Abstract
Unlike mouse embryonic stem cells (ESCs), which are closely related to the inner cell mass, human ESCs appear to be more closely related to the later primitive ectoderm. For example, human ESCs and primitive ectoderm share a common epithelial morphology, growth factor requirements, and the potential to differentiate to all three embryonic germ layers. However, it has previously been shown that human ESCs can also differentiate to cells expressing markers of trophoblast, an extraembryonic lineage formed before the formation of primitive ectoderm. Here, we show that phorbol ester 12-O-tetradecanoylphorbol 13-acetate causes human ESCs to undergo an epithelial mesenchymal transition and to differentiate into cells expressing markers of parietal endoderm, another extraembryonic lineage. We further confirmed that this differentiation is through the activation of protein kinase C (PKC) pathway and demonstrated that a particular PKC subtype, PKC-δ, is most responsible for this transition.
Keywords: Human embryonic stem cells, TPA, Extraembryonic endoderm, PKC
Introduction
Similar to mouse embryonic stem cells (ESCs) and the more recently derived mouse epiblast stem cells (EpiSCs) [1, 2], human ESCs proliferate without limit and maintain the potential to differentiate into advanced derivatives of all three embryonic germ layers: ectoderm, mesoderm and endoderm [3]. In mice, ESCs closely resemble the inner cell mass from which they are derived, and EpiSCs closely resemble the later postimplantation epiblast from which they are derived [1, 2, 4, 5]. Human ESC growth in polarized, epithelial colonies, and the particular growth factors that promote human ESC self-renewal, such as transforming growth factor β/nodal/activin and basic fibroblast growth factor [6], suggest that human ESCs more closely resemble the primitive ectoderm than the earlier inner cell mass stage. However, although generally similar, the gene expression profiles and growth factor responses of human ESCs do differ in some significant ways from mouse EpiSCs [2, 7].
Interestingly, under some culture conditions, the addition of bone morphogenetic proteins (BMPs) cause human ESCs to differentiate into cells that uniformly express trophoblast-specific genes, secrete progesterone and estradiol into the culture medium, and form syncytia with hundreds of nuclei with a concomitant upregulation of chorionic gonadotropin, suggesting a homogeneous trophoblast differentiation [8, 9]. This is somewhat surprising because if human ESCs more closely resemble the primitive ectoderm than the earlier inner cell mass stage, they should have lost the capability of trophoblast differentiation. A recent study calls into question the identity of the in vitro produced trophoblast-like cells, and an extraembryonic mesoderm cell has been proposed as a more likely in vivo counterpart [10]. However, although that study raises significant questions about the identity of the BMP-induced cells, because it has not been possible to study the molecular signatures of these peri-implantation human lineages in appropriately staged intact embryos, the actual in vivo counterpart still remains somewhat in doubt. Thus, further elucidating the developmental status and differentiation potential of human ESCs remains important to understand the stage of their in vivo counterpart. Here, we show that activation of the protein kinase C (PKC) pathway causes human ESCs to differentiate to a second extraembryonic lineage, the primitive endoderm, which provides another example suggesting that human ESCs can differentiate into not only three germ layers but also some extraembryonic tissues.
PKC is a serine-threonine kinase family that consists of 11 different isotypes [11]. These PKC isozymes are further divided into three subclasses based on their second messenger requirements: conventional, novel, and atypical. PKC isozymes have different tissue distribution, subcellular localization, cofactor dependence, and substrate specificity; therefore, they exert various roles in cell proliferation, differentiation, apoptosis, and angiogenesis. Phorbol ester 12-O-tetradecanoylphorbol 13-acetate (TPA) activates conventional and novel PKCs, but not atypical PKCs, by mimicking the function of the endogenous secondary messenger, diacylglycerol. Interestingly, in some situations, TPA can also reduce PKC activity by enhancing the degradation of PKC [12]. By increasing or decreasing overall PKC activity, TPA induces a wide range of biological effects in vitro and in vivo, including the induction of epithelial-mesenchymal transition (EMT) [13].
EMT is a process in which cells transition from a polarized, tightly connected epithelial phenotype to an individualized, nonpolarized, and migratory mesenchymal phenotype. EMT is a fundamental process for both normal development and carcinoma progression. In the development of the mouse embryo, the earliest EMTs lead to the formation of the three germ layers through gastrulation and differentiation of parietal endoderm from primitive endoderm [14]. Parietal endoderm is one of the two sublineages of primitive endoderm, which exhibits distinct differences in morphology and marker gene expression compared with the other lineage, visceral endoderm [15]. In recent studies of induced pluripotent stem cells, it was also found that EMT blocks reprogramming while the opposite process of EMT, mesenchymal-epithelial transition, not only facilitates but also is required for the reprogramming of fibroblasts [16]. Previously, it has also been shown that TPA disrupts gap junction communication in human ESCs [17], an early event in EMT.
To examine the effect of PKC activation on human ESCs, we treated the cells with TPA and examined the gene expression profile along a 7-day time course. TPA induced fast EMT of human ESCs, accompanied by dramatic downregulation of pluripotency gene OCT4 and upregulation of multiple endoderm markers without previously expressing primitive streak markers such as brachyury [18]. We further identified that only certain PKC subtype plays essential role in this TPA-induced differentiation, which suggests that the 11 PKC isotypes may have distinct functions in human ESCs. Overall, this and some previous reports [9, 15, 19–22] suggest that human ESCs have the ability to differentiate to both extraembryonic lineages normally differentiating from the embryo prior to implantation, the trophoblast and the extraembryonic endoderm.
Materials and Methods
Cell Culture
Human ESCs (H9 cell line) were cultured in human ESC culture medium preconditioned with irradiated mouse embryonic fibroblasts on Matrigel (BD Biosciences, Bedford, MA, www.bdbiosciences.com/) as described [23]. For routine maintenance, cells were passaged with 1 mg/ml Collagenase (Invitrogen Corporation, Carlsbad, CA, www.invitrogen.com). For the TPA treatment time course experiment, cells were individualized with 0.05% trypsin-EDTA, and seeded at 2 × 106 cells per 10-cm tissue culture dish. Twenty-four hours later, the cells formed small colonies with relatively uniform size (20–30 cells per colony).
Chemical Treatment
TPA (Sigma-Aldrich, St. Louis, MO, www.sigmaaldrich.com) was used at 50 nM. GF 109203X and Bisindolylmaleimide V (Calbiochem, LA Jolla, CA, http://www.emdchemicals.com) were used at 5 µM. For cotreatment experiments, the cells were exposed to GF 109203X or Bisindolylmaleimide V for 40 minutes before adding TPA.
Immunofluorescence
After fixation, cells were permeabilized and blocked in phosphate buffered saline (PBS) containing 0.5% Triton X-100 and 10% horse serum for 30 minutes at room temperature. Cells were then incubated overnight with ZO1 antibody (Invitrogen) or vimentin antibody (Sigma) at 4°C. After washing three times, the cells were stained with Alexa Fluor 488 conjugated anti-mouse IgG (Invitrogen) for 1 hour at room temperature. In terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay, human ESCs were treated with or without 50 nm TPA for 2 days before fixation. TUNEL staining was conducted using In Situ Cell Death Detection Kit, Fluorescein (Roche Applied Science, Indianapolis, IN, www.roche-applied-science.com/) according to the manufacturer’s instructions. Cells treated with DNase I (136 U/ml in 50 mM Tris-HCl, pH 7.5, 1 mg/ml bovine serum albumin) for 10 minutes were used as positive control (data not shown).
E-Cadherin-EGFP Expression and Imaging
The expression plasmid of Ecadherin-enhanced green fluorescent protein (EGFP) fusion protein (pEGFP-N1-Ecad) was provided by Dr. W. James Nelson (Stanford University). The fragment containing E-cadherin-EGFP (3,461 bp) was cut out and ligated into an episomal expression vector and stably expressed in human ESCs [24]. Fluorescence was documented using a Leica confocal TCS SP2 AOBS microscope.
Reverse Transcription Polymerase Chain Reaction (RT-PCR)
Total RNA was prepared with RNeasy Mini Kit (Qiagen, Inc., Valencia, CA, www.qiagen.com) and reverse transcribed (RT) with Omniscript Reverse Transcription Kit (Qiagen). The following quantitative PCR (Q-PCR) was performed with Power SYBR Green PCR Master Mix or TaqMan Gene Expression Assay on the AB7300 Real Time PCR system (Applied Biosystems, Foster City, CA, http://www.appliedbiosystems.com). Glyceraldehyde-3-phosphate dehydrogenase was used as an endogenous reference gene for normalization. Sequences of some primers are listed in the Supporting Information Table S1.
Flow Cytometry
Individualized human ESCs were fixed, permeabilized, and resuspended in fluorescence-activated cell sorting (FACS) buffer. The OCT4 antibody or GATA6 antibody and corresponding isotype control (all from Santa Cruz Biotechnology, Inc., Santa Cruz, CA, www.scbt.com) were added at a final concentration of 4 µg/ml. After overnight incubation, the cells were stained with Alexa Fluor 488 conjugated anti-mouse IgG (for OCT4) or Alexa Fluor 488 conjugated anti-rabbit IgG (for GATA6) for 1 hour at room temperature in dark. The cells finally were analyzed on a BD FACSAria flow cytometer (BD Biosciences) using BD FACSDiva software (BD Biosciences). The final data and graphs were analyzed and prepared in FlowJo software (Tree Star, Inc., Ashland, www.treestar.com/). For the flow cytometry analysis of THBD (CD141), individualized cells were stained with PE-conjugated Mouse Anti-Human CD141 (BD Biosciences) at room temperature for 1 hour in dark without fixation or permeabilization. The cells were analyzed on Accuri flow cytometer C6 (BD Accuri Cytometers Inc, Ann Arbor, MI, www.accuricytometer-s.com) and the graphs were prepared in FCS Express (De novo Software, Los Angeles, CA, www.denovosoftware.com/).
Western Blot
Cells were lysed in 2% SDS lysis buffer containing Protease Inhibitor Cocktail (Sigma) and 100 µM Na3VO4. Protein concentrations were determined by BCA reagent (Pierce, Rockford, IL, www.piercenet.com/). Western blot was performed according to standard protocols with phospho-myristoylated alanine-rich C-kinase substrate (phospho-MARCKS; Ser152/156) antibody (Cell Signaling Technology, Boston, MA, www.cellsignal.com/). The membrane was stripped and reblotted with β-actin antibody (Sigma) as a loading control.
Time Course Microarray Analysis of TPA-Treated Human ESCs
Sample Preparation
Cells were harvested at 0, 1, 3, 6, 12 18, 24, 30, 36, 42, 48, 72, 96, and 168 hours after TPA treatment for RNA purification. One microgram of total RNA of each sample was amplified, coupled to Cy5-NHS ester (Amersham, Piscataway, NJ, www.gelifesciences.com), and fragmented using RNA Fragmentation Reagents (Ambion, Austin, TX, www.invitrogen.com/site/us/en/home/brands/ambion.html). Sonicated genomic DNA (gDNA) was primed with random octamers and labeled with 5-(3-aminoallyl)-dUTP. The resulting product was coupled to Cy3-NHS ester. The NimbleGen whole human genome expression array (NimbleGen Systems, Madison, WI, http://www.nimblegen.com/) used here consists of approximately 380,000 60-mer probes covering 35,952 transcripts from NCBI build 35.
Microarray Data Analysis
Gene expression raw data were extracted using NimbleScan software v2.1 (NimbleGen Systems). The signal intensities from both the RNA and DNA channels in all the arrays were separately normalized with Robust Multiple-chip Analysis algorithm [25]. For a given gene, the relative RNA level was calculated as (intensity from RNA channel)/(intensity from gDNA channel + median intensity of all genes from gDNA channel). Fold changes were then calculated for each gene based on their relative RNA levels in TPA-treated samples over the control sample. A minimum of threefold change in at least one time point was used as a cut-off to identify differentially expressed genes before and after TPA treatment. The microarray data are available in the Gene Expression Omnibus database (http://www.ncbi.nlm.nih.gov/geo) under the accession number GSE33180.
Gene Functional Annotation
The functional annotation (GO terms) of the differentially expressed genes (7,256 in total) during TPA treatment time course was extracted by FatiGo [26]. Hierarchical cluster analysis on genes belonging to selected functional classes was conducted with the MeV program (http://www.tm4.org/mev.html).
Knockdown of PKCs by siRNA
Cells were plated at 6 × 104 per well in 12-well plate 1 day before transfection. PKC isoform-specific knockdown was performed by transfecting the cells with 70 pmol ON-TARGETplus SMARTpool siRNA (Dharmacon, Lafayette, CO, http://www.dharmacon.com) plus 2 µl Dharmacon siRNA Transfection Reagent 1, following the manufacturer’s protocol. Knockdown efficiency and specificity were evaluated by quantitative RT-PCR with total RNA extracted at 72 hours after transfection. Q-PCR reactions were conducted with TaqMan Gene Expression Assays (Applied Biosystems). To examine the effect of PKC isoform-specific knockdown on TPA-induced differentiation, cells were treated with 50 nM TPA at 48 hours after transfection for another 48 hours.
Overexpression of Constitutively Active PKC
The constitutively active PKC-δ was generated by fusing the first nine amino acids of p60 c-Src myristoylation sequence (MGSNKSKPK) to its N terminus [27]. A Flag epitope was added to the C terminus to facilitate the detection of the protein. The Myr–PKC-δ–FLAG cDNA was expressed from an EF1α promoter and followed by an internal ribosome entry site driving a puromycin resistance gene. Human ESCs were transfected with Fugene HD (Roche Applied Science) as specified by the manufacturer and were lysed for RNA extraction 3 days after drug selection. Cells overexpressing EGFP from the same plasmid were included as control.
Results
TPA Induces Human ESCs to Undergo EMT with the Concomitant Downregulation of ESC-Specific Genes
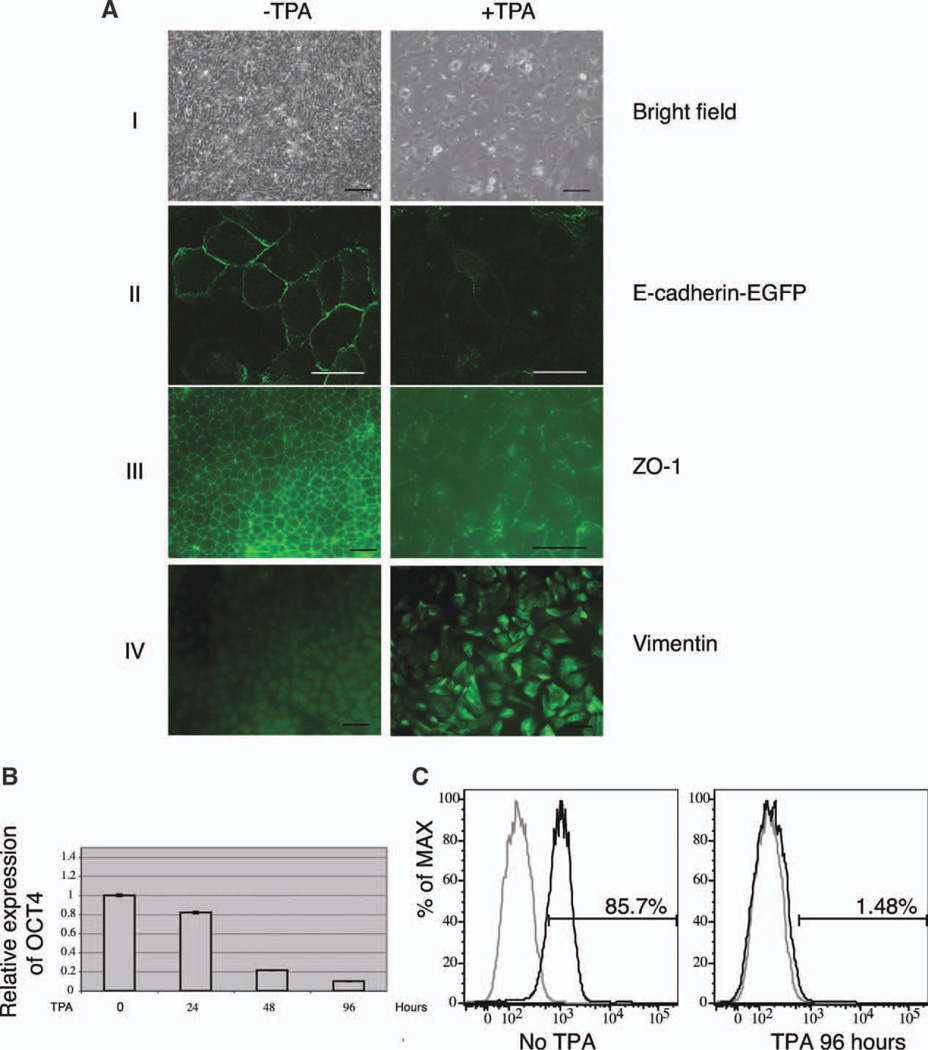
Human ESCs exhibit the hallmarks of epithelial cells. They grow as compact colonies with distinct borders; adjacent cells are coupled by tight junctions [28], gap junctions [29], and adherens junctions [30]; they exhibit a basal-apical polarity [28], and they undergo a specialized form of cell death termed anoikis when dissociated [31]. After TPA treatment, human ESCs became elongated, junction complexes were disrupted, and cells migrated individually (Fig. 1A, I–III; Supporting Information Movie S1 and S2). Vimentin, a mesenchymal-specific cytoskeleton protein, became highly expressed (Fig. 1A, IV).
Figure 1.
TPA induces epithelial-mesenchymal transition of human embryonic stem cells (ESCs) with the concomitant downregulation of OCT4. (A): (I) Morphological changes induced by TPA. Human ESCs were treated with or without TPA for 48 hours. Scale bar = 100 µm. (II) Loss of E-cadherin during TPA-induced cell-cell dissociation. Cells stably expressing EGFP-E-cadherin fusion protein were treated with TPA. Before treatment, EGFP-E-cadherin was localized at the plasma membrane. After exposure to TPA, EGFP-E-cadherin disappeared from the membrane. Scale bar = 25 µm. (III) Disruption of tight junction during TPA-induced cell-cell dissociation. Scale bar = 100 µm. (IV) Expression of vimentin in TPA stimulated cells. Scale bar = 100 µm. (B): The expression of OCT4 mRNA in 0, 24, 48, and 96-hour TPA-treated cells examined by quantitative PCR. (C): Fluorescence-activated cell sorting analysis of OCT4 expression in 96-hour TPA-treated cells. Gray line: isotype control; black line: antibody staining. Abbreviations: EGFP, enhanced green fluorescent protein; % of MAX, % of maximum; TPA, 12-O-tetradecanoylphorbol 13-acetate; and PCR, polymerase chain reaction.
Downregulation of OCT4 could be detected as early as 24 hours after TPA treatment, and after 96-hour treatment, less than 10% of the initial OCT4 mRNA level remained (Fig. 1B). FACS analysis of OCT4 protein at the individual cell level also suggests that almost all cells were OCT4 negative at this time point (Fig. 1C). To exclude the possibility that TPA did not direct differentiation, but only provided a selective advantage for the cells that were already differentiated in the original population, the fate of individual cells was tracked by time-lapse microscopy with 2-day TPA treatment (see Supporting Information Movie S3 and S4). The cells used in this experiment expressed histone H2B.b-mOrange fusion protein [32], which allowed for more effective tracking under a fluorescence microscope. Single cell fate tracking indicated that the death rate of TPA-treated cells was similar to that of the control cells (Supporting Information Table S2, 12.2% and 13.6%, respectively), while in TPA-treated cells the frequency of mitosis was much lower. The result of TUNEL assay also confirmed that exposure to TPA did not cause massive cell death (Supporting Information Fig. S1). Thus, these observations are incompatible with a mere selective killing of the ESCs (which constitutes the majority of the starting population) and the rescue and expansion of a pre-existing differentiated population. We concluded that TPA treatment had an instructive effect on the differentiation of human ESCs.
TPA Differentiated Human ESCs Express Markers of EMT and of Parietal Endoderm
To examine the identity of TPA differentiated cells, we performed microarray analysis of gene expression during TPA-induced differentiation, selecting multiple time points to detect both transient gene expression changes and the terminal differentiation. During the entire time series, out of 35,952 transcripts represented on the microarrays, a total of 7,256 genes were differentially expressed for at least one time point, and the number of genes that showed differential expression at each individual time point increased with time (Supporting Information Table S3).
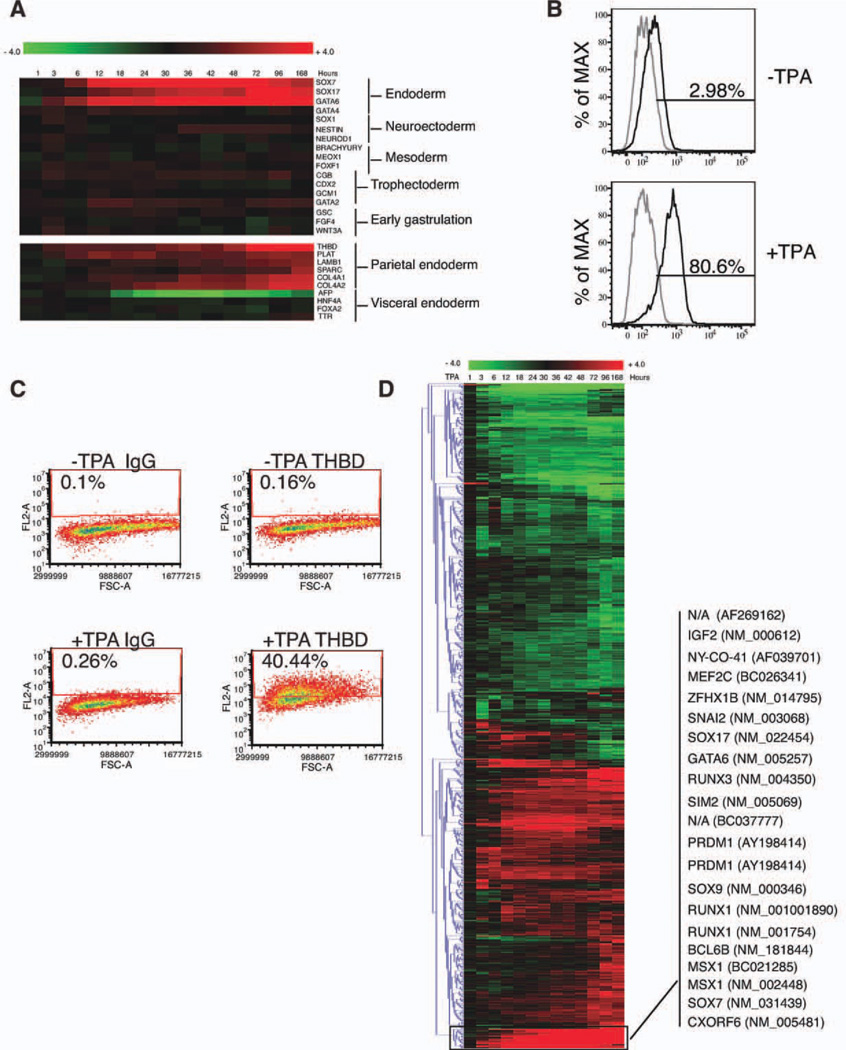
During TPA-induced differentiation, genes pivotal in promoting endoderm differentiation (SOX7, SOX17, and GATA6) [33, 34] were dramatically upregulated, while markers of neuroectoderm (SOX1, NESTIN, and NEUROD1) [35], mesoderm (BRACHYURY, MEOX1, and FOXF1) [18, 36, 37], and trophectoderm (CGB, CDX2, GCM1, and GATA2) [9] remained relatively unchanged (Fig. 2A, upper panel). During the entire time course, marker genes characteristic of primitive streak/mesendoderm formation (BRACHYURY, GSC, FGF4, and WNT3A) were not increased (Fig. 2A, upper panel), suggesting that TPA-induced cells did not progress through gastrulation. Thus, the upregulation of endoderm markers likely reflected extraembryonic endoderm differentiation. Further examination indicated that parietal endoderm-specific genes (THBD, PLAT, LAMB1, SPARC, and COL4A) [15, 38] were dramatically upregulated, while visceral endoderm-specific genes (AFP, HNF4A, FOXA2, and TTR) [34] were not increased (Fig. 2A, lower panel). FACS analysis of cells treated with TPA for 48 hours indicated a uniform shift toward GATA6 expression (Fig.2B) and the expression of THBD protein was detected in 5-day TPA-treated cells (Fig. 2C). Notably, a similar pattern of marker gene expression was obtained from both H9 ES line and H1 ES line (data not shown), suggesting that the response to TPA is not a specific attribute of H9 ESCs.
Figure 2.
TPA differentiated human embryonic stem cells express markers of parietal endoderm. (A): Marker gene expression along TPA treatment time course. Heatmap produced in MeV program was used to display the changes of selected lineage markers in TPA-treated cells over untreated cells. The color of the tiles represented the fold change of each gene in a log2 scale. Upper panel: The expression of different embryonic and extraembryonic tissue markers. Lower panel: The expression of two extraembryonic endoderm sublineage markers. (B): Fluorescence-activated cell sorting (FACS) analysis of GATA6 expression in 0-hour and 48-hour TPA-treated cells. Gray line: isotype control; black line: antibody staining. (C): FACS analysis of THBD expression in 0-day and 5-day TPA-treated cells. (D): Identification of genes that exhibited earliest and strongest upregulation during TPA treatment time course. Hierarchical cluster analysis of the 685 genes that belonged to selected functional classes was conducted based on the Euclidean Distance. The fold changes were displayed in a log2 ratio. Abbreviations:% ofMAX,% of maximum; TPA, 12-O-tetradecanoylphorbol 13-acetate.
Further hierarchical cluster analysis of 685 genes of selected functional classes (transcription factors, transcription cofactors/repressors, RNA binding proteins, and chromatin modifiers) indicated that a small cluster of 21 transcripts (representing 18 different genes) were dramatically upregulated starting just 3–6 hours after TPA treatment and continued to be upregulated through day 7. These 18 genes included not only the essential transcription factors for endoderm differentiation but also genes that are known to be involved in EMT in different processes, such as IGF2, SNAI2, SOX9, MSX1, and ZFHX1 [39–41], suggesting the importance of EMT in both early commitment and subsequent differentiation (Fig. 2D).
TPA Induces Human ESC Differentiation Through Activation of PKC
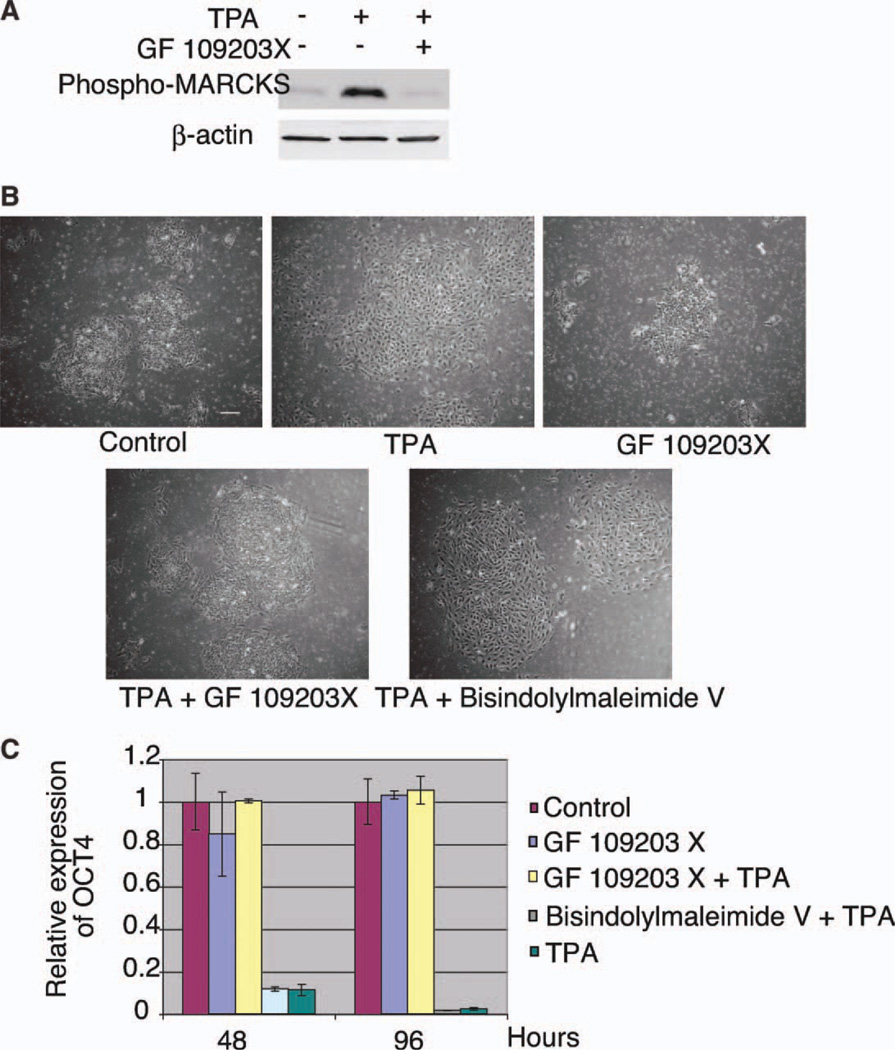
Although TPA is a well-known PKC activator, PKC is not the only TPA target [12]. To determine the role of PKC in TPA-induced differentiation, we first examined the phosphorylation of MARCKS protein, a PKC-specific substrate [42] (Fig. 3A). Within 15 minutes of TPA treatment, the phosphorylation level of MARCKS protein was dramatically upregulated. This phosphorylation was inhibited by GF 109203X, a specific PKC inhibitor. In addition, when cells were cotreated with TPA and GF 109203X, both the morphological changes and the downregulation of OCT4 induced by TPA were inhibited (Fig. 3B, 3C). The inactive analog of GF 109203X, Bisindolylmaleimide V, did not have this effect. Thus, TPA induces differentiation through activation of the PKC pathway.
Figure 3.
Protein kinase C (PKC)-specific inhibitor blocks TPA-induced differentiation. (A): Western blot analysis of phosphorylated MARCKS protein in 0-minute and 15-minute TPA or TPA plus GF 109203X-treated cells. (B): TPA-induced morphological changes were inhibited by PKC inhibitor. Scale bar = 200 µm. (C): TPA-induced downregulation of OCT4 was inhibited by PKC inhibitor. Abbreviations: MARCKS, myristoylated alanine-rich C-kinase substrate; TPA, 12-O-tetradecanoylphorbol 13-acetate.
Identification of Specific PKC Subtype(s) Involved in TPA-Induced Differentiation
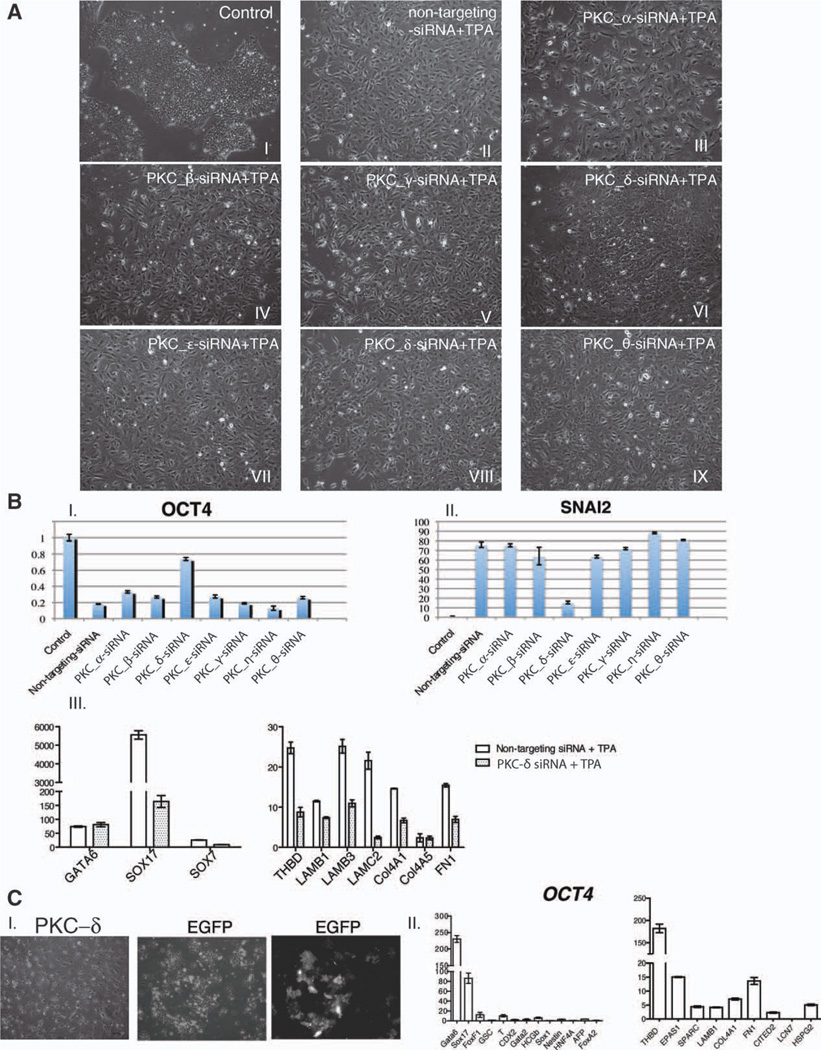
Different PKC subenzymes can have different, even opposite functions, depending on the cell type and culture condition. Based on the results of Serial Analysis of Gene Expression (SAGE) and a large-scale Western blot screen all seven conventional and novel PKCs that can be activated by TPA are expressed in human ESCs [43, 44]. To explore which PKC(s) played major roles in TPA-induced differentiation, we did a knockdown on each PKC individually and then examined how this affects TPA-induced differentiation. First, the efficiency and specificity of each of the PKC isotype-specific siRNAs was evaluated. Three days after transfection, the mRNAs of all the targeted PKCs were downregulated for at least 60%, while the expression of the nontargeting PKCs did not show obvious change (Supporting Information Fig. S2). Upon TPA treatment, only PKC-δ knockdown cells were able to maintain the morphology of compact colony with some flattened cells while cells transfected with other PKC siRNAs exhibited a scattered morphology (Fig. 4A). This difference in cell morphology was consistent with the expression of OCT4: after exposure to TPA for 48 hours, cells transfected with PKC-δ siRNA maintained ~80% of the OCT4 level of non-TPA-treated cells, while in cells transfected with other PKC siRNAs, OCT4 was downregulated to a similar level to cells transfected with nontargeting siRNAs (Fig. 4B–I). These results suggested that although PKC-δ may not be the only subtype involved in differentiation, it plays a major role in TPA-induced loss of pluripotency.
Figure 4.
PKC-δ plays dominant role in TPA-induced differentiation. (A): Morphologies of cells transfected with nontargeting or PKC isoform-specific siRNA followed by TPA treatment. (B): (I and II) Quantitative PCR analysis of OCT4 and SNAI2 in cells transfected with nontargeting or PKC isoform-specific siRNA followed by TPA treatment. (III) Quantitative PCR analysis of extraembryonic endoderm markers in PKC-δ knockdown cells. The data are presented as mean ± SD (n = 3). Cells were treated with 50 nM TPA at 48 hours after transfection for another 48 hours. The fold changes are normalized to non-TPA-treated cells. (C): (I) Overexpression of PKC-δ generated the same morphology as TPA treatment. Cells overexpressing EGFP was included as control. Scale bar = 100 µM. (II) Quantitative PCR analysis of lineage-specific markers in PKC-δ overexpressed cells. The data are normalized to EGFP overexpressed cells and presented as mean ± SD (n = 3). Abbreviations: EGFP, enhanced green fluorescent protein; PKC, protein kinase C; TPA, 12-O-tetradecanoylphorbol 13-acetate; and PCR, polymerase chain reaction.
Since one prominent phenomenon in TPA-induced differentiation was EMT, we examined whether TPA-induced upregulation of EMT related genes was also blocked by the downregulation of PKC-δ. We selected SNAI2 as a representative gene since it was upregulated upon EMT of human ESCs [45]. As expected, only knockdown of PKC-δ efficiently inhibited the upregulation of SNAI2 induced by TPA (Fig. 4B–II). This result further suggested that activation of PKC-δ is not only important for the downregulation of pluripotent genes but also important for the upregulation of differentiation related genes.
If PKC-δ is critical in TPA-induced extraembryonic endoderm differentiation, its knockdown should inhibit this lineage formation while its overexpression should lead the cells into this lineage. Interestingly, we found that TPA-induced upregulation of SOX17 and SOX7, but not GATA6, was efficiently inhibited in PKC-δ knockdown cells (Fig. 4B-III, left panel). The expression of parietal endoderm markers, such as THBD, COL4A1, LAMB3, and FN1 was also much lower in PKC-δ siRNA transfected cells than in nonspecific siRNA transfected cells (Fig. 4B-III, right panel). These results are consistent with the critical roles of SOX17 in extraembryonic endoderm differentiation in mouse ESC-derived embryoid bodies and the observations that SOX17 directly binds the promoters of multiple parietal endoderm genes, such as COL4A1 and LAMB1, and regulates their expression [46–48].
Next, we examined whether overexpression of PKC-δ can mimic TPA-induced differentiation. Overexpression of the constitutively active form of PKC-δ induced a morphology very similar to TPA-treated cells (Fig. 4C-I). These cells not only highly expressed transcription factors characteristic of extraembryonic endoderm (Fig. 4C-II, left panel) but also upregulated genes associated with parietal endoderm differentiation (Fig. 4C-II, right panel) [15, 49]. Thus, this data further confirms the dominant effect of PKC-δ in TPA-induced differentiation.
Discussion
In this article, we demonstrated that TPA causes human ESCs to undergo an EMT and differentiate into parietal endoderm-like cells through the activation of PKC pathway. We further demonstrated that, among a variety of PKC subtypes, PKC-δ is the most important one in mediating this differentiation, by both gene knockdown and overexpression. In early mouse embryonic development, the 11 isoforms of PKC are expressed with a distinct pattern and inhibiting the activity of certain PKC isoforms delays blastocyst formation [50, 51]. Although it was reported that overexpression of PKCα or TPA treatment enhances retinoic acid-induced extraembryonic endoderm differentiation in mouse embryonic carcinoma cells [52, 53], a role for PKC alone in the differentiation of ESCs or extraembryonic endoderm formation in the embryo has not been described. PKC isozyme-specific knockout mice do not show defects in early lineage segregation [54, 55], but this could well reflect a redundancy of function.
Activating the PKC pathway differentiates human ESCs to a cell type that expresses genes characteristic of mouse parietal endoderm; however, the exact population of extraembryonic cells in the human embryo that these cells represent is uncertain because of the extensive morphological differences between mouse and human extraembryonic endoderm [56, 57], and because of the lack of molecular studies on early postimplantation human embryos. The primary endoderm of the human bilayered embryonic disc gives rise to the yolk sac/umbilical vesicle, which appears initially as a mesh of cell strands with the primary yolk sac cavity at the center [58]. Although both the wall of the primary umbilical vesicle and the surrounding meshwork are thought to be derived from the extraembryonic endoderm [58], direct evidence for this is lacking. However, as these mesh-like mesenchymal cells are the earliest cells thought to be derived from the primary endoderm, and they have an appropriate morphology, they are the cells most likely to be represented by the TPA differentiated human ESCs.
In general, the major strength of using human ESCs to model early events in human development is that appropriate material is often completely unavailable from any other source; the major weakness is the extreme difficulty in demonstrating that in vitro results truly reflect in vivo biology. This difficulty is highlighted by a recent study reevaluating whether human ESCs give rise to trophoblast when treated with BMP4 [10]. Although the authors of that study described some markers in BMP-treated ESCs that were discordant with later in vivo trophoblast populations, and proposed as an alternative extraembryonic mesoderm, neither of these lineages have been studied in early human postimplantation development as the appropriately staged human embryos are simply not available. Thus, the true in vivo molecular signatures of early postimplantation human lineages remain uncertain. Very selective experiments on nonhuman primate embryos provide a partial solution [59], and given the recent completion of multiple nonhuman primate genomes [60, 61] and an increased ability to examine global gene expression in very small samples, there is a compelling need to more accurately map gene expression patterns in early nonhuman primate embryos so that the relationships between these early lineages can be better established.
Summary
In summary, our data demonstrated that activation of the PKC pathway efficiently differentiated human ESCs into parietal endoderm-like extraembryonic endoderm cells. Not all PKC subtypes played equal roles in this process, but a particular subtype, PKC-δ, had the most critical function. This study not only generates new understandings of extraembryonic endoderm formation but also provides further evidence that human ESCs are capable of forming extraembryonic lineages.
Supplementary Material
Acknowledgments
This study was supported by The University of Wisconsin Foundation, NIH Grant P01 GM081629 and NIH Grant P51 RR000167. The authors thank Dr. W. James Nelson for providing the pEGFP-N1-Ecad construct, Christine A. Daigh for identifying TPA as an efficient differentiation inducer for human ESCs, and Clay Glennon for making the time-lapse movies. We also thank Gudrun A. Jonsdottir for performing the microarray hybridization, Victor Ruotti for analyzing the expression of PKC isozymes in human ESCs by SAGE, and Krista Eastman for critical reading of the manuscript.
Footnotes
Author contributions: X.F.: conception and design, collection and/or assembly of data, data analysis and interpretation, and manuscript writing; J.Z.: collection and/or assembly of data and data analysis and interpretation; K.S.-O.: collection and/or assembly of data; S.T. and R.S.: data analysis and interpretation; J.Y.: provision of study material or patients; and J.A.T.: conception and design, financial support, and final approval of manuscript.
Disclosure of Potential Conflicts of Interest
The authors do not declare any conflicts of interest.
See www.StemCells.com for supporting information available online.
References
- 1.Brons IG, Smithers LE, Trotter MW, et al. Derivation of pluripotent epiblast stem cells from mammalian embryos. Nature. 2007;448:191–195. doi: 10.1038/nature05950. [DOI] [PubMed] [Google Scholar]
- 2.Tesar PJ, Chenoweth JG, Brook FA, et al. New cell lines from mouse epiblast share defining features with human embryonic stem cells. Nature. 2007;448:196–199. doi: 10.1038/nature05972. [DOI] [PubMed] [Google Scholar]
- 3.Thomson JA, Itskovitz-Eldor J, Shapiro SS, et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 4.Toyooka Y, Shimosato D, Murakami K, et al. Identification and characterization of subpopulations in undifferentiated ES cell culture. Development. 2008;135:909–918. doi: 10.1242/dev.017400. [DOI] [PubMed] [Google Scholar]
- 5.Nichols J, Chambers I, Taga T, et al. Physiological rationale for responsiveness of mouse embryonic stem cells to gp130 cytokines. Development. 2001;128:2333–2339. doi: 10.1242/dev.128.12.2333. [DOI] [PubMed] [Google Scholar]
- 6.Vallier L, Alexander M, Pedersen RA. Activin/nodal and FGF pathways cooperate to maintain pluripotency of human embryonic stem cells. J Cell Sci. 2005;118:4495–4509. doi: 10.1242/jcs.02553. [DOI] [PubMed] [Google Scholar]
- 7.Nichols J, Silva J, Roode M, et al. Suppression of Erk signalling promotes ground state pluripotency in the mouse embryo. Development. 2009;136:3215–3222. doi: 10.1242/dev.038893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yu P, Pan G, Yu J, et al. FGF2 sustains NANOG and switches the outcome of BMP4-induced human embryonic stem cell differentiation. Cell Stem Cell. 2011;8:326–334. doi: 10.1016/j.stem.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xu RH, Chen X, Li DS, et al. BMP4 initiates human embryonic stem cell differentiation to trophoblast. Nat Biotechnol. 2002;20:1261–1264. doi: 10.1038/nbt761. [DOI] [PubMed] [Google Scholar]
- 10.Bernardo AS, Faial T, Gardner L, et al. BRACHYURY and CDX2 mediate BMP-induced differentiation of human and mouse pluripotent stem cells into embryonic and extraembryonic lineages. Cell Stem Cell. 2011;9:144–155. doi: 10.1016/j.stem.2011.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nishizuka Y. Protein kinase C and lipid signaling for sustained cellular responses. FASEB J. 1995;9:484–496. [PubMed] [Google Scholar]
- 12.Newton AC. Protein kinase C: Structure, function, and regulation. J Biol Chem. 1995;270:28495–28498. doi: 10.1074/jbc.270.48.28495. [DOI] [PubMed] [Google Scholar]
- 13.Batlle E, Verdu J, Dominguez D, et al. Protein kinase C-alpha activity inversely modulates invasion and growth of intestinal cells. J Biol Chem. 1998;273:15091–15098. doi: 10.1074/jbc.273.24.15091. [DOI] [PubMed] [Google Scholar]
- 14.Thiery JP. Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer. 2002;2:442–454. doi: 10.1038/nrc822. [DOI] [PubMed] [Google Scholar]
- 15.Seguin CA, Draper JS, Nagy A, et al. Establishment of endoderm progenitors by SOX transcription factor expression in human embryonic stem cells. Cell Stem Cell. 2008;3:182–195. doi: 10.1016/j.stem.2008.06.018. [DOI] [PubMed] [Google Scholar]
- 16.Li R, Liang J, Ni S, et al. A mesenchymal-to-epithelial transition initiates and is required for the nuclear reprogramming of mouse fibroblasts. Cell Stem Cell. 2010;7:51–63. doi: 10.1016/j.stem.2010.04.014. [DOI] [PubMed] [Google Scholar]
- 17.Wong RC, Pebay A, Nguyen LT, et al. Presence of functional gap junctions in human embryonic stem cells. Stem Cells. 2004;22:883–889. doi: 10.1634/stemcells.22-6-883. [DOI] [PubMed] [Google Scholar]
- 18.Wilkinson DG, Bhatt S, Herrmann BG. Expression pattern of the mouse T gene and its role in mesoderm formation. Nature. 1990;343:657–659. doi: 10.1038/343657a0. [DOI] [PubMed] [Google Scholar]
- 19.Pera MF, Andrade J, Houssami S, et al. Regulation of human embryonic stem cell differentiation by BMP-2 and its antagonist noggin. J Cell Sci. 2004;117:1269–1280. doi: 10.1242/jcs.00970. [DOI] [PubMed] [Google Scholar]
- 20.Darr H, Benvenisty N. Genetic analysis of the role of the reprogramming gene LIN-28 in human embryonic stem cells. Stem Cells. 2009;27:352–362. doi: 10.1634/stemcells.2008-0720. [DOI] [PubMed] [Google Scholar]
- 21.Hyslop L, Stojkovic M, Armstrong L, et al. Downregulation of NANOG induces differentiation of human embryonic stem cells to extraembryonic lineages. Stem Cells. 2005;23:1035–1043. doi: 10.1634/stemcells.2005-0080. [DOI] [PubMed] [Google Scholar]
- 22.Zhang P, Li J, Tan Z, et al. Short-term BMP-4 treatment initiates mesoderm induction in human embryonic stem cells. Blood. 2008;111:1933–1941. doi: 10.1182/blood-2007-02-074120. [DOI] [PubMed] [Google Scholar]
- 23.Xu C, Inokuma MS, Denham J, et al. Feeder-free growth of undifferentiated human embryonic stem cells. Nat Biotechnol. 2001;19:971–974. doi: 10.1038/nbt1001-971. [DOI] [PubMed] [Google Scholar]
- 24.Kameda T, Smuga-Otto K, Thomson JA. A severe de novo methylation of episomal vectors by human ES cells. Biochem Biophys Res Commun. 2006;349:1269–1277. doi: 10.1016/j.bbrc.2006.08.175. [DOI] [PubMed] [Google Scholar]
- 25.Irizarry RA, Hobbs B, Collin F, et al. Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics. 2003;4:249–264. doi: 10.1093/biostatistics/4.2.249. [DOI] [PubMed] [Google Scholar]
- 26.Al-Shahrour F, Diaz-Uriarte R, Dopazo J. FatiGO: A web tool for finding significant associations of Gene Ontology terms with groups of genes. Bioinformatics. 2004;20:578–580. doi: 10.1093/bioinformatics/btg455. [DOI] [PubMed] [Google Scholar]
- 27.Chou MM, Hou W, Johnson J, et al. Regulation of protein kinase C zeta by PI 3-kinase and PDK-1. Curr Biol. 1998;8:1069–1077. doi: 10.1016/s0960-9822(98)70444-0. [DOI] [PubMed] [Google Scholar]
- 28.Krtolica A, Genbacev O, Escobedo C, et al. Disruption of apical-basal polarity of human embryonic stem cells enhances hematoendothelial differentiation. Stem Cells. 2007;25:2215–2223. doi: 10.1634/stemcells.2007-0230. [DOI] [PubMed] [Google Scholar]
- 29.Huettner JE, Lu A, Qu Y, et al. Gap junctions and connexon hemi-channels in human embryonic stem cells. Stem Cells. 2006;24:1654–1667. doi: 10.1634/stemcells.2005-0003. [DOI] [PubMed] [Google Scholar]
- 30.Ullmann U, In’t Veld P, Gilles C, et al. Epithelial-mesenchymal transition process in human embryonic stem cells cultured in feeder-free conditions. Mol Hum Reprod. 2007;13:21–32. doi: 10.1093/molehr/gal091. [DOI] [PubMed] [Google Scholar]
- 31.Chen G, Hou Z, Gulbranson DR, et al. Actin-myosin contractility is responsible for the reduced viability of dissociated human embryonic stem cells. Cell Stem Cell. 2010;7:240–248. doi: 10.1016/j.stem.2010.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shaner NC, Campbell RE, Steinbach PA, et al. Improved monomeric red, orange and yellow fluorescent proteins derived from Discosoma sp. red fluorescent protein. Nat Biotechnol. 2004;22:1567–1572. doi: 10.1038/nbt1037. [DOI] [PubMed] [Google Scholar]
- 33.D’Amour KA, Agulnick AD, Eliazer S, et al. Efficient differentiation of human embryonic stem cells to definitive endoderm. Nat Biotechnol. 2005;23:1534–1541. doi: 10.1038/nbt1163. [DOI] [PubMed] [Google Scholar]
- 34.Yasunaga M, Tada S, Torikai-Nishikawa S, et al. Induction and monitoring of definitive and visceral endoderm differentiation of mouse ES cells. Nat Biotechnol. 2005;23:1542–1550. doi: 10.1038/nbt1167. [DOI] [PubMed] [Google Scholar]
- 35.Chambers SM, Fasano CA, Papapetrou EP, et al. Highly efficient neural conversion of human ES and iPS cells by dual inhibition of SMAD signaling. Nat Biotechnol. 2009;27:275–280. doi: 10.1038/nbt.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mahlapuu M, Ormestad M, Enerback S, et al. The forkhead transcription factor Foxf1 is required for differentiation of extra-embryonic and lateral plate mesoderm. Development. 2001;128:155–166. doi: 10.1242/dev.128.2.155. [DOI] [PubMed] [Google Scholar]
- 37.Candia AF, Hu J, Crosby J, et al. Mox-1 and Mox-2 define a novel homeobox gene subfamily and are differentially expressed during early mesodermal patterning in mouse embryos. Development. 1992;116:1123–1136. doi: 10.1242/dev.116.4.1123. [DOI] [PubMed] [Google Scholar]
- 38.Brown K, Legros S, Artus J, et al. A comparative analysis of extra-embryonic endoderm cell lines. PLoS One. 2010;5:e12016. doi: 10.1371/journal.pone.0012016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Takeyama Y, Sato M, Horio M, et al. Knockdown of ZEB1, a master epithelial-to-mesenchymal transition (EMT) gene, suppresses anchorage-independent cell growth of lung cancer cells. Cancer Lett. 2010;296:216–224. doi: 10.1016/j.canlet.2010.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sakai D, Suzuki T, Osumi N, et al. Cooperative action of Sox9, Snail2 and Pka signaling in early neural crest development. Development. 2006;133:1323–1333. doi: 10.1242/dev.02297. [DOI] [PubMed] [Google Scholar]
- 41.Morali OG, Delmas V, Moore R, et al. IGF-II induces rapid beta-catenin relocation to the nucleus during epithelium to mesenchyme transition. Oncogene. 2001;20:4942–4950. doi: 10.1038/sj.onc.1204660. [DOI] [PubMed] [Google Scholar]
- 42.Hartwig JH, Thelen M, Rosen A, et al. MARCKS is an actin filament crosslinking protein regulated by protein kinase C and calcium-cal-modulin. Nature. 1992;356:618–622. doi: 10.1038/356618a0. [DOI] [PubMed] [Google Scholar]
- 43.Hirst M, Delaney A, Rogers SA, et al. LongSAGE profiling of nine human embryonic stem cell lines. Genome Biol. 2007;8:R113. doi: 10.1186/gb-2007-8-6-r113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lawrence DS. Functional proteomics: Large-scale analysis of protein kinase activity. Genome Biol. 2001;2 doi: 10.1186/gb-2001-2-2-reviews1007. REVIEWS1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Eastham AM, Spencer H, Soncin F, et al. Epithelial-mesenchymal transition events during human embryonic stem cell differentiation. Cancer Res. 2007;67:11254–11262. doi: 10.1158/0008-5472.CAN-07-2253. [DOI] [PubMed] [Google Scholar]
- 46.Niakan KK, Ji H, Maehr R, et al. Sox17 promotes differentiation in mouse embryonic stem cells by directly regulating extraembryonic gene expression and indirectly antagonizing self-renewal. Genes Dev. 2010;24:312–326. doi: 10.1101/gad.1833510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shimoda M, Kanai-Azuma M, Hara K, et al. Sox17 plays a substantial role in late-stage differentiation of the extraembryonic endoderm in vitro. J Cell Sci. 2007;120:3859–3869. doi: 10.1242/jcs.007856. [DOI] [PubMed] [Google Scholar]
- 48.Niimi T, Hayashi Y, Futaki S, et al. SOX7 and SOX17 regulate the parietal endoderm-specific enhancer activity of mouse laminin alpha1 gene. J Biol Chem. 2004;279:38055–38061. doi: 10.1074/jbc.M403724200. [DOI] [PubMed] [Google Scholar]
- 49.Chadalavada RS, Korkola JE, Houldsworth J, et al. Constitutive gene expression predisposes morphogen-mediated cell fate responses of NT2/D1 and 27X-1 human embryonal carcinoma cells. Stem Cells. 2007;25:771–778. doi: 10.1634/stemcells.2006-0271. [DOI] [PubMed] [Google Scholar]
- 50.Eckert JJ, McCallum A, Mears A, et al. Specific PKC isoforms regulate blastocoel formation during mouse preimplantation development. Dev Biol. 2004;274:384–401. doi: 10.1016/j.ydbio.2004.07.027. [DOI] [PubMed] [Google Scholar]
- 51.Dehghani H, Hahnel AC. Expression profile of protein kinase C isozymes in preimplantation mouse development. Reproduction. 2005;130:441–451. doi: 10.1530/rep.1.00571. [DOI] [PubMed] [Google Scholar]
- 52.Cho Y, Klein MG, Talmage DA. Distinct functions of protein kinase Calpha and protein kinase Cbeta during retinoic acid-induced differentiation of F9 cells. Cell Growth Differ. 1998;9:147–154. [PubMed] [Google Scholar]
- 53.Kindregan HC, Rosenbaum SE, Ohno S, et al. Characterization of conventional protein kinase C (PKC) isotype expression during F9 terato-carcinoma differentiation. Overexpression of PKC alpha alters the expression of some differentiation-dependent genes. J Biol Chem. 1994;269:27756–27761. [PubMed] [Google Scholar]
- 54.Abeliovich A, Chen C, Goda Y, et al. Modified hippocampal long-term potentiation in PKC gamma-mutant mice. Cell. 1993;75:1253–1262. doi: 10.1016/0092-8674(93)90613-u. [DOI] [PubMed] [Google Scholar]
- 55.Tan SL, Parker PJ. Emerging and diverse roles of protein kinase C in immune cell signalling. Biochem J. 2003;376:545–552. doi: 10.1042/BJ20031406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Enders AC, Schlafke S, Hendrickx AG. Differentiation of the embryonic disc, amnion, and yolk sac in the rhesus monkey. Am J Anat. 1986;177:161–185. doi: 10.1002/aja.1001770205. [DOI] [PubMed] [Google Scholar]
- 57.Gardner RL. Origin and differentiation of extraembryonic tissues in the mouse. Int Rev Exp Pathol. 1983;24:63–133. [PubMed] [Google Scholar]
- 58.Luckett WP. Origin and differentiation of the yolk sac and extraembryonic mesoderm in presomite human and rhesus monkey embryos. Am J Anat. 1978;152:59–97. doi: 10.1002/aja.1001520106. [DOI] [PubMed] [Google Scholar]
- 59.Zheng P, Patel B, McMenamin M, et al. The primate embryo gene expression resource: A novel resource to facilitate rapid analysis of gene expression patterns in non-human primate oocytes and preimplantation stage embryos. Biol Reprod. 2004;70:1411–1418. doi: 10.1095/biolreprod.103.023788. [DOI] [PubMed] [Google Scholar]
- 60.Gibbs RA, Rogers J, Katze MG, et al. Evolutionary and biomedical insights from the rhesus macaque genome. Science. 2007;316:222–234. doi: 10.1126/science.1139247. [DOI] [PubMed] [Google Scholar]
- 61.Mikkelsen TH, Hillier LW, Eichler EE. Initial sequence of the chimpanzee genome and comparison with the human genome. Nature. 2005;437:69–87. doi: 10.1038/nature04072. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.