Abstract
Acute compartment syndrome (ACS) is a surgical emergency. Diagnosis depends on a high clinical suspicion and an understanding of risk factors, pathophysiology and subtle physical exam findings. The typical high risk scenario for ACS is a male patient younger than 35 years of age, involved in a high energy sport or roadway collision, resulting in a tibial shaft fracture. He will go on to develop acute compartment syndrome of the leg in less than 10 hours and require emergent fasciotomy. Diagnosis of ACS in this patient is primarily a clinical one but can be confirmed with invasive intracompartmental pressure monitoring or non-invasive near infrared spectroscopy (NIRS). Delaying the diagnosis will likely result in some degree of permanent disability and places the surgeon at high risk for litigation. This article reviews the salient features of acute compartment syndrome that should be understood by all orthopaedic residents and surgeons.
Keywords: Compartment syndrome, Acute compartment syndrome, Pediatric compartment syndrome, Missed compartment syndrome, Intracompartmental pressure monitoring, Muscle death, Pathophysiology, Lower extremity fasciotomy, Upper extremity fasciotomy, Fasciotomy, Tibia fracture, Lower extremity trauma, Upper extremity trauma, Pressure, Intracompartmental pressure, Stryker needle, Slit catheter, Medicolegal, Wound closure, Negative pressure wound therapy, Near infrared spectroscopy, Trauma
Introduction
Evolving acute compartment syndrome (ACS) is a condition most orthopaedic surgeons will encounter during their career. The diagnosis of compartment syndrome is often based on subtle changes in symptoms and vague clinical exam findings. If left untreated, compartment syndrome can be a devastating injury resulting in loss of function and potentially loss of limb. The orthopaedic surgeon must have an understanding of high-risk patients and injuries, pathophysiology, natural history, and surgical management. While there are several facets to the care of the patient with an evolving compartment syndrome, by reviewing the most common aspects of this injury and focusing on detection and treatment, we hope to prevent future complications that may arise. In addition, future directions of non-invasive intracompartmental pressure (ICP) monitoring will be offered, along with the medicolegal aspects of compartment syndrome and its impact on orthopaedic surgery. Pediatric patients can also develop ACS, but their presentation differs dramatically from that of adults.
Epidemiology
Acute compartment syndrome commonly develops in traumatized patients with distracting or neurologically inhibiting injuries. The physician must have a high degree of suspicion when treating these patients. Time to diagnosis is the most important prognostic factor for these patients. Insufficient understanding of the natural history and limited evaluation of signs and symptoms primarily account for delays in diagnosis. Several risk factors can aid in making the diagnosis. Age is a major risk factor for developing ACS. Patients younger than 35 years of age are more likely than older patients to develop ACS following the same type of injury [1]. ACS is most commonly seen in tibial shaft fractures, accounting for one third of all cases. Isolated soft tissue injuries account for a quarter of ACS cases and forearm fractures account for a fifth of them (Table 1). ACS is ten times more common in males. There is no difference in incidence of ACS in open compared to closed fractures. There are a multitude of less common causes of ACS including snake bites, nephrotic syndrome, IV infiltrations, and other volume expanding disorders [1–5].
Table 1.
Epidemiology of ACS in adults
| 91 % of cases are males – average age 32 years | |
| 9 % of cases are females – average age 44 years | |
| Tibial diaphyseal fracture | 36.0 % |
| Soft-tissue injury | 23.2 % |
| Distal radial fracture | 9.8 % |
| Crush syndrome | 7.9 % |
| Diaphyseal fracture of the radius/ulna | 7.9 % |
| Femoral fracture | 3.0 % |
| Tibial plateau fracture | 3.0 % |
Adapted from [1]
Pathophysiology
ACS occurs as a result of two factors occurring in isolation or simultaneously: an increase in the contents of an enclosed space (e.g. bleeding) and/or a decrease in the volume of a space (e.g. tight cast). Compartment syndrome occurs when the interstitial pressure within the compartment exceeds the perfusion pressure at the level of the capillary beds. Elevated intracompartmental pressure (ICP) leads to increased pressure at the venous end of the capillary beds causing increased hydrostatic pressure and further increase in ICP, eventually leading to arteriolar compression [6]. Loss of the perfusion pressure gradient results in the onset of ischemia and ultimately leads to cellular anoxia and death.
At the cellular level, diminishing ATP levels correlate closely with worsening muscle necrosis. In a canine study, after six hours of ischemia, only 20 % of pre-ischemic ATP remained which led to complete muscle necrosis [7]. Histologic analysis revealed central muscle necrosis with a surrounding zone of partial ischemia and peripheral tissue edema, often within areas of incomplete injury [8].
Lindsay et al showed that over time ischemia of muscle results in ATP breakdown. They further suggested that the degree of energy depletion during ischemia determines the extent of the ischemic damage [9].
Skeletal muscle is more sensitive to ischemia than other tissue types [10]. Red muscle fibers (e.g. anterior compartment of the leg), which rely predominantly on aerobic metabolism, are more vulnerable to ischemia, while white muscle fibers (e.g. gastroc-soleus complex), which rely predominantly on anaerobic metabolism, are less vulnerable to ischemia [11]. Ischemic duration and fiber type are important determinants of ischemic injury. The amount of muscle necrosis can also be influenced by available residual blood flow and temperature at which ischemia takes place. Increased collateral blood flow and decreased ischemic temperature lead to decreased amounts of muscle necrosis [12]. This translates to functional outcomes that are inversely related to the time to diagnosis and definitive treatment, as the result of progressive skeletal muscle death [13]. In summary, the degree of skeletal muscle injury correlates directly with the severity and duration of ischemia.
Clinical diagnosis
Compartment syndrome is, for the most part, a clinical diagnosis. It is a diagnosis that is made over time as the evolution of signs and symptoms are assessed, rather than a diagnosis made in isolation at a single time point. Serial examinations should always be performed, preferably by the same, experienced examiner.
The classic “P’s” described in compartment syndrome are pain, paresthesia, paralysis/paresis, pulselessness, and pallor [14]. Although all have a role in the diagnosis of compartment syndrome, the constellation of signs and symptoms and overall clinical picture are more important than the presence or absence of any particular finding (Fig. 1).
Fig. 1.
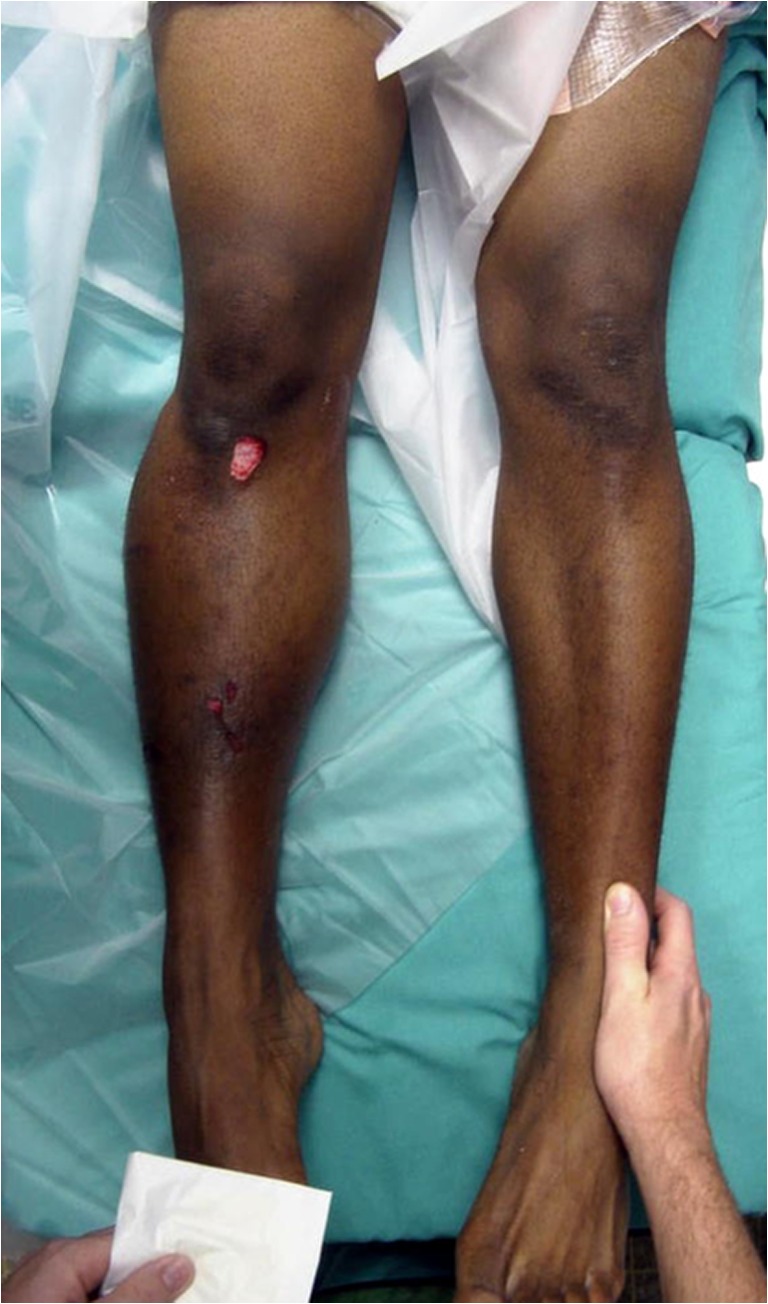
Clinical example of Acute Compartment Syndrome (Right Leg). Notice the markedly increased swelling compared to the contralateral side, as well as skin changes. This patient was the victim of a gunshot injury to the leg
Pain in ACS is described as out of proportion to the clinical examination or injury. Pain with passive stretch and rest pain are both usually present; however, pain can be absent in established or late-stage compartment syndrome. In addition, pain can be absent in the setting of central or peripheral nerve deficit or regional anesthesia [15].
Paresthesias are often the first indication of nerve ischemia. Altered sensation in the first dorsal web space of the foot could be the first indication of increased pressure in the anterior compartment related to ischemia of the deep peroneal nerve. If ischemia continues, paresthesia progresses to hypoesthesia and finally anesthesia.
Paresis or paralysis is often present in ACS but can be misleading as it could be the result of muscle ischemia, nerve ischemia, guarding secondary to pain, or a combination. True paralysis is a late finding indicative of prolonged nerve compression or irreversible muscle damage.
The loss of distal pulses with compartment syndrome is a late finding. It is rare for compartment pressure to be high enough to occlude arterial inflow and the absence of pulses could indicate an arterial injury. In addition, capillary refill is routinely present even in established ACS. Pallor is only present if arterial inflow is severely compromised or a vascular injury is present.
Increased firmness of compartment may be the only objective finding in developing compartment syndrome; however, Shuler et al revealed that manual detection of compartment pressure associated with critical elevations in ICP is poor, even by senior or experienced surgeons. [16••].
Overall, the absence of symptoms is more useful in excluding ACS, than the presence of symptoms is in to diagnosing ACS [2, 17]. (Table 2).
Table 2.
Diagnosis of Acute Compartment Syndrome Using the Ps (Pain, Paresthesia, Pain with Passive Stretch, and Paresis)
| Sensitivity | 13 %-19 % |
| Positive Predictive Value | 11 %-15 % |
| Specificity | 97 %-98 % |
| Negative Predictive Value | 97 %-98 % |
Adapted from [17]
Compartment pressure monitoring
ICP monitoring is a controversial component in evaluating the patient with suspected ACS. Normal resting ICP is around 8 mmHg in adults and slightly higher (13 to 16 mmHg) in children [2, 18]. A number of different techniques have been described for ICP monitoring including the slit catheter, the side portal needle (Stryker needle), and a regular 18-gauge needle with a setup similar to an arterial line. When compared, there was no significant difference between compartment pressures measured by slit catheters and side portal needles. There was, however, on average a 20 mmHg increase in measured compartment pressures when using an 18-gauge needle [19].
Compartment pressures also vary by location both within normal compartments and in relationship to an injury. Seiler et al demonstrated that there is significant intracompartmental variability within normal compartments [20] and Heckman et al showed that ICP measurements also show variability within an injured compartment. In fact, there is a relationship between ICP and distance from the fracture site. In closed tibial fractures, 89 % of compartments had the highest ICP measured at the fracture site, while 5 % had the highest pressure measured 5 cm distal to the fracture site, and 2 % had the highest pressure 5 cm proximal to the fracture site [21]. This data suggests that all pressure measurements should be performed within all compartments and at multiple sites within 5 cm distal and proximal to the injury.
Various compartment pressure thresholds have been described as an indication for decompressive fasciotomy. An absolute value of 45 mmHg was used by Matsen et al, while Mubarak et al used an absolute value of 30 mmHg[22, 23]. McQueen and Court-Brown reported a prospective clinical series with continuous compartment monitoring using the difference between the diastolic blood pressure (DBP) and measured ICP. If the difference between DBP and ICP was greater than or equal to 30 mmHg, a decompressive fasciotomy was not performed. All patients in this group had normal muscle function at follow up [24] (Table 3).
Table 3.
Intracompartmental pressure (in mmHg) as indicator for decompressive fasciotomy
Heckman et al developed a canine model of compartment syndrome in which irreversible histologic changes with infarction and fibrosis were seen in all compartments if tissue pressures came within 10 mmHg of DBP. No irreversible changes were seen if compartment pressures were greater than 30 mmHg from the mean arterial pressure (MAP) or greater than 20 mmHg from DBP. In fact, a mean compartment pressure of 59 mmHg was tolerated for 8 hours with no evidence of infarction. This lead to the conclusion that irreversible tissue damage from ischemia is directly related to the absolute difference between compartment pressure and blood pressure rather than a set ICP [25]. Matava et al similarly used a canine model and found the threshold for muscle necrosis to be 20 mmHg less than DBP [26].
Tissue that has been previously subjected to intervals of ischemia is especially sensitive to increased pressure. Bernot et al showed that tissue previously compromised by ischemia prior to elevated ICP has a lower threshold for metabolic deterioration and irreversible damage. Hypoxic metabolic changes occurred in postischemic limbs in all compartments when the difference between MAP and compartment pressure was less than 40 mmHg, while previously normally perfused limbs did not become ischemic until the difference was less than 30 mmHg [27]. In addition, anesthesia can lead to decreased DBP, which is often used to determine whether or not to proceed with decompressive fasciotomy.
Patients under general anesthesia or who are otherwise sedated or obtunded need to be monitored with extreme vigilance, as most early clinical predictors are unobtainable. Patients receiving postoperative epidurals for lower extremity surgery must be followed closely. Patients undergoing tibial fracture fixation who received postoperative epidurals are 4 times as likely to develop neurologic complications or missed compartment syndrome compared to those receiving only narcotics [28, 29]. In addition to analgesia, epidurals are known to increase local blood flow secondary to sympathetic blockade and can lead to increased swelling of an injured limb. Patient controlled analgesia (PCA) and regional anesthesia can also contribute to the masking of symptoms of a developing compartment syndrome [30].
In the awake and alert patient, compartment syndrome can be diagnosed based on clinical signs and symptoms with or without confirmatory intracompartmental pressure data. In the obtunded, sedated, or unresponsive patient, diagnosis relies on a high index of suspicion and a low threshold for compartment pressure monitoring.
Fasciotomy
ACS is an orthopaedic emergency definitively managed in the operating room. Upon diagnosis, the operating room and staff should be mobilized promptly. While preparations are in place for the patient to go to the operating theater, several non-operative techniques can be utilized to delay the onset of ischemia and preserve soft tissues. All restrictive dressings should be loosened and removed if possible. The extremity should be elevated to maximize venous return and minimize edema. Fracture reduction should be attempted to limit ongoing soft tissue damage.
The most common surgical approach to ACS of the leg is two-incision fasciotomy with anterolateral and posteromedial incisions. The anterolateral incision is made between the tibial crest and the fibula to access the anterior and lateral compartments. It extends from 5 cm inferior to the fibular head to 5 cm superior to the lateral malleolus. Both the skin and fascia should be released. The superficial peroneal nerve is at risk with this incision as it exits through the fascia approximately 10–12 cm proximal to the lateral malleolus. The posteromedial incision is made 2 cm posterior to the medial border of the tibia. The deep and superficial posterior compartments are released via this incision to access the superficial and deep posterior compartments. In order to adequately decompress the deep posterior compartment, the soleus insertion must be released. The saphenous nerve and vein are at risk with this incision as they travel in the subcutaneous tissue about the anteromedial aspect of the leg [2].
The single incision fasciotomy is less commonly used but appears as effective in experienced hands. Maheshwari et al, in their case series of 58 legs undergoing single incision fasciotomy, showed that all compartments, including the deep posterior compartment, can be reliably released through one incision with excellent outcomes. A longitudinal incision is made in line with the fibula along the posterolateral aspect of the leg from 5 cm inferior to and superior to the fibular head and lateral malleolus. Large, full thickness skin flaps are raised and the anterior, lateral and superficial posterior compartments are released through longitudinal incisions. The deep posterior compartment is released at the posterolateral fibular insertion of the lateral intermuscular septum and incised along its entire length. Entrance into the deep posterior compartment via this incision places the peroneal neurovascular structures at risk, as they course medial to the fibula. Care must be taken to incise the lateral intermuscular septum at its fibular insertion. Fibulectomy through a single lateral incision was once a common approach to 4-compartment fasciotomy of the leg. This technique has fallen out of favor as one and two incision fasciotomies have been shown to be effective and less morbid procedures [31].
The forearm is the second most common location of ACS in both adults and children and many techniques have been described for fasciotomy of the forearm. The forearm consists of 3 to 4 compartments; volar, dorsal, mobile wad of three, and (as recent case reports suggest) the pronator quadratus may be a separate compartment [3, 4, 32, 33]. In contrast to the leg, the forearm compartments are not completely independent from one another. As a result, not all compartments must be decompressed through their own fascial incisions, and intra-operative compartment measurements must be utilized [34]. The volar compartment is decompressed first and several incision patterns have been described in the literature. The median nerve and radial artery are very superficial structures;therefore, the distal aspect of the incision should stay ulnar, so that these structures are not left exposed in the wound. The volar incision should always be carried distally in order to release the carpal tunnel. After releasing the flexor digitorum superficialis, dissection should be carried down to the deep volar musculature such that the flexor digitorum profundus and pronator quadratus may be released. Once the volar compartment is released, the dorsal and mobile wad compartments’ pressures should be measured as this release is often sufficient to decompress the remaining compartments. The dorsal forearm and mobile wad are released through a single, straight, longitudinal incision from 3–4 cm distal to the lateral epicondyle to Lister’s tubercle.
Wound closure
After decompressive fasciotomies are performed, the open surgical wounds that result must be managed. Primary closure can be considered, however residual edema, skin retraction, and poor tissue quality may preclude closure or mandate skin closure under tension. Closure most often takes place in a delayed or staged manner as wounds should not be completely closed until all necrotic tissue is débrided and swelling has been controlled.
Countless methods have been described for fasciotomy wound closures including dermatotraction with vessel loops or pre-positioned suture. This can lead to increased risk of wound edge necrosis and increased compartment pressures. By using this type of progressive closure, multiple trips to the operating room can be avoided. The avoidance of additional procedures leads to reductions in the need for anesthesia, repeat surgical procedures, nursing care, and length of hospital stay [35–37].
Negative pressure wound therapy (NPWT) is also very commonly used in the management of fasciotomy wounds. A recent retrospective chart review of the use of NPWT in fasciotomy wound closure revealed that when the NPWT dressing was compared to traditional wet-to-dry dressing changes, there were higher rates of primary closure, decreased times to primary closure, decreased time to skin grafting, decreased length of hospitalization, decreased time to initiation of rehabilitation, and increased patient satisfaction [38]. Negative pressure wound therapy has also been used in conjunction with hyperbaric oxygen therapy (HBO) to expedite healing of fasciotomy wounds. In a limited series, the use of both a NPWT device and HBO accelerated the reduction of edema and permitted earlier closure of fasciotomy wounds [39]. Some techniques have combined pre-positioned dermatotraction sutures with negative pressure wound therapy [40].
Other novel techniques include the use of a silicon sheet that can be tightened to suture, serial application of tensioned steri-strips, serial application of tensioned plaster strips, adhesive skin anchors with bridging elastomer fibers, and commercially available devices such as the Sure-closure device which uses tensioned dermal pins [41–45].
There remains a significant morbidity associated with fasciotomy wounds. Fitzgerald at al detailed wound complications following closure including painful scars, altered sensation, dry scaly skin, pruritus, wound discoloration, swollen limbs, tethered scars, recurrent ulceration, muscle herniation, and tethered tendons. These side effects led to patients keeping their wound covered, changing hobbies, and even changing occupation [46]. Lower extremity fasciotomies, underlying vascular injuries, prophylactic fasciotomies, and an elapsed time from injury to fasciotomy greater than eight hours all lead to increased wound complication rates. All these factors are associated with increased risk for soft tissue necrosis, wound dehiscence after closure, skin graft related problems, need for further débridement, and increased need for skin grafting [47].
Split thickness skin grafting (STSG) is often used when the wound is thought to be too large to primarily close or when other methods have failed to close the wound; however, this necessitates additional procedures and can lead to insensate areas after grafting, donor site morbidity, and aesthetically unappealing scarring and graft appearance.
Pediatric compartment syndrome
ACS in the young patient can be difficult to diagnose, leading to delays. As with adults, the most common location for ACS in children is the leg. The classic signs and symptoms often present later in children or are absent all together. Nearly one-third of patients will present only with pain [13]. Age and development dictate the child’s ability to comply with physical examination and communicate pain sensation. The physician must be exceedingly vigilant in looking for the unique signs and symptoms generally not seen in adults. These features include increasing narcotic requirement, restlessness, and anxiety. Increasing narcotic requirement may be the first indication of impending compartment syndrome and may precede neurovascular changes by up to seven hours. Recent data from Flynn et al showed that the average time from injury to diagnosis was 18.2 hours and from injury to fasciotomy was 20.5 hours [48••]. Another pediatric series from Europe showed that time from presentation to fasciotomy was 27 hours [49]. Vaillancourt et al reported an average time from injury to fasciotomy in adult trauma patients of 9.5 hours [50]. These data not only highlight the difficulty in diagnosing ACS in young patients but also suggest that the evolution of compartment syndrome may be a slower process in children as compared to adults. It also appears that children can tolerate longer ischemic times with little or no permanent sequelae.
Medicolegal aspects
ACS is one of only a handful of true orthopaedic emergencies where delayed diagnosis often has limb and life threatening consequences. Despite the relative frequency with which ACS is seen by orthopaedic surgeons, the diagnosis is difficult and leads to delays in definitive management. The evolving medicolegal climate in large urban areas where polytraumatized patients are often managed definitively has added an additional layer of complexity to the management of these patients. Bhattacharyya reviewed all malpractice claims related to ACS filed with a large insurer over a 23 year period [51]. That data show that 6 % of all malpractice claims against orthopaedic surgeons are related to ACS and greater than 50 % are ruled in favor of the patient. This is significantly greater than the 25 % of overall claims against orthopaedic surgeons that resolve in favor of the patient. There is also a linear relationship between the number of cardinal signs and the time from presentation to fasciotomy and payment size. In addition, Shadgan et al, suggest that poor communication between physician, nursing staff and the patient is associated with unfavorable outcomes [2].
New methods of diagnosis/monitoring
Clinical compartment monitoring is highly variable across examiners and is not always reliable in the diagnosis of ACS [16••]. Published reports on the clinical diagnosis of compartment syndrome suggest that sensitivity of clinical findings for diagnosing compartment syndrome is low, as is the positive predictive value. Clinical features of compartment syndrome are more suggestive in their absence in ruling out the diagnosis than in confirming the diagnosis by their presence [17]. In addition, compartment pressure monitoring techniques have shown to vary by user, instrument, and location. ICP monitoring is also invasive, requiring multiple needle sticks that give limited data at only one point in time. Near infrared spectroscopy (NIRS) is a technique that is both noninvasive and continuous and has been adapted to aid in the diagnosis of ACS. Monitoring is based on the transmission and absorption of light in the near-infrared spectrum at wavelengths that correspond with the absorption of oxygenated and deoxygenated hemoglobin. Tissue oxygenation is assessed by comparing the concentrations of venous blood oxyhemoglobin and deoxyhemoglobin.
Garr et showed an inverse correlation between compartment pressure and oxygenation as well as a correlation between perfusion pressure and oxygenation in an animal model. These indicate that oxygenation surveillance by NIRS may ultimately be an effective tool to monitor patients with increased compartment pressure and evolving compartment syndrome [52]. Furthermore, NIRS is able to identify decreased tissue perfusion secondary to increased compartment pressure even in the setting of hypotension and hypoxemia, making NIRS useful in the setting of critically ill patients [53].
NIRS has been validated in humans as a noninvasive, continuous technique to evaluate tissue oxygenation. A human model of compartment syndrome showed that both tissue oxygenation and compartment pressures significantly correlated with a decrease in deep peroneal conduction, cutaneous peroneal sensitivity, and pain. NIRS was shown to be at least as good as ICP monitoring for detecting developing compartment syndromes in this particular model [54, 55].
Multiple case reports have been published detailing the use of NIRS as an adjunct for continuous lower extremity perfusion monitoring. Shuler et al published a series on patients who underwent fasciotomies based on ICP monitoring with simultaneous NIRS monitoring. All compartments showed decreasing tissue oxygenation with decreasing perfusion pressure [56, 57, 58••].
Giannotti et al published a series of case reports on patients who underwent fasciotomy that showed tissue oxygenation levels in patients with compartment syndrome were significantly lower than a matched control group with lower extremity injuries and no compartment syndrome. The measured tissue perfusions of the compartment syndrome group were also significantly lower than post fasciotomy values [59].
NIRS has also been used to characterize changes in perfusion following tibial fracture in patients without ACS, as compared to subjects’ contralateral extremity and uninjured extremities in other controls. A predictable 15.4 % increase in tissue oxygenation was seen in injured extremities. A lack in this post-traumatic hyperemia may actually represent a compartment syndrome even if oxygenation in the injured leg is equivalent to that in the uninjured extremity [60].
Conclusion
Compartment syndrome is a potentially devastating condition and all physicians should remain hypervigilant in their monitoring of at risk patients. Late or missed diagnosis of acute compartment syndrome can be catastrophic; hence there should be a low threshold for decompressive fasciotomy. Multiple ICPs have been used as cutoffs for surgical intervention and clinical indicators are proving to be less reliable than once thought. In the future, new methods of noninvasive and continuous monitoring of ICP and muscle perfusion should continue to be studied.
Acknowledgments
Disclosure
RM Taylor: none; MP Sullivan: none; S Mehta: consultant for Synthes, Smith & Nephew; receives payment for lectures from AO North America, Zimmer, Smith & Nephew.
Contributor Information
Ryan M. Taylor, FAX: +1-215-3495890, Email: Ryan.Taylor@uphs.upenn.edu
Matthew P. Sullivan, FAX: +1-215-3495890, Email: Matthew.Sullivan2@uphs.upenn.edu
Samir Mehta, FAX: +1-215-3495890, Email: Samir.Mehta@uphs.upenn.edu.
References
Papers of particular interest, published recently, have been highlighted as: •• Of major importance
- 1.McQueen MM, Gaston P, Court-Brown CM. Acute compartment syndrome. Who is at risk? J Bone Joint Surg Br. 2000;82:200–203. doi: 10.1302/0301-620X.82B2 .9799. [DOI] [PubMed] [Google Scholar]
- 2.Shadgan B, Menon M, Sanders D, et al. Current thinking about acute compartment syndrome of the lower extremity. Can J Surg. 2010;53:329–334. [PMC free article] [PubMed] [Google Scholar]
- 3.Kalyani BS, Fisher BE, Roberts CS, et al. Compartment Syndrome of the Forearm: A Systematic Review. J Hand Surg Am. 2011;36:535–543. doi: 10.1016/j.jhsa.2010.12.007. [DOI] [PubMed] [Google Scholar]
- 4.Leversedge FJ, Moore TJ, Peterson BC, Seiler JG, III, et al. Compartment Syndrome of the Upper Extremity. J Hand Surg. 2011;36:544–560. doi: 10.1016/j.jhsa.2010.12.008. [DOI] [PubMed] [Google Scholar]
- 5.McQueen MM, Christie J, Court-Brown CM. Compartment pressures after intramedullary nailing of the tibia. J Bone Joint Surg Br. 1990;72:395–397. doi: 10.1302/0301-620X.72B3.2341435. [DOI] [PubMed] [Google Scholar]
- 6.Tiwari A, Hag AI, Myint F, et al. Acute compartment syndromes. Br J Surg. 2002;89:397–412. doi: 10.1046/j.0007-1323.2002.02063.x. [DOI] [PubMed] [Google Scholar]
- 7.Hayes G, Liauw S, Romaschin A, et al. Separation of reperfusion injury from ischemia-induced necrosis. Surg Forum. 1988;39:306–308. [Google Scholar]
- 8.Har-Shai Y, Silbermann M, Reis ND, et al. Muscle microcirculatory impairment following acute compartment syndrome in the dog. Plast Reconstr Surg. 1992;89:283–289. doi: 10.1097/00006534-199289010-00012. [DOI] [PubMed] [Google Scholar]
- 9.Lindsay T, Liauw F, Romaschin S, et al. The effect of ischemia/reperfusion on adenine nucleotide metabolism and xanthine oxidase production in skeletal muscle. J Vasc Surg. 1990;12:8–15. doi: 10.1067/mva.1990.19946. [DOI] [PubMed] [Google Scholar]
- 10.Blaisdell FW. The pathophysiology of skeletal muscle ischemia and the reperfusion syndrome: A review. Cardiovasc Surg. 2002;10:620–630. doi: 10.1016/S0967-2109(02)00070-4. [DOI] [PubMed] [Google Scholar]
- 11.Jennische E. Ischemia-induced injury in glycogen-depleted skeletal muscle: selective vulnerability of the FG-fibres. Acta Physiol Scand. 1985;125:727–734. doi: 10.1111/j.1748-1716.1985.tb07776.x. [DOI] [PubMed] [Google Scholar]
- 12.Petrasek PF, Homer-Vanniasinkam S, Walker PM. Determinants of ischemic injury to skeletal muscle. J Vasc Surg. 1994;19:623–631. doi: 10.1016/S0741-5214(94)70035-4. [DOI] [PubMed] [Google Scholar]
- 13.Bae DS, Kadiyala RK, Waters PM. Acute Compartment Syndrome in Children: Contemporary Diagnosis, Treatment, and Outcomes. J Pediatr Orthop. 2001;21:680–688. doi: 10.1097/00004694-200109000-00025. [DOI] [PubMed] [Google Scholar]
- 14.McQueen MM, Christie J, Court-Brown CM. Acute compartment syndrome in tibial diaphyseal fractures. J Bone Joint Surg Br. 1996;78:95–98. [PubMed] [Google Scholar]
- 15.Badhe S, Baiju D, Elliot R, et al. The ‘silent’ compartment syndrome. Injury. 2009;40:220–222. doi: 10.1016/j.injury.2008.07.023. [DOI] [PubMed] [Google Scholar]
- 16.Shuler FD, Dietz MJ. Physicians’ ability to manually detect isolated elevations in leg ICP. J Bone Joint Surg Am. 2010;90:361–367. doi: 10.2106/JBJS.I.00411. [DOI] [PubMed] [Google Scholar]
- 17.Ulmer T. The Clinical Diagnosis of Compartment Syndrome of the Lower Leg: Are Clinical Findings Predictive of the Disorder? J Orthop Trauma. 2002;16:572–577. doi: 10.1097/00005131-200209000-00006. [DOI] [PubMed] [Google Scholar]
- 18.Staudt JM, Smeulders MJ, Horst CM. Normal compartment pressures of the lower leg in children. J Bone Joint Surg Br. 2008;90:215–9. doi: 10.1302/0301-620X.90B2.19678. [DOI] [PubMed] [Google Scholar]
- 19.Moed BR, Thorderson PK. Measurement of ICP: A comparison of the slit catheter, side-ported needle, and simple needle. J Bone Joint Surg Am. 1993;75:231–235. [PubMed] [Google Scholar]
- 20.Seiler JG, III, Womack S, L’Aune WR, et al. Intracompartmental pressure measurements in the normal forearm. J Orthop Trauma. 1993;7:414–416. doi: 10.1097/00005131-199310000-00003. [DOI] [PubMed] [Google Scholar]
- 21.Heckman MM, Whitesides TE, Jr, Grewe SR, et al. Compartment pressure in association with closed tibial fractures: The relationship between tissue pressure, compartment, and the distance from the site of the fracture. J Bone Joint Surg Am. 1994;76:1285–1292. doi: 10.2106/00004623-199409000-00002. [DOI] [PubMed] [Google Scholar]
- 22.Matsen FA, Winquist RA, Krugmire RB. Diagnosis and Management of Compartmental Syndromes. J Bone Joint Surg Am. 1980;62:286–291. [PubMed] [Google Scholar]
- 23.Mubarak SJ, Owen CA, Hargens AR, et al. Acute compartment syndromes: diagnosis and treatment with the aid of the wick catheter. J Bone Joint Surg Am. 1978;60:1091–1095. [PubMed] [Google Scholar]
- 24.McQueen MM, Court-Brown CM. Compartment monitoring in tibial fractures: The pressure threshold for decompression. J Bone Joint Surg Br. 1996;78:99–104. [PubMed] [Google Scholar]
- 25.Heckman MM, Whitesides TE, Jr, Grewe SR, et al. Histologic determination of the ischemic threshold of muscle in the canine compartment syndrome model. J Orthop Trauma. 1993;7:199–210. doi: 10.1097/00005131-199306000-00001. [DOI] [PubMed] [Google Scholar]
- 26.Matava MJ, Whitesides TE, Jr, Seiler JG, III, et al. Determination of the compartment pressure threshold of muscle ischemia in a canine model. J Trauma. 1994;37:50–58. doi: 10.1097/00005373-199407000-00010. [DOI] [PubMed] [Google Scholar]
- 27.Bernot M, Gupta R, Dobrasz J, et al. The effect of antecedent ischemia on the tolerance of skeletal muscle to increased interstitial pressure. J Orthop Trauma. 1996;10:555–559. doi: 10.1097/00005131-199611000-00008. [DOI] [PubMed] [Google Scholar]
- 28.Iaquinto JM, Pienkowski D, Thornsberry R, et al. Increased neurologic complications associated with postoperative epidural analgesia after tibial fracture fixation. Am J Orthop. 1997;26:604–608. [PubMed] [Google Scholar]
- 29.Strecker WB, Wood MB, Bieber EJ. Compartment syndrome masked by epidural anesthesia for postoperative pain. Report of a case. J Bone Joint Surg Am. 1986;68:1447–1448. [PubMed] [Google Scholar]
- 30.Harrington P, Bunola J, Jennings AJ, et al. Acute compartment syndrome masked by intravenous morphine from a patient-controlled analgesia pump. Injury. 2000;31:387–389. doi: 10.1016/S0020-1383(99)00308-3. [DOI] [PubMed] [Google Scholar]
- 31.Maheshwari R, Taitsman LA, Barei DP. Single-Incision Fasciotomy for Compartmental Syndrome of the Leg in Patients With Diaphyseal Tibial Fractures. J Orthop Trauma. 2008;22:723–730. doi: 10.1097/BOT.0b013e31818e43f9. [DOI] [PubMed] [Google Scholar]
- 32.Chloros GD, Papadonikolakis A, Ginn S, et al. Pronator quadratus space and compartment syndrome after low-energy fracture of the distal radius: a case report. J Surg Orthop Adv. 2008;17:102–106. [PubMed] [Google Scholar]
- 33.Summerfield SL, Folberg CR, Weiss AP. Compartment syndrome of the pronator quadratus: a case report. J Hand Surg. 1997;22:266–268. doi: 10.1016/S0363-5023(97)80162-1. [DOI] [PubMed] [Google Scholar]
- 34.Gelbernam RH, Zakaib GS, Mubarak SJ, et al. Decompression of Forearm Compartment Syndromes. Clin Orthop Relat Res. 1978;134:225–229. [PubMed] [Google Scholar]
- 35.Janzing HM, Broos PL. Dermatotraction: An Effective Technique for the Closure of Fasciotomy Wounds: A Preliminary Report of Fifteen Patients. J Orthop Trauma. 2001;15:438–441. doi: 10.1097/00005131-200108000-00010. [DOI] [PubMed] [Google Scholar]
- 36.Zorilla P, Mariin A, Gomez LA, et al. Shoelace Technique for Gradual Closure of Fasciotomy Wounds. J Trauma. 2005;59:1515–1517. doi: 10.1097/01.ta.0000199242.24511.30. [DOI] [PubMed] [Google Scholar]
- 37.Chiverton N, Redden JF. A new technique for delayed primary closure of fasciotomy wounds. Injury. 2000;31:21–24. doi: 10.1016/S0020-1383(99)00193-X. [DOI] [PubMed] [Google Scholar]
- 38.Zannis J, Angobaldo J, Marks M, et al. Comparison of Fasciotomy Wound Closures Using Traditional Dressing Changes and the Vacuum-Assisted Closure Device. Ann Plast Surg. 2009;62:407–409. doi: 10.1097/SAP.0b013e3181881b29. [DOI] [PubMed] [Google Scholar]
- 39.Weiland DE. Fasciotomy closure using simultaneous vacuum-assisted closure and hyperbaric oxygen. Am Surg. 2007;73:261–266. doi: 10.1177/000313480707300313. [DOI] [PubMed] [Google Scholar]
- 40.Velde M, Hudson DA. VADER (Vacuum-Assisted Dermal Recruitment) A New Method of Wound Closure. Ann Plast Surg. 2005;6:660–664. doi: 10.1097/01.sap.0000187181.59748.19. [DOI] [PubMed] [Google Scholar]
- 41.Walker T, Gruler M, Ziemer G, et al. The use of a silicon sheet for gradual wound closure after fasciotomy. J Vasc Surg. 2012;55:1826–8 [DOI] [PubMed]
- 42.Harrah J, Gates R, Carl J, et al. A Simpler Less Expensive Technique for Delayed Primary Closure of Fasciotomies. Am J Surg. 2000;180:55–57. doi: 10.1016/S0002-9610(00)00409-8. [DOI] [PubMed] [Google Scholar]
- 43.Mbubaegbu CE, Stallard MC. A method of fasciotomy wound closure. Injury. 1996;27:613–615. doi: 10.1016/S0020-1383(96)00111-8. [DOI] [PubMed] [Google Scholar]
- 44.Taylor RC, Reitsma BJ, Sarazin S, et al. Early Results Using a Dynamic Method for Delayed Primary Closure of Fasciotomy Wounds. J Am Coll Surg. 2003;197:872–878. doi: 10.1016/S1072-7515(03)00646-X. [DOI] [PubMed] [Google Scholar]
- 45.Narayanan K, Latenser BA, Jones LM, et al. Simultaneous primary closure of four fasciotomy wounds in a single setting using the Sure-Closure™ device. Injury. 1996;27:449–451. doi: 10.1016/0020-1383(96)00029-0. [DOI] [PubMed] [Google Scholar]
- 46.Fitzgerald AM, Gaston P, Wilson Y, et al. Long-term sequelae of fasciotomy wounds. Br J Plast Surg. 2000;53:690–693. doi: 10.1054/bjps.2000.3444. [DOI] [PubMed] [Google Scholar]
- 47.Velmahos GC, Theodorou D, Demetriades D, et al. Complications and Nonclosure Rates of Fasciotomy for Trauma and Related. Risk World J. Surg. 1997;21:247–253. doi: 10.1007/s002689900224. [DOI] [PubMed] [Google Scholar]
- 48.Flynn JM, Bashyal RK, Yeger-McKeever M, et al. Acute Traumatic Compartment Syndrome of the Leg in Children: Diagnosis and Outcome. J Bone Joint Surg Am. 2011;93:937–941. doi: 10.2106/JBJS.J.00285. [DOI] [PubMed] [Google Scholar]
- 49.Erdos J, Dlaska C, Szatmary P, et al. Acute compartment syndrome in children: a case series in 24 patients and review of the literature. Int Orthop. 2010;35:569–57. doi: 10.1007/s00264-010-1016-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vaillancourt C, Shrier I, Falk M, et al. Quantifying delays in the recognition and management of acute compartment syndrome. CJEM. 2001;3:26–30. doi: 10.1017/s148180350000511x. [DOI] [PubMed] [Google Scholar]
- 51.Bhattacharyya T, Vrahas MS. The Medical-Legal Aspects Of Compartment Syndrome. J Bone Joint Surg Am. 2004;86:864–868. doi: 10.2106/00004623-200404000-00029. [DOI] [PubMed] [Google Scholar]
- 52.Garr J, Gentilello LM, Cole PA, et al. Monitoring for Compartmental Syndrome Using Near-Infrared Spectroscopy: A Noninvasive, Continuous, Transcutaneous Monitoring Technique. J Trauma. 1999;46:613–618. doi: 10.1097/00005373-199904000-00009. [DOI] [PubMed] [Google Scholar]
- 53.Arbabi S, Brundate SI, Gentilello LM. Near-Infrared Spectroscopy: A Potential Method for Continuous, Transcutaneous Monitoring for Compartmental Syndrome in Critically Injured Patients. J Trauma. 1999;47:829–833. doi: 10.1097/00005373-199911000-00002. [DOI] [PubMed] [Google Scholar]
- 54.Mancini DM, Bolinger L, Li H, et al. Validation of near-infrared spectroscopy in humans. J Appl Physiol. 1994;77:2740–2747. doi: 10.1152/jappl.1994.77.6.2740. [DOI] [PubMed] [Google Scholar]
- 55.Gentilello LM, Sanzone A, Wang L, et al. Near-infrared spectroscopy versus compartment pressure for the diagnosis of lower extremity compartmental syndrome using electromyography-determined measurements of neuromuscular function. J Trauma. 2001;51:1–8. doi: 10.1097/00005373-200107000-00001. [DOI] [PubMed] [Google Scholar]
- 56.Sanchez de Toledo J, Chrysostomou C, Wearden PD. Acute compartment syndrome in a Patient on Extracorporeal Support: Use of Near-Infrared Spectroscopy. J Cardiothorac Vasc Anesth. 2011;25:836–837. doi: 10.1053/j.jvca.2010.06.023. [DOI] [PubMed] [Google Scholar]
- 57.Shuler MS, Reisman WM, Cole AL, et al. Near-infrared spectroscopy in acute compartment syndrome: Case report. Injury. 2011;42:1506–1508. doi: 10.1016/j.injury.2011.03.022. [DOI] [PubMed] [Google Scholar]
- 58.Shuler MS, Reisman WM, Kinsey TL, et al. Correlation Between Muscle Oxygenation and Compartment Pressures in Acute Compartment Syndrome of the Leg. J Bone Joint Surg Am. 2010;92:863–870. doi: 10.2106/JBJS.I.00816. [DOI] [PubMed] [Google Scholar]
- 59.Giannotti G, Cohn SM, Brown M, et al. Utility of near-infrared spectroscopy in the diagnosis of lower extremity compartment syndrome. J Trauma. 2000;48:396–399. doi: 10.1097/00005373-200003000-00005. [DOI] [PubMed] [Google Scholar]
- 60.Shuler MS, Reisman WM, Whitesides TE, et al. Near-Infrared Spectroscopy in Lower Extremity Trauma. J Bone Joint Surg Am. 2009;91:1360–1368. doi: 10.2106/JBJS.H.00347. [DOI] [PubMed] [Google Scholar]
- 61.Ouellette EA. Compartment syndromes in obtunded patients. Hand Clin. 1998;14:431–50. [PubMed] [Google Scholar]


