Introduction
Atrial fibrillation (AF) is the most common arrhythmia seen in clinical practice and is associated with increased risk of stroke, heart failure and death.1 However, currently available treatments for AF are less than satisfactory. Many drugs have been tried with very limited success.2 On the other hand, the demonstration of AF triggers in the atrial sleeves of the pulmonary veins (PVs)3 has led to a significant improvement in therapy. Today PV isolation by means of radiofrequency (RF) ablation is a gold standard treatment for paroxysmal AF.4 However, the success rate of RF ablation in the more prevalent and highly heterogeneous persistent and long-term persistent AF populations has been disappointing.5 The reasons are probably multifactorial, but undoubtedly incomplete understanding of the mechanism(s) underlying this complex arrhythmia has contributed substantially to such a poor outcome.
Increased knowledge of cardiac arrhythmias in general has come from studies aimed at elucidating cardiac impulse formation and propagation at several levels of integration, from computer modeling, through electrophysiological experiments in cells, tissues and the whole animal, and the human patient.6 In clinical electrophysiology (EP), progress has been driven, to a large extent, by available technology designed for the detection of electrical signals on the endocardial and epicardial surfaces of the heart. Although the surface ECG is the obvious first and compulsory starting point in the clinical diagnostic ladder, programmed cardiac stimulation, combined with intracardiac activation mapping, enables electrophysiologic characterization, and is the cornerstone for successful diagnosis and treatment of most types of arrhythmias.7
In contrast to what happens in most other arrhythmic problems, electrical impulses during AF often are highly irregular, changing in number, direction, and width on a beat-to-beat basis with significant temporal variations in timing and morphology of the intracardiac atrial electrograms. Consequently, time-domain analysis of the signals recorded by electrodes positioned concurrently at different locations in the atria reveals extremely complex spatio-temporal activation patterns that preclude meaningful interpretation. It is therefore fair to state that understanding the mechanisms of AF maintenance has been hampered in part by the inability to reproducibly quantifying activation patterns during ongoing AF. Consequently, the demonstration in the late 1990’s that the PVs are the most common site of triggers for AF, led the clinical EP community to adopt an “anatomical” approach to the RF treatment of AF involving the creation of circumferential lesions around the right and the left PV ostia,8 which probably impacts not only the triggers but also the substrate of AF. This was in great part responsible for reducing the interest in performing extensive mapping of the atria or immersing into the study of AF mechanisms.
Perhaps more important, the relatively poor outcomes of pharmacologic AF therapy, together with the apparently greater effectiveness of RF ablation in controlling the arrhythmia, has contributed to significantly reduce the interest of clinical EPs for mechanistically based approaches to AF therapy. On the other hand, basic EPs have focused on scientific and technological innovations in biology, which have provided unprecedented opportunities for the discovery of the genetic and molecular bases of both acquired and inheritable arrhythmogenic diseases, with the idea of searching for novel therapeutic targets.9 Unfortunately, the unintended consequence of the above success stories was that clinical and basic EPs stopped speaking the same “language”. Probably, with very few exceptions, basic and clinical EPs seem to be “lost in translation” and appear unaware of each other’s work. In other words, the reduced communication between the two groups over the last 20 years has engendered a void separating on one side the world of the clinical electrophysiologist, whose primary immediate interest is the health and quality of life of his/her patient, and on the other, the world of the basic electrophysiologist whose novel scientific discoveries may take years to reach that patient.
During the last 20 years, and until very recently, mechanistically oriented EP studies in the clinic have been relatively scarce.10–13 Much of the insight into EP mechanisms and complex dynamics of wave propagation in AF have come from detailed investigation in numerical and animal models of AF14 where there is substantially greater flexibility to explore the AF process from the molecule to the organism, and where high spatiotemporal resolution of the fibrillatory dynamics can be achieved.15
Excellent reviews have appeared in the recent literature on the consensus among clinicians for treatment,1 the epidemiology and the genetic, molecular and pathophysiological bases of AF and atrial remodeling9, 16–20 Therefore, our aim here is not to present an exhaustive discussion on fundamental AF mechanisms. Instead, this article focuses on attempts by clinicians and basic scientists to find potential AF diagnostic tools and treatments based on the pathophysiologic mechanisms responsible for AF initiation and maintenance. By discussing the evidence, we hope to promote reflection and to encourage both clinical and basic electrophysiology communities to re-acquaint with each other’s work, find common ground and collaborate toward improving knowledge and advance therapy of AF.
Finding our Way in Translation
Translation of knowledge derived from the experimental setting to clinical practice plays a critical role in scientific advancement,21, 22 and the importance of building clinical research on the solid foundations provided by scientifically rigorous basic research has been long recognized. Basic research can advance clinical research and practice in several ways:22 1) it informs clinical research by building the foundation of biological knowledge; 2) it guides clinicians in their practice by indicating not only what direction to pursue but what directions are possible; 3) it contributes to the development and testing of medical devices, surgical procedures and candidate drugs. In fact, most medical diagnostic tests and therapies in use today were initially developed and tried in animals. For example, the development, testing and clinical application of ACE-inhibitors constitutes a paradigmatic example of successful translational research from bench to bedside.23 However, many results of animal experiments often never undergo clinical testing or eventually fail to be replicated when tested in rigorous human trials.21, 24 Francis Collins, Director of the National Institutes of Health has commented that despite a “dizzying rate” of basic science discoveries, “far too often promising diagnostic devices and treatments are not making it to market”. In a systematic review of highly cited animal studies, only 37% were replicated in human randomized trials, and 45% remained untested.21 According to an article published recently in Nature24 “…’translational research’ is seen by many as a solution to this disparity that could ensure that many fundamental discoveries are effectively ‘translated’ into benefits in medicine”.
Diagnosis and treatment of AF is at a point at which it would greatly benefit from translational research. Despite many years of basic and clinical research, fundamental mechanisms governing AF initiation and maintenance, and the transition from paroxysmal to permanent forms of AF are far from being understood,15 and we have not learned how to treat AF effectively. The inexorable progression from paroxysmal to persistent and permanent AF reflects the progressive electrophysiological and structural remodeling occurring in both atria,25–28 making the sources of the arrhythmia more stable and long-lasting. A long-term follow-up study reported a 30-year cumulative probability risk of progression from paroxysmal/persistent to permanent AF of 29%, with most of the transitions occurring within the first 15 years after diagnosis.29 Yet, it is unknown which factors contribute to that progression, let alone whether or not we have the appropriate tools to prevent it.
Fortunately, the demonstration of AF triggers in the atrial sleeves of the pulmonary veins (PVs) has led to a significant improvement in therapy.3 Today PV isolation by means of RF ablation is curative in about ~70–80% of patients with paroxysmal AF who are treated in the EP lab.4 However, PV isolation is a relatively new procedure and the very long term outcomes are not known. In addition, AF ablation is reserved for the subset of patients with symptoms and paroxysmal or persistent AF. Moreover, the financial burden of AF ablation therapy may be significant and is unlikely to be cost effective for patients who have preserved quality of life despite their AF or for patients whose quality of life is not expected to improve substantially despite elimination of AF.30 Therefore, there is much room for improvement despite the great success. As will be further discussed later, here we and others see opportunities for translation toward the discovery of novel and effective therapies.31 Interestingly, research into the fundamental understanding of some of the potential targets for therapy began almost 40 years ago with the discovery of the cellular mechanisms of delayed-afterdepolarization (DAD)-induced triggered activity32–34 and the mechanisms of rotor initiation and maintenance.35
Unfortunately, the success rate of RF ablation in the more prevalent and highly heterogeneous persistent and long-term persistent AF populations is ~30% or less.36 Moreover, a slow but steady decline in arrhythmia-free survival after 5 years is noted despite successful ablation treatment, especially among persistent AF patients.37 Perhaps our current incomplete understanding of the mechanisms involved in the maintenance and perpetuation of AF has prevented us from being able to generate more specific prevention and/or treatment of this dangerous and debilitating disease. It may be argued, however, that one important reason for such disappointing outcomes is the paucity of communication that has existed, and continues to exist, between the basic scientists and the clinicians during the last 20 years. But there is a glimmer of hope. Academic investigators on both sides of the aisle now have access to scientific resources and technology that should enable them to participate in translational research and collaborate in unprecedented ways. Advances have occurred in the basic understanding of the molecular mechanisms of AF induced remodeling, including both electrical and structural remodeling, on the one hand, and in the development of highly sophisticated MRI imaging and high-resolution electrode mapping technologies capable of identifying AF sources in the clinical EP lab on the other hand. Such advances should help place basic and clinical investigators in a new position to partner together with industry and play a more powerful part in therapeutic development in both paroxysmal and persistent AF. In the following lines we review the evolution of the principal ideas that have attempted to explain AF maintenance mechanisms, from the perspective of how translation of the results of experimental studies might influence future clinical practice.
Atrial Fibrillation Maintenance Mechanisms
The principal competing hypotheses attempting to explain AF mechanisms have been around more than 100 years, and their historical evolution has been recently reviewed.15 From the beginning of the past century, two such hypotheses were proposed to explain AF maintenance mechanisms: single source focus and circus movement reentry. According to these hypotheses, AF may be caused either by a rapidly discharging, spontaneously active atrial ectopic focus, or by a single functional re-entrant circuit. In either case, the activation wavefronts spawned at high frequency were thought to interact with the heterogeneous atrial muscle to give rise to what we know now as “fibrillatory conduction”. While investigators at the time saw these two hypotheses as mutually exclusive, common to both hypotheses was the idea that AF was maintained by a localized source of atrial activity, and required the continuous presence of the ectopic focus, or alternatively, the reentrant circuit. A third hypothesis, proposed subsequently by Moe et al, postulated that AF depended on the sustained, but random propagation of multiple wavelets, and that maintenance of the arrhythmia was self-sustaining and independent of the initiating event.38
To our knowledge, not a single one of the above competing hypotheses has reached consensus among basic or clinical scientists. Over the years, arguments for or against each of the above schools of thought have been forcefully advanced by one or the other group, and the debate continues even today. Undoubtedly the debate will continue for years to come. Below we briefly review the pathophysiologic nature of each of these mechanisms and their respective implications for potential therapy, as well as the treatment strategies that have been devised according to each conceptual hypothesis.
The Multiple Wavelet Hypothesis
The multiple wavelet hypothesis was the dominant mechanistic model of AF during the second half of the 20th century. In 1962, Moe postulated that AF was the result of randomly propagating multiple electrical wavelets, ever changing in number and direction, with local excitation limited by the heterogeneous distribution of refractory periods throughout the atria.38, 39 In Moe’s model, the number of wavelets was a function of the mass of the tissue and inversely related to the product of the duration of refractory period and the conduction velocity (i.e., the wavelength). The model predicted that a critical number of 23 to 40 wavelets was necessary for the maintenance of AF.39 Any factor that increased the total number of wavelets (e.g., wavelength shortening) would serve to perpetuate the arrhythmia while, conversely, any factor that decreased the total number of wavelets would favor termination.38 This hypothesis was later reinforced by high-resolution electrode mapping experiments in dogs.40 In support of Moe’s model,39 Allessie et al40 suggested that AF was maintained by 6 to 7 wavelets that propagated through relatively refractory tissue in which there was no “fully excitable gap”, enabling the coexistence of multiple simultaneous wavelets in a minimum amount of tissue (i.e. critical mass). However, translation of the multiple wavelet hypothesis into human persistent AF diagnosis and therapy has been difficult and has not yet resulted in widely accepted strategies to effectively terminate AF. Nevertheless, the idea is widespread in the literature, even though both experimental and clinical studies have raised important questions and argue against the applicability of the multiple wavelet hypothesis to explain sustained AF. Examples follow:
First, the surgical Maze procedure is able to successfully treat some cases of chronic AF, which has been attributed to a reduction the tissue mass and thus the number of wavelets that the atria can sustain.41 However, other factors may explain the success of this intervention, including PV isolation,3 cardiac denervation42 and empiric elimination of sites responsible for AF maintenance.43 Moreover, several studies challenged the critical mass hypothesis, by demonstrating that fibrillation can be sustained by tissues of very small size. Chen et al showed that the minimal length that allows for the sustenance of reentry in the left atrium (LA) of the sheep heart is about 4 mm.44 Gray et al demonstrated theoretically that rodent hearts can accommodate 1 or 2 reentrant sources (rotors), whereas in larger hearts, e.g., sheep, dogs, and humans, a greater number of rotors may co-exist.45 Such a theoretical prediction has been borne out by the demonstration that in mammalian hearts from mouse to horse the ventricular fibrillation (VF) cycle length scales with the forth power of the body mass.46 Finally, clinical studies have shown that AF can also be sustained by small portions of tissue, such as remnants of receptor atrial tissue after orthotopic heart transplantation (Figure S1, Online Supplement),47 which also argues against the idea of a critical mass for AF. Second, wavelength shortening would be expected to increase the number and instability of randomly propagating wavelets as a means to perpetuate the arrhythmia and increase its complexity. Instead, Schuessler et al.48 found in a canine model of acute AF that, with increasing concentrations of acetylcholine, and below a critical level of refractory period (<95 msec), the rapid repetitive responses initially characterized by multiple reentrant circuits stabilized to a single stable circuit that became stable and dominated activation.48
Third, the absence of a fully excitable gap should make the arrhythmia amenable for termination by pharmacologic interventions that increase the refractory period (and thereby the wavelength). It should also limit the number of wavelets that may coexist simultaneously. Thus, several antiarrhythmic drugs capable of prolonging atrial ERP increase both wavelength and the size of reentry circuits during AF have been developed and used with variable success.49, 50 However, the above prediction is incompatible with the antiarrhythmic effect of Na+ channel blockade which, by decreasing conduction velocity and thereby wavelength, should promote AF instead of terminating it, such as occurs in clinical practice.51 Finally, since there is no anatomically determined circuit, there should be no theoretical possibility of interrupting the arrhythmia by disrupting the circuit through the application of localized lesions. However, several experimental and clinical studies have demonstrated that AF can terminate by limited radiofrequency applications directed to localized sources in the atria.52–54
In what appears to be a new variant of the multiple wavelet hypothesis, Allessie et al recently put forth the idea that the electric dissociation of neighboring atrial muscle bundles is a key element in the electropathological substrate of long-standing persistent atrial fibrillation in patients with structural heart disease.55 In addition, from experiments in the goat heart, the same investigators have suggested that, during AF, pronounced dissociation of electrical activity occurs between the epicardial layer and the endocardial bundle network, which they attributed to progressive uncoupling between the epicardial layer and the endocardial bundles.56 This interesting idea might lead to identification of novel molecular targets for therapeutic agents and the design of more effective antifibrillatory drugs. However, it is unclear why such dissociation would occur in the absence of dissociation within any of the two layers. Moreover, even if longitudinal dissociation occurs, unless otherwise demonstrated, the phenomena described by the Allessie group55, 56 are fully compatible with fibrillatory conduction maintained by a single high-frequency source, either focal or reentrant, generating waves from a remote location.
Single Source Hypothesis
Experimental evidence showing that AF may be the result of a single localized electrical source, acting as a rapidly discharging ectopic focus, dates back to the beginning of the 20th century.57 However, this observation fell largely in disfavor due to the convincing evidence provided at that time by the circus movement defenders, particularly Sir Thomas Lewis.58 Nevertheless, Lewis recognized that “[atrial] fibrillation, like flutter, may also on occasion be terminated in the auricle by cold or pressure very locally applied”.58 In 1949, Scherf and Terranova induced atrial tachycardia or fibrillation by applying crystals aconitine on the epicardial surface of the right atrial (RA) appendage of the dog.59 The arrhythmia could be terminated by cooling the area of injection, suggesting that the mechanism of AF was compatible with an ectopic focus.59 More recently, Yamazaki et al provided direct demonstration that reentrant ventricular tachycardia and ventricular fibrillation may be terminated by local cooling at nor near the center of rotation.60 This argues against being able to distinguish between arrhythmogenic mechanisms based on changes in temperature alone.
Nevertheless, there is no question that although focalized atrial ectopy can result from micro-reentry, enhanced atrial automaticity (spontaneous diastolic depolarization) or triggered activity (afterdepolarizations) are important mechanisms involved in AF initiation.61 The vast majority (~90%) of ectopic discharges that trigger AF localize in the PVs,3 and are thought to result from either early (EADs) or delayed (DADs) afterdepolarizations. EADs occur when action potential duration (APD) is excessively prolonged, allowing Ca2+ channels to recover from inactivation. Ca2+ enters into the cell and initiates a new action potential upstroke62. DADs may be the consequence of intracellular Ca2+ handling dysfunction, secondary to abnormal Ca2+ leak from the sarcoplasmic reticulum (SR) through the type-2 ryanodine receptors (RyR2) channels63. Clinical observation indicates that autonomic blockade with propranolol suppresses bursts of atrial fibrillation.64 On the other hand, isoproterenol infusion, which facilitates calcium release from the SR and promotes DADs and triggered activity, has a high sensitivity and specificity for induction of AF in patients with PAF.65 Figure S2 (Online Supplement) shows a possible mechanism of induction of triggered activity at the PV by the intravenous infusion of isoproterenol, as routinely done in the clinical EP lab in combination with rapid pacing.
Rotors and Fibrillatory Conduction
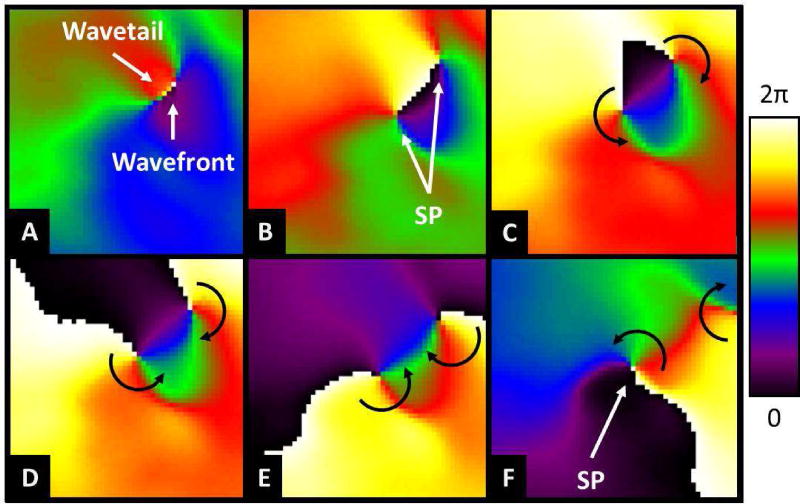
A significant deviation from the prevailing concepts used to explains AF maintenance was derived a number of years ago from the theory of wave propagation in excitable media35 and from studies using voltage-sensitive probes and high-resolution video imaging. The increased understanding through mathematical modeling of the manner in which waves propagate in a wide variety of physical, chemical and biological excitable media, together with the ability to record electrical wave propagation on the surface of isolated hearts with an unprecedented spatial and temporal resolution, lead to a better understanding of the dynamics of AF and of its nature.44, 45, 66 Such an advanced technology has confirmed that the turbulent electrical activity seen in the electrogram recordings of the atria may in some cases be explained by a single or a small number of rapidly spinning rotors.67, 68 Rotor formation or its maintenance does not require a permanent anatomical obstacle; a transient functional heterogeneity that is able to generate a wavebreak suffices to generate a singularity around which the newly formed wavefront with extreme curvature rotates (Figure 1).69 These studies demonstrated that the self-organized functional reentrant sources (i.e., the rotors) that maintain AF adopt the appearance of a pinwheel, which rotates rhythmically at high rates and sheds large numbers of spiral waves (Figure S3A, Online Supplement). A rotor resembles a hurricane or a tornado, and its vortex-like activity is organized by a phase singularity near its center at which all phases (colors) of the excitation–recovery cycle converge (Figure S3B).35, 45 Accordingly, the spiral waves emitted by the rotor propagate through the heterogeneous cardiac muscle and interact with anatomical and functional obstacles, leading to wavefront fragmentation and fibrillatory conduction.70
Figure 1.
Wavebreak and singularity point formation. A. The front of a wave (green) moves rapidly to the neighborhood of the refractory tail of a slowly dissipating wave tail of a preceeding wave. B. When the wave front reaches the wave tail, it attaches to it and divides into two daughter waves that propagate forward as they encounter excitable tissue. C through F, under appropriate conditions of reduced excitability the two wave fronts detach from the wave tail forming a singularity point at their respective broken tip. At each singularity point the curvature of the wave front is extreme, which forces the rest of the wave front to curl around it and spin. In this case, two counter revolving rotors would be formed.
Wave Fractionation and CFAEs
Just as the advancement of clinical medicine requires insights from basic research (i.e. “forward translational research”), clinical work also helps clarify unresolved biological behaviors. This notion is known as “backward translational research”.71, 72 In fact, several proposals for basic research steaming from the Atrial Fibrillation Guidelines have been recently proposed.72 Although these proposals are yet to be resolved, in the following lines we describe several examples of backward translational research that provided significant insights into the mechanisms of AF.
In 2004, Nademanee et al. proposed that areas with complex fractionated electrograms or CFAEs were critical sites for perpetuation of AF and their elimination with radiofrequency ablation was associated with a high probability of sinus rhythm maintenance.43 Recent studies have targeted CFAEs at a range of atrial sites as a means to increase AF ablation success following circumferential PV isolation.1, 53 However, clinical results have been controversial at best, calling into question the role of CFAEs in AF maintenance, especially in patients with paroxysmal AF.
Although some fractionated signals might represent critical zones related to AF maintenance (i.e., high-frequency sources “driving” AF), others might be passive and unrelated to the primary arrhythmia mechanism (i.e., wave front collision or overlapping).1, 10, 73 Still others appear to be located in areas surrounding the autonomic ganglionated plexi.74, 75 In fact, the pathophysiological relationship between high-frequency PV discharges and CFAEs on the posterior left atrial wall is far from being understood. Experimental and clinical studies have shown that fractionated signals need not be critical for AF maintenance. Kalifa et al found that the periphery of high-frequency AF drivers is the area at which most fractionation occurs.76 That is the area where the phenomenon of fibrillatory conduction is most manifest. Rotor meandering might also underlie, at least in part, the electrogram fractionation at close proximity of the source.73 This might explain the success of some CFAE ablation procedures that by chance produce an anatomic obstacle around the highest dominant frequency (DF) site. However, other studies suggest that CFAEs might be unrelated to the primary arrhythmia mechanism and simply represent transient pivoting, wavefront collision, or wave fractionation.10, 13, 77 The latter has been confirmed by a recent study with typical bench to bedside design showing that, in paroxysmal AF, electrogram fractionation at the posterior left atrial wall is a reflection of fibrillatory conduction and a consequence of the dynamic interaction between high-frequency reentrant sources and the atrial anatomy (Figure S4, Online Supplement).78, 79
Validation of experiments using optical mapping
Not surprisingly, most experimentally derived information on mechanisms of cardiac fibrillation has come from large animal models.14 In general, fibrillation in small animals like mice or rats is difficult to initiate or maintain for relatively long periods of time. Nevertheless, as discussed above, there might be a strong similarity in the underlying mechanisms of fibrillation in most, if not all, mammalian species,46 which may be of considerable fundamental and practical significance and provides some validation for the translational of data derived from experimental studies to arrhythmias in humans, including AF. We summarize some of these results in the next section.
Left-to-right atrial frequency gradients
Several methods have been used to analyze and compare AF signals. On the one hand, time domain analysis is routinely used in the clinical EP laboratory to record the electrical signals of the atria during AF. However, a few catheter-based electrodes positioned at different locations in the atria commonly reveal extremely complex spatio-temporal activation patterns that often preclude straightforward interpretation. On the other hand, analyses in the frequency domain, while imperfect,66 enables reliable quantization of the local frequency of activation which reveals a high degree of spatiotemporal organization.80 Using these signal processing methods, experimental studies in the isolated sheep heart have demonstrated that the highest frequency rotors that maintain acute cholinergic AF are usually localized in the LA, and that propagation of the waves that emanate from them undergo spatially distributed intermittent block processes that give rise to fibrillatory conduction result in a left-to-right gradient of excitation frequencies.71,73,79,80 More recently, a significant number of studies have characterized the spatial distribution of DFs during AF in patients, confirming the existence of a similar hierarchical organization in the rate of atrial activation in both paroxysmal and chronic AF patients.52, 54, 81, 82 Simultaneous time- and frequency-domain analyses have demonstrated a high correlation between the location of maximal DF areas and the origin of incoming waves, which led investigators to infer that during organized AF episodes the posterior wall of the LA is passively activated from high-frequency sources located at the pulmonary vein-LA junction.7, 79 As a natural consequence of these studies, real-time spectral mapping techniques have been used to identify and ablate high-frequency sites in patients with AF,54 and recent work has demonstrated that an unequal distribution of inward rectifier28 and other K+ currents27 underlies this spatial distribution of frequencies, which may represent new therapeutic targets.
An important limitation of using dominant frequency mapping in human patients is the very low number of intracardiac electrodes that can be used simultaneously in the clinical EP lab, which makes it very hard to localize the highest dominant frequency sources.81 Optical mapping techniques on the other hand enable high-resolution mapping and relatively easy localization of the highest frequency sources, as well as the determination of the frequency gradients and their quantification. In fact, cardiac optical mapping has evolved to become a powerful tool that can provide new mechanistic insights into the most complex atrial and ventricular arrhythmias.83 In the ventricles, its submillimeter spatial resolution enabled the demonstration that VF in isolated human hearts is associated with wavebreaks and singularity point formation and is maintained by high-frequency rotors and fibrillatory conduction.84 With the development of new infrared voltage-sensitive dyes,85 optical mapping based on sophisticated catheter-based endoscopic mapping protocols (Figure S5) may someday be used in the clinical EP lab to localize AF sources in the human heart.86 However, until that day comes, catheter electrode mapping will have to suffice. As discussed below, important advances even in this area have created new excitement about the possibility of using mechanistic approaches to effectively terminate atrial fibrillation using novel RF ablation strategies.
The role of high frequency sites in human AF maintenance
Using a canine chronic AF model, Morillo et al. demonstrated that AF cycle lengths were consistently shorter in the LA than in the RA.87 The shortest cycle lengths were measured in the area of the posterior left atrial wall near the PV-LA junctions. This suggested that AF in that model was maintained by high frequency drivers in the posterior LA that gave rise to consistent left-to-right frequency gradient.69 Moreover, cryoablation of this area significantly prolonged atrial cycle length and successfully restored sinus rhythm in most dogs, confirming the crucial role of localized sites with high activation frequency. The observations of Morillo et al87 were amply confirmed and expanded upon by large numbers of experiments in the sheep heart.67, 70 Confirmation of a hierarchical organization of AF frequencies in humans, however, came later with seminal work of Haissaguerre et al3 demonstrating that ectopic foci in the pulmonary veins were the most common triggers, that they were capable of initiating and even maintaining AF, and that they could be eliminated by treatment with radiofrequency ablation.
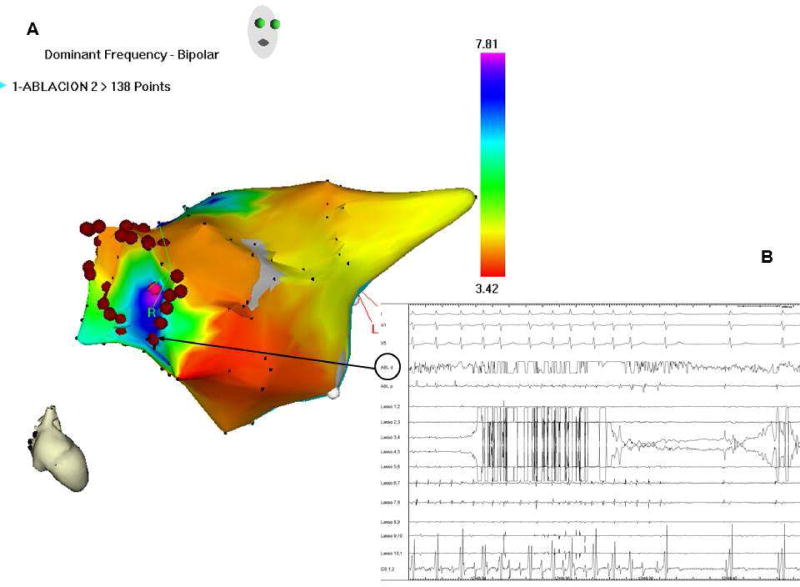
Recently, we used a combination of real-time DF mapping and RF ablation to determine the safety and long-term outcome of targeting maximal DF (DFmax) sites in patients.54 We demonstrated that real-time spectral analysis of AF was safe and that it enables identification and elimination of sources responsible for AF maintenance (Figure 2). Most important, we showed that targeting such sources followed by circumferential pulmonary vein isolation resulted in long-term SR maintenance in 75% of paroxysmal and 50% of persistent AF patients.54
Figure 2.
A: Real-time atrial DF map (right anterior view; CARTO system) in a paroxysmal AF patient. Purple, high DF site on right superior PV antrum. Red dots, circumferential ablation line. B: Surface ECG leads and intracardiac lasso catheter electrograms within RSPV; CS and ablation catheter (black arrow) during radiofrequency delivery, with sinus rhythm conversion, prior to isolation of the RSPV, confirming the reentrant nature of AF, incompatible with the multiple wavelet theory. CS: coronary sinus; DF: dominant frequency; ECG: electrocardiogram; PV: pulmonary vein. (Unpublished).
As discussed above, most experimentally derived information on the existence of rotors or drivers as a mechanism of cardiac fibrillation has come from large animal models using high-resolution video imaging.14 This is not surprising and not only due to conceptual differences but also to substantial differences of mapping techniques used in the clinic as well as in various experimental laboratories. Not only the number of electrodes is highly variable, but the electrodes may be unipolar or bipolar, the mapped area may be too small, and while some investigators use high-resolution cameras others use photodiode arrays.83 The type of voltage sensitive dyes and the software specifically designed to look for AF sources also varies. Undoubtedly all of the above have contributed to the inconsistent detection of rotors in both animal models and humans. Therefore, perhaps the most remarkable development in the field of AF therapy since the discovery of the PV triggers, has been the recent direct demonstration by Narayan et al that AF in humans may result of long-standing rotors with fibrillatory conduction to the surrounding atrium.88 They used novel computational mapping approach via two basket catheters, one in the right atrium and the other in the left atrium, each with 64 electrodes, to reveal AF rotors in the left or right atrium. Most important, brief radiofrequency ablation at or near the center of rotation alone acutely terminated AF,88 which supported the conclusion that rotors are the primary drivers of AF, at least in some patients. The results further suggest that heterogeneity in the atrium in the form of spatially distributed refractory period gradients constitutes a likely arrhythmogenic substrate in which rotors remain relatively stable as a result of such gradients, and that waves emanating at high frequency result in the turbulent electrical activation which manifests as fibrillatory conduction. While the results of Narayan et al88 need to be confirmed by other laboratories, they are nevertheless an exciting mechanistically based approach to AF therapy which derives from translation of basic science knowledge into clinical practice.
Conclusions
There is an urgent need for enhanced communication between basic scientists and clinical electrophysiologists. Clearly both communities are interested in preventing the expansion of the AF epidemic by improving outcomes in AF therapy. Together both communities, in collaboration with industry, should endeavor to identify strategies to pursue the translation of their discoveries into clinical practice. While RF ablation has been relatively successful in improving the quality of life of some patients with AF, it is clear that current ablation strategies are insufficient at controlling the arrhythmia in the majority of patients. Mechanistically based approaches to AF therapy offer a potentially exciting alternative but this requires greater communication and collaboration. We submit that generating insights into AF mechanisms through novel theory and experimentation will require the use of relevant numerical and biological models, where translation of basic science concepts into clinical practice should be the critical final step. Translational electrophysiology will be essential in our future attempts to improve patient care and develop new, safe, and more successful therapies.
Supplementary Material
Acknowledgments
Funding Sources: This work was supported by the Spanish Society of Cardiology (FA); NHLBI grants P01-HL039707 and P01-HL87226 (JJ); the Leducq Foundation (JJ); the Fédération Francaise de Cardiologie (RPM); and Centro Nacional de Investigaciones Cardiovasculares (proyecto CNIC-13) to (FA, JJ).
Footnotes
Conflict of Interest Disclosures: Felipe Atienza, MD receives a research grants from St Jude Medical and is on the Advisory Board of Medtronic, Inc. Raphael P Martins, MD has no conflict to declare. José Jalife, M.D receives a research grant from Gilead, Inc; he is on the Scientific Advisory Board of Topera, Inc (stock options), and the Scientific Advisory Board of Rhythm Solutions, Inc (stock options).
References
- 1.Calkins H, Kuck KH, Cappato R, Brugada J, Camm AJ, Chen SA, Crijns HJ, Damiano RJ, Jr, Davies DW, DiMarco J, Edgerton J, Ellenbogen K, Ezekowitz MD, Haines DE, Haissaguerre M, Hindricks G, Iesaka Y, Jackman W, Jalife J, Jais P, Kalman J, Keane D, Kim YH, Kirchhof P, Klein G, Kottkamp H, Kumagai K, Lindsay BD, Mansour M, Marchlinski FE, McCarthy PM, Mont JL, Morady F, Nademanee K, Nakagawa H, Natale A, Nattel S, Packer DL, Pappone C, Prystowsky E, Raviele A, Reddy V, Ruskin JN, Shemin RJ, Tsao HM, Wilber D. 2012 hrs/ehra/ecas expert consensus statement on catheter and surgical ablation of atrial fibrillation: Recommendations for patient selection, procedural techniques, patient management and follow-up, definitions, endpoints, and research trial design. Europace. 2012;14:528–606. doi: 10.1093/europace/eus027. [DOI] [PubMed] [Google Scholar]
- 2.Dobrev D, Nattel S. New antiarrhythmic drugs for treatment of atrial fibrillation. Lancet. 2010;375:1212–1223. doi: 10.1016/S0140-6736(10)60096-7. [DOI] [PubMed] [Google Scholar]
- 3.Haissaguerre M, Jais P, Shah DC, Takahashi A, Hocini M, Quiniou G, Garrigue S, Le Mouroux A, Le Metayer P, Clementy J. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med. 1998;339:659–666. doi: 10.1056/NEJM199809033391003. [DOI] [PubMed] [Google Scholar]
- 4.Cappato R, Calkins H, Chen SA, Davies W, Iesaka Y, Kalman J, Kim YH, Klein G, Natale A, Packer D, Skanes A, Ambrogi F, Biganzoli E. Updated worldwide survey on the methods, efficacy, and safety of catheter ablation for human atrial fibrillation. Circ Arrhythm Electrophysiol. 2010;3:32–38. doi: 10.1161/CIRCEP.109.859116. [DOI] [PubMed] [Google Scholar]
- 5.Weerasooriya R, Khairy P, Litalien J, Macle L, Hocini M, Sacher F, Lellouche N, Knecht S, Wright M, Nault I, Miyazaki S, Scavee C, Clementy J, Haissaguerre M, Jais P. Catheter ablation for atrial fibrillation: Are results maintained at 5 years of follow-up? J Am Coll Cardiol. 2011;57:160–166. doi: 10.1016/j.jacc.2010.05.061. [DOI] [PubMed] [Google Scholar]
- 6.Packer DL. Evolution of mapping and anatomic imaging of cardiac arrhythmias. J Cardiovasc Electrophysiol. 2004;15:839–854. doi: 10.1046/j.1540-8167.2004.04275.x. [DOI] [PubMed] [Google Scholar]
- 7.Wellens HJ. Forty years of invasive clinical electrophysiology: 1967–2007. Circ Arrhythm Electrophysiol. 2008;1:49–53. doi: 10.1161/CIRCEP.108.770529. [DOI] [PubMed] [Google Scholar]
- 8.Pappone C, Oreto G, Rosanio S, Vicedomini G, Tocchi M, Gugliotta F, Salvati A, Dicandia C, Calabro MP, Mazzone P, Ficarra E, Di Gioia C, Gulletta S, Nardi S, Santinelli V, Benussi S, Alfieri O. Atrial electroanatomic remodeling after circumferential radiofrequency pulmonary vein ablation: Efficacy of an anatomic approach in a large cohort of patients with atrial fibrillation. Circulation. 2001;104:2539–2544. doi: 10.1161/hc4601.098517. [DOI] [PubMed] [Google Scholar]
- 9.Iwasaki YK, Nishida K, Kato T, Nattel S. Atrial fibrillation pathophysiology: Implications for management. Circulation. 2011;124:2264–2274. doi: 10.1161/CIRCULATIONAHA.111.019893. [DOI] [PubMed] [Google Scholar]
- 10.Konings KT, Kirchhof CJ, Smeets JR, Wellens HJ, Penn OC, Allessie MA. High-density mapping of electrically induced atrial fibrillation in humans. Circulation. 1994;89:1665–1680. doi: 10.1161/01.cir.89.4.1665. [DOI] [PubMed] [Google Scholar]
- 11.Konings KT, Smeets JL, Penn OC, Wellens HJ, Allessie MA. Configuration of unipolar atrial electrograms during electrically induced atrial fibrillation in humans. Circulation. 1997;95:1231–1241. doi: 10.1161/01.cir.95.5.1231. [DOI] [PubMed] [Google Scholar]
- 12.Kumagai K, Khrestian C, Waldo AL. Simultaneous multisite mapping studies during induced atrial fibrillation in the sterile pericarditis model. Insights into the mechanism of its maintenance. Circulation. 1997;95:511–521. doi: 10.1161/01.cir.95.2.511. [DOI] [PubMed] [Google Scholar]
- 13.Ortiz J, Niwano S, Abe H, Rudy Y, Johnson NJ, Waldo AL. Mapping the conversion of atrial flutter to atrial fibrillation and atrial fibrillation to atrial flutter. Insights into mechanisms. Circ Res. 1994;74:882–894. doi: 10.1161/01.res.74.5.882. [DOI] [PubMed] [Google Scholar]
- 14.Nishida K, Michael G, Dobrev D, Nattel S. Animal models for atrial fibrillation: Clinical insights and scientific opportunities. Europace : European pacing, arrhythmias, and cardiac electrophysiology : journal of the working groups on cardiac pacing, arrhythmias, and cardiac cellular electrophysiology of the European Society of Cardiology. 2010;12:160–172. doi: 10.1093/europace/eup328. [DOI] [PubMed] [Google Scholar]
- 15.Jalife J. Deja vu in the theories of atrial fibrillation dynamics. Cardiovasc Res. 2011;89:766–775. doi: 10.1093/cvr/cvq364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sinner MF, Ellinor PT, Meitinger T, Benjamin EJ, Kaab S. Genome-wide association studies of atrial fibrillation: Past, present, and future. Cardiovasc Res. 2011;89:701–709. doi: 10.1093/cvr/cvr001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dobrev D, Nattel S. New insights into the molecular basis of atrial fibrillation: Mechanistic and therapeutic implications. Cardiovasc Res. 2011;89:689–691. doi: 10.1093/cvr/cvr021. [DOI] [PubMed] [Google Scholar]
- 18.Wakili R, Voigt N, Kaab S, Dobrev D, Nattel S. Recent advances in the molecular pathophysiology of atrial fibrillation. J Clin Invest. 2011;121:2955–2968. doi: 10.1172/JCI46315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Van Wagoner DR, Pond AL, McCarthy PM, Trimmer JS, Nerbonne JM. Outward k+ current densities and kv1.5 expression are reduced in chronic human atrial fibrillation. Circ Res. 1997;80:772–781. doi: 10.1161/01.res.80.6.772. [DOI] [PubMed] [Google Scholar]
- 20.Dun W, Boyden PA. Aged atria: Electrical remodeling conducive to atrial fibrillation. J Interv Card Electrophysiol. 2009;25:9–18. doi: 10.1007/s10840-008-9358-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hackam DG, Redelmeier DA. Translation of research evidence from animals to humans. JAMA. 2006;296:1731–1732. doi: 10.1001/jama.296.14.1731. [DOI] [PubMed] [Google Scholar]
- 22.Musch TI, Carroll RG, Just A, Lane PH, Talman WT. A broader view of animal research. BMJ. 2007;334:274. doi: 10.1136/bmj.39115.390984.1F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moye LA, Pfeffer MA, Braunwald E. Rationale, design and baseline characteristics of the survival and ventricular enlargement trial. Save investigators. Am J Cardiol. 1991;68:70D–79D. doi: 10.1016/0002-9149(91)90263-k. [DOI] [PubMed] [Google Scholar]
- 24.Wadman N. The bridge between lab and clinic. Nature. 2010:468. doi: 10.1038/468877a. [DOI] [PubMed] [Google Scholar]
- 25.Voigt N, Maguy A, Yeh YH, Qi X, Ravens U, Dobrev D, Nattel S. Changes in i k, ach single-channel activity with atrial tachycardia remodelling in canine atrial cardiomyocytes. Cardiovasc Res. 2008;77:35–43. doi: 10.1093/cvr/cvm051. [DOI] [PubMed] [Google Scholar]
- 26.Bosch RF, Zeng X, Grammer JB, Popovic K, Mewis C, Kuhlkamp V. Ionic mechanisms of electrical remodeling in human atrial fibrillation. Cardiovasc Res. 1999;44:121–131. doi: 10.1016/s0008-6363(99)00178-9. [DOI] [PubMed] [Google Scholar]
- 27.Caballero R, de la Fuente MG, Gomez R, Barana A, Amoros I, Dolz-Gaiton P, Osuna L, Almendral J, Atienza F, Fernandez-Aviles F, Pita A, Rodriguez-Roda J, Pinto A, Tamargo J, Delpon E. In humans, chronic atrial fibrillation decreases the transient outward current and ultrarapid component of the delayed rectifier current differentially on each atria and increases the slow component of the delayed rectifier current in both. J Am Coll Cardiol. 2010;55:2346–2354. doi: 10.1016/j.jacc.2010.02.028. [DOI] [PubMed] [Google Scholar]
- 28.Voigt N, Trausch A, Knaut M, Matschke K, Varro A, Van Wagoner DR, Nattel S, Ravens U, Dobrev D. Left-to-right atrial inward rectifier potassium current gradients in patients with paroxysmal versus chronic atrial fibrillation. Circ Arrhythm Electrophysiol. 2010;3:472–480. doi: 10.1161/CIRCEP.110.954636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jahangir A, Lee V, Friedman PA, Trusty JM, Hodge DO, Kopecky SL, Packer DL, Hammill SC, Shen WK, Gersh BJ. Long-term progression and outcomes with aging in patients with lone atrial fibrillation: A 30-year follow-up study. Circulation. 2007;115:3050–3056. doi: 10.1161/CIRCULATIONAHA.106.644484. [DOI] [PubMed] [Google Scholar]
- 30.Kautzner J, Bulkova V, Hindricks G, Maniadakis N, Della Bella P, Jais P, Kuck KH. Atrial fibrillation ablation: A cost or an investment? Europace. 2011;13(Suppl 2):ii39–43. doi: 10.1093/europace/eur084. [DOI] [PubMed] [Google Scholar]
- 31.Dobrev D, Carlsson L, Nattel S. Novel molecular targets for atrial fibrillation therapy. Nat Rev Drug Discov. 2012;11:275–291. doi: 10.1038/nrd3682. [DOI] [PubMed] [Google Scholar]
- 32.Rosen MR, Gelband H, Merker C, Hoffman BF. Mechanisms of digitalis toxicity. Effects of ouabain on phase four of canine purkinje fiber transmembrane potentials. Circulation. 1973;47:681–689. doi: 10.1161/01.cir.47.4.681. [DOI] [PubMed] [Google Scholar]
- 33.Hashimoto K, Moe GK. Transient depolarizations induced by acetylstrophanthidin in specialized tissue of dog atrium and ventricle. Circ Res. 1973;32:618–624. doi: 10.1161/01.res.32.5.618. [DOI] [PubMed] [Google Scholar]
- 34.Ferrier GR, Moe GK. Effect of calcium on acetylstrophanthidin-induced transient depolarizations in canine purkinje tissue. Circ Res. 1973;33:508–515. doi: 10.1161/01.res.33.5.508. [DOI] [PubMed] [Google Scholar]
- 35.Winfree AT. Spiral waves of chemical activity. Science. 1972;175:634–636. doi: 10.1126/science.175.4022.634. [DOI] [PubMed] [Google Scholar]
- 36.Chao TF, Tsao HM, Lin YJ, Tsai CF, Lin WS, Chang SL, Lo LW, Hu YF, Tuan TC, Suenari K, Li CH, Hartono B, Chang HY, Ambrose K, Wu TJ, Chen SA. Clinical outcome of catheter ablation in patients with nonparoxysmal atrial fibrillation: Results of 3-year follow-up. Circ Arrhythm Electrophysiol. 2012;5:514–520. doi: 10.1161/CIRCEP.111.968032. [DOI] [PubMed] [Google Scholar]
- 37.Tzou WS, Marchlinski FE, Zado ES, Lin D, Dixit S, Callans DJ, Cooper JM, Bala R, Garcia F, Hutchinson MD, Riley MP, Verdino R, Gerstenfeld EP. Long-term outcome after successful catheter ablation of atrial fibrillation. Circ Arrhythm Electrophysiol. 2010;3:237–242. doi: 10.1161/CIRCEP.109.923771. [DOI] [PubMed] [Google Scholar]
- 38.Moe G. On the multiple wavelet hypothesis of atrial fibrillation. Arch Int Pharmacodyn Ther. 1962;140:183–188. [Google Scholar]
- 39.Moe GK, Rheinboldt WC, Abildskov JA. A computer model of atrial fibrillation. Am Heart J. 1964;67:200–220. doi: 10.1016/0002-8703(64)90371-0. [DOI] [PubMed] [Google Scholar]
- 40.Allessie MA, Lammers WJEP, Bonke FIM, Hollen J. Experimental evaluation of moe’s multiple wavelet hypothesis of atrial fibrillation. In: Zipes DP, Jalife J, editors. Cardiac Electrophysiology and Arrhythmias. Orlando: Grune & Straton; 1985. pp. 265–275. [Google Scholar]
- 41.Cox JL, Boineau JP, Schuessler RB, Ferguson TB, Jr, Cain ME, Lindsay BD, Corr PB, Kater KM, Lappas DG. Successful surgical treatment of atrial fibrillation. Review and clinical update. JAMA. 1991;266:1976–1980. [PubMed] [Google Scholar]
- 42.Sheng X, Scherlag BJ, Yu L, Li S, Ali R, Zhang Y, Fu G, Nakagawa H, Jackman WM, Lazzara R, Po SS. Prevention and reversal of atrial fibrillation inducibility and autonomic remodeling by low-level vagosympathetic nerve stimulation. J Am Coll Cardiol. 57:563–571. doi: 10.1016/j.jacc.2010.09.034. [DOI] [PubMed] [Google Scholar]
- 43.Nademanee K, McKenzie J, Kosar E, Schwab M, Sunsaneewitayakul B, Vasavakul T, Khunnawat C, Ngarmukos T. A new approach for catheter ablation of atrial fibrillation: Mapping of the electrophysiologic substrate. J Am Coll Cardiol. 2004;43:2044–2053. doi: 10.1016/j.jacc.2003.12.054. [DOI] [PubMed] [Google Scholar]
- 44.Chen J, Mandapati R, Berenfeld O, Skanes AC, Gray RA, Jalife J. Dynamics of wavelets and their role in atrial fibrillation in the isolated sheep heart. Cardiovasc Res. 2000;48:220–232. doi: 10.1016/s0008-6363(00)00177-2. [DOI] [PubMed] [Google Scholar]
- 45.Gray RA, Pertsov AM, Jalife J. Spatial and temporal organization during cardiac fibrillation. Nature. 1998;392:75–78. doi: 10.1038/32164. [DOI] [PubMed] [Google Scholar]
- 46.Noujaim SF, Berenfeld O, Kalifa J, Cerrone M, Nanthakumar K, Atienza F, Moreno J, Mironov S, Jalife J. Universal scaling law of electrical turbulence in the mammalian heart. Proc Natl Acad Sci U S A. 2007;104:20985–20989. doi: 10.1073/pnas.0709758104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Waldo A, Atienza F. Atrial flutter. In: Zipes DPJJ, editor. Cardiac electrophysiology: From cell to bedside. Philadelphia: Saunders Elsevier; 2009. pp. 567–576. [Google Scholar]
- 48.Schuessler RB, Grayson TM, Bromberg BI, Cox JL, Boineau JP. Cholinergically mediated tachyarrhythmias induced by a single extrastimulus in the isolated canine right atrium. Circ Res. 1992;71:1254–1267. doi: 10.1161/01.res.71.5.1254. [DOI] [PubMed] [Google Scholar]
- 49.Wijffels MC, Dorland R, Mast F, Allessie MA. Widening of the excitable gap during pharmacological cardioversion of atrial fibrillation in the goat: Effects of cibenzoline, hydroquinidine, flecainide, and d-sotalol. Circulation. 2000;102:260–267. doi: 10.1161/01.cir.102.2.260. [DOI] [PubMed] [Google Scholar]
- 50.Hayashi H, Fujiki A, Tani M, Usui M, Inoue H. Different effects of class ic and iii antiarrhythmic drugs on vagotonic atrial fibrillation in the canine heart. J Cardiovasc Pharmacol. 1998;31:101–107. doi: 10.1097/00005344-199801000-00015. [DOI] [PubMed] [Google Scholar]
- 51.Nattel S, Khairy P, Roy D, Thibault B, Guerra P, Talajic M, Dubuc M. New approaches to atrial fibrillation management: A critical review of a rapidly evolving field. Drugs. 2002;62:2377–2397. doi: 10.2165/00003495-200262160-00005. [DOI] [PubMed] [Google Scholar]
- 52.Sanders P, Berenfeld O, Hocini M, Jais P, Vaidyanathan R, Hsu LF, Garrigue S, Takahashi Y, Rotter M, Sacher F, Scavee C, Ploutz-Snyder R, Jalife J, Haissaguerre M. Spectral analysis identifies sites of high-frequency activity maintaining atrial fibrillation in humans. Circulation. 2005;112:789–797. doi: 10.1161/CIRCULATIONAHA.104.517011. [DOI] [PubMed] [Google Scholar]
- 53.Oral H, Chugh A, Good E, Sankaran S, Reich SS, Igic P, Elmouchi D, Tschopp D, Crawford T, Dey S, Wimmer A, Lemola K, Jongnarangsin K, Bogun F, Pelosi F, Jr, Morady F. A tailored approach to catheter ablation of paroxysmal atrial fibrillation. Circulation. 2006;113:1824–1831. doi: 10.1161/CIRCULATIONAHA.105.601898. [DOI] [PubMed] [Google Scholar]
- 54.Atienza F, Almendral J, Jalife J, Zlochiver S, Ploutz-Snyder R, Torrecilla EG, Arenal A, Kalifa J, Fernandez-Aviles F, Berenfeld O. Real-time dominant frequency mapping and ablation of dominant frequency sites in atrial fibrillation with left-to-right frequency gradients predicts long-term maintenance of sinus rhythm. Heart Rhythm. 2009;6:33–40. doi: 10.1016/j.hrthm.2008.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Allessie MA, de Groot NM, Houben RP, Schotten U, Boersma E, Smeets JL, Crijns HJ. Electropathological substrate of long-standing persistent atrial fibrillation in patients with structural heart disease: Longitudinal dissociation. Circ Arrhythm Electrophysiol. 2010;3:606–615. doi: 10.1161/CIRCEP.109.910125. [DOI] [PubMed] [Google Scholar]
- 56.Eckstein J, Maesen B, Linz D, Zeemering S, van Hunnik A, Verheule S, Allessie M, Schotten U. Time course and mechanisms of endo-epicardial electrical dissociation during atrial fibrillation in the goat. Cardiovasc Res. 2011;89:816–824. doi: 10.1093/cvr/cvq336. [DOI] [PubMed] [Google Scholar]
- 57.Rothberger C, Winterberg H. Vorhofflimmern und arhythmia perpetua. Wiener Klinische Wochenschrift. 1909;22:839–844. [Google Scholar]
- 58.Lewis T. Oliver-sharpey lectures on the nature of flutter and fibrillation of the auricle. Br Med J. 1921;1:590–593. doi: 10.1136/bmj.1.3147.590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Scherf D, Terranova R. Mechanism of auricular flutter and fibrillation. Am J Physiol. 1949;159:137–142. doi: 10.1152/ajplegacy.1949.159.1.137. [DOI] [PubMed] [Google Scholar]
- 60.Yamazaki M, Honjo H, Ashihara T, Harada M, Sakuma I, Nakazawa K, Trayanova N, Horie M, Kalifa J, Jalife J, Kamiya K, Kodama I. Regional cooling facilitates termination of spiral-wave reentry through unpinning of rotors in rabbit hearts. Heart Rhythm. 2012;9:107–114. doi: 10.1016/j.hrthm.2011.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hoffman BF, Dangman KH. Mechanisms for cardiac arrhythmias. Experientia. 1987;43:1049–1056. doi: 10.1007/BF01956038. [DOI] [PubMed] [Google Scholar]
- 62.Ming Z, Nordin C, Aronson RS. Role of l-type calcium channel window current in generating current-induced early afterdepolarizations. J Cardiovasc Electrophysiol. 1994;5:323–334. doi: 10.1111/j.1540-8167.1994.tb01169.x. [DOI] [PubMed] [Google Scholar]
- 63.Hove-Madsen L, Llach A, Bayes-Genis A, Roura S, Rodriguez Font E, Aris A, Cinca J. Atrial fibrillation is associated with increased spontaneous calcium release from the sarcoplasmic reticulum in human atrial myocytes. Circulation. 2004;110:1358–1363. doi: 10.1161/01.CIR.0000141296.59876.87. [DOI] [PubMed] [Google Scholar]
- 64.Dixit S, Gerstenfeld EP, Callans DJ, Marchlinski FE. Mechanisms underlying sustained firing from pulmonary veins: Evidence from pacing maneuvers and pharmacological manipulation. Pacing Clin Electrophysiol. 2004;27:1120–1129. doi: 10.1111/j.1540-8159.2004.00594.x. [DOI] [PubMed] [Google Scholar]
- 65.Oral H, Crawford T, Frederick M, Gadeela N, Wimmer A, Dey S, Sarrazin JF, Kuhne M, Chalfoun N, Wells D, Good E, Jongnarangsin K, Chugh A, Bogun F, Pelosi F, Jr, Morady F. Inducibility of paroxysmal atrial fibrillation by isoproterenol and its relation to the mode of onset of atrial fibrillation. J Cardiovasc Electrophysiol. 2008;19:466–470. doi: 10.1111/j.1540-8167.2007.01089.x. [DOI] [PubMed] [Google Scholar]
- 66.Berenfeld O, Ennis S, Hwang E, Hooven B, Grzeda K, Mironov S, Yamazaki M, Kalifa J, Jalife J. Time- and frequency-domain analyses of atrial fibrillation activation rate: The optical mapping reference. Heart Rhythm. 2011;8:1758–1765. doi: 10.1016/j.hrthm.2011.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mandapati R, Skanes A, Chen J, Berenfeld O, Jalife J. Stable microreentrant sources as a mechanism of atrial fibrillation in the isolated sheep heart. Circulation. 2000;101:194–199. doi: 10.1161/01.cir.101.2.194. [DOI] [PubMed] [Google Scholar]
- 68.Mansour M, Mandapati R, Berenfeld O, Chen J, Samie FH, Jalife J. Left-to-right gradient of atrial frequencies during acute atrial fibrillation in the isolated sheep heart. Circulation. 2001;103:2631–2636. doi: 10.1161/01.cir.103.21.2631. [DOI] [PubMed] [Google Scholar]
- 69.Jalife J, Berenfeld O, Mansour M. Mother rotors and fibrillatory conduction: A mechanism of atrial fibrillation. Cardiovasc Res. 2002;54:204–216. doi: 10.1016/s0008-6363(02)00223-7. [DOI] [PubMed] [Google Scholar]
- 70.Berenfeld O, Mandapati R, Dixit S, Skanes AC, Chen J, Mansour M, Jalife J. Spatially distributed dominant excitation frequencies reveal hidden organization in atrial fibrillation in the langendorff-perfused sheep heart. J Cardiovasc Electrophysiol. 2000;11:869–879. doi: 10.1111/j.1540-8167.2000.tb00066.x. [DOI] [PubMed] [Google Scholar]
- 71.Mankoff SP, Brander C, Ferrone S, Marincola FM. Lost in translation: Obstacles to translational medicine. J Transl Med. 2004;2:14. doi: 10.1186/1479-5876-2-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nattel S. From guidelines to bench: Implications of unresolved clinical issues for basic investigations of atrial fibrillation mechanisms. Can J Cardiol. 2011;27:19–26. doi: 10.1016/j.cjca.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 73.Zlochiver S, Yamazaki M, Kalifa J, Berenfeld O. Rotor meandering contributes to irregularity in electrograms during atrial fibrillation. Heart Rhythm. 2008;5:846–854. doi: 10.1016/j.hrthm.2008.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lin J, Scherlag BJ, Zhou J, Lu Z, Patterson E, Jackman WM, Lazzara R, Po SS. Autonomic mechanism to explain complex fractionated atrial electrograms (cfae) J Cardiovasc Electrophysiol. 2007;18:1197–1205. doi: 10.1111/j.1540-8167.2007.00976.x. [DOI] [PubMed] [Google Scholar]
- 75.Jackman WM, Scherlag BJ. Nature of electrogram fractionation during atrial fibrillation. Circ Arrhythm Electrophysiol. 2012;5:5–7. doi: 10.1161/CIRCEP.111.969279. [DOI] [PubMed] [Google Scholar]
- 76.Kalifa J, Tanaka K, Zaitsev AV, Warren M, Vaidyanathan R, Auerbach D, Pandit S, Vikstrom KL, Ploutz-Snyder R, Talkachou A, Atienza F, Guiraudon G, Jalife J, Berenfeld O. Mechanisms of wave fractionation at boundaries of high-frequency excitation in the posterior left atrium of the isolated sheep heart during atrial fibrillation. Circulation. 2006;113:626–633. doi: 10.1161/CIRCULATIONAHA.105.575340. [DOI] [PubMed] [Google Scholar]
- 77.Berenfeld O, Zaitsev AV, Mironov SF, Pertsov AM, Jalife J. Frequency-dependent breakdown of wave propagation into fibrillatory conduction across the pectinate muscle network in the isolated sheep right atrium. Circ Res. 2002;90:1173–1180. doi: 10.1161/01.res.0000022854.95998.5c. [DOI] [PubMed] [Google Scholar]
- 78.Klos M, Calvo D, Yamazaki M, Zlochiver S, Mironov S, Cabrera JA, Sanchez-Quintana D, Jalife J, Berenfeld O, Kalifa J. Atrial septopulmonary bundle of the posterior left atrium provides a substrate for atrial fibrillation initiation in a model of vagally mediated pulmonary vein tachycardia of the structurally normal heart. Circ Arrhythm Electrophysiol. 2008;1:175–183. doi: 10.1161/CIRCEP.107.760447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Atienza F, Calvo D, Almendral J, Zlochiver S, Grzeda KR, Martinez-Alzamora N, Gonzalez-Torrecilla E, Arenal A, Fernandez-Aviles F, Berenfeld O. Mechanisms of fractionated electrograms formation in the posterior left atrium during paroxysmal atrial fibrillation in humans. J Am Coll Cardiol. 2011;57:1081–1092. doi: 10.1016/j.jacc.2010.09.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dibs SR, Ng J, Arora R, Passman RS, Kadish AH, Goldberger JJ. Spatiotemporal characterization of atrial activation in persistent human atrial fibrillation: Multisite electrogram analysis and surface electrocardiographic correlations--a pilot study. Heart Rhythm. 2008;5:686–693. doi: 10.1016/j.hrthm.2008.01.027. [DOI] [PubMed] [Google Scholar]
- 81.Atienza F, Almendral J, Moreno J, Vaidyanathan R, Talkachou A, Kalifa J, Arenal A, Villacastin JP, Torrecilla EG, Sanchez A, Ploutz-Snyder R, Jalife J, Berenfeld O. Activation of inward rectifier potassium channels accelerates atrial fibrillation in humans: Evidence for a reentrant mechanism. Circulation. 2006;114:2434–2442. doi: 10.1161/CIRCULATIONAHA.106.633735. [DOI] [PubMed] [Google Scholar]
- 82.Sahadevan J, Ryu K, Peltz L, Khrestian CM, Stewart RW, Markowitz AH, Waldo AL. Epicardial mapping of chronic atrial fibrillation in patients: Preliminary observations. Circulation. 2004;110:3293–3299. doi: 10.1161/01.CIR.0000147781.02738.13. [DOI] [PubMed] [Google Scholar]
- 83.Herron TJ, Lee P, Jalife J. Optical imaging of voltage and calcium in cardiac cells & tissues. Circ Res. 2012;110:609–623. doi: 10.1161/CIRCRESAHA.111.247494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Nanthakumar K, Jalife J, Masse S, Downar E, Pop M, Asta J, Ross H, Rao V, Mironov S, Sevaptsidis E, Rogers J, Wright G, Dhopeshwarkar R. Optical mapping of langendorff-perfused human hearts: Establishing a model for the study of ventricular fibrillation in humans. Am J Physiol Heart Circ Physiol. 2007;293:H875–880. doi: 10.1152/ajpheart.01415.2006. [DOI] [PubMed] [Google Scholar]
- 85.Matiukas A, Mitrea BG, Qin M, Pertsov AM, Shvedko AG, Warren MD, Zaitsev AV, Wuskell JP, Wei MD, Watras J, Loew LM. Near-infrared voltage-sensitive fluorescent dyes optimized for optical mapping in blood-perfused myocardium. Heart Rhythm. 2007;4:1441–1451. doi: 10.1016/j.hrthm.2007.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kalifa J, Klos M, Zlochiver S, Mironov S, Tanaka K, Ulahannan N, Yamazaki M, Jalife J, Berenfeld O. Endoscopic fluorescence mapping of the left atrium: A novel experimental approach for high resolution endocardial mapping in the intact heart. Heart Rhythm. 2007;4:916–924. doi: 10.1016/j.hrthm.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Morillo CA, Klein GJ, Jones DL, Guiraudon CM. Chronic rapid atrial pacing. Structural, functional, and electrophysiological characteristics of a new model of sustained atrial fibrillation. Circulation. 1995;91:1588–1595. doi: 10.1161/01.cir.91.5.1588. [DOI] [PubMed] [Google Scholar]
- 88.Narayan SM, Krummen DE, Rappel WJ. Clinical mapping approach to diagnose electrical rotors and focal impulse sources for human atrial fibrillation. J Cardiovasc Electrophysiol. 2012;23:447–454. doi: 10.1111/j.1540-8167.2012.02332.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.