Abstract
OBJECTIVES:
We recently identified a six-gene methylation-based biomarker panel suitable for early detection of colorectal cancer (CRC). In this study, we compared the performance of this novel epi-panel with that of previously identified DNA methylation markers in the same clinical tissue sample sets.
METHODS:
Quantitative methylation-specific PCR was used to analyze the promoter region of SEPT9 and VIM in a total of 485 tissue samples, divided into test and validation sets. ITGA4, NTRK2, OSMR, and TUBG2 were also included in the analyses. Receiver operating characteristic (ROC) curves were used to compare the performances of the individual biomarkers with that of the novel epi-panel.
RESULTS:
SEPT9 and VIM were methylated in 82 and 67% of CRCs (n=169) and in 88 and 54% of the adenomas (n=104). Only 3% of the normal mucosa samples (n=107) were methylated for these genes, confirming that the methylation was highly cancer-specific. Areas under the ROC curve (AUC), distinguishing CRCs from normal mucosa, were 0.94 for SEPT9 and 0.81 for VIM. AUC values for separating adenomas from normal mucosa samples were 0.96 and 0.81 for the same genes. In comparison, the novel epi-panel achieved an AUC of 0.98 (CRC) and 0.97 (adenomas).
ITGA4, OSMR, NTRK2, and TUBG2 were methylated in 90, 78, 7, and 1% of the CRCs, and in 76, 77, 3, and 0% of the adenomas. Between 0 and 2% of the normal mucosa samples were methylated for the same genes. ITGA4 and OSMR achieved an AUC of 0.96 and 0.92 (CRC vs. normal mucosa), and 0.93 and 0.92 (adenomas vs. normal mucosa).
CONCLUSIONS:
We have confirmed the high performance of some of the previously identified DNA methylation markers. Furthermore, we showed that a recently reported epi-panel performed better than the individual DNA methylation biomarkers when analyzed in the same tissue samples. This observation was also true for VIM and SEPT9, which are included in commercially available noninvasive tests for CRC. These results further underscore the value of combining a manageable number of individual markers into a panel, which in addition to having a higher sensitivity and specificity might provide a more profound robustness to a noninvasive test compared with single markers.
INTRODUCTION
Colorectal cancer (CRC) is one of the most common malignancies worldwide with an estimated incidence of 1.2 million cases leading to ∼600,000 deaths per year.1 As CRC develops from premalignant lesions that can be removed during endoscopy, early detection of the disease can decrease the incidence and reduce CRC-related mortality.2 However, the invasive nature of this method underscores the need for a noninvasive primary screening tool for early detection of this disease. Cancer-specific DNA methylation are common in a variety of cancer types3, 4 and such aberrations have been shown to also be present in body fluids including stool and plasma from CRC patients.5, 6 Furthermore, promoter DNA hypermethylation has been shown to be an early event in colorectal tumorigenesis, making methylation-based biomarkers relevant candidates for detection of pre-malignant lesions.7
Several biomarkers have so far been reported to be highly methylated in CRC;8, 9, 10, 11 however, VIM and SEPT9 are the only ones presently included in commercially available noninvasive tests.12 These genes are promising biomarkers for detecting colorectal neoplasms in stool and blood, respectively.5, 6, 13, 14 ColoSure (Laboratory Corporation of America, Burlington, NC, USA) is a single-marker stool DNA test for detection of CRC-associated VIM methylation, and a sensitivity range of 72–77% and a specificity range of 83–94% have been reported.15 The Epi proColon blood test, with a recent company-reported sensitivity of 68% and specificity of 80%, is based on detection of methylated SEPT9 in plasma from CRC patients.16 In addition to these genes, several others have been suggested as biomarkers for noninvasive detection of CRC due to seemingly high performances, including ALX4, GATA4, HLTF, MGMT, OSMR, TMEFF2 TPEF/HPP1, and NEUROG1.8, 17
We recently identified a panel of six promising biomarkers for early detection of CRC and adenomas (CNRIP1, FBN1, INA, MAL, SNCA, and SPG20; referred to as the “novel epi-panel”).11 The aim of this study was to compare the performance of a number of previously reported methylated genes in CRC with that of the novel epi-panel, using the same clinical test and validation series. Our a priori hypothesis is that a panel will outperform individual biomarkers with regard to sensitivity, specificity, and areas under the receiver operating characteristic (ROC) curve values.
METHODS
Selecting candidate genes for promoter methylation analysis
SEPT9 and VIM were included in the study as they are presently the only methylation markers used in commercially available noninvasive tests for CRC.6, 18 We were also interested in analyzing some of the most highly methylated CRC genes reported in the literature, recently reviewed in Kim et al.8 From this gene list, we selected candidates that had been analyzed using real-time quantitative methylation-specific PCR (qMSP) with resulting sensitivity and specificity measurements in tissue samples of at least 80% and 90%, respectively. The candidate genes that met these criteria included ITGA4, NTRK2, OSMR, and TUBG28, 19, 20 and these were subjected to quantitative DNA methylation analysis in the Norwegian sample sets along with SEPT9 and VIM.
Cancer cell lines
In this study, 20 colon cancer cell lines were included comprising 11 microsatellite stable cell lines (MSS; ALA, Colo320, EB, FRI, HT29, IS1, IS2, IS3, LS1034, SW480, and V9P) and 9 microsatellite unstable cell lines (MSI; Co115, HCT15, HCT116, LoVo, LS174T, RKO, SW48, TC7, and TC71). The commercially available cell lines have recently been authenticated using the AmpFLSTR Identifiler PCR Amplification Kit (Applied Biosystems, Foster City, CA, USA) following manufacturer's instructions. Their genotypes are given in the Supplementary Table 1 online.
Tissue samples
The DNA from tissue samples analyzed in this study was derived from fresh-frozen material and was divided into a test and a validation set consisting of 485 tissue samples. Also included in the analyses were 20 colon cancer cell lines. The test set comprised 64 CRC samples, 61 adenomas, and 51 normal colorectal mucosa samples. The carcinomas were collected at seven hospitals in the South-Eastern region of Norway in the time period 1987–1989.21 The median patient age in the sample set was 71 (range 33–92), and consisted of 23 tumors with a MSI phenotype and 41 with a MSS phenotype. The adenomas were collected from a population-based sigmoidoscopy-based screening study (Telemark, Norway22). Two were MSI, whereas the remaining 59 were MSS. Median adenoma size was 8 mm (range 5–50 mm), whereas median age at adenoma removal was 67 years (range 62–72 years). The normal samples were autopsy material derived from deceased CRC-free individuals collected at the institute of Forensic Medicine, University of Oslo, and median age was 55 years (range 22–86 years).
The validation set comprised 105 CRC samples with an equal amount of matching normal mucosa samples collected from the resection margins, 43 adenomas, and 56 normal mucosa samples. The CRCs with corresponding normal mucosa samples were collected in the time period 2005–2007 by Department of Surgery at Aker hospital, Oslo University Hospital. The median patient age at time of surgery was 71 years (range 29–93): 22 of the tumors were MSI, whereas the remaining 83 samples were MSS. The 43 adenomas were derived from a second sigmoidoscopy-based screening study,23 and median adenoma size at time of removal was 12 mm (range 7–40 mm) while median age was 58 years (range 50–64 years). The normal samples consisted of rectal mucosal biopsies from individuals with a negative sigmoidoscopy derived from the first screening study mentioned above.22 Median age was 67 years (range 63–72 years). The tissue sample set in this study include the majority of the samples analyzed in a recent report from our lab where the novel epi-panel for early detection or CRC and adenomas was identified.11 However, six MSI tumor samples and eight adenomas from the original sample test set were not included here due to lack of sufficient amounts of DNA.
Ethics
All samples belong to approved research biobanks registered at the Norwegian Institute of Public Health and approvals are given by the Regional Ethics Committee and National Data Inspectorate of Norway.
Bisulfite treatment
The DNA included in this study was isolated using either a standard phenol/chloroform procedure or a magnetic beads approach (the Maxwell 16 DNA Purification kits; Promega, Madison, WI, USA and MagAttract DNA Mini M48 kit; Qiagen, Valencia, CA, USA). DNA was subjected to bisulfite conversion using the EpitTect bisulfite kit from Qiagen (Qiagen). DNA (1.3 μg) was used as input material, and the conversion procedure was performed according to the manufacturer's protocol. The MJ Mini Personal Thermal Cycler from Bio-Rad (Bio-Rad, Hercules, CA, USA) was used to perform the conversion, whereas the Qiacube automated pipette system (Qiagen) was used to perform the purification and elution of converted DNA.
Quantitative methylation-specific PCR
To generate comparative data for the genes analyzed in this study, we aimed at using assays that were as overlapping as possible with the original assays.6, 8, 18, 19, 20 However, primers and probes were adjusted to fulfill the criteria for minor groove binder nonfluorescent quencher qMSP assays set by the Primer Express Software 3.0 (Applied Biosystems; Table 1). Primers were purchased from Medprobe (Oslo, Norway) and 5-prime 6-FAM-labeled probes were purchased from Applied Biosystems.
Table 1. Primer and probe sequences for qMSP.
| Assay | Forward primer (5′–3′) | Reverse primer (5′–3′) | Probe | Refseq | Reference no. |
|---|---|---|---|---|---|
| ITGA4 | GCGGTTCGTATTCGGAGAAG | TCTACCGCCAACCGAAAACT | 6FAM-AGCGCGAGTATTC-MGB | NM_000885 | 19 |
| NTRK2 | CGTTGGTTGCGTATTTGGC | GCAATACCTAACACTTCCGAAAACTC | 6FAM-TGTTCGTCGCGATTC-MGB | NM_006180 | 20 |
| OSMR | CGTCGGTTGGTTCGTGC | CCGAACTTTACGAACGAACGA | 6FAM-CGGTCGTCGAGTTTT-MGB | NM_03999 | 20 |
| TUBG2 | TAACGAGTTTTATTGCGTAGGCG | CAACTAAAATTCCGCACAAACG | 6FAM-GGAGCGGGCGTGGT-MGB | NM_016437 | 20 |
| SEPT9 | CGCGCGATTCGTTGTTTATTA | CCAACCCAACACCCACCTT | 6FAM-GGATTTCGCGGTTAAC-MGB | NM_001113493 | 6 |
| VIM | GGTCGAGTTTTAGTCGGAGTTACGT | CCCGAAAACGAAACGTAAAAACTA | 6FAM-CGTATTTATAGTTTGGGTAGCGC-MGB | NM_003380 | 18 |
MGB, minor groove binder; qMSP, quantitative methylation-specific PCR.
The qMSP reactions were carried out in triplicates in a 10-μl volume including 1 × Taqman Universal PCR Mastermix (No AmpErase UNG, Applied Biosystems, Foster City, CA, USA), 0.45 μℳ of forward and reverse primers, 0.1 μℳ probe, and 30 ng of bisulfite-treated DNA template. The PCR conditions were as follows: one step at 95 °C for 10 min, 45 cycles at 95 °C for 15 s and finally 60 °C for 1 min using the 7900 HT Fast Real-Time PCR machine (Applied Biosystems). The PCR reactions were carried out in 384-well plates, and the median value from the triplicates was used for data analysis. Both test and validation sets were analyzed using the SDS 2.3 software (Applied Biosystems). In addition to patient samples, each plate included a standard curve generated from a 1:5 dilution series (32.5–0.052 ng) using a commercially available fully methylated positive DNA control (CpGenome Universal Methylated DNA; Millipore Billerica, MA, USA), multiple water blanks, positive controls (CpGenome Universal Methylated DNA), unmodified DNA, as well as bisulfite-treated normal blood as negative controls. To normalize for input level of DNA, the ALU-C4 repetitive element was used as an internal reference24 and the resulting data output was calculated as percent of methylated reference (PMR) values, where the median value of GENE:ALU ratio for each sample was divided by the median GENE:ALU ratio of the positive control and multiplied by 100. All samples were censored after cycle 35 according to the protocol from Applied Biosystems. To ensure high specificity, the highest PMR value across the normal mucosa test set was used to set an individual scoring threshold for each gene (ITGA4, 2; NTRK2, 0; OSMR, 10; SEPT9, 9; TUBG2, 0; and VIM, 2). All samples with PMR values greater than the thresholds were scored as positive for methylation. One normal mucosa sample had an outlier PMR value (OSMR, 19.2), and was consequently excluded when determining the threshold.
Cloning and DNA bisulfite sequencing
Owing to the apparent discrepancy in methylation frequencies generated in this study and the previously reported data for NTRK2, this gene was subjected to promoter region bisulfite sequencing in representative tissue samples (CRC, n=4; adenomas, n=2; and normal mucosa n=2) as well as in colon cancer cell lines (n=2). The analyzed CRCs comprised two MSI and two MSS samples. Both subtypes included one sample with medium/high PMR value and one with a low PMR value from the NTRK2 qMSP analysis. The MSI cell line SW48 was selected for analysis (high PMR value) along with the MSS cell line LS1034 (low PMR value). Following the initial PCR using bisulfite sequencing primers flanking the methylation sites of interest, 4 μl PCR product was used in TOPO TA cloning system (Invitrogen, Carlsbad, CA, USA) according to the instructions by the manufacturer. From each sample, plasmid DNA was isolated from 12 individual colonies using Qiaprep Spin Miniprep Kit (Qiagen) and the isolated DNA was sequenced using M13 forward primer and T3 reverse primer included in the TOPO TA Kit. The sequencing reaction included dGTP BigDye Terminator v.3.0 Cycle Sequencing Ready Reaction Kit (Applied Biosystems) and the oligonucleotide fragments were separated by ABI PRISM 3730 Sequencer (Applied Biosystems). The BISMA program was used for analysis of the resulting bisulfite sequencing data using default filtering thresholds.25
The bisulfite sequencing primers were designed to amplify regions with preferably none and maximum 1 CpG site in the forward and reverse primers and to flank the area amplified by the qMSP assay. The NTRK2 fragment (Refseq NM_006180) covered bases −365 to −64 relative to the transcription start site. All together 40 CpG sites, including the 17 included in the original NTRK2 assay,20 were covered by the bisulfite sequencing analysis (forward primer: 5′-TTGCGGGTAGATTAGTGATTAT-3′ reverse primer: 5′-CATTTACAAACCTTATCTAAAAA-TCC-3′). The primers were designed using the Methyl Primer Express 1.0 from Applied Biosystems.
Statistical Analyses
Statistical analyses were performed using SPSS 16.0 (IBM Corporation, Armonk, NY, USA). Comparisons of categorical variables were carried out using χ2 or Fisher's exact test, and findings were considered statistically significant if the P-value was ≤0.05 (5%). Student t-test and regression were used to analyze a potential association between methylation status and patient age, whereas Mann–Whitney U-test was used to analyze a potential association between adenoma size and promoter hypermethylation. PMR values were used to generate receiver operating characteristic curves.
RESULTS
Methylation frequencies of target genes in clinical test and validation set
The selected biomarkers were analyzed in clinical tissue samples. When comparing the methylation frequencies with that of the novel epi-panel, the latter was shown to outperform the previously identified DNA methylation markers. These results are summarized in Table 2 and illustrated in Figure 1. No statistical difference in methylation frequencies was seen between the test and the validation sets. For the normal mucosa samples, the validation set comprised only rectum samples, which could introduce a possible bias. However, also here, comparable methylation frequencies were found between the test and validation series.
Table 2. Promoter methylation frequencies of selected target genes.
| Assay | Cell lines |
Carcinoma |
Adenoma |
Normal mucosa |
Cancer normals | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Test | Validation | Combined | Test | Validation | Combined | Test | Validation | Combined | |||
| ITGA4 | 19/20 (95%) | 54/64 (84%) | 98/105 (93%) | 152/169 (90%) | 48/61 (79%) | 31/43 (72%) | 79/104 (76%) | 0/51 (0%) | 1/56 (2%) | 1/107 (1%) | 8/105 (8%) |
| NTRK2 | 2/20 (10%) | 5/64 (8%) | 6/105 (6%) | 11/169 (7%) | 2/61 (3%) | 1/43 (2%) | 3/104 (3%) | 0/51 (0%) | 0/56 (0%) | 0/107 (0%) | 1/105 (1%) |
| OSMR | 13/20 (65%) | 48/64 (75%) | 83/105 (79%) | 131/169 (78%) | 51/61 (84%) | 29/43 (67%) | 80/104 (77%) | 1/51 (2%) | 1/56 (2%) | 2/107 (2%) | 32/105 (30%) |
| SEPT9 | 20/20 (100%) | 48/64 (75%) | 90/105 (86%) | 138/169 (82%) | 55/61 (90%) | 37/43 (86%) | 92/104 (88%) | 0/51 (0%) | 3/56 (5%) | 3/107 (3%) | 11/105 (10%) |
| TUBG2 | 1/20 (5%) | 0/64 (0%) | 2/105 (2%) | 2/169 (1%) | 0/61 (0%) | 0/43 (0%) | 0/104 (0%) | 0/51 (0%) | 0/56 (0%) | 0/107 (0%) | 0/105 (0%) |
| VIM | 17/20 (85%) | 40/64 (63%) | 73/105 (70%) | 113/169 (67%) | 38/61 (62%) | 18/43 (42%) | 56/104 (54%) | 0/51 (0%) | 3/56 (5%) | 3/107 (3%) | 8/105 (8%) |
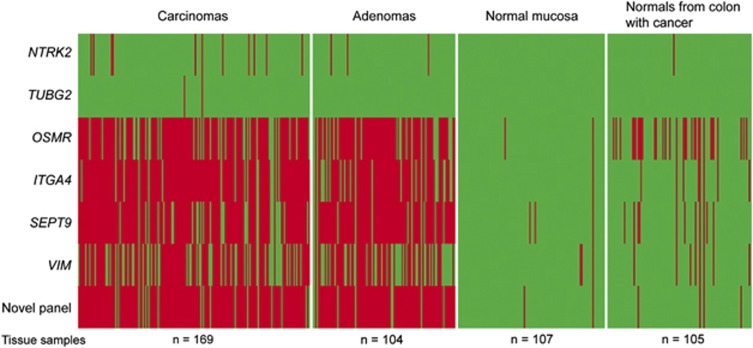
Figure 1.
Promoter methylation status of the previously identified DNA methylation markers in normal and tumor tissue samples. The figure illustrates the methylation status of the previously identified DNA methylation biomarkers across test and validation sets of colorectal carcinomas (n=169), adenomas (n=104), normal mucosa samples from colorectal cancer (CRC)-free individuals (n=107), and normal mucosa from CRC patients (n=105). For comparison the methylation status of the novel epi-panel for early detection of CRC is included.11 Green color indicates unmethylated sample, whereas red color indicates methylated sample.
Associations between the methylated genes and known genetic and clinicopathological features
The DNA methylation status of the majority of the investigated gene promoters in CRC was independent of MSI status, BRAF mutation, tumor localization, tumor stage as well as gender and age of the patients. Promoter methylation was more frequent among MSI CRCs than seen among MSS samples for VIM (91% vs. 58% P=3.2E−5) and NTRK2 (22% vs. 1% P=6.0E−7). Promoter hypermethylation of VIM and NTRK2 was further associated with mutation in exon 15 of the BRAF gene (P=0.002 and P=9.0E−9). CRC methylation of VIM was additionally associated with proximal location in the colon (P=0.004), and with increased patient age (mean age 72 years for patients with methylated tumors, and 66 years for patients with unmethylated tumors; P=0.005; logistic regression). Methylation of NTRK2 was slightly more common among female than male patients (11% vs. 3% Fisher's exact test P=0.029). In adenomas, the methylation status of the analyzed genes was independent of age, MSI status, tumor location, gender, and adenoma size.
Comparing sensitivities and specificities of the previously identified DNA methylation markers with the novel epi-panel
The methylation frequencies achieved for the individual genes across the tumor test and validation set were lower compared with the performance of the novel panel for early detection of CRC.11 The panel, consisting of the genes CNRIP1, FBN1, INA, MAL, SNCA, and SPG20, had initially a combined sensitivity of 94% for CRC and 93% for adenoma, with a specificity of 98%. Positive samples were defined to be methylated in at least two out of these six genes. Restricting the measurements to the samples included in this study, where six MSI CRC and eight adenoma samples were omitted due to shortage of DNA, resulted in a sensitivity of 93% in CRC and 92% in adenoma, and a combined specificity of 98%, which is significantly higher than for VIM (CRC, P=2.4E−4; adenoma, P=0.013) and SEPT9 (CRC: P=2.3E−5; adenoma: P=3.3E−12). Only ITGA4 achieved comparable specificity and sensitivity measurements, although the methylation frequency among the adenomas was significantly lower (P=4.9E−4) than for the panel (Table 2). When combining the six previously identified DNA methylation markers, ITGA4, NTRK2, OSMR, TUBG2, SEPT9, and VIM, and using the same scoring threshold as for the novel epi-panel (two out of six positive genes to call a sample as methylated), this panel achieved a sensitivity of 89% for CRC and 84% for adenoma, with a specificity of 99%. The resulting lower performance of this panel compared with that of the novel epi-panel underscored that the choice of markers to combine is important and not merely the combinatorial approach itself.
The novel epi-panel outperformed VIM and SEPT9 in tissue samples
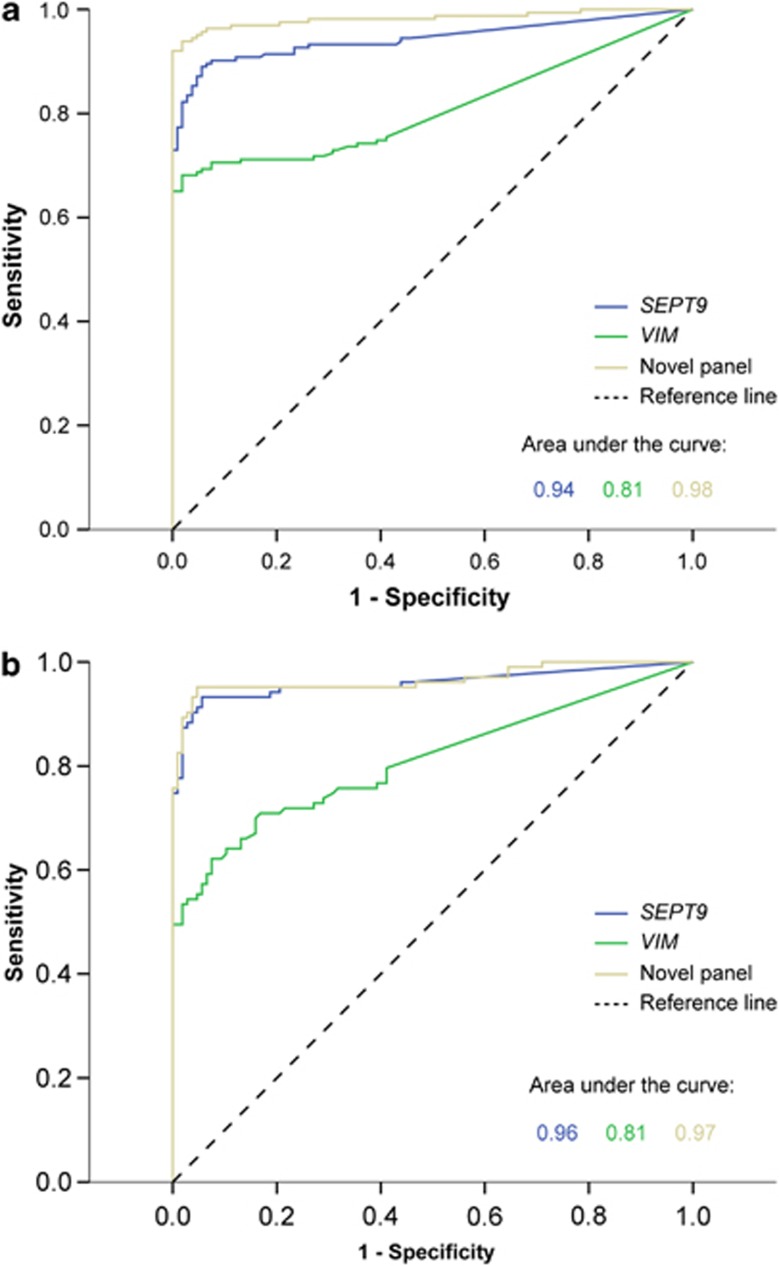
As VIM and SEPT9 are currently the only clinically available biomarkers for noninvasive diagnostic use, we used ROC curves to compare the performance of these biomarkers with that of the novel epi-panel, using data generated from tissue samples. SEPT9 and VIM achieved an area under the ROC curve of 0.94 and 0.81, respectively, for discriminating CRCs from normal mucosa and 0.96 and 0.81 for discriminating adenomas from normal mucosa (Figure 2). In comparison, the novel epi-panel achieved areas under the ROC curve values of 0.98 and 0.97, respectively. Across the test and validation sets, we showed that SEPT9 and VIM were methylated in 82% and 67% of CRCs (n=169) and in 88% and 54% of the adenomas (n=104), respectively, whereas 3% of the normal mucosa samples (n=107) were methylated for these genes.
Figure 2.
Receiver operating characteristic (ROC) curves for the novel epi-panel and the previously identified DNA methylation markers. The area under the curve conveys the accuracy of the biomarkers in distinguishing colorectal carcinoma (a) or adenoma (b) from normal mucosa.
Interestingly, a small number of CRC samples (7/169) were unmethylated for all six genes included in the novel epi-panel. Five of these samples (71%) were unmethylated for both SEPT9 and VIM, whereas the remaining two samples (29%) displayed promoter methylation of either VIM or SEPT9. Among the CRC samples that were scored as unmethylated for VIM (n=52), 83% could be detected using the novel epi-panel. Furthermore, 75% of the CRCs unmethylated in the SEPT9 promoter (n=28) were positive when adding this information, underscoring that the novel epi-panel could significantly improve the diagnostic sensitivity without compromising the specificity.
Bisulfite sequencing confirmed the promoter methylation status of NTRK2
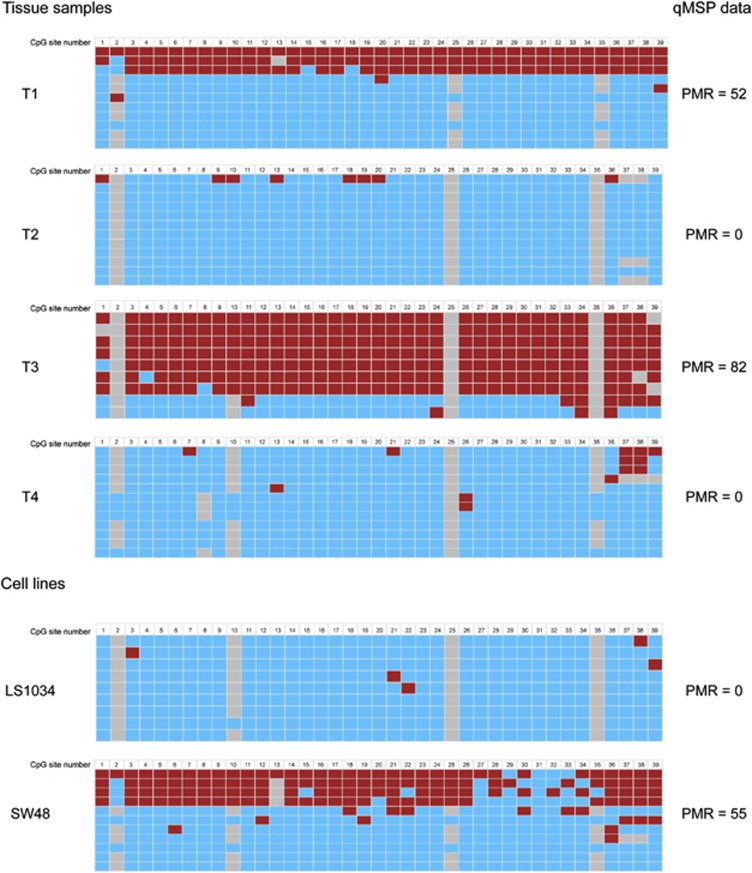
Bisulfite sequencing following cloning of selected cancer cell lines and tissue samples confirmed the promoter methylation status of NTRK2 as assessed by qMSP (Figure 3). As expected, samples with a low PMR value displayed few methylated CpG sites from the bisulfite sequencing analysis, whereas several of the clones from samples with higher PMR values had methylated CpG sites.
Figure 3.
Bisulfite sequencing of the NTRK2 promoter confirmed the methylation status as assessed by quantitative methylation-specific PCR (qMSP). The figure illustrates the bisulfite sequencing results for the NTRK2 promoter in four colorectal cancer samples and two colon cancer cell lines. Rightmost column reflects the percent of methylated reference (PMR) values for the same samples assessed by qMSP. Red square illustrates methylated CpG site, blue square represents unmethylated site, whereas gray squares represent unknown or undetermined CpG sites.
DISCUSSION
In this study, we have evaluated the performance of six previously identified DNA methylation markers for CRC, including VIM and SEPT9, in Norwegian clinical sample sets. The results were compared with the performance of a novel epi-panel consisting of CNRIP1, FBN1, INA, MAL, SNCA, and SPG20.11 To achieve methylation frequencies that were directly comparable, all analyses have been performed in the same lab, using the same experimental procedures and the same tissue sample sets. From analyses of 485 tissue samples, we showed that the novel epi-panel had a higher sensitivity and specificity than all the individual biomarkers.6, 18, 20
The vast majority of the CRC samples analyzed in this study were positive for the novel epi-panel including a substantial number of tumors that were unmethylated for both VIM and SEPT9. These results underscore the value of combining several promising candidates into a robust panel for CRC detection. A higher resulting area under the ROC curve of the novel epi-panel compared with VIM and SEPT9 further supported this in tissue samples, and further indicated that the panel has the potential to perform at least as good, possibly even better than these single markers in a noninvasive test.
A small number of CRC samples were unmethylated for all six genes included in the novel epi-panel. Combining methylation markers with genetic markers could in principle further increase the fraction of detected tumors. Indeed, two of the seven negative cancers had a mutation in the PTEN gene, and one of these mutated cancers was additionally of the MSI phenotype. The rest of the methylation negative cancers were MSS and wild type for all the genetic CRC markers tested, including BRAF, KRAS, PTEN, TP53, and PIK3CA (data not shown).
We also analyzed the methylation frequencies of ITGA4, OSMR, NTRK2, and TUBG2 in this study. For the two first genes, we observed methylation frequencies that were comparable with previously reported data.19, 20 With a sensitivity of 90% and a specificity of 99%, ITGA4 represents one of the most promising biomarkers for CRC. Indeed, methylation of this gene has already been evaluated in stool specimens with a reported sensitivity of 69% and a specificity of 79% for colorectal adenomas.19 Another study has reported ITGA4 methylation in patients with CRC (37%) as well as in individuals with adenoma (16%) and combining ITGA4 with p16 and SFRP2 resulted in an increased sensitivity in the same noninvasive material (70% in CRC and 72% in adenoma, with a specificity of 97%).26
OSMR had slightly lower tumor methylation frequencies than ITGA4 in this study. Several studies have previously analyzed this gene in CRC samples resulting in a range of methylation frequencies from 32 to 90%.20, 27, 28 Interestingly, OSMR promoter hypermethylation has also been identified in stool DNA from CRC patients.20
Surprisingly, for NTRK2 and TUBG2 the methylation frequencies observed in this study were much lower than what have been reported previously.20 A general source of errors when comparing methylation frequencies between various studies can be the choice of methodology and even variations in primer and probe design within the same method. Furthermore, the scoring threshold is also an important source for deviating results between studies. In our analyses, we chose to set individual scoring thresholds for each gene based on the highest PMR value achieved among the normal mucosa samples in the test set to ensure optimal specificities. By lowering these threshold values, the sensitivity may be increased, but at the expense of the specificity. In this qMSP-based study, we aimed at including as many overlapping CpG sites as possible from the original assays to generate comparable data. With the exception of the NTRK2 forward primer, where only one out of three CpG sites in the original assay were included in the modified assay, all remaining CpG sites for the primers and probes for this gene as well as for TUBG2 were included in the qMSP assays used in this study. Owing to the discrepant results, particularly for NTRK2, we cloned and bisulfite sequenced parts of its promoter to determine whether the observed differences were a consequence of technical issues. The bisulfite sequencing data confirmed that the NTRK2 promoter region indeed was rarely methylated in both cell lines and CRC tissue samples, indicating that the quantitative methylation data generated in this study across a large set of Norwegian tissue samples were valid. Ethnicity has previously been found to be associated with differences in cancer incidence and mortality, exemplified by African American men who have greater risk of developing testicular germ cell tumors and prostate cancer compared with Caucasian men.29, 30 In this study, we cannot exclude that the observed discrepancy of NTRK2 and TUBG2 may be due to differences in the population of the included patients. However, to the best of our knowledge, these genes have previously only been analyzed in a relatively small sample set,20 which may contribute to the discrepancy in the results.
It should be noted that the evaluations and comparisons of the biomarker performance have been done in tissue samples and not in noninvasive material, such as stool or blood. A handful studies have shown that methylation frequencies can vary from tissue samples to noninvasive samples.5, 20, 31, 32 This is exemplified by promoter hypermethylation of NDRG4 and VIM, which both have been shown to perform well in tissue samples. When analyzed in fecal DNA samples, the sensitivity of both biomarkers decreased, whereas the specificity for NDRG4 methylation remained high.5, 31 When tested in noninvasive samples, we expect a decrease in the performance also of the novel epi-panel. Although the data presented here show that the panel outperforms individual biomarkers in tissue-based samples, it remains to be seen if this is the case also in noninvasive material.
Although our analyses have been based on the exact same tissue sample sets the space in time between the analyses of the novel epi-panel and the previously identified DNA methylation markers may provide a potential bias. Preferably all markers should have been investigated at the same time, using the exact same bisulfate-treated DNA. Although we have used the same DNA stock for all analyses as well as the same standardized experimental procedures, we cannot exclude differences in results due to the time delay in the experiments. However, we recently analyzed the novel epi-panel in another independent clinical series of CRCs (n=197) and achieved a comparable and even slightly higher sensitivity than previously reported (data not shown), which shows that the previously established data are highly reproducible.
So far, only a few epigenetic biomarkers with a diagnostic potential have been identified and subsequently validated in blood and/or stool samples from CRC patients. In addition to the previously mentioned VIM and SEPT9, TFPI2 is a promising candidate in stool from patients with stage I−III CRC.32 This gene was not included in the previously mentioned review Kim et al.8 and was consequently not analyzed in this study. Interestingly, TFP12 methylation has recently also been detected in serum, however, with a low sensitivity (18%).33 Although blood-based detection of CRC could be more cost effective as well as more accepted among the general population, the high sensitivity observed in stool compared with serum indicates that TFPI2 could potentially be more useful in a stool-based test. Hypermethylation of GATA4/5 has also been shown to have a high sensitivity and specificity in stool DNA from patients with CRC.34 Combining these with other promising biomarkers may give a robust epigenetic panel with a high sensitivity and specificity for noninvasive CRC detection.
In conclusion, using tissue samples the performance of a novel epi-panel outperformed some of the most highly methylated genes from the literature, including VIM and SEPT9. This underscores the value of combining a manageable number of individually promising markers into a panel, which at least on a tissue level can achieve more robustness in addition to a high sensitivity and specificity. From this comparison, we conclude that the novel epi-panel is highly suitable for developing into a noninvasive test for early detection of CRC.
Study Highlights

Acknowledgments
We are grateful to Merete Hektoen, Hilde Honne, and Zeremariam Yohannes, Department of Cancer Prevention, Institute for Cancer Research, The Norwegian Radium Hospital, Oslo University Hospital, Oslo, for technical assistance.
Guarantor of the article: Guro E. Lind, Dr Philos.
Specific author contributions: GEL conceived and was responsible for study design. DA, THA, and SAD carried out the experimental analyses. DA and GEL carried out data interpretation and statistical analyses and drafted the manuscript. MB, ETE, GH, TOR, AN, and RAL contributed with biobank material, molecular data, and patient data. RAL contributed in designing the study and interpretation of results. All authors have participated in manuscript preparation and approved the final manuscript.
Financial support: This work was supported by grants from the Norwegian Cancer Society (GEL: PR-2008–0163 and PR-2009-0307: funding DA as PhD student; RAL: PR-2006-0442 funding SAD as a post doc), and grants from the Norwegian Health Region South East (RAL: no. 2011024 “Genome medicine of colorectal cancer”, funding THÅ as post doc).
Potential competing interests: A patent application has been filed covering the novel epi-panel for early detection of CRC (Patent Cooperation Treaty, international no. PCT/EP2008/052156). The biomarker panel was recently licensed to “Oxford Gene Technology” (February 2012).
Footnotes
Supplementary Information accompanies this paper on the Clinical and TranslationalGastroenterology website (http://www.nature.com/ctg)
Supplementary Material
References
- Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- Atkin WS, Edwards R, Kralj-Hans I, et al. Once-only flexible sigmoidoscopy screening in prevention of colorectal cancer: a multicentre randomised controlled trial. Lancet. 2010;375:1624–1633. doi: 10.1016/S0140-6736(10)60551-X. [DOI] [PubMed] [Google Scholar]
- Feinberg AP, Tycko B. The history of cancer epigenetics. Nat Rev Cancer. 2004;4:143–153. doi: 10.1038/nrc1279. [DOI] [PubMed] [Google Scholar]
- Esteller M. Epigenetics in cancer. N Engl J Med. 2008;358:1148–1159. doi: 10.1056/NEJMra072067. [DOI] [PubMed] [Google Scholar]
- Chen WD, Han ZJ, Skoletsky J, et al. Detection in fecal DNA of colon cancer-specific methylation of the nonexpressed vimentin gene. J Natl Cancer Inst. 2005;97:1124–1132. doi: 10.1093/jnci/dji204. [DOI] [PubMed] [Google Scholar]
- Lofton-Day C, Model F, Devos T, et al. DNA methylation biomarkers for blood-based colorectal cancer screening. Clin Chem. 2008;54:414–423. doi: 10.1373/clinchem.2007.095992. [DOI] [PubMed] [Google Scholar]
- Baylin SB, Ohm JE. Epigenetic gene silencing in cancer—a mechanism for early oncogenic pathway addiction. Nat Rev Cancer. 2006;6:107–116. doi: 10.1038/nrc1799. [DOI] [PubMed] [Google Scholar]
- Kim MS, Lee J, Sidransky D. DNA methylation markers in colorectal cancer. Cancer Metastasis Rev. 2010;29:181–206. doi: 10.1007/s10555-010-9207-6. [DOI] [PubMed] [Google Scholar]
- Lind GE, Ahlquist T, Kolberg M, et al. Hypermethylated MAL gene - a silent marker of early colon tumorigenesis. J Transl Med. 2008;6:13. doi: 10.1186/1479-5876-6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lind GE, Raiborg C, Danielsen SA, et al. SPG20, a novel biomarker for early detection of colorectal cancer, encodes a regulator of cytokinesis. Oncogene. 2011;30:3967–3978. doi: 10.1038/onc.2011.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lind GE, Danielsen SA, Ahlquist T, et al. Identification of an epigenetic biomarker panel with high sensitivity and specificity for colorectal cancer and adenomas. Mol Cancer. 2011;10:85. doi: 10.1186/1476-4598-10-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lao VV, Grady WM. Epigenetics and colorectal cancer. Nat Rev Gastroenterol Hepatol. 2011;. p. 10. [DOI] [PMC free article] [PubMed]
- Itzkowitz SH, Jandorf L, Brand R, et al. Improved fecal DNA test for colorectal cancer screening. Clin Gastroenterol Hepatol. 2007;5:111–117. doi: 10.1016/j.cgh.2006.10.006. [DOI] [PubMed] [Google Scholar]
- Grutzmann R, Molnar B, Pilarsky C, et al. Sensitive detection of colorectal cancer in peripheral blood by septin 9 DNA methylation assay. PLoS One. 2008;3:e3759. doi: 10.1371/journal.pone.0003759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ned RM, Melillo S, Marrone M.Fecal DNA testing for Colorectal Cancer Screening: the ColoSure test PLoS Curr 20113RRN1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epigenomics AG. Epigenomics AG Completes US Clinical Validation Study for Colorectal Cancer Blood Test Epi proColon; Provides Update on US Regulatory Plans. 9-12-2011.
- Herbst A, Rahmig K, Stieber P, et al. Methylation of NEUROG1 in serum is a sensitive marker for the detection of early colorectal cancer. Am J Gastroenterol. 2011;106:1110–1118. doi: 10.1038/ajg.2011.6. [DOI] [PubMed] [Google Scholar]
- Zou H, Harrington JJ, Shire AM, et al. Highly methylated genes in colorectal neoplasia: implications for screening. Cancer Epidemiol Biomarkers Prev. 2007;16:2686–2696. doi: 10.1158/1055-9965.EPI-07-0518. [DOI] [PubMed] [Google Scholar]
- Ausch C, Kim YH, Tsuchiya KD, et al. Comparative analysis of PCR-based biomarker assay methods for colorectal polyp detection from fecal DNA. Clin Chem. 2009;55:1559–1563. doi: 10.1373/clinchem.2008.122937. [DOI] [PubMed] [Google Scholar]
- Kim MS, Louwagie J, Carvalho B, et al. Promoter DNA methylation of oncostatin m receptor-beta as a novel diagnostic and therapeutic marker in colon cancer. PLoS One. 2009;4:e6555. doi: 10.1371/journal.pone.0006555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meling GI, Lothe RA, Borresen AL, et al. Genetic alterations within the retinoblastoma locus in colorectal carcinomas. Relation to DNA ploidy pattern studied by flow cytometric analysis. Br J Cancer. 1991;64:475–480. doi: 10.1038/bjc.1991.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiis-Evensen E, Hoff GS, Sauar J, et al. Population-based surveillance by colonoscopy: effect on the incidence of colorectal cancer. Telemark Polyp Study I. Scand J Gastroenterol. 1999;34:414–420. doi: 10.1080/003655299750026443. [DOI] [PubMed] [Google Scholar]
- Bretthauer M, Gondal G, Larsen K, et al. Design, organization and management of a controlled population screening study for detection of colorectal neoplasia: attendance rates in the NORCCAP study (Norwegian Colorectal Cancer Prevention) Scand J Gastroenterol. 2002;37:568–573. doi: 10.1080/00365520252903125. [DOI] [PubMed] [Google Scholar]
- Weisenberger DJ, Campan M, Long TI, et al. Analysis of repetitive element DNA methylation by MethyLight. Nucleic Acids Res. 2005;33:6823–6836. doi: 10.1093/nar/gki987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohde C, Zhang Y, Reinhardt R, et al. BISMA--fast and accurate bisulfite sequencing data analysis of individual clones from unique and repetitive sequences. BMC Bioinformatics. 2010;11:230. doi: 10.1186/1471-2105-11-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang E, Park DI, Kim YJ, et al. Detection of colorectal neoplasm using promoter methylation of ITGA4, SFRP2, and p16 in stool samples: a preliminary report in Korean patients. Hepatogastroenterology. 2010;57:720–727. [PubMed] [Google Scholar]
- Deng G, Kakar S, Okudiara K, et al. Unique methylation pattern of oncostatin m receptor gene in cancers of colorectum and other digestive organs. Clin Cancer Res. 2009;15:1519–1526. doi: 10.1158/1078-0432.CCR-08-1778. [DOI] [PubMed] [Google Scholar]
- Hibi K, Goto T, Sakuraba K, et al. Methylation of OSMR gene is frequently observed in non-invasive colorectal cancer. Anticancer Res. 2011;31:1293–1295. [PubMed] [Google Scholar]
- Sun M, Abdollah F, Liberman D, et al. Racial disparities and socioeconomic status in men diagnosed with testicular germ cell tumors: a survival analysis. Cancer. 2011;117:4277–4285. doi: 10.1002/cncr.25969. [DOI] [PubMed] [Google Scholar]
- Moses KA, Paciorek AT, Penson DF, et al. Impact of ethnicity on primary treatment choice and mortality in men with prostate cancer: data from CaPSURE. J Clin Oncol. 2010;28:1069–1074. doi: 10.1200/JCO.2009.26.2469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melotte V, Lentjes MH, Van den Bosch SM, et al. N-Myc downstream-regulated gene 4 (NDRG4): a candidate tumor suppressor gene and potential biomarker for colorectal cancer. J Natl Cancer Inst. 2009;101:916–927. doi: 10.1093/jnci/djp131. [DOI] [PubMed] [Google Scholar]
- Glockner SC, Dhir M, Yi JM, et al. Methylation of TFPI2 in stool DNA: a potential novel biomarker for the detection of colorectal cancer. Cancer Res. 2009;69:4691–4699. doi: 10.1158/0008-5472.CAN-08-0142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibi K, Goto T, Shirahata A, et al. Detection of TFPI2 methylation in the serum of colorectal cancer patients. Cancer Lett. 2011;311:96–100. doi: 10.1016/j.canlet.2011.07.006. [DOI] [PubMed] [Google Scholar]
- Hellebrekers DM, Lentjes MH, Van den Bosch SM, et al. GATA4 and GATA5 are potential tumor suppressors and biomarkers in colorectal cancer. Clin Cancer Res. 2009;15:3990–3997. doi: 10.1158/1078-0432.CCR-09-0055. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.