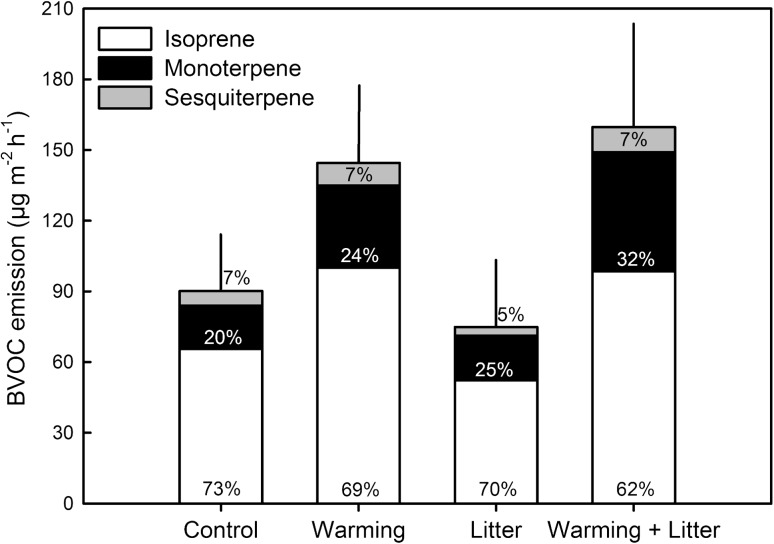
It seems that the subarctic BVOC emissions increase more per unit temperature increase than the emissions from vegetation at lower latitudes (Holst et al. 2010; Faubert et al. 2010). Warming of the wet heath (Fig. 1b) has been shown to double the emissions of monoterpenes and sesquiterpenes (Faubert et al. 2010) and to increase the isoprene emissions by 50–80 % (Tiiva et al. 2008; Fig. 5). Furthermore, especially when combined with mountain birch litter addition, warming increased the relative emission of the highly reactive sesquiterpenes (Fig. 5). The warming-induced increase in BVOC emission could be due to direct stimulation of BVOC production or release by higher air temperature, or due to indirect effects via long-term changes in vegetation composition or biomass in response to warming. The first explanation is most likely, because the warming effect was not significant when the open-top greenhouses were removed (Tiiva et al. 2008; Faubert et al. 2010).
Fig. 5.
Volatile organic compound emissions from mixed, wet dwarf shrub heath with warming by open-top chambers and litter addition plots. The figure shows average emissions (± SE, n = 6) from July 2006 and 2007, after 8–9 years of treatment. Data modified from Tiiva et al. (2008), and Faubert et al. (2010). Percentage of isoprene, monoterpene and sesquiterpene emissions of the total BVOC emission in each treatment is shown
Increased BVOC emissions in response to warming may influence climate via complex atmospheric processes (Peñuelas and Staudt 2010). In the arctic areas free of anthropogenic pollutants, the most relevant process is probably the formation of secondary organic aerosols with further effects on radiation scatter and cloud formation.
Long-Term Fate of Subarctic Tundra Ecosystems
Subarctic heath ecosystems are experiencing rapidly advancing climatic warming, which is shaping the vegetation cover of the tundra landscape with, e.g., mountain birch spreading to higher elevation and further north. The long-term experiments suggest that deciduous and evergreen shrubs will expand their cover at the expense of mosses and lichens. This affects the C and nutrient cycling in heath ecosystems, with consequences for C pools and greenhouse gas emissions. Warming will probably lead to enhanced emission of CO2 and a whole range of reactive volatile organic compounds, which affects atmospheric environment. It is important to evaluate the consequences of climate change for the soil C stocks, because the large amount of C stored in the high latitude soils has a potential to intensify global warming if released to the atmosphere. The long-term field experiments also suggest that warming leads to reduced microbial biomass C, which may be due to top-down regulation by microbivores, and possibly lower organic matter quality due to progressive N limitation. Mycorrhizal fungi play an important role in the tight nutrient cycling, organic matter turnover and plant nutrient uptake in these inherently N deficient systems. Warming might increase soil nutrient availability, which leads to higher biomass of dwarf shrubs, but as mosses and lichens are strongly reduced when the plant canopy closes, associated N2 fixation diminishes, which will moderate the rate of expansion of canopy forming shrubs in the longer run. As both vegetation and organic matter turnover in subarctic ecosystems respond slowly to changes and experimental results from the first and second decade of long-lasting field experiments may differ, it is essential to continue climate change related experiments over a long time span to be able to make reliable predictions of the truly long-term effects of climatic warming.
Acknowledgments
We are grateful to the staff at the Abisko Scientific Research Station for excellent facilities and support, and to the Director through many years, Prof. Terry V. Callaghan, for his encouragement and enthusiastic work in arctic ecological research. The studies have been supported by multiple grants from The Danish Council for Independent Research. We also wish to thank The Danish National Research Foundation for funding the activities within the Center for Permafrost (CENPERM). Numerous colleagues, students and field assistants are thanked for enthusiastic collaboration during the field and analytical work.
Biographies
Anders Michelsen
is professor in physiolocal ecology. His field of research is biogeochemistry and plant ecology, with particular focus on high latitude ecosystems and impacts of climate change.
Riikka Rinnan
is associate professor in ecophysiology. She is working on volatile organic compound emission and plant–soil–microbe interactions in relation to climate change.
Sven Jonasson
is professor emeritus in plant ecology. He has worked for nearly 30 years on the interplay between plants, microbes and soil, mainly in northern latitude ecosystems.
Contributor Information
Anders Michelsen, Email: andersm@bio.ku.dk.
Riikka Rinnan, Email: riikkar@bio.ku.dk.
Sven Jonasson, Email: svenj@bio.ku.dk.
References
- Aerts R. The freezer defrosting: Global warming and litter decomposition rates in cold biomes. Journal of Ecology. 2006;94:713–724. doi: 10.1111/j.1365-2745.2006.01142.x. [DOI] [Google Scholar]
- Aerts R, Cornelissen JHC, Dorrepaal E. Plant performance in a warmer world: General responses of plants from cold, northern biomes and the importance of winter and spring events. Plant Ecology. 2006;182:65–77. [Google Scholar]
- Andresen LC, Michelsen A, Ström L, Jonasson S. Uptake of pulse injected nitrogen by soil microbes and mycorrhizal and non-mycorrhizal plants in a species-diverse subarctic heath ecosystem. Plant and Soil. 2008;313:283–295. doi: 10.1007/s11104-008-9700-7. [DOI] [Google Scholar]
- Arroniz-Crespo M, Gwynn-Jones MD, Callaghan TV, Nunez-Olivera E, Martınez-Abaigar J, Horton P, Phoenix GK. Impacts of long-term enhanced UV-B radiation on bryophytes in two sub-Arctic heathland sites of contrasting water availability. Annals of Botany. 2011;108:557–565. doi: 10.1093/aob/mcr178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjerke JW, Bokhorst S, Zielke M, Callaghan TV, Bowles FC, Phoenix GK. Contrasting sensitivity to extreme winter warming events of dominant sub-Arctic heathland bryophyte and lichen species. Journal of Ecology. 2011;99:1481–1488. doi: 10.1111/j.1365-2745.2011.01859.x. [DOI] [Google Scholar]
- Björk RG, Majdi H, Klemedtsson L, Lewis-Jonsson L, Molau U. Long-term warming effects on root morphology, root mass distribution, and microbial activity in two dry tundra plant communities in northern Sweden. New Phytologist. 2007;176:862–873. doi: 10.1111/j.1469-8137.2007.02231.x. [DOI] [PubMed] [Google Scholar]
- Bokhorst S, Bjerke JW, Street LE, Callaghan TV, Phoenix GK. Impacts of multiple extreme winter warming events on sub-Arctic heathland: Phenology, reproduction, growth, and CO2 flux responses. Global Change Biology. 2011;17:2817–2830. doi: 10.1111/j.1365-2486.2011.02424.x. [DOI] [Google Scholar]
- Callaghan TV, Björn LO, Chernov Y, Chapin T, Christensen TR, Huntley B, Ims RA, Johansson M, et al. Effects on the function of Arctic ecosystems in the short- and long-term perspectives. AMBIO. 2004;33:448–458. doi: 10.1579/0044-7447-33.7.448. [DOI] [PubMed] [Google Scholar]
- Christensen TR, Johansson T, Olsrud M, Ström L, Lindroth A, Mastepanov M, Malmer N, Friborg T, et al. A catchment-scale carbon and greenhouse gas budget of a subarctic landscape. Philosophical Transactions of the Royal Society A-Mathematical Physical and Engineering Sciences. 2007;365:1643–1656. doi: 10.1098/rsta.2007.2035. [DOI] [PubMed] [Google Scholar]
- Christensen TR, Michelsen A, Jonasson S. Exchange of CH4 and N2O in a subarctic heath soil: Effects of inorganic N and P and amino acid addition. Soil Biology & Biochemistry. 1999;31:637–641. doi: 10.1016/S0038-0717(98)00166-7. [DOI] [Google Scholar]
- Christensen TR, Michelsen A, Jonasson S, Schmidt IK. Carbon dioxide and methane exchange of a subarctic heath in response to climate change related environmental manipulations. Oikos. 1997;79:34–44. doi: 10.2307/3546087. [DOI] [Google Scholar]
- Clemmensen KE, Michelsen A. Integrated long-term responses of an arctic-alpine willow and associated ectomycorrhizal fungi to an altered environment. Canadian Journal of Botany. 2006;84:831–843. doi: 10.1139/b06-039. [DOI] [Google Scholar]
- Clemmensen KE, Michelsen A, Jonasson S, Shaver GR. Increased ectomycorrhizal fungal abundance after long-term fertilization and warming of two arctic tundra ecosystems. New Phytologist. 2006;171:391–404. doi: 10.1111/j.1469-8137.2006.01778.x. [DOI] [PubMed] [Google Scholar]
- Clemmensen KE, Sørensen PL, Michelsen A, Jonasson S, Ström L. Site-dependent N uptake from N-form mixtures by arctic plants, soil microbes and ectomycorrhizal fungi. Oecologia. 2008;155:771–783. doi: 10.1007/s00442-008-0962-9. [DOI] [PubMed] [Google Scholar]
- Craine JM, Elmore AJ, Aidar MPM, Bustamante M, Dawson TE, Hobbie EA, Kahmen A, Mack MC, et al. Global patterns of foliar nitrogen isotopes and their relationships with climate, mycorrhizal fungi, foliar nutrient concentrations, and nitrogen availability. New Phytologist. 2009;183:980–992. doi: 10.1111/j.1469-8137.2009.02917.x. [DOI] [PubMed] [Google Scholar]
- Dorrepaal E, Toet S, Logtestijn RSP, Swart E, Weg MJ, Callaghan TV, Aerts R. Carbon respiration from subsurface peat accelerated by climate warming in the subarctic. Nature. 2009;460:616–619. doi: 10.1038/nature08216. [DOI] [Google Scholar]
- Ekberg A, Arneth A, Hakola H, Hayward S, Holst T. Isoprene emission from wetland sedges. Biogeosciences. 2009;6:601–613. doi: 10.5194/bg-6-601-2009. [DOI] [Google Scholar]
- Ekberg A, Arneth A, Holst T. Isoprene emission from Sphagnum species occupying different growth positions above the water table. Boreal Environment Research. 2011;16:47–59. [Google Scholar]
- Elmendorf S, Henry G, Hollister R, Björk R, Bjorkman A, Callaghan TV, Collier L, Cooper E, et al. Global assessment of experimental climate warming on tundra vegetation: Heterogeneity over space and time. Ecology Letters. 2012;15:164–175. doi: 10.1111/j.1461-0248.2011.01716.x. [DOI] [PubMed] [Google Scholar]
- Fahnestock JT, Jones MH, Welker JM. Wintertime CO2 efflux from Arctic soils: Implications for annual carbon budgets. Global Biogeochemical Cycles. 1999;13:775–779. doi: 10.1029/1999GB900006. [DOI] [Google Scholar]
- Faubert, P., P. Tiiva., A. Michelsen, Å. Rinnan, H. Ro-Poulsen, and R. Rinnan. 2012. The shift in plant species composition in a subarctic mountain birch forest floor due to climate change would modify the biogenic volatile organic compound emission profile. Plant and Soil 352: 199–215. doi:10.1007/s11104-011-0989-2.
- Faubert P, Tiiva P, Rinnan Å, Michelsen A, Holopainen JK, Rinnan R. Doubled volatile organic compound emissions from subarctic tundra under simulated climate warming. New Phytologist. 2010;187:199–208. doi: 10.1111/j.1469-8137.2010.03270.x. [DOI] [PubMed] [Google Scholar]
- Fox AM, Huntley B, Lloyd CR, Williams M, Baxter R. Net ecosystem exchange over heterogeneous Arctic tundra: Scaling between chamber and eddy covariance measurements. Global Biogeochemical Cycles. 2008;22:GB2027. doi: 10.1029/2007GB003027. [DOI] [Google Scholar]
- Graglia E, Jonasson S, Michelsen A, Schmidt IK, Havström M, Gustavsson L. Effects of environmental perturbations on abundance of subarctic plants after three, seven and ten years of treatments. Ecography. 2001;24:5–12. doi: 10.1034/j.1600-0587.2001.240102.x. [DOI] [Google Scholar]
- Grogan P, Illeris L, Michelsen A, Jonasson S. Respiration of recently-fixed plant carbon dominates mid-winter ecosystem CO2 production in sub-arctic heath tundra. Climatic Change. 2001;50:129–142. doi: 10.1023/A:1010610131277. [DOI] [Google Scholar]
- Grogan P, Jonasson S. Controls on annual nitrogen cycling in the understory of a subarctic birch forest. Ecology. 2003;84:202–218. doi: 10.1890/0012-9658(2003)084[0202:COANCI]2.0.CO;2. [DOI] [Google Scholar]
- Haapanala S, Ekberg A, Hakola H, Tarvainen V, Rinne J, Hellen H, Arneth A. Mountain birch—potentially large source of sesquiterpenes into high latitude atmosphere. Biogeosciences. 2009;6:2709–2718. doi: 10.5194/bg-6-2709-2009. [DOI] [Google Scholar]
- Hansen AH, Jonasson S, Michelsen A, Julkunen-Tiitto R. Long-term experimental warming, shading and nutrient addition affect the concentration of phenolic compounds in subarctic deciduous and evergreen dwarf shrubs. Oecologia. 2006;147:1–11. doi: 10.1007/s00442-005-0233-y. [DOI] [PubMed] [Google Scholar]
- Hartley AE, Neill C, Melillo JM, Crabtree R, Bowles FP. Plant performance and soil nitrogen mineralization in response to simulated climate change in subarctic dwarf shrub heath. Oikos. 1999;86:331–343. doi: 10.2307/3546450. [DOI] [Google Scholar]
- Haugwitz MS, Michelsen A. Long-term addition of fertilizer, labile carbon and fungicide alters the biomass of plant functional groups in a subarctic-alpine community. Plant Ecology. 2011;212:715–726. doi: 10.1007/s11258-010-9857-z. [DOI] [Google Scholar]
- Haugwitz MS, Schmidt IK, Michelsen A. Long-term microbial control of nutrient availability and plant biomass in a subarctic-alpine heath after addition of carbon, fertilizer and fungicide. Soil Biology & Biochemistry. 2011;43:179–187. doi: 10.1016/j.soilbio.2010.09.032. [DOI] [Google Scholar]
- Holst T, Arneth A, Hayward S, Ekberg A, Mastepanov M, Jackowicz-Korczynski M, Friborg T, Crill PM, Bäckstrand K. BVOC ecosystem flux measurements at a high latitude wetland site. Atmospheric Chemistry and Physics. 2010;10:1617–1634. doi: 10.5194/acp-10-1617-2010. [DOI] [Google Scholar]
- Illeris L, König SM, Grogan P, Jonasson S, Michelsen A, Ro-Poulsen H. Growing season carbon dioxide flux in a dry subarctic heath: Responses to long-term manipulations. Arctic, Antarctic, and Alpine Research. 2004;36:456–463. doi: 10.1657/1523-0430(2004)036[0456:GCDFIA]2.0.CO;2. [DOI] [Google Scholar]
- Jonasson S, Castro J, Michelsen A. Interactions between plants, litter and microbes in cycling of nitrogen and phosphorus in the arctic. Soil Biology & Biochemistry. 2006;38:526–532. doi: 10.1016/j.soilbio.2005.05.024. [DOI] [Google Scholar]
- Jonasson S, Michelsen A. Plant nutrition and nutrient cycling in the Subarctic, with special reference to the Abisko and Torneträsk area. Ecological Bulletins. 1996;45:45–52. [Google Scholar]
- Jonasson S, Michelsen A, Schmidt IK, Nielsen EV. Responses in microbes and plants to changed temperature, nutrient, and light regimes in the Arctic. Ecology. 1999;80:1828–1843. doi: 10.1890/0012-9658(1999)080[1828:RIMAPT]2.0.CO;2. [DOI] [Google Scholar]
- Karlsson G.P., C. Akselsson, S. Hellsten, P.E. Karlsson, and G. Malm. 2009. Övervakning av luftföroreningar norra Sverige – mätningar och moddellering. Svenska Miljöinstitut IVL rapport B1851. Lund Universitet (in Swedish).
- Kjøller R, Olsrud M, Michelsen A. Co-existing ericaceous plant species in a subarctic heath community share fungal root endophytes. Fungal Ecology. 2010;3:205–214. doi: 10.1016/j.funeco.2009.10.005. [DOI] [Google Scholar]
- Konestabo SH, Michelsen A, Holmstrup M. Responses of springtail and mite populations to prolonged periods of soil freeze–thaw cycles in a sub-arctic ecosystem. Applied Soil Ecology. 2007;36:136–146. doi: 10.1016/j.apsoil.2007.01.003. [DOI] [Google Scholar]
- Krab EJ, Cornelissen JHC, Lang SI, Logtestijn RSP. Amino acid uptake among wide-ranging moss species may contribute to their strong position in higher-latitude ecosystems. Plant and Soil. 2008;304:199–208. doi: 10.1007/s11104-008-9540-5. [DOI] [Google Scholar]
- Kullman L, Öberg L. Post-Little Ice Age tree line rise and climate warming in the Swedish Scandes: A landscape ecological perspective. Journal of Ecology. 2009;97:415–429. doi: 10.1111/j.1365-2745.2009.01488.x. [DOI] [Google Scholar]
- Larsen KS, Grogan P, Jonasson S, Michelsen A. Respiration and microbial dynamics in two sub-arctic ecosystems during winter and spring thaw: Effects of increased snow depth. Arctic, Antarctic, and Alpine Research. 2007;39:268–276. doi: 10.1657/1523-0430(2007)39[268:RAMDIT]2.0.CO;2. [DOI] [Google Scholar]
- Larsen KS, Ibrom A, Jonasson S, Michelsen A, Beier C. Significance of cold-season respiration and photosynthesis in a subarctic heath ecosystem in Northern Sweden. Global Change Biology. 2007;13:1498–1508. doi: 10.1111/j.1365-2486.2007.01370.x. [DOI] [Google Scholar]
- Mack MC, Schuur EAG, Bret-Harte MS, Shaver GR, Chapin FS., III Ecosystem carbon storage in arctic tundra reduced by long-term nutrient fertilization. Nature. 2004;431:440–443. doi: 10.1038/nature02887. [DOI] [PubMed] [Google Scholar]
- McKane RB, Johnson. LC, Shaver GR, Nadelhoffer KJ, Rastetter EB, Fry B, Giblin AE, Kielland K, et al. Resource-based niches provide a basis for plant species diversity and dominance in arctic tundra. Nature. 2002;415:68–71. doi: 10.1038/415068a. [DOI] [PubMed] [Google Scholar]
- Michelsen A, Graglia E, Schmidt IK, Jonasson S, Quarmby C, Sleep D. Differential responses of grass and a dwarf shrub to long-term changes in soil microbial biomass C, N and P by factorial NPK fertilizer, fungicide and labile carbon addition to a heath. New Phytologist. 1999;143:523–538. doi: 10.1046/j.1469-8137.1999.00479.x. [DOI] [PubMed] [Google Scholar]
- Michelsen A, Jonasson S, Sleep D, Havström M, Callaghan TV. Shoot biomass, δ13C, nitrogen and chlorophyll responses of two arctic dwarf-shrubs to in situ shading, nutrient application and warming simulating climatic change. Oecologia. 1996;105:1–12. doi: 10.1007/BF00328785. [DOI] [PubMed] [Google Scholar]
- Michelsen A, Quarmby C, Sleep D, Jonasson S. Vascular plant 15N natural abundance in heath and forest tundra ecosystems is closely correlated with presence and type of mycorrhizal fungi in roots. Oecologia. 1998;115:406–418. doi: 10.1007/s004420050535. [DOI] [PubMed] [Google Scholar]
- Molau U. Long-term impacts of observed and induced climate change on tussock tundra near its southern limit in northern Sweden. Plant Ecology and Diversity. 2010;3:29–34. doi: 10.1080/17550874.2010.487548. [DOI] [Google Scholar]
- Olsrud M, Carlsson BÅ, Svensson BM, Michelsen A, Melillo JM. Responses of fungal root colonization, plant cover and leaf nutrients to long-term exposure to elevated atmospheric CO2 and warming in a subarctic birch forest understory. Global Change Biology. 2010;16:1820–1829. doi: 10.1111/j.1365-2486.2009.02079.x. [DOI] [Google Scholar]
- Olsrud M, Michelsen A. Effects of shading on photosynthesis, plant organic nitrogen uptake and root fungal colonization in a subarctic mire ecosystem. Botany. 2009;87:463–474. doi: 10.1139/B09-021. [DOI] [Google Scholar]
- Olsrud M, Michelsen A, Wallander H. Ergosterol content in ericaceous hair roots correlates with dark septate endophytes but not with ericoid mycorrhizal colonization. Soil Biology & Biochemistry. 2007;39:1218–1221. doi: 10.1016/j.soilbio.2006.11.018. [DOI] [Google Scholar]
- Peñuelas K, Staudt M. BVOCs and global change. Trends in Plant Science. 2010;15:133–144. doi: 10.1016/j.tplants.2009.12.005. [DOI] [PubMed] [Google Scholar]
- Press MC, Potter JA, Burke MJW, Callaghan TV, Lee JA. Responses of a subarctic dwarf shrub heath community to simulated environmental change. Journal of Ecology. 1998;86:315–327. doi: 10.1046/j.1365-2745.1998.00261.x. [DOI] [Google Scholar]
- Rinnan R, Michelsen A, Bååth E. Long-term warming of a subarctic heath decreases soil bacterial community growth but has no effects on its temperature adaptation. Applied Soil Ecology. 2011;47:217–220. doi: 10.1016/j.apsoil.2010.12.011. [DOI] [Google Scholar]
- Rinnan R, Michelsen A, Bååth E, Jonasson S. Fifteen years of climate change manipulations alter soil microbial communities in a subarctic heath ecosystem. Global Change Biology. 2007;13:28–39. doi: 10.1111/j.1365-2486.2006.01263.x. [DOI] [Google Scholar]
- Rinnan R, Michelsen A, Bååth E, Jonasson S. Mineralization and carbon turnover in subarctic heath soil as affected by warming and additional litter. Soil Biology & Biochemistry. 2007;39:3014–3023. doi: 10.1016/j.soilbio.2007.05.035. [DOI] [Google Scholar]
- Rinnan R, Michelsen A, Jonasson S. Effects of litter addition and warming on soil carbon, nutrient pools and microbial communities in a subarctic heath ecosystem. Applied Soil Ecology. 2008;39:271–281. doi: 10.1016/j.apsoil.2007.12.014. [DOI] [Google Scholar]
- Rinnan R, Rinnan Å, Faubert P, Tiiva P, Holopainen JK, Michelsen A. Few long-term effects of simulated climate change on volatile organic compound emissions and leaf chemistry of three subarctic dwarf shrubs. Environmental and Experimental Botany. 2011;72:377–386. doi: 10.1016/j.envexpbot.2010.11.006. [DOI] [Google Scholar]
- Robinson CH, Wookey PA, Parsons AN, Potter JA, Callaghan TV, Lee JA, Press MC, Welker JM. Responses of plant litter decomposition and nitrogen mineralisation to simulated environmental change in a high arctic polar semi-desert and a subarctic dwarf shrub heath. Oikos. 1995;74:503–512. doi: 10.2307/3545996. [DOI] [Google Scholar]
- Ruess L, Michelsen A, Schmidt IK, Jonasson S. Simulated climate change affecting microorganisms, nematode density and biodiversity in subarctic soils. Plant and Soil. 1999;212:63–73. doi: 10.1023/A:1004567816355. [DOI] [Google Scholar]
- Rundqvist S, Hedenås H, Sandström A, Emanuelsson U, Eriksson H, Jonasson C, Callaghan TV. Tree and shrub expansion over the past 34 years at the tree-line near Abisko, Sweden. AMBIO. 2011;40:683–692. doi: 10.1007/s13280-011-0174-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt IK, Jonasson S, Shaver GR, Michelsen A, Nordin A. Mineralization and distribution of nutrients in plants and microbes in four arctic ecosystems: Responses to warming. Plant and Soil. 2002;242:93–106. doi: 10.1023/A:1019642007929. [DOI] [Google Scholar]
- Sjögersten S, Wookey PA. The impact of climate change on ecosystem carbon dynamics at the Scandinavian mountain birch forest–tundra heath ecotone. AMBIO. 2009;38:2–10. doi: 10.1579/0044-7447-38.1.2. [DOI] [PubMed] [Google Scholar]
- Sjursen H, Michelsen A, Jonasson S. Effects of long-term soil warming and fertilisation on microarthropod abundances in three sub-arctic ecosystems. Applied Soil Ecology. 2005;30:148–161. doi: 10.1016/j.apsoil.2005.02.013. [DOI] [Google Scholar]
- Solheim B, Johanson U, Callaghan TV, Lee JA, Gwynn-Jones D, Björn LO. The nitrogen fixation potential of arctic cryptogram species is influenced by enhanced UV-B radiation. Oecologia. 2002;133:90–93. doi: 10.1007/s00442-002-0963-z. [DOI] [PubMed] [Google Scholar]
- Sorensen PL, Lett S, Michelsen A. Moss specific changes in nitrogen fixation following two decades of warming, shading and fertilizer addition. Plant Ecology. 2012;213:695–706. doi: 10.1007/s11258-012-0034-4. [DOI] [Google Scholar]
- Sorensen PL, Michelsen A. Long-term warming and litter addition affects nitrogen fixation in subarctic heath. Global Change Biology. 2011;17:528–537. doi: 10.1111/j.1365-2486.2010.02234.x. [DOI] [Google Scholar]
- Sorensen PL, Michelsen A, Jonasson S. Nitrogen fixation, denitrification and ecosystem nitrogen pools in relation to vegetation development in the Subarctic. Arctic, Antarctic, and Alpine Research. 2006;38:263–272. doi: 10.1657/1523-0430(2006)38[263:NFDAEN]2.0.CO;2. [DOI] [Google Scholar]
- Sorensen PL, Michelsen A, Jonasson S. Ecosystem partitioning of 15N-glycine after long-term climate manipulations, plant clipping and addition of labile carbon in a subarctic heath tundra. Soil Biology & Biochemistry. 2008;40:2344–2350. doi: 10.1016/j.soilbio.2008.05.013. [DOI] [Google Scholar]
- Sorensen PL, Michelsen A, Jonasson S. Nitrogen uptake during one year in subarctic plant functional groups and in microbes after long-term warming and fertilization. Ecosystems. 2008;11:223–233. doi: 10.1007/s10021-008-9204-6. [DOI] [Google Scholar]
- Tiiva P, Faubert P, Michelsen A, Holopainen T, Holopainen JK, Rinnan R. Climatic warming increases isoprene emission from a subarctic heath. New Phytologist. 2008;180:853–863. doi: 10.1111/j.1469-8137.2008.02587.x. [DOI] [PubMed] [Google Scholar]
- Urcelay C, Bret-Harte MS, Diaz S, Chapin III FS. Mycorrhizal colonization mediated by species interactions in arctic tundra. Oecologia. 2003;137:399–404. doi: 10.1007/s00442-003-1349-6. [DOI] [PubMed] [Google Scholar]
- Wijk MT, Clemmensen KE, Shaver GR, Williams M, Callaghan TV, Chapin FS, III, Cornelissen JHC, Gough L, et al. Long-term ecosystem level experiments at Toolik Lake, Alaska, and at Abisko, Northern Sweden: Generalisations and differences in ecosystem and plant type responses to global change. Global Change Biology. 2004;10:105–123. doi: 10.1111/j.1365-2486.2003.00719.x. [DOI] [Google Scholar]
- Welker JM, Fahnestock JT, Henry GHR, O’Dea KW, Chimner RA. CO2 exchange in three Canadian High Arctic ecosystems: Response to long-term experimental warming. Global Change Biology. 2004;10:1981–1995. doi: 10.1111/j.1365-2486.2004.00857.x. [DOI] [Google Scholar]
- Welker JM, Fahnestock JT, Jones MH. Annual CO2 flux in dry and moist arctic tundra: Field responses to increases in summer temperatures and winter snow depth. Climatic Change. 2000;44:139–150. doi: 10.1023/A:1005555012742. [DOI] [Google Scholar]
- Yano Y, Shaver GR, Giblin AE, Rastetter EB. Depleted 15N in hydrolysable-N of arctic soils and its implication for mycorrhizal fungi–plant interaction. Biogeochemistry. 2010;97:183–194. doi: 10.1007/s10533-009-9365-1. [DOI] [Google Scholar]