Abstract
OBJECTIVES:
The role of T lymphocytes in the pathogenesis of Celiac disease (CD) is well established. However, the mechanisms of T-cell involvement remain elusive. Little is known on the distribution of T subpopulations: T-regulatory (Treg), Th17, CD103, and CD62L cells at disease onset and after gluten-free diet (GFD). We investigated the involvement of several T subpopulations in the pathogenesis of CD.
METHODS:
We studied T cells both in the peripheral blood (PB) and the tissue-infiltrating lymphocytes (TILs) from the mucosa of 14 CD patients at presentation and after a GFD, vs. 12 controls.
RESULTS:
Our results extend the involvement of Treg, Th1, and Th17 cells in active CD inflammation both in the PB and at the TILs. At baseline, Tregs, Th1, and Th17 cells are significantly higher in active CD patients in TILs and PB. They decreased after diet. Moreover, CD62L+ TILs were increased at diagnosis as compared with GFD patients.
CONCLUSIONS:
Our data show significant modifications of the above-mentioned subpopulations both in the PB and TILs. The increase of suppressive Tregs in active CD both in the PB and TILs is intriguing. T lymphocytes are known to have a crucial role in the pathogenesis of CD. We have shown that gluten trigger results in systemic recruitment of T lymphocytes, the unbalance between pro-inflammatory and anti-inflammatory populations and the increase of CD62L+ T cells in TILs. Our results delineate a more complete picture of T-cell subsets in active vs. GFD disease. Our data of T-cell subpopulations, combined with known data on cytokine production, support the concept that duodenal micro-environment acts as an immunological niche and this recognition may have an important role in the diagnosis, prognosis and therapeutical approach of CD.
INTRODUCTION
Celiac disease (CD) is a relatively common pathology, characterized by an abnormal immune response to gliadin in genetically predisposed individuals.1, 2, 3, 4 Gluten intolerance causes inflammation to the mucosa of the small intestine resulting in villous atrophy. There is no specific treatment, but the symptoms can be ameliorated or resolved by eliminating gluten from the diet. The reversibility of the disease is closely linked to the intake by celiac patients of gluten-free food.5
T lymphocytes are known to have a crucial role in the pathogenesis of CD. So far, the main actors of inflammation were thought to be played by 1) cytotoxic CD8 and NK cells among intraepithelial lymphocytes6 in an environment with elevated production of IFN-gamma.7 CD8 cells express FasL and therefore induce apoptosis of mucosal cells8, 9 and by 2) Th1-secreting CD4 cells in the lamina propria (LP).10, 11 Thus, CD4 cells participate to developing inflammation through cytokine production with a prevalence of a Th1 pattern of pro-inflammatory cytokines. T-box transcription factor T-bet has been identified as a key transcription factor for the development of Th1 cells and has also been shown to be involved in the regulation of CD8+ T cells (Tc1) and CD19+ B cells (Be1). T-bet induces the production of IFN-gamma and orchestrates the Th1 cell–migratory program by regulating the expression of chemokines and chemokine receptors. CD4 cells massively infiltrate the LP12 CD4 clones responding to gliadin-deaminated immunodominant peptides have been shown to produce IFN-gamma.13
Recently, the focus of CD pathogenesis has shifted to the role of novel subsets of T lymphocytes: T-regulatory (Tregs) and Th17 cells. Although heterogeneity of CD4 cells has been reported some decades ago,14, 15 only recently novel CD4+ subpopulations such as Tregs and Th17 cells have been described (review in Pandolfi et al.16).
Tregs are CD3/CD4+/CD25+/FoxP3+ cells that exert a strong inhibitory effect on the immune responses.17, 18 Recently, after the description of an inverse relationship between the expression of FoxP3 and CD127,17 it has become possible to define an alternative phenotype of Tregs as CD3/CD4/CD25high/CD127− cells. Previous works have reported that both Tregs and Th17 cells are involved in active CD.19, 20, 21 Tregs perform an anti-inflammatory activity and can downregulate the immune response. Despite their suppressor activity on the immune response, Tregs are increased in the inflamed mucosa of active CD. However, only few studies on the role of Treg in CD are available.22, 23, 24
Th17 cells are characterized by the synthesis and production of IL (interleukin)-17A and F, by the activation of nuclear transcription factor STAT-3, and by the induction of retinoic acid-related nuclear transcription factor ROR-gammat.16 In active CD, severe inflammation is present and it is not surprising that Th17 cells have been found to be increased.21, 25, 26 Tregs have been localized in the LP.22 Data on Th17 cells localization are preliminary. Sapone et al. 27 showed IL-17 mRNA mostly in the LP, but their immunofluorescence stains do not allow to determine their localization in the CD mucosa.
There are only few works analyzing a wide panel of lymphocyte subpopulations in both peripheral blood (PB) and intestinal mucosa of patients with CD at diagnosis and after gluten-free diet (GFD), compared with non-CD patients.19, 22, 26, 28 Therefore, we compared the distribution of several subsets of T lymphocytes (Treg, Th17 and naïve CD62L cells) in both PB and in the duodenal mucosa of CD patients at diagnosis and after a GFD diet, as compared with non-CD controls. Finally, as T cells expressing the adhesion molecule CD103 are known to home in the intestine, we also evaluated CD103+ cells to get insights in the homing of T cells during the disease.
These intricate interactions between several T-cell subpopulations described above, reinforce the idea of a complex regulation of tissue immune system in duodenal mucosa of CD patients. Cytokines also have an important role and can modulate immune response in a pro-inflammatory or anti-inflammatory pathway.
Therefore, duodenal micro-environment can be considered as an “immunological niche”, that is, a definite anatomo-functional region that hosts the components of a complex immune functional tissue. Immunological niche has a direct role in the homeostasis of local immune system, regulating inflammation, apoptosis, immune T-cell differentiation, peripheral tolerance and anergy.29 The complex functional and cellular structure of the immunological niche of duodenal mucosa in CD includes pro-inflammatory cytokines (such as, IFN-gamma, IL-12, IL-15, IL-23), anti-inflammatory cytokines (such as, TGF-beta, IL-10), gluten-specific naïve and memory T cells, Tregs, and Th17 cells.29
The concept of “niche” was first formulated by Schofield,30 in the 1970s, defining the “haematopoietic stem cell niche”, as a region within the bone marrow containing functional cell types that can maintain haematopoietic stem cell potency throughout life. Haematopoietic stem cell niche, such as immunological niche here examined, are specialized microenvironments composed by numerous cell types and a plethora of signals that dictate their behavior with respect to homeostatic requirements and exogenous stresses in a complex picture, with functional crosstalk between cells. The immunological niche constitutes a basic unit of tissue physiology, integrating signals that mediate the balanced cellular response to the needs of organisms.31
METHODS
Patients' recruitment
Fourteen consecutive patients (11 females and 3 males, age range: 21–51 years, mean age 41.14 years) with diagnosis of CD (diagnosed in accordance with the latest international recommendation32) were recruited. This study has been approved by the Ethical Committee of Catholic University. All patients, were evaluated both before (Time 0 or T0) and after 12 months (T1) of GFD. They underwent upper gastrointestinal tract endoscopy and duodenal biopsies to evaluate histological and immunohistochemical findings and T-subpopulation analysis. All patients were analyzed for anti-endomysium antibodies (EMAs) and anti-tissue transglutaminase (anti-tTG) antibodies that are landmarks of serological diagnosis even if their pathogenetical role is not completely understood.33 We included in our study only untreated patients with total villous atrophy and with EMA and anti-tTG antibody positivity. Dietary compliance was established on dietary history and negative EMA and anti-tTG antibodies. After 12-month GFD (T1), CD patients underwent upper gastrointestinal tract endoscopy and duodenal biopsies to evaluate the histological and immunohistochemical findings and T-subpopulation analysis.
The control population consisted of 12 non-celiac subjects, age- and sex- matched (9 females, 3 males, age range 22–53 years, mean age 41.08 years), with no family history of CD or other autoimmune diseases, who were selected randomly from the same geographical area as the CD patients, who underwent a diagnostic esophageal-gastro-duodenoscopy resulting negative for celiac, neoplastic and inflammatory ulcerative disease.
Lymphocytes immunophenotyping from PB lymphocytes and from duodenal biopsy
At baseline, eligible patients and controls were submitted to a venous puncture of 5 cc of PB in lithium heparin tube. In CD patients, two samples at T0 and T1 were obtained.
Peripheral blood mononuclear cells (PBMC) were separated according to standard procedures.34 Tissue samples obtained from the duodenal mucosa were incubated in 24 multiwell Falcon dishes within 3 h from collection. Incubation took place at 37 °C in complete RPMI 1640 supplemented with gentamicin 50 μg/ml and 10% fetal calf serum. Human recombinant IL-2 (100 UI/ml) was added to allow lymphocyte growth, as previously reported.35 Cells were observed with an inverted microscope until a number sufficient to perform the immunophenotype was reached (usually after 48 h of culture). After washing, surface markers were studied by immunofluorescence. Tregs were identified by four-color immunofluorescence, performed on a FACSCalibur (Becton Dickinson, Franklin Lakes, NJ, USA) and characterized by the following phenotype: CD3 positive-fluorescein isothiocyanate, CD4-positive peridinin chlorophyll protein, CD25-positive phycoerythrin (PE), CD127-negative Alexa Fluor 647 (Becton Dickinson).16, 17, 18 For the detection of Foxp3 expression, PBMC were also analyzed by three-color intracellular flow cytometry using anti-CD4-PE-CY5 (Beckman Coulter, Miami, FL, USA), anti-CD25-fluorescein isothiocyanate (Beckman Coulter) and anti-Foxp3-PE-matched monoclonal antibody (mAb) (clone 236A/E7, eBioscience, San Diego, CA, USA).
Th17 cells were evaluated on fixed permeabilized cells by staining with an anti-IL-17A-PE mAb (Becton Dickinson). Their phenotype was identified as CD4-peridinin chlorophyll or CD8-fluorescein isothiocyanate according to positivity with three respective antibodies, flow cytometric evaluation was performed immediately on these cells. The percentage of positive cells was calculated by background staining assessed by isotype-matched fluorochrome-conjugated irrelevant mAb.
All stainings, both for surface molecules and the expression of intracytoplasmic cytokines and or transcription factors, have been performed on fresh samples by incubating the cells with monoclonal antibodies variously combined; for the intracytoplasmic staining, the surface marking was followed by membrane fixation and permeabilization using Cytofix-Cytoperm kit (BD Bioscience, Franklin Lakes, NJ, USA).
For the detection of T-bet expression, PBMCs were analyzed using a double-labeling procedure staining with an anti-CD4-PE-Cy5, anti-CD8-PE-Cy5, and anti-CD19-PE-Cy5 (Beckman Coulter). After fixation, cells were permeabilized using a commercially available perm/wash kit (BD Biosciences/Pharmingen, Franklin Lakes, NJ, USA). Upon permeabilization, 5 × 105 PBMCs and 2 × 105 tissue lymphocytes were re-suspended in 100 μl of PBS and incubated for 30 min with anti-pSTAT1(A-2)-PE antibody, anti-pSTAT3(B-7)-PE antibody, and anti-T-bet(4B10)-PE antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA). Appropriate fluorochrome-conjugated isotype-mAb (Beckman Coulter) was used as control for background staining in each flow acquisition.
Each analysis was performed using at least 10,000 cells that were gated in the region of the lymphocyte–monocyte population, as determined by light-scatter properties (forward scatter vs. side scatter). To analyze the expression of transcription factors in lymphocytes (CD4+, CD8+ T cells and CD19+ B cells), cells were gated in both the lymphocyte and CD4+/CD8+/CD19+ regions.
Quadrants of dot plot were set using appropriate isotype controls for each intra- and extra-cellular antibody. Appropriate fluorochrome-conjugated isotype-matched mAbs (Beckman Coulter) were used as control for background staining in each flow acquisition. In these assays, careful color compensation was performed before cell analysis. Mean fluorescence intensity (MFI) was calculated only for positive events after subtraction of specific isotype control MFI.
Immunohistochemical analysis for Foxp3 and IL-17
Immunohistochemical analysis for Foxp3 and IL-17 was performed on 3 mm tissues slides using the antihuman mouse monoclonal Foxp3 antibody (eBioscience; dilution 1/250) and antihuman rabbit polyclonal antibody IL-17 (H132) (Santa Cruz Biotechnology, clone 236A/E7, dilution 1/100), after a step of antigen retrieval in microwave oven for 10 min in citrate buffer (0.01 ℳ; pH 6), at 750 W. Hydrogen peroxide, serum-biotinylated immunoglobulins, and avidin–biotin complexes were used according to the manufacturer's instructions (Dako LSAB; Dakopatts, Golstrup, Denmark). Slides were then incubated overnight at 4 °C (1.5 mg/ml concentration of primary antibody). After induction of the color reaction with freshly made diaminobenzidine solution (Dakopatts), slides were counterstained with hematoxylin. The FoxP3 showed a nuclear and perinuclear staining, whereas IL-17 showed a cytoplasmatic staining.
Statistical analysis
Differences between groups were compared using Student's t-tests. Differences were considered as statistically significant for P<0.05.
RESULTS
Lymphocytes in the PB
Percentages of peripheral lymphocytes in CD patients and controls pre- and post-diet are listed in Table 1. At baseline, the percentage of Tregs (evaluated on the basis of a four-color staining CD3+/CD4+/CD25+/CD127−) was higher in untreated CD patients as compared with both in GFD CD patients and healthy subjects.
Table 1. Expression of markers in peripheral lymphocytes in celiac disease patients and controls.
|
Celiac disease patients |
|||
|---|---|---|---|
| Peripheral blood | Controls | T0 (pre-diet) | T1 (after-diet) |
| Tregs | 6.19 (1.80–9.78) | 7.27 (3.60–11.65) | 5.66 (2.84–9.93) |
| Th17 cells | 0.39 (0.03–1.40) | 1.05* (0.08–1.80) | 0.14# (0.04–0.34) |
| CD4+/IL17A+ | 0.24 (0.00–1.60) | 0.23 (0.03–1.01) | 0.07 (0.00–0.15) |
| CD8+/IL17A+ | 0.28 (0.00–1.11) | 0.67 (0.08–2.23) | 0.04# (0.00–0.10) |
| CD103+ | 1.13 (0.33–3.94) | 1.14 (0.26–2.80) | 0.55 (0.18–1.58) |
| CD4+/CD103+ | 0.38 (0.10–1.10) | 0.79 (0.08–4.89) | 0.26 (0.09–0.75) |
| CD8+/CD103+ | 0.46 (0.08–2.14) | 0.55 (0.13–1.73) | 0.17 (0.04–0.34) |
| CD62L+ | 54.34 (22.11–81.00) | 50.16 (8.35–81.70) | 59.25 (16.07–86.90) |
| CD4+/CD62L+ | 20.66 (6.44–44.13) | 22.66 (2.35–53.70) | 35.28 (4.08–53.70) |
| CD8+/CD62L+ | 11.16 (0.34–24.42) | 13.34 (2.57–22.80) | 10.62 (4.37–18.37) |
| CD19+ | 1.71 (0.10–3.71) | 2.45 (0.01–5.33) | 3.38 (0.39–8.24) |
| CD4+/T-bet+ | 2.81 (0.26–6.29) | 7.64*# (1.29–14.02) | 0.98 (0.06–1.98) |
| CD8+/T-bet+ | 1.41 (1.06–10.37) | 13.87*# (1.57–22.83) | 3.95 (0.98–9.03) |
| CD19+/T-bet+ | 4.42 (2.77–6.71) | 7.81*# (3.68–16.10) | 4.05 (3.90–7.21) |
GFD, gluten-free diet; Treg, T-regulatory.
Results are expressed as a percentage of positive cells (range in parentheses).
*P<0.05 pre-GFD vs. controls levels; #P<0.05 post-GFD vs. pre-GFD levels. Calculated by Student's t-test.
Th17 cells were significantly increased in CD patients at T0 and decreased at T1 (P=0.00 01), cells with the most significant reduction being observed in the subset of CD8+/Th17+ cells (P=0.004).
In patients after GFD, CD103+ T cells, double-positive CD4+/CD103+ and CD8+/CD103+ decreased compared with active CD, showing values inferior even to controls.
CD62L+ T cells and double-positive CD8+/CD62L+ T cells did not significantly change after treatment. On the other side, CD4+/CD62L+ increased after GFD with respect to controls and pre-diet (P=0.07).
T-bet expression in circulating T cells and B cells
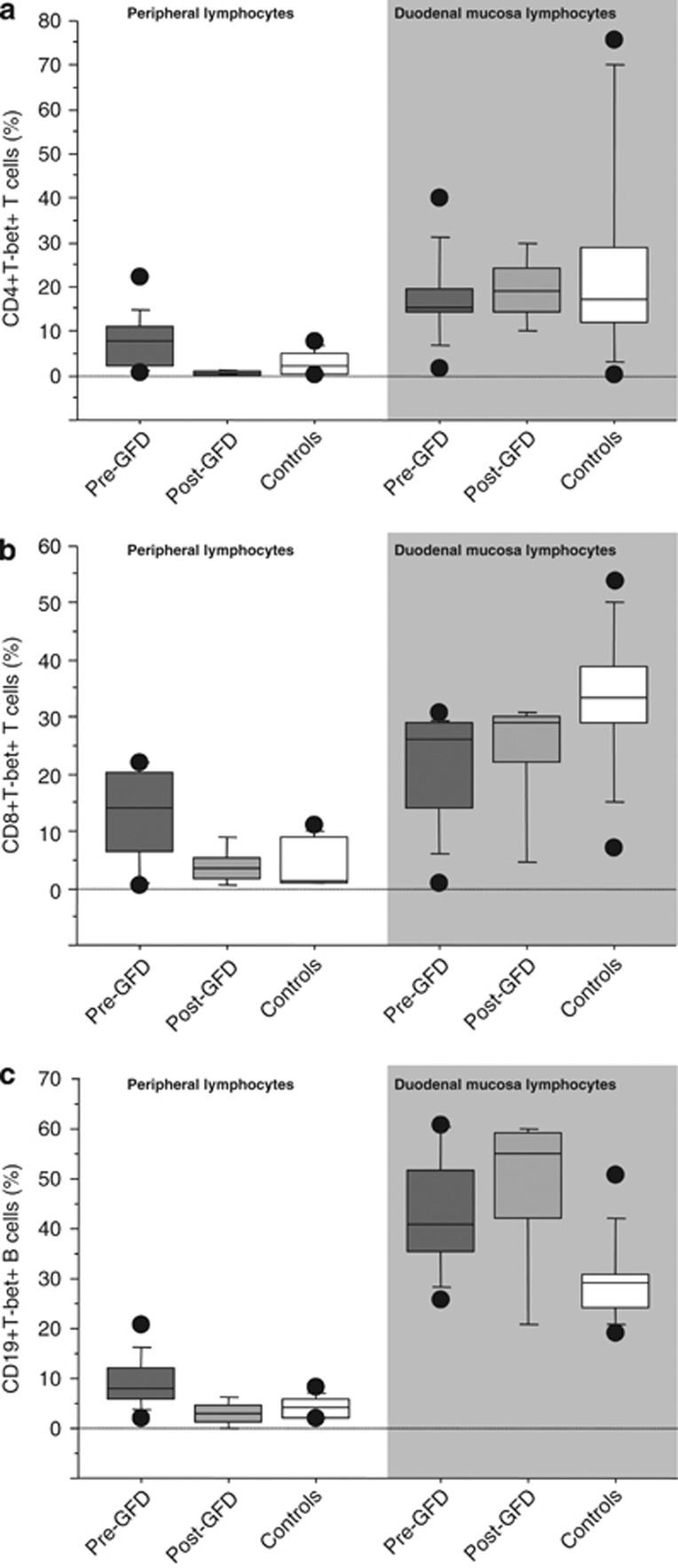
We observed higher percentages of circulating T-bet+ CD4+, CD8+ T cells and CD19+ B cells from untreated than from treated CD patients (P=0.0076, P=0.0090, and P=0.0052, respectively) and healthy subjects (P=0.0206, P=0.0023, and P=0.0055, respectively). No significant difference in the percentages of circulating T-bet+ CD4+, CD8+ T cells, and CD19+ B cells was observed between treated CD patients and controls. We didn't find any significant difference between the MFI of T-bet in the circulating CD4+, CD8+ T cells, and CD19+ B cells among treated and untreated CD patients and controls (Figure 1).
Figure 1.
T-bet expression in PBMC and TIL from circulating T cells and B cells. Mean percentage of CD4+ T-bet (a), CD8+ T-bet (b) T cells, and CD19+ T-bet B cells (c) in peripheral blood and in duodenal mucosa of healthy subjects and celiac disease. Box plots express the first (Q1) and third (Q3) quartiles within a given data set by the upper and lower horizontal lines in a rectangular box, in which there is a horizontal line showing the median. The whiskers extend upwards and downwards to the highest or lowest observation within the upper (Q3+1.5 × interquartile range) and lower (Q1–1.5 × interquartile range) limits. P-values indicate statistical significances (<0.05) between the different groups. GFD, gluten-free diet; MFI, mean fluorescence intensity.
Lymphocytes in tissue-infiltrating lymphocytes (TILs) of duodenal mucosa
Percentages of lymphocytes subpopulations in duodenal mucosa of CD patients and in normal duodenal mucosa are reported in Table 2. At baseline, Tregs (evaluated on the basis of a four-color staining CD3+/CD4+/CD25+/CD127−) were significantly higher than controls (P=0.0009). After diet, the levels of Tregs were significantly lower than pre-diet (P=0.0004), showing values similar to control group. Total Th17 cells were significantly decreased after diet vs. pre-diet (P=0.02). The decreased values are the result of a more marked reduction of CD8+/Th17 (P=0.03).
Table 2. Expression of markers in duodenal mucosa lymphocytes in celiac disease patients and controls.
|
Celiac disease patients |
|||
|---|---|---|---|
| Duodenal mucosa | Controls | T0 (pre-diet) | T1 (after-diet) |
| Tregs | 5.57 (3.00–6.91) | 16.43* (10.43–21.70) | 4.60# (1.83–11.30) |
| Th17 cells | 1.56 (0.82–2.18) | 2.00 (0.90–3.77) | 1.09# (0.10–2.34) |
| CD4+/IL17A+ | 0.96 (0.14–2.06) | 0.75 (0.00–2.00) | 0.82 (0.00–3.62) |
| CD8+/IL17A+ | 1.70 (0.17–5.10) | 1.12 (0.08–2.75) | 0.50# (0.01–1.20) |
| CD103+ | 55.73 (29.17–76.00) | 43.72 (9.75–97.0) | 42.04 (14.80–85.00) |
| CD4+/CD103+ | 25.20 (4.64–66.00) | 8.99* (0.71–26.63) | §7.02 (1.90–17.35) |
| CD8+/CD103+ | 36.46 (12.40–69.53) | 21.50* (3.07–47.80) | 24.60 (0.84–57.29) |
| CD62L+ | 25.85 (1.30–74.89) | 11.30 (0.93–26.15) | §5.90# (0.25–24.20) |
| CD4+/CD62L+ | 3.20 (0.40–6.87) | 4.70 (0.39–15.39) | 1.63# (0.18–7.78) |
| CD8+/CD62L+ | 6.61 (0.85–25.57) | 4.74 (0.45–9.13) | 3.02 (0.16–11.60) |
| CD19+ | 2.22 (0.10–2.82) | 1.61 (0.99–2.70) | 2.59 (0.20–8.08) |
| CD4+/T-bet | 17.85 (3.78–71.49) | 14.52 (7.18–32.10) | 18.65 (10.8–30.51) |
| CD8+/T-bet | 34.81 (25.63–50.73) | 26.74* (6.42–29.05) | 29.01 (4.89–30.15) |
| CD19+/T-bet | 29.85 (20.93–43.32) | 40.52* (27.80–60.25) | 54.16§ (20.26–59.89) |
GFD, gluten-free diet; Treg, T-regulatory.
Results are expressed as a percentage of positive cells (range in parentheses).
*P<0.05 pre-GFD vs. controls levels; #P<0.05 post-GFD vs. pre-GFD levels; §P<0.05 post-GFD vs. controls levels. Calculated by Student's t-test.
CD103+ in CD patients (pre- and post-diet) and controls were not statistically different. In CD patients pre and post diet, there was a decrease in CD4+/CD103+ cells (P=0.03 and 0.01, respectively) with respect to controls; the CD8+/CD103+ cells were significantly reduced in active CD patients vs. controls (P=0.04). In conclusion, in active CD, CD103+ are significantly reduced in the tissue vs. the controls. In addition, they are still reduced after GFD. However, this datum is only significant for CD4+/CD103+.
CD62L+ cells were significantly reduced in CD patients after GFD with respect to controls and active CD patients P=0.02 and 0.007, respectively), with particular regard to CD4+/CD62L+ subpopulations in which there is a significantly reduction post diet vs. active patients (P=0.03).
T-bet expression in TILs of duodenal mucosa
Among TILs, there was no significant difference between the percentage of T-bet+ CD4+, CD8+, and CD19+ between treated and untreated CD patients. Untreated CD patients showed increased percentages of T-bet+ CD8+ and CD19+ TILs then controls (P=0.0133 and P=0.0066, respectively), but no significant difference was observed between the percentages of T-bet+ CD4+ TILs. The percentages of T-bet+ CD19+ TILs were higher in treated CD patients than in controls (P=0.0051). We found no significant difference between the percentages of T-bet+ CD4+ and CD8+ TILs from untreated than from treated CD patients (Figure 1).
We observed no significant difference between the MFI of T-bet in the CD4+, CD8+, and CD19+ TILs among treated and untreated CD patients and controls.
DISCUSSION
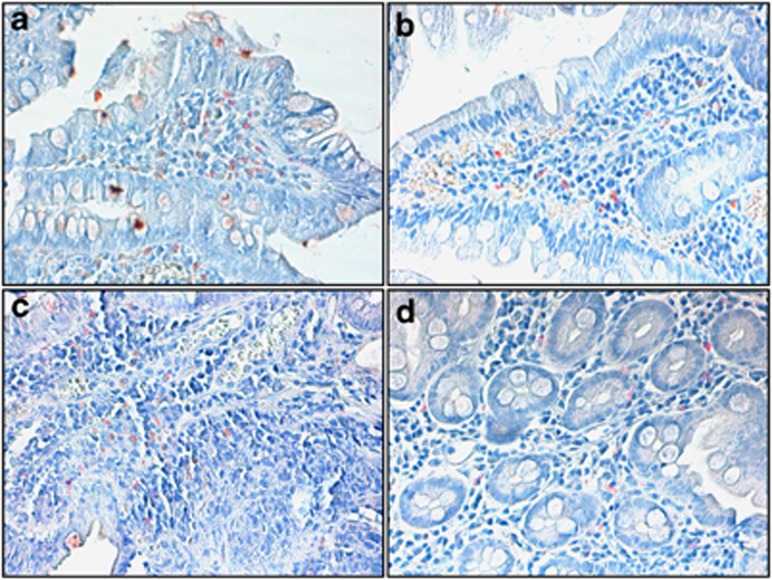
In this report, we show that the combination of several immunological techniques allows a better understanding of complex immunological modifications present in CD. Our analysis included lymphocyte characterization both in the periphery and in mucosa-derived TILs, by surface markers and intracytoplasmic staining (IL-17, FoxP3, T-bet). Data were also compared in a few number of cases with immunohistochemestry (Figure 2). To our knowledge, this is the first time that such a vast approach is used to characterize CD patients at diagnosis and after 12 months GFD.
Figure 2.
Immunohistochemistry for FoxP3 and IL-17 of gut mucosa in CD patients. Immunohistochemistry for FoxP3 and IL-17 proteins in a representative case of celiac disease (a for FoxP3 and c for IL-17) and in normal mucosa (b for FoxP3 and d for IL-17) (Avidin–Biotin–Peroxidase complex method in paraffin section lightly counterstained with ematoxylin. Original magnification, × 100 (a,b and d); × 200 (c).
Our data show significant modifications of several T-cell subsets both in the PB and in TILs in active CD as compared with both normal controls and CD patients after GFD, confirming and expanding with a more detailed analysis previous data showing that high levels of inflammatory activity are reduced after GFD.
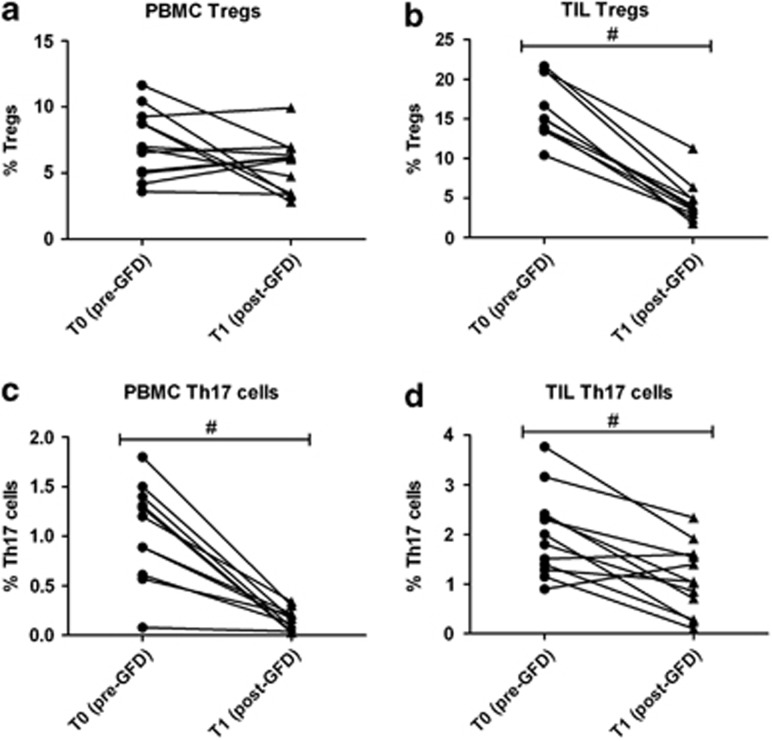
Tregs were significantly increased in active CD TILs. Th17 cells were also significantly increased both PB and TILs. Notably both subpopulations returned to normal values after 12 months GFD (Figure 3).
Figure 3.
Tregs and Th17 expression in PBMC and TIL from CD patients before and after GFD. PMBC Tregs (CD3+/CD4+/CD25+/CD127−) (a) are augmented in CD patients and decrease after GFD, returning to similar value of control patients. TIL Tregs (b) are significantly augmented in CD patients and significantly decrease after GFD. PMBC Th17 cells (c) are significantly augmented in CD patients and significantly decrease after GFD. TIL Th17 cells (d) are augmented in CD patients and significantly decrease after GFD. #P<0.05 post-GFD vs. pre-GFD levels. GFD, gluten-free diet; PBMC, peripheral blood mononuclear cell; TIL, tissue-infiltrating lymphocytes; Treg, T-regulatory.
Our data showing high levels of Tregs in TILs of active CD with high inflammation are intriguing on the light that Tregs are usually considered anti-inflammatory cells. On the basis of their common function, high levels of Tregs would be easier to understand in an immunopathological micro-environment with low levels of inflammation. However, in CD, we experience the opposite. We therefore hypothesize that naive T cells are recruited by gluten at the tissue level, where because of the tissue micro-environment (cytokines and interactions with other T-cell subsets) differentiate into Tregs.
The increased level of Tregs in duodenal mucosa of CD patients support our idea of an immunological niche, where naïve T cells are recruited by gluten trigger from PB home to local tissue and differentiate into Tregs in the presence of anti-inflammatory cytokine (TGF-beta, IL-10).29 However, their number although increased is insufficient to reduce the inflammatory damage. Thus, the increase of Tregs in active CD may suggest insufficient recruitment. Recent data have shown that IL-15 interferes with suppressor activity of Tregs, and this is probably related to enhanced expression of IL-15 receptor.22 In addition, an alternative explanation for lack of T-regulatory activity may be found in the data by Hmida et al.,24 who demonstrated that IL-15 could make human T cells resistant to Tregs suppression.
Th17 cells showed a similar modification trend as Tregs. In fact, both CD4+ and CD8+ Th17+ subpopulations decreased after GFD both in PB and at tissue level, confirming the presence of increased level of pro-inflammatory Th17 cells in active CD patients.
Th17 cells have a role in the pathogenesis of the disease. Th17 cells can produce pro-inflammatory cytokines (such as, IL-17, IFN-gamma and IL-21).21 However, Th17 cells also produce mucosa-protective IL-22 and TGF-beta, which actively modulates IL-17A production.26
The increase of pro-inflammatory Th17 cells and of T-bet cells supports the common idea that a major inflammation is present in active CD disease. Although Th17 cells are commonly observed after in vitro activation of T cells, it is worth noting that in active CD, Th17 cells were present among TILs, suggesting their presence in vivo. This is also supported by our immunochemistry data showing both Tregs and Th17 cells located to the LP adjacent to the submucosa. It is interesting to note that CD4+ T-bet+ (Th1), CD8+ T-bet+ (Tc1) e CD19+ T-bet+ (Be1) show a similar pattern to Th17 cells (i.e., increased in active disease and reduced after GFD).
Only anecdotal reports are available on CD103+ cell in the mucosa of CD patients. Kolkowsky et al.36 showed an expansion of CD8+ CD103+ clones with reduced production of IL-10. However, these conclusions were based on clonal analysis from only two CD biopsies. Others have reported the presence of abnormal CD103+ CD7+ cells lacking membrane expression of the TCR.37 We showed that CD103+ cells were slightly reduced in PB of GFD CD patients vs. active CD patients. This suggests the systemic recruitment of growing numbers of T cells to support ongoing inflammation in active CD intestine-homing cells, a pattern similar to that observed by us in a different inflammatory bowel disease.34 Among TILs, CD103+ cells in CD patients pre- and post-diet and controls were similar, as expected in cells homing to the gut. In CD patients, CD4+/CD103+ cells were decreased (P=0.03 and 0.01, respectively) with respect to controls. The CD8+/CD103+ cells were significantly reduced in CD patients pre-diet vs controls (P=0.04).
On the basis of available information, several hypotheses can be formulated to explain the reduction of CD103 cells in active CD reported by us in 14 patients. This reduction may be related to ongoing apoptosis or cell damage associated to villous damage. Alternatively, it could be the result of decreased TGF-beta activity. In fact, CD103 has been described as both a homing receptor and an activation marker.38 CD103 expression is increased by TGF-beta. In CD, TGF-beta production is defective39 and its activity is inhibited by IL-15.40
Integrins alpha4-beta7 (LPAM-1) and alphaE-beta7 (CD103) are adhesion molecules generally considered responsible for intestinal homing.41, 42, 43 LPAM-1 binds to the mucosa-addressing cell adhesion molecule (MadCaM-1), whereas CD103 binds to cadherin-E widely expressed on epithelial cells. A crucial role for both integrins in gut homing has been suggested by several studies and in knockout mice. In fact, both beta7 −/− and alphaE −/− mice show severe impairment of gut-associated lymphoid tissue, including lamina propria and intraepithelial lymphocytes.44 Gut-homing CD103+ (and/or LPAM-1) cells have an essential role in the regulation of experimental colitis and are involved in several diseases resulting in colonic inflammation. These data suggest that CD103 cells are involved in the pathogenesis of T-cell-mediated inflammatory conditions of the gastrointestinal tract. CD8+/CD103+ intraepithelial lymphocytes are activated in inflammatory bowel diseases, and may exert a regulatory function.45
Naive cells identified by CD62L were significantly reduced at tissue level after diet. We suggest that GFD contributes to increase memory T-cell subsets, as the percentages of naive T cells (CD62L+) decreased. Further data on the characterization of memory cells might allow the identification of these cells as part of the effector memory pool ready to react against non-self antigenic stimuli. Once the gluten is removed, naive CD62L cells are reduced possibly as a result of decreased recruitment.
In a very short summary, there is a gluten-related systemic recruitment of T cells at duodenal site. After 12 months GFD, these T-cell subpopulations are deeply modified.
Our data therefore confirm the importance of studying the many actors playing their roles in the duodenal immunological niche to understand the complex immunopathogenesis of CD: the close relationship between T cells and their related pattern of secreted cytokines in duodenal micro-environment may have important role in the diagnostic, prognostic, and therapeutical approach of CD.
Study Highlights
Guarantor of the article: Franco Pandolfi, MD.
Specific author contribution: Rossella Cianci ideated the experiment, coordinated the project, analyzed samples, analyzed data, and wrote and corrected the paper. Giovanni Cammarota contributed to the ideation of the experiment, recluted patients, performed gastroscopies, collected histological samples, provided financial support, and corrected the article. Giovanni Frisullo contributed to the ideation of the experiment, analyzed samples, analyzed data, and wrote and corrected part of the paper. Danilo Pagliari contributed to the ideation of the experiment, analyzed samples, analyzed data, and wrote and corrected the paper. Gianluca Ianiro collected clinical data and histological samples. Maurizio Martini performed Immunohistochemistry and participated to the writing of the article. Domenico Plantone participated to the analysis of samples and results. Valentina Damato participated to the analysis of samples and results. Fabio Casciano participated to the analysis of some samples. Raffaele Landolfi participated to the discussion of results, to the writing and correcting the article. Anna Paola Batocchi participated to the discussion of results, to the writing and correcting the article, and provided financial support. Franco Pandolfi contributed to the ideation and supervised the study, provided financial support, and wrote and corrected the paper. Simona Frosali analyzed several samples, contributed significantly to data analysis, wrote and corrected the paper.
Financial support: This work was supported in part by a Linea D1 grant from the Catholic University to F.P.
Potential competing interests: None.
References
- Sollid LM, Thorsby E. HLA susceptibility genes in celiac disease: genetic mapping and role in pathogenesis. Gastroenterology. 1993;105:910–922. doi: 10.1016/0016-5085(93)90912-v. [DOI] [PubMed] [Google Scholar]
- Green PH, Jabri B. Coeliac disease. Lancet. 2003;362:383–391. doi: 10.1016/S0140-6736(03)14027-5. [DOI] [PubMed] [Google Scholar]
- Giambra V, Cianci R, Lolli S, et al. Allele *1 of HS1.2 enhancer associates with selective IgA deficiency and IgM concentration. J Immunol. 2009;183:8280–8285. doi: 10.4049/jimmunol.0902426. [DOI] [PubMed] [Google Scholar]
- Green PH, Cellier C. Celiac disease. N Engl J Med. 2007;357:1731–1743. doi: 10.1056/NEJMra071600. [DOI] [PubMed] [Google Scholar]
- Ludvigsson JF, Green PH. Clinical management of coeliac disease. J Intern Med. 2011;269:560–571. doi: 10.1111/j.1365-2796.2011.02379.x. [DOI] [PubMed] [Google Scholar]
- Ciccocioppo R, Finamore A, Mengheri E, et al. Isolation and characterization of circulating tissue transglutaminase-specific T cells in coeliac disease. Int J Immunopathol Pharmacol. 2010;23:179–191. doi: 10.1177/039463201002300116. [DOI] [PubMed] [Google Scholar]
- Olaussen RW, Johansen FE, Lundin KE, et al. Interferon-gamma-secreting T cells localize to the epithelium in coeliac disease. Scand J Immunol. 2002;56:652–664. doi: 10.1046/j.1365-3083.2002.01195.x. [DOI] [PubMed] [Google Scholar]
- Ciccocioppo R, D'Alo S, Di Sabatino A, et al. Mechanisms of villous atrophy in autoimmune enteropathy and coeliac disease. Clin Exp Immunol. 2002;128:88–93. doi: 10.1046/j.1365-2249.2002.01795.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovannetti A, Pierdominici M, Di Iorio A, et al. Apoptosis in the homeostasis of the immune system and in human immune mediated diseases. Curr Pharm Des. 2008;14:253–268. doi: 10.2174/138161208783413310. [DOI] [PubMed] [Google Scholar]
- Sollid LM. Coeliac disease: dissecting a complex inflammatory disorder. Nat Rev Immunol. 2002;2:647–655. doi: 10.1038/nri885. [DOI] [PubMed] [Google Scholar]
- Jabri B, Sollid LM. Tissue-mediated control of immunopathology in coeliac disease. Nat Rev Immunol. 2009;9:858–870. doi: 10.1038/nri2670. [DOI] [PubMed] [Google Scholar]
- Frisullo G, Nociti V, Iorio R, et al. T-bet and pSTAT-1 expression in PBMC from coeliac disease patients: new markers of disease activity. Clin Exp Immunol. 2009;158:106–114. doi: 10.1111/j.1365-2249.2009.03999.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsen EM, Lundin KE, Krajci P, et al. Gluten specific, HLA-DQ restricted T cells from coeliac mucosa produce cytokines with Th1 or Th0 profile dominated by interferon gamma. Gut. 1995;37:766–776. doi: 10.1136/gut.37.6.766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandolfi F, Corte G, Quinti I, et al. Defect of T helper lymphocytes, as identified by the 5/9 monoclonal antibody, in patients with common variable hypogammaglobulinaemia. Clin Exp Immunol. 1983;51:470–474. [PMC free article] [PubMed] [Google Scholar]
- Hishii M, Kurnick JT, Ramirez-Montagut T, et al. Studies of the mechanism of cytolysis by tumour-infiltrating lymphocytes. Clin Exp Immunol. 1999;116:388–394. doi: 10.1046/j.1365-2249.1999.00879.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandolfi F, Cianci R, Pagliari D, et al. Cellular mediators of inflammation: tregs and TH17 cells in gastrointestinal diseases. Mediators Inflamm. 2009;2009:132028. doi: 10.1155/2009/132028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W, Putnam AL, Xu-Yu Z, et al. CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ T reg cells. J Exp Med. 2006;203:1701–1711. doi: 10.1084/jem.20060772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaguchi S, Miyara M, Costantino CM, et al. FOXP3+ regulatory T cells in the human immune system. Nat Rev Immunol. 2010;10:490–500. doi: 10.1038/nri2785. [DOI] [PubMed] [Google Scholar]
- Brazowski E, Cohen S, Yaron A, et al. FOXP3 expression in duodenal mucosa in pediatric patients with celiac disease. Pathobiology. 2010;77:328–334. doi: 10.1159/000322049. [DOI] [PubMed] [Google Scholar]
- Delabie J, Holte H, Vose JM, et al. Enteropathy-associated T-cell lymphoma: clinical and histological findings from the international peripheral T-cell lymphoma project. Blood. 2011;118:148–155. doi: 10.1182/blood-2011-02-335216. [DOI] [PubMed] [Google Scholar]
- Monteleone I, Sarra M, Del Vecchio Blanco G, et al. Characterization of IL-17A-producing cells in celiac disease mucosa. J Immunol. 2010;184:2211–2218. doi: 10.4049/jimmunol.0901919. [DOI] [PubMed] [Google Scholar]
- Zanzi D, Stefanile R, Santagata S, et al. IL-15 interferes with suppressive activity of intestinal regulatory T cells expanded in Celiac disease. Am J Gastroenterol. 2011;106:1308–1317. doi: 10.1038/ajg.2011.80. [DOI] [PubMed] [Google Scholar]
- Frisullo G, Nociti V, Iorio R, et al. Increased CD4+CD25+Foxp3+ T cells in peripheral blood of celiac disease patients: correlation with dietary treatment. Hum Immunol. 2009;70:430–435. doi: 10.1016/j.humimm.2009.04.006. [DOI] [PubMed] [Google Scholar]
- Hmida NB, Ben Ahmed M, Moussa A, et al. Impaired control of effector T cells by regulatory T cells: a clue to loss of oral tolerance and autoimmunity in celiac disease. Am J Gastroenterol. 2012;107:604–611. doi: 10.1038/ajg.2011.397. [DOI] [PubMed] [Google Scholar]
- Castellanos-Rubio A, Santin I, Irastorza I, et al. TH17 (and TH1) signatures of intestinal biopsies of CD patients in response to gliadin. Autoimmunity. 2009;42:69–73. doi: 10.1080/08916930802350789. [DOI] [PubMed] [Google Scholar]
- Fernandez S, Molina IJ, Romero P, et al. Characterization of gliadin-specific Th17 cells from the mucosa of celiac disease patients. Am J Gastroenterol. 2011;106:528–538. doi: 10.1038/ajg.2010.465. [DOI] [PubMed] [Google Scholar]
- Sapone A, Lammers KM, Mazzarella G, et al. Differential mucosal IL-17 expression in two gliadin-induced disorders: gluten sensitivity and the autoimmune enteropathy celiac disease. Int Arch Allergy Immunol. 2010;152:75–80. doi: 10.1159/000260087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kivling A, Nilsson L, Falth-Magnusson K, et al. Diverse foxp3 expression in children with type 1 diabetes and celiac disease. Ann N Y Acad Sci. 2008;1150:273–277. doi: 10.1196/annals.1447.018. [DOI] [PubMed] [Google Scholar]
- Cianci R, Pagliari D, Landolfi R, et al. New Insights on the role of T-cells in the pathogenesis of Celiac disease. J Biol Regul Homeost Agents. 2012;26:171–179. [PubMed] [Google Scholar]
- Schofield R. The relationship between the spleen colony-forming cell and the haemopoietic stem cell. Blood Cells. 1978;4:7–25. [PubMed] [Google Scholar]
- Scadden DT. The stem-cell niche as an entity of action. Nature. 2006;441:1075–1079. doi: 10.1038/nature04957. [DOI] [PubMed] [Google Scholar]
- Rostom A, Murray JA, Kagnoff MF. American Gastroenterological Association (AGA) Institute technical review on the diagnosis and management of celiac disease. Gastroenterology. 2006;131:1981–2002. doi: 10.1053/j.gastro.2006.10.004. [DOI] [PubMed] [Google Scholar]
- Cianci R, Cammarota G, Lolli S, et al. Abnormal synthesis of IgA in coeliac disease and related disorders. J Biol Regul Homeost Agents. 2008;22:99–104. [PubMed] [Google Scholar]
- Cianci R, Iacopini F, Petruzziello L, et al. Involvement of central immunity in uncomplicated diverticular disease. Scand J Gastroenterol. 2009;44:108–115. doi: 10.1080/00365520802321204. [DOI] [PubMed] [Google Scholar]
- Pandolfi F, Boyle LA, Trentin L, et al. Expression of HLA-A2 antigen in human melanoma cell lines and its role in T-cell recognition. Cancer Res. 1991;51:3164–3170. [PubMed] [Google Scholar]
- Kolkowski EC, Fernandez MA, Pujol-Borrell R, et al. Human intestinal alphabeta IEL clones in celiac disease show reduced IL-10 synthesis and enhanced IL-2 production. Cell Immunol. 2006;244:1–9. doi: 10.1016/j.cellimm.2007.01.006. [DOI] [PubMed] [Google Scholar]
- Sanchez-Munoz LB, Santon A, Cano A, et al. Flow cytometric analysis of intestinal intraepithelial lymphocytes in the diagnosis of refractory celiac sprue. Eur J Gastroenterol Hepatol. 2008;20:478–487. doi: 10.1097/MEG.0b013e3282f16a4b. [DOI] [PubMed] [Google Scholar]
- Woodberry T, Suscovich TJ, Henry LM, et al. Alpha E beta 7 (CD103) expression identifies a highly active, tonsil-resident effector-memory CTL population. J Immunol. 2005;175:4355–4362. doi: 10.4049/jimmunol.175.7.4355. [DOI] [PubMed] [Google Scholar]
- Lionetti P, Pazzaglia A, Moriondo M, et al. Differing patterns of transforming growth factor-beta expression in normal intestinal mucosa and in active celiac disease. J Pediatr Gastroenterol Nutr. 1999;29:308–313. doi: 10.1097/00005176-199909000-00013. [DOI] [PubMed] [Google Scholar]
- Benahmed M, Meresse B, Arnulf B, et al. Inhibition of TGF-beta signaling by IL-15: a new role for IL-15 in the loss of immune homeostasis in celiac disease. Gastroenterology. 2007;132:994–1008. doi: 10.1053/j.gastro.2006.12.025. [DOI] [PubMed] [Google Scholar]
- Cianci R, Pagliari D, Pietroni V, et al. Tissue infiltrating lymphocytes: the role of cytokines in their growth and differentiation. J Biol Regul Homeost Agents. 2010;24:239–249. [PubMed] [Google Scholar]
- Hu MC, Crowe DT, Weissman IL, et al. Cloning and expression of mouse integrin beta p(beta 7): a functional role in Peyer's patch-specific lymphocyte homing. Proc Natl Acad Sci USA. 1992;89:8254–8258. doi: 10.1073/pnas.89.17.8254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner N, Lohler J, Kunkel EJ, et al. Critical role for beta7 integrins in formation of the gut-associated lymphoid tissue. Nature. 1996;382:366–370. doi: 10.1038/382366a0. [DOI] [PubMed] [Google Scholar]
- Schon MP, Arya A, Murphy EA, et al. Mucosal T lymphocyte numbers are selectively reduced in integrin alpha E (CD103)-deficient mice. J Immunol. 1999;162:6641–6649. [PubMed] [Google Scholar]
- Allez M, Brimnes J, Dotan I, et al. Expansion of CD8+ T cells with regulatory function after interaction with intestinal epithelial cells. Gastroenterology. 2002;123:1516–1526. doi: 10.1053/gast.2002.36588. [DOI] [PubMed] [Google Scholar]