Abstract
Problem
Preterm premature rupture of fetal membranes (pPROM) occurs in 30–40% of spontaneous preterm births (PTB) and is associated with intra-amniotic infection and inflammation. The membranes may sense and respond to microbes via Toll-like receptors (TLRs), however, little is known about their expression and regulation in this tissue. The objective of this study was to evaluate the expression of TLR1-10 in fetal membranes after exposure to pathogens associated with intra-amniotic infection and PTB.
Method of study
Normal human term fetal membrane explants were exposed to various bacteria. After 24hrs RNA was extracted and quantitative RT-PCR performed for TLRs 1–10.
Results
Treatment of fetal membranes with Mycoplasma hominis increased expression of TLR4, TLR6, and TLR8 mRNA. Ureaplasma parvum upregulated TLR8 mRNA, and Porphyromonas gingivalis significantly increased fetal membrane TLR7 expression. In contrast, treatment with Gram-negative Escherichia. coli (and its cell wall component LPS) downregulated TLR10 mRNA. No effect was detected for Ureaplasma urealyticum, Gardnerella vaginalis, or Group B Streptococcus.
Conclusions
These findings demonstrate that different types of bacteria have distinct effects on fetal membrane TLR expression patterns. Moreover, these findings highlight the disparity of fetal membrane responses to infection and thus, suggest heterogeneity in the mechanisms by which infection-associated pregnancy complications, such as pPROM and PTB, arise.
Keywords: Bacteria, Chorioamnion, Infection, Preterm birth, Toll-like Receptor
INTRODUCTION
Preterm delivery (< 37 weeks gestation) affects 12.3% of live births in the US1, the highest rate in the developed world, and is a major cause of neonatal morbidity and mortality. Approximately 60% of these deliveries are spontaneous where the causality is as yet, unclear. However, inflammation of the chorioamniotic membranes (chorioanmionitis) is a strong indicator of pathology and is common in spontaneous preterm births (PTB), particularly where there is preterm premature rupture of membranes (pPROM). pPROM occurs in 30 – 40% of all PTB2–4. There is an established clinical association between infection and preterm labor5,6, and an intrauterine bacterial infection poses a significant threat to pregnancy by gaining access to gestational tissues, such as the decidua, the placenta, and the fetal membranes7. The fetal membranes are likely the first tissue colonized by an ascending infection8, and while most normal term deliveries have evidence of bacteria in the fetal membranes, it is the association with inflammation that correlates with pathology and adverse pregnancy outcome4.
Normal term fetal membranes stimulated in vitro with various bacteria or bacterial components produce elevated levels of pro-inflammatory cytokines, such as IL-1β, TNFα, IL-6, and IL-88–12, an inflammatory profile also found clinically in preterm fetal membranes13. Furthermore, IL-1β and TNFα are known to be damaging to the fetal membranes by upregulating degradative matrix metalloproteinases (MMPs)14–16 and by inducing apoptosis17–19. This leads to weakening of the membranes, predisposing them to pPROM2,13. The way in which an infection can lead to inflammation and PTB is thought to be mediated by innate immune pattern recognition receptors (PRRs), such as the Toll-like receptors (TLRs)20. To date, 10 human TLR genes have been identified to encode protein, and each sense distinct infectious products. TLR4 recognizes Gram-negative bacterial lipopolysaccharide (LPS)21,22. TLR2 recognizes bacterial lipoproteins, Gram-positive bacterial peptidoglycan (PDG) and lipoteichoic acid, and fungal zymosan23–25; and forms heterodimers with either TLR1, TLR6, or TLR1026–28. TLR3 senses viral dsRNA, TLR5 senses bacterial flagellin, TLR8 senses viral ssRNA, and TLR9 senses bacterial CpG DNA29–31. While the natural ligand for human TLR7 is unknown, in the mouse, TLR7 recognizes the human TLR8 ligand, viral ssRNA29.
The chorioamniotic membranes may sense and respond to microbes via TLRs; however, little is known about their expression, regulation or function in this tissue. TLR2 and TLR4 are expressed by normal fetal membranes32–34, and their expression is increased in chorioamnionitis33. This suggests that inflammation is associated with altered fetal membrane TLR expression, and perhaps changes in pathogen sensing by this tissue. Different fetal immune responses to different intra-amniotic and cervico-vaginal pathogens associated with PTB, suggests that each pathogen may elicit a unique response through specific TLR mediated signaling mechanisms12. Therefore, the objective of this current study was to assess the expression levels of fetal membrane TLRs 1–10 after exposure to various bacteria associated with PTB. In this study we examined 7 different bacteria belonging to the following categories: genital mycoplasmas (Mycoplasma hominis, Ureaplasma urealyticum and Ureaplasma parvum); Gram-negative urogenital tract infectious agents (Escherichia coli and Gardnerella vaginalis), a Gram-negative periodontal pathogen (Porphyromonas gingivalis); and a Gram-positive genital tract colonizer (Group B Streptococci), all of which are associated with PTB and pPROM.
MATERIALS AND METHODS
Patients and collection of fetal membranes
The study was approved by Western Institutional Review Board, Seattle, WA (for The Perinatal Research Center) and IRB at The University of Texas Medical Branch at Galveston. Placentas were obtained from elective repeat Cesarean sections at term (3 37 weeks of gestation) prior to onset of labor (n=11). Maternal ages were between 20 and 32 years. Fetal membranes were dissected from placenta under sterile conditions. Decidua and adhering blot clots were removed from the membranes using saline and sterile cotton gauze. These tissues were placed in Hanks balanced salt solution (HBSS, Sigma Chemical Co., St. Louis, MO) containing penicillin/streptomycin (10μl/ml) and 1μl/ml amphotericin B (Sigma). Women with a prior history of pPROM or PTB were excluded, as were patients with obstetrical and medical complications, cervico-vaginal Group B streptococcus colonization, infections (sub-clinical infections as indicated by high CRP levels, foul smelling vaginal discharge, fever, or those undergoing antibiotic treatment). A sample of fetal membranes from each patient was examined by light microscopy (H&E staining of paraffin-embedded sections) to rule out subclinical chorioamnionitis (> 5 PMNs/high power field).
Tissue-culture of normal term fetal membranes
Fetal membranes were washed twice with HBSS as described above and cut into 6 mm-circles using a biopsy punch and washed in HBSS before placing into a tissue culture system. The details of this method can be found elsewhere 35,36. Briefly, tissue biopsies were placed in a Falcon cell culture insert (Becton-Dickinson Labware, Franklin Lakes, NJ) containing 200μl Dulbecco’s Modified Eagle’s Medium: F12 Ham’s mixture. These inserts were placed in a Falcon cell culture plate with 500μl medium. Media contained 15% (v/v) heat-inactivated fetal bovine serum (FBS), 1% (v/v) glutamine solution, 1% (v) penicillin/streptomycin solution and 1μl/ml amphotericin B (all from Sigma Chemical Co., St. Louis, MO). Cultures were incubated at 37 °C, 5% CO for 48 hours. Culture media was changed every 24 hours.
Preparation of bacteria
Stock cultures of Ureaplasma urealyticum (ATCC #27816; U. urealyticum, UU), Ureaplasma parvum (ATCC #27813; U. parvum, UP), Mycoplasma hominis (ATCC #14027; M. hominis, MH), Gardnerella vaginalis (ATCC #49145; G. vaginalis, GV), Polyporhorans gingivalis (ATCC# BAA-1703; P. gingivalis, PG), Group B Streptococci (ATCC#BAA-25; Streptococcus agalactiae, GBS), Escherichia coli (ATCC #33908; E. coli, EC) were purchased from the American Type Culture Collection (Manassas, VA). U. urealyticum and U. parvum were cultivated in Beef Heart Infusion Broth supplemented with 20% (v/v) Horse Serum + 10% (w/v) Yeast extract + 0.2% (w/v) Urea + 1000U/ml Penicillin G + 40 mg/ml Phenol Red (pH = 6.0). M. hominis was cultivated in PPLO broth supplemented with 20% (v/v) Horse Serum + 10% (w/v) Yeast extract + 0.2% Arginine (w/v)+ 1000U/ml Penicillin G. G. vaginalis was cultivated in Casman’s Broth + 5% (v/v) defibrinated sheep blood. S. agalatiae was grown in Brain Heart Infusion Broth supplemented with 5% (v/v) defibrinated sheep blood. E. coli was cultured in nutrient broth and P. gingivalis was cultivated anaerobically in Chopped Meat Broth. To prepare bacteria for the studies below, organisms were cultured to late log-phase, concentrated by centrifugation and re-suspended in RPMI 1640. Quantitative cultures were then established for color changing unit (CCU) or CFU determination by serial dilution and plating on appropriate agar. Bacteria were then heat-killed by heating at 80 °C for 1 h. Stocks of heat-killed bacteria were then stored at −80 °C until use.
Stimulation of fetal membranes with heat inactivated bacteria and LPS
Tissue cultures were stimulated with either 10 CFU/CCU of heat-inactivated bacterial suspension or 100ng/ml LPS (E. coli O55:B5). Dilutions of the bacterial suspensions and LPS were made in tissue culture media. Tissue cultures were stimulated for 24 hours. Culture and stimulation conditions are chosen based on our prior reports12 and also based on other published studies. Tissue samples from both stimulated and unstimulated controls (hereafter referred as controls) were harvested and stored at −80 °C for further analysis.
Quantitative Real-Time RT-PCR
Fetal membrane tissues were homogenized in 1ml of Trizol (Invitrogen, Grand Island, NY), and RNA was extracted following the manufacturer’s instructions using chloroform and phenol (Invitrogen). Extracted RNA was quantified using a Nanodrop 2000 (Thermo Scientific, Wilmington, DE), after which the RNA was treated with amplification grade DNase I from Invitrogen. Complementary DNA (cDNA) was synthesized from 1μg of RNA using the First-Strand cDNA Synthesis SuperScript II reverse transcriptase kit (Invitrogen). Relative mRNA expression levels of TLRs 1–10 and the housekeeping gene, GAPDH, were measured using SYBR-Green based quantitative real-time PCR using the KAPA SYBR Fast qPCR kit (Kapa Biosystems, Woburn, MA). PCR amplification was performed on the BioRad CFX Connect Real Time System (BioRad, Hercules, CA) for 50 cycles using the following cycling conditions: 95 C for 3 min, followed by 50 cycles of 95 C for 10 s and 58 C for 45 s. The primer pairs obtained from Real Time Primers (Elkins Park, PA) were as follows: TLR1, FWD (5′-ATT CCG CAG TAC TCC ATT CC-3′) and REV (5′-TTT GCT TGC TCT GTC AGC TT-3′); TLR2 FWD (5′-ATG CCT ACT GGG TGG AGA AC-3′) and REV (5′-TGC ACC ACT CAG TCT TCA CA-3′); TLR3, FWD (5′-GAA AGG CTA GCA GTC ATC CA-3′) and REV (5′-CAT CGG GTA CCT GAG TCA AC-3′); TLR4, FWD (5′-CAG CTC TTG GTG GAA GTT GA-3′) and REV, (5′-GCA AGA AGC ATC AGG TGA AA-3′); TLR5, FWD (5′-GGA ACC AGC TCC TAG CTC CT-3′) and REV (5′-AAG AGG GAA ACC CCA GAG AA-3′); TLR6, FWD (5′-CCT CCC AGG ATC AAG GTA CT-3′) and REV (5′-GGA TTG TCC CCT GCT TTT AT-3′); TLR8, FWD (5′-GGG CAT TGC ATT TAA GAG GT-3′) and REV (5′-TCC GGA TAT GAC GTT GAA AA-3′); TLR10, FWD (5′-ACT TTG CCC ACC ACA ATC TC-3′) and REV (5′-CCC AGA AAA GCC CAC ATT TA-3′). Primer pairs ordered from Yale Keck facility were as follows: TLR7, FWD (5′-TTT ACC TGG ATG GAA ACC AGC TA-3′) and REV (5′-TCA AGG CCT GAG AAG CTG TAA GCT A-3′); TLR9, FWD (5′-GGA CCT CTG GTA CTG CTT CCA-3′) and REV (5′-AAG CTC GTT GTA CAC CCA GTC T-3′)37; and GAPDH, FWD (5′-GGG GAA GGT GAA GGT CGG AGT-3′) and REV (5′-GGA GGG ATC TCG CTC CTG GAA-3′). The lack of DNA contamination was confirmed by amplifying mock controls of RNA that was not reverse-transcribed. Amplification (Ct) for each reaction was estimated using the instrument’s software (BioRad CFX Manager 2.1, BioRad) and exported to Statistical Analysis Software (SAS) for analysis.
Statistical analysis
TLR1-10 expression was analyzed using a block design where tissues from a given subject were considered the blocking factor. Ct levels were normalized to the housekeeping GAPDH value by calculating fold change to the housekeeping gene expression. Fold change values were used for further analysis. Data were analyzed using PROC MIXED for mixed effects models in Statistical Analysis Software (SAS), version 9.2 (SAS Institute Inc., Cary, NC). Analyses evaluated the effect of bacterial treatment (LPS, EC, GV, PG, GBS, MH, UP, UU) on TLR production for each receptor (TLR1, TLR2, TLR3, TLR4, TLR5, TLR6, TLR7, TLR8, TLR9, TLR10). Mathematical models evaluated effects due to exposure (bacterial treatment) as a fixed effect and tissue donor as a random effect. Such a model fit a random intercept for each patient to normalize subject-to-subject variability in basal TLR expression. There was no adjustment for multiple comparisons as only a limited number of planned comparisons between control and treatments were conducted 38. Data that did not meet the assumptions of normality and/or homoscedasticity of errors were log-transformed and re-checked as appropriate. Differences between individual treatments and the control (no treatment) were evaluated by conducting comparisons of least squares means estimates. Basic descriptive statistics, least squares mean estimates, along with corresponding p-values for least squares mean differences are presented in the tables below. Least squares means and standard errors of untransformed data are shown for clarity of presentation. Differences were declared statistically significant at p<0.05.
RESULTS
Effect of the treatment of normal human term fetal membrane explants with genital M. hominis is shown in Figure 1. M. hominis significantly increased expression of TLR4, TLR6, and TLR8 mRNA (p= 0.01, 0.03, and 0.02, respectively). Treatment with U. parvum, significantly upregulated TLR8 mRNA expression (p=0.02) (Figure 1). All other changes in TLR expression after exposure to either M. hominis or U. parvum were not significant (Figure 1 and supplemental data). Treatment with U. urealyticum, had no significant effect on the expression of any of the TLRs (see supplemental data).
Figure 1. Effect of mycoplasmas on fetal membrane TLR Expression.
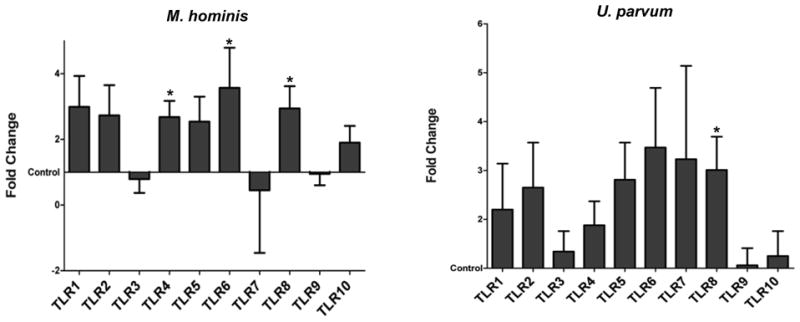
Fetal membrane explants were cultured with and without M. hominis or U. parvum. Bar chart shows TLR1-10 mRNA expression as the least squares mean (LSM) and Standard Error of fold changes relative to the control. Values were graphed using the control (no treatment) value as a baseline.
P. gingivalis significantly increased fetal membrane TLR7 expression 25-fold compared to controls (p=0.01) (Figure 2). In contrast, treatment of fetal membranes with the Gram-negative bacterial component, LPS, significantly down regulated TLR10 mRNA expression (p=0.01) compared to unstimulated controls, as did the Gram-negative bacterium E. coli (p=0.02) (Figure 2). Treatment of fetal membranes with G. vaginalis had no significant effect on the expression levels of any of the TLRs (Figure 2 and supplemental data).
Figure 2. Effect of Gram-negative bacteria and LPS on fetal membrane TLR Expression.

Fetal membrane explants were cultured with and without LPS, E. coli, G. vaginalis or P. gingivalis. Bar charts show fetal membrane TLR1-10 mRNA expression in response to each bacterial treatment as the least squares mean (LSM) and Standard Error of fold changes relative to the control. Values were graphed using the control (no treatment) value as a baseline.
Treatment of fetal membranes with the Gram-positive bacterium Group B streptococci had no significant effect on TLR1-10 expression (Figure 3 and supplemental data).
Figure 3. Effect of Gram-positive bacteria on fetal membrane TLR Expression.
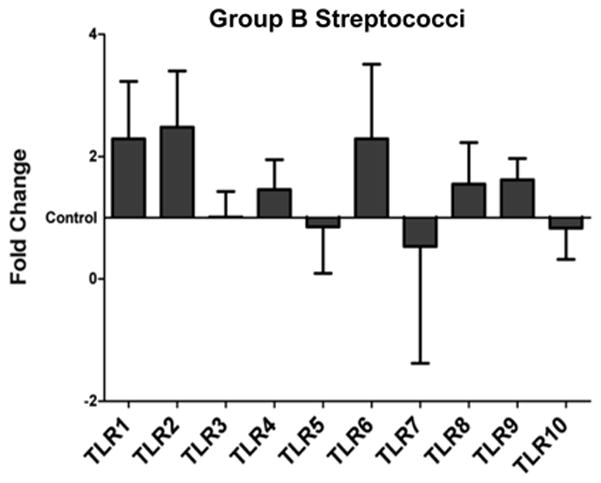
Fetal membrane explants were cultured with and without Group B Streptococci. Bar charts show fetal membrane TLR1-10 mRNA expression in response to each bacterial treatment as the least squares mean (LSM) and Standard Error of fold changes relative to the control. Values were graphed using the control (no treatment) value as a baseline.
DISCUSSION
Preterm labor and pPROM have been associated with a number of Gram-positive and Gram-negative bacteria from the lower genital tract, as well as pathogenic mycoplasmas and oral cavity microrganisms6,39–41. In this study we show that exposure of normal term human term fetal membranes to a range of heat-inactivated bacteria, associated with PTB, generate distinct TLR expression patterns. While the ability of the fetal membranes to generate an inflammatory response towards bacterial infection is known8–12, the precise role of innate immune PRRs, such as the TLRs, is still uncertain. However, that the chorioamnion can express TLRs and their expression can change under pathological conditions32–34, suggests they may play an important role in mediating inflammatory responses towards intrauterine infections, and thus PTB and pPROM. Therefore, TLR stimulation by bacterial infection, may in turn, modulate fetal membrane TLR expression.
Mycoplasma are frequently isolated from the human reproductive tract and are often associated with membrane rupture and PTB41. U. parvum activates TLR2, TLR1 and TLR642, U. urealyticum, is sensed by TLR2 and TLR443 and M. hominis is, at present, only known to activate TLR244,45. Although these three bacterial species can all be sensed by TLR2, they had quite different effects on fetal membrane TLR expression. M. hominis significantly increased fetal membrane expression of TLR4, TLR6 and TLR8. U. parvum, which has little effect on fetal membrane pro-inflammatory cytokine production12, also significantly upregulated TLR8 expression. In contrast, U. urealyticum had no significant effect on the expression of any of the fetal membrane TLRs. The only other bacterium tested to significantly upregulate fetal membrane TLR expression was the oral Gram-negative bacterium, P. gingivialis, which can activate TLR4 and TLR246,47. P. gingivalis can disseminate to the placenta and is associated with preterm labor and chorioamnionitis48. Moreover, in pregnant mice P. gingivalis increases placental TLR4 expression, reduces fetal weight and reduces fecundity49. In our current study we found that exposure of fetal membranes to P. gingivalis strikingly and significantly increased TLR7 expression by 25 fold. These findings suggest that different bacteria, all with the capacity to activate TLR2, have distinct effects on fetal membrane TLR expression and thus, the capacity of the chorioamnion to subsequently respond to other types of infection. Through the upregulation of TLR7 and TLR8, M. hominis, U. parvum and P. gingivalis may augment the ability of the fetal membrane to subsequently sense and respond to a viral infection. Indeed, in THP-1 monocytic cells, exposure to Gram-positive S. aureus or E. coli, which activate TLR2 and TLR4, respectively, strongly increase expression of the viral sensors, TLR7 and TLR850. The opposite has been reported in trophoblast cells, whereby after a viral infection, TLR2 and TLR4 expression is increased51. Similarly, through the upregulation of TLR4 and the TLR2 co-receptor, TLR627, M. hominis may allow the chorioamnion to generate a heightened response towards Gram-positive and Gram-negative bacterial infections.
The other Gram-negative bacterium tested were G. vaginalis, which modestly triggers fetal membrane inflammation, and E. coli, which robustly activate fetal membranes to produce inflammatory cytokines and prostaglandins12. However, G. vaginalis had no significant effect on the expression of any TLRs and E. coli, instead of upregulating TLR expression, downregulated TLR10 mRNA levels, as did the Gram-negative bacterial component, LPS. Since TLR10 is a co-receptor for TLR228, exposure of fetal membranes to E. coli may limit or alter subsequent responses to bacterium that can stimulate TLR2.
The Gram-positive bacterium, Group B Streptococcus (GBS), similar to G. vaginalis, modestly triggers fetal membrane inflammation12, yet it too had no significant effect on fetal membrane TLR expression levels. Previous studies have demonstrated that while GBS can activate TR2 and TLR6 via a secreted factor, heat-killed GBS is unable to stimulate TLR1, TLR2, TLR4 or TLR652. Thus, it is possible that we might observe different TLR expression patterns and cytokine responses of the fetal membrane after exposure to live bacteria.
While significance was not reached, we did observe additional changes in TLR expression patterns that were greater than 2 fold. The mycoplasmas, M. hominis, increased TLR1, TLR2, and TLR5 expression in the fetal membranes, and U. parvum, increased TLR1, TLR2, TLR5, TLR6 and TLR7 mRNA levels (Figure 1 and supplemental data). In response to Gram-negative bacterium we observed a non-significant >2 fold increase in: TLR1 and TLR6 by LPS and P. gingivalis; TLR2 by LPS, G. vaginalis and P. gingivalis; and TLR7 by P. gingivalis (Figure 2 and supplemental data). Treatment of fetal membranes with the Gram-positive bacterium Group B streptococci increased TLR1, TLR2 and TLR6 expression levels (Figure 3 and supplemental data). The lack of significance for these changes may be due to our sample size not giving us sufficient power. Nevertheless, these observations may indicate a greater ability for various bacterial species to regulate fetal membrane TLR expression.
In summary, we have demonstrated that various genitourinary tract and oral bacteria, associated with PTB, even from the same genus, have distinct effects on fetal membrane TLR expression patterns. These findings highlight the disparity of fetal membrane responses to infection and thus, suggest heterogeneity in the mechanisms by which infection-associated pregnancy complications, such as pPROM and PTB, arise.
Supplementary Material
Acknowledgments
This research was supported in part by NIH/NICHD grants RO1HD049446 and PO1HD054713 (to VMA) and NIH grant R03HD067446 (to RM).
References
- 1.Martin JA, Osterman MJ, Sutton PD. Are preterm births on the decline in the United States? Recent data from the National Vital Statistics System. NCHS Data Brief. 2010;39:1–8. [PubMed] [Google Scholar]
- 2.Menon R, Fortunato SJ. Infection and the role of inflammation in preterm premature rupture of the membranes. Best Pract Res Clin Obstet Gynaecol. 2007;21:467–78. doi: 10.1016/j.bpobgyn.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 3.Menon R, Taylor RN, Fortunato SJ. Chorioamnionitis--a complex pathophysiologic syndrome. Placenta. 2010;31:113–20. doi: 10.1016/j.placenta.2009.11.012. [DOI] [PubMed] [Google Scholar]
- 4.Romero R, Espinoza J, Goncalves LF, Kusanovic JP, Friel L, Hassan S. The role of inflammation and infection in preterm birth. Semin Reprod Med. 2007;25:21–39. doi: 10.1055/s-2006-956773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lamont RF. The role of infection in preterm labour and birth. Hosp Med. 2003;64:644–7. doi: 10.12968/hosp.2003.64.11.2343. [DOI] [PubMed] [Google Scholar]
- 6.Goldenberg RL, Hauth JC, Andrews WW. Intrauterine infection and preterm delivery. N Engl J Med. 2000;342:1500–7. doi: 10.1056/NEJM200005183422007. [DOI] [PubMed] [Google Scholar]
- 7.Espinoza J, Erez O, Romero R. Preconceptional antibiotic treatment to prevent preterm birth in women with a previous preterm delivery. Am J Obstet Gynecol. 2006;194:630–7. doi: 10.1016/j.ajog.2005.11.050. [DOI] [PubMed] [Google Scholar]
- 8.Herve R, Schmitz T, Evain-Brion D, Cabrol D, Leroy MJ, Mehats C. The PDE4 inhibitor rolipram prevents NF-kappaB binding activity and proinflammatory cytokine release in human chorionic cells. J Immunol. 2008;181:2196–202. doi: 10.4049/jimmunol.181.3.2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fortunato SJ, Lombardi SJ, Menon R. Immunoreactivity of human fetal membranes to peptidoglycan polysaccharide (PGPS): cytokine response. J Perinat Med. 1998;26:442–7. doi: 10.1515/jpme.1998.26.6.442. [DOI] [PubMed] [Google Scholar]
- 10.Zaga V, Estrada-Gutierrez G, Beltran-Montoya J, Maida-Claros R, Lopez-Vancell R, Vadillo-Ortega F. Secretions of interleukin-1beta and tumor necrosis factor alpha by whole fetal membranes depend on initial interactions of amnion or choriodecidua with lipopolysaccharides or group B streptococci. Biol Reprod. 2004;71:1296–302. doi: 10.1095/biolreprod.104.028621. [DOI] [PubMed] [Google Scholar]
- 11.Zaga-Clavellina V, Garcia-Lopez G, Flores-Herrera H, Espejel-Nunez A, Flores-Pliego A, Soriano-Becerril D, Maida-Claros R, Merchant-Larios H, Vadillo-Ortega F. In vitro secretion profiles of interleukin (IL)-1beta, IL-6, IL-8, IL-10, and TNF alpha after selective infection with Escherichia coli in human fetal membranes. Reprod Biol Endocrinol. 2007;5:46. doi: 10.1186/1477-7827-5-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Menon R, Peltier MR, Eckardt J, Fortunato SJ. Diversity in cytokine response to bacteria associated with preterm birth by fetal membranes. Am J Obstet Gynecol. 2009;201:306, e1–6. doi: 10.1016/j.ajog.2009.06.027. [DOI] [PubMed] [Google Scholar]
- 13.Patni S, Flynn P, Wynen LP, Seager AL, Morgan G, White JO, Thornton CA. An introduction to Toll-like receptors and their possible role in the initiation of labour. Bjog. 2007;114:1326–34. doi: 10.1111/j.1471-0528.2007.01488.x. [DOI] [PubMed] [Google Scholar]
- 14.Arechavaleta-Velasco F, Ogando D, Parry S, Vadillo-Ortega F. Production of matrix metalloproteinase-9 in lipopolysaccharide-stimulated human amnion occurs through an autocrine and paracrine proinflammatory cytokine-dependent system. Biol Reprod. 2002;67:1952–8. doi: 10.1095/biolreprod.102.004721. [DOI] [PubMed] [Google Scholar]
- 15.So T, Ito A, Sato T, Mori Y, Hirakawa S. Tumor necrosis factor-alpha stimulates the biosynthesis of matrix metalloproteinases and plasminogen activator in cultured human chorionic cells. Biol Reprod. 1992;46:772–8. doi: 10.1095/biolreprod46.5.772. [DOI] [PubMed] [Google Scholar]
- 16.Zaga-Clavellina V, Lopez GG, Estrada-Gutierrez G, Martinez-Flores A, Maida-Claros R, Beltran-Montoya J, Vadillo-Ortega F. Incubation of human chorioamniotic membranes with Candida albicans induces differential synthesis and secretion of interleukin-1beta, interleukin-6, prostaglandin E, and 92 kDa type IV collagenase. Mycoses. 2006;49:6–13. doi: 10.1111/j.1439-0507.2005.01171.x. [DOI] [PubMed] [Google Scholar]
- 17.Fortunato SJ, Menon R. IL-1 beta is a better inducer of apoptosis in human fetal membranes than IL-6. Placenta. 2003;24:922–8. doi: 10.1016/s0143-4004(03)00160-7. [DOI] [PubMed] [Google Scholar]
- 18.Luo G, Abrahams VM, Tadesse S, Funai EF, Hodgson EJ, Gao J, Norwitz ER. Progesterone inhibits basal and TNF-alpha-induced apoptosis in fetal membranes: a novel mechanism to explain progesterone-mediated prevention of preterm birth. Reprod Sci. 2010;17:532–9. doi: 10.1177/1933719110363618. [DOI] [PubMed] [Google Scholar]
- 19.Menon R, Lombardi SJ, Fortunato SJ. TNF-alpha promotes caspase activation and apoptosis in human fetal membranes. J Assist Reprod Genet. 2002;19:201–4. doi: 10.1023/A:1014898130008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abrahams VM. Pattern recognition at the maternal-fetal interface. Immunol Invest. 2008;37:427–47. doi: 10.1080/08820130802191599. [DOI] [PubMed] [Google Scholar]
- 21.Medzhitov R, Preston-Hurlburt P, Janeway CA., Jr A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature. 1997;388:394–7. doi: 10.1038/41131. [DOI] [PubMed] [Google Scholar]
- 22.Poltorak A, He X, Smirnova I, Liu MY, Van Huffel C, Du X, Birdwell D, Alejos E, Silva M, Galanos C, Freudenberg M, Ricciardi-Castagnoli P, Layton B, Beutler B. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science. 1998;282:2085–8. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- 23.Aliprantis AO, Yang RB, Mark MR, Suggett S, Devaux B, Radolf JD, Klimpel GR, Godowski P, Zychlinsky A. Cell activation and apoptosis by bacterial lipoproteins through toll-like receptor-2. Science. 1999;285:736–9. doi: 10.1126/science.285.5428.736. [DOI] [PubMed] [Google Scholar]
- 24.Ozinsky A, Underhill DM, Fontenot JD, Hajjar AM, Smith KD, Wilson CB, Schroeder L, Aderem A. The repertoire for pattern recognition of pathogens by the innate immune system is defined by cooperation between toll-like receptors. Proc Natl Acad Sci U S A. 2000;97:13766–71. doi: 10.1073/pnas.250476497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schwandner R, Dziarski R, Wesche H, Rothe M, Kirschning CJ. Peptidoglycan-and lipoteichoic acid-induced cell activation is mediated by toll-like receptor 2. J Biol Chem. 1999;274:17406–9. doi: 10.1074/jbc.274.25.17406. [DOI] [PubMed] [Google Scholar]
- 26.Krutzik SR, Ochoa MT, Sieling PA, Uematsu S, Ng YW, Legaspi A, Liu PT, Cole ST, Godowski PJ, Maeda Y, Sarno EN, Norgard MV, Brennan PJ, Akira S, Rea TH, Modlin RL. Activation and regulation of Toll-like receptors 2 and 1 in human leprosy. Nat Med. 2003;9:525–32. doi: 10.1038/nm864. [DOI] [PubMed] [Google Scholar]
- 27.Takeuchi O, Kawai T, Muhlradt PF, Morr M, Radolf JD, Zychlinsky A, Takeda K, Akira S. Discrimination of bacterial lipoproteins by Toll-like receptor 6. Int Immunol. 2001;13:933–40. doi: 10.1093/intimm/13.7.933. [DOI] [PubMed] [Google Scholar]
- 28.Hasan U, Chaffois C, Gaillard C, Saulnier V, Merck E, Tancredi S, Guiet C, Briere F, Vlach J, Lebecque S, Trinchieri G, Bates EE. Human TLR10 is a functional receptor, expressed by B cells and plasmacytoid dendritic cells, which activates gene transcription through MyD88. J Immunol. 2005;174:2942–50. doi: 10.4049/jimmunol.174.5.2942. [DOI] [PubMed] [Google Scholar]
- 29.Heil F, Hemmi H, Hochrein H, Ampenberger F, Kirschning C, Akira S, Lipford G, Wagner H, Bauer S. Species-specific recognition of single-stranded RNA via toll-like receptor 7 and 8. Science. 2004;303:1526–9. doi: 10.1126/science.1093620. [DOI] [PubMed] [Google Scholar]
- 30.Takeda K, Kaisho T, Akira S. Toll-like receptors. Annu Rev Immunol. 2003;21:335–76. doi: 10.1146/annurev.immunol.21.120601.141126. [DOI] [PubMed] [Google Scholar]
- 31.Zhang D, Zhang G, Hayden MS, Greenblatt MB, Bussey C, Flavell RA, Ghosh S. A toll-like receptor that prevents infection by uropathogenic bacteria. Science. 2004;303:1522–6. doi: 10.1126/science.1094351. [DOI] [PubMed] [Google Scholar]
- 32.Adams KM, Lucas J, Kapur RP, Stevens AM. LPS induces translocation of TLR4 in amniotic epithelium. Placenta. 2007;28:477–81. doi: 10.1016/j.placenta.2006.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim YM, Romero R, Chaiworapongsa T, Kim GJ, Kim MR, Kuivaniemi H, Tromp G, Espinoza J, Bujold E, Abrahams VM, Mor G. Toll-like receptor-2 and -4 in the chorioamniotic membranes in spontaneous labor at term and in preterm parturition that are associated with chorioamnionitis. Am J Obstet Gynecol. 2004;191:1346–55. doi: 10.1016/j.ajog.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 34.Leroy MJ, Dallot E, Czerkiewicz I, Schmitz T, Breuiller-Fouche M. Inflammation of choriodecidua induces tumor necrosis factor alpha-mediated apoptosis of human myometrial cells. Biol Reprod. 2007;76:769–76. doi: 10.1095/biolreprod.106.058057. [DOI] [PubMed] [Google Scholar]
- 35.Fortunato SJ, Menon R, Swan KF, Lyden TW. Organ culture of amniochorionic membrane in vitro. Am J Reprod Immunol. 1994;32:184–7. doi: 10.1111/j.1600-0897.1994.tb01112.x. [DOI] [PubMed] [Google Scholar]
- 36.Menon R, Swan KF, Lyden TW, Rote NS, Fortunato SJ. Expression of inflammatory cytokines (interleukin-1 beta and interleukin-6) in amniochorionic membranes. Am J Obstet Gynecol. 1995;172:493–500. doi: 10.1016/0002-9378(95)90562-6. [DOI] [PubMed] [Google Scholar]
- 37.Patni S, Wynen LP, Seager AL, Morgan G, White JO, Thornton CA. Expression and activity of Toll-like receptors 1–9 in the human term placenta and changes associated with labor at term. Biol Reprod. 2009;80:243–8. doi: 10.1095/biolreprod.108.069252. [DOI] [PubMed] [Google Scholar]
- 38.Rothman KJ. No adjustments are needed for multiple comparisons. Epidemiology. 1990;1:43–6. [PubMed] [Google Scholar]
- 39.Romero R, Espinoza J, Kusanovic JP, Gotsch F, Hassan S, Erez O, Chaiworapongsa T, Mazor M. The preterm parturition syndrome. Bjog. 2006;113 (Suppl 3):17–42. doi: 10.1111/j.1471-0528.2006.01120.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sanu O, Lamont RF. Periodontal disease and bacterial vaginosis as genetic and environmental markers for the risk of spontaneous preterm labor and preterm birth. J Matern Fetal Neonatal Med. 2011;24:1476–85. doi: 10.3109/14767058.2010.545930. [DOI] [PubMed] [Google Scholar]
- 41.Taylor-Robinson D, Lamont RF. Mycoplasmas in pregnancy. Bjog. 2011;118:164–74. doi: 10.1111/j.1471-0528.2010.02766.x. [DOI] [PubMed] [Google Scholar]
- 42.Shimizu T, Kida Y, Kuwano K. Ureaplasma parvum lipoproteins, including MB antigen, activate NF-{kappa}B through TLR1, TLR2 and TLR6. Microbiology. 2008;154:1318–25. doi: 10.1099/mic.0.2007/016212-0. [DOI] [PubMed] [Google Scholar]
- 43.Peltier MR, Freeman AJ, Mu HH, Cole BC. Characterization of the macrophage-stimulating activity from Ureaplasma urealyticum. Am J Reprod Immunol. 2007;57:186–92. doi: 10.1111/j.1600-0897.2006.00460.x. [DOI] [PubMed] [Google Scholar]
- 44.Takeyama K, Mitsuzawa H, Shimizu T, Konishi M, Nishitani C, Sano H, Kunishima Y, Matsukawa M, Takahashi S, Shibata K, Tsukamoto T, Kuroki Y. Prostate cell lines secrete IL-8 in response to Mycoplasma hominis through Toll-like receptor 2-mediated mechanism. Prostate. 2006;66:386–91. doi: 10.1002/pros.20358. [DOI] [PubMed] [Google Scholar]
- 45.Peltier MR, Freeman AJ, Mu HH, Cole BC. Characterization and partial purification of a macrophage-stimulating factor from Mycoplasma hominis. Am J Reprod Immunol. 2005;54:342–51. doi: 10.1111/j.1600-0897.2005.00316.x. [DOI] [PubMed] [Google Scholar]
- 46.Darveau RP, Pham TT, Lemley K, Reife RA, Bainbridge BW, Coats SR, Howald WN, Way SS, Hajjar AM. Porphyromonas gingivalis lipopolysaccharide contains multiple lipid A species that functionally interact with both toll-like receptors 2 and 4. Infect Immun. 2004;72:5041–51. doi: 10.1128/IAI.72.9.5041-5051.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang PL, Azuma Y, Shinohara M, Ohura K. Toll-like receptor 4-mediated signal pathway induced by Porphyromonas gingivalis lipopolysaccharide in human gingival fibroblasts. Biochem Biophys Res Commun. 2000;273:1161–7. doi: 10.1006/bbrc.2000.3060. [DOI] [PubMed] [Google Scholar]
- 48.Katz J, Chegini N, Shiverick KT, Lamont RJ. Localization of P. gingivalis in preterm delivery placenta. J Dent Res. 2009;88:575–8. doi: 10.1177/0022034509338032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Arce RM, Barros SP, Wacker B, Peters B, Moss K, Offenbacher S. Increased TLR4 expression in murine placentas after oral infection with periodontal pathogens. Placenta. 2009;30:156–62. doi: 10.1016/j.placenta.2008.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zarember KA, Godowski PJ. Tissue expression of human Toll-like receptors and differential regulation of Toll-like receptor mRNAs in leukocytes in response to microbes, their products, and cytokines. J Immunol. 2002;168:554–61. doi: 10.4049/jimmunol.168.2.554. [DOI] [PubMed] [Google Scholar]
- 51.Cardenas I, Means RE, Aldo P, Koga K, Lang SM, Booth C, Manzur A, Oyarzun E, Romero R, Mor G. Viral infection of the placenta leads to fetal inflammation and sensitization to bacterial products predisposing to preterm labor. J Immunol. 2010;185:1248–57. doi: 10.4049/jimmunol.1000289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Henneke P, Takeuchi O, van Strijp JA, Guttormsen HK, Smith JA, Schromm AB, Espevik TA, Akira S, Nizet V, Kasper DL, Golenbock DT. Novel engagement of CD14 and multiple toll-like receptors by group B streptococci. J Immunol. 2001;167:7069–76. doi: 10.4049/jimmunol.167.12.7069. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


