Abstract
Young patients with cartilage defects in the hip present a complex problem for the treating physician with limited treatment modalities available. Cartilage repair/replacement techniques have shown promising results in other joints, however, the literature regarding the hip joint is limited. The purpose of the current study is to conduct a systematic review of clinical outcomes following various treatments for chondral lesions of the hip and define the techniques for the treatment of these cartilage defects. The full manuscripts of 15 studies were reviewed for this systematic review including case studies, case series, and clinical studies. A variety of techniques have been reported for the treatment of symptomatic chondral lesions in the hip. Microfracture, cartilage repair, autologous chondrocyte implantation, mosaicplasty, and osteochondral allografting have all been used in very limited case series. Although good results have been reported, most studies lack both a control group and a large number of patients. However, the reported results in this article do provide a good foundation for treatments and stimulant for further study in an inherently difficult to treat young patient population with articular cartilage defects in the hip.
Keywords: Hip, Cartilage, Sports, Musculoskeletal, Operative treatment
Introduction
Cartilage damage in the hip presents a challenge to the surgeon due to its limited ability for spontaneous regeneration [1], friability [2], and the difficult-to-replace properties of biological hyaline cartilage [3]. Outcome studies of procedures developed in the knee have been encouraging and there has been a considerable amount of benchtop research into artificial cartilage replacement, but to date, there is still no “perfect” solution to reproducibly replicate the load-bearing capacity and durability of native joint cartilage [3]. While long-term outcome studies on knee cartilage replacement and repair are becoming more prevalent in the literature, definitive evidence-based treatment guidelines for chondral lesions of the hip have lagged behind [3, 4••, 5].
Causes of cartilage damage in the hip are numerous and include trauma, femoroacetabular impingement (FAI), labral tears, osteonecrosis, osteochondritis dessicans, degenerative joint disease, loose bodies, slipped capital femoral epiphysis, and hip dysplasia, among others [6, 7]. Arthroplasty remains the gold standard treatment for diffuse osteoarthritis [3] and is considered a failure endpoint in most studies, but the current trend is to treat the underlying morphological pathology in younger patients in an attempt to prevent progression to end-stage disease. A major leap in both the diagnosis and treatment of intra-articular hip pathology occurred with the advent of the “lateral approach to hip arthroscopy” in 1984, and the 2002 defining of femoroacetabular impingement syndrome highlighted that underlying morphological conflicts are a major contributing factor to osteoarthritis in the hip [2]. The implications in treating these morphological conflicts, as well as the chondral lesions themselves, are profound. A recent study, for instance, showed that 36 % of Olympic or professional athletes undergoing hip arthroscopy required decompression of a cam or pincer impingement [8]. It has also been demonstrated that labral tears can cause progression of osteoarthritis by increasing joint contact stress by up to 92 % [8], and that most cartilage injuries of the hip are associated with a torn acetabular labrum [9].
Advances in the understanding of femoroacetabular impingement syndrome are progressing the understanding of cartilage damage in the hip. CAM lesions, in which the anterior femoral head/neck junction has an abnormal protrusion causing impingement on the anterior acetabulum, have been shown to cause chondral damage to the anterior acetabulum near the rim in a fairly predictable and progressive manner [8]. Pincer deformity, in which a retroverted or deep acetabulum makes contact with a normal femoral neck, also has a recognized pattern of chondral damage to the femur and a posterior-medial acetabular countercoup injury [6, 8]. As more information has been gathered through arthroscopy on the patterns of chondral injury in these hips, new classification systems have recently been published, which attempt to grade acetabular chondral defects in a way that more accurately guides treatment [2, 10]. Konan et al [10] tested the validity of a classification system based on the work of Ilizaliturri et al [11] that divided the acetabulum into 6 anatomical zones with varying degrees of cartilage injury and location in each zone. They found good intra- and inter-observer reliability with this method.
In diagnosing chondral injuries of the hip, there is no specific physical examination maneuver to assess for them [6]. The typical presentation of intra-articular pathology is anterior groin pain [8], and accompanying lesions may present with more specific signs and symptoms. Clicking or other mechanical symptoms are common in a labral tear [6], while the impingement test, in which the hip is flexed, internally rotated, and adducted, nearly always elicits pain in femoroacetabular impingement [8]. In imaging the patient with hip pain, radiographs are the most useful initial tool and can reveal most bone pathology [6]. While MRI is useful for looking at soft tissue and can provide information regarding the status of the cartilage [6], an MRI arthrogram is a more useful modality for identifying these lesions. However, it has been shown to accurately diagnose only 76 % of acetabular labral tears and 62.7 % of articular cartilage lesions when compared with arthroscopy [12]. Diagnostic joint injection with lidocaine during MRI arthrogram can help further delineate whether the pain originates intra-articularly [2]. Variations such as delayed gadolinium-enhanced MRI have the potential to accurately diagnose focal chondral defects in a non-invasive manner [3, 7], but the gold standard is still direct visualization by arthroscopy.
Importantly, Suzuki et al [13] showed that cartilage lesions will often not improve after correction of the underlying pathologic mechanism. The authors completed second look arthroscopy after pelvic osteotomies and found no improvement in the majority of cases with regard to articular cartilage lesions present at the time of the index operation. Thus, numerous joint-preserving treatments exist for chondral and osteochondral lesions of the hip, mirroring the treatment options in the knee. These include debridement [8, 14••, 15], microfracture [6-8, 15, 16, 17•, 18, 19], autologous chondrocyte transplantation [14••, 20], osteochondral autograft transplantation (OATS) and mosaicplasty [4••, 17•, 21, 22], osteochondral allograft transplantation [5, 23•], partial resurfacing prostheses [24], and recently, suturing techniques [7] as well as fixation with a fibrin adhesive for delamination lesions [25••, 26]. Indications vary between the procedures. Microfracture, for instance, requires intact subchondral bone on which a stable marrow clot can form, and is most commonly used in full-thickness lesions [6-8]. Osteochondral autografts and allografts, on the other hand, have been used for defects which involve a combination of both cartilage and subchondral bone destruction [4••, 5, 17•, 23•]. While hip arthroplasty offers both good pain relief and function, younger patients face the likely prospect of revision in the future and thus our review is limited to non-arthroplasty techniques [5].
There are a multitude of treatment options available for chondral defects in the hip; however, a surgeon who encounters such a lesion is left with little guidance on the best manner in which to proceed. No recent systematic reviews currently exist in the literature to provide the surgeon with evidence-based recommendations on treating these cartilage defects. Additionally, due to the young nature of the field, many innovative techniques have made their way into the literature in the past few years.
The object of this study was to conduct: (1) systematic review of clinical outcomes following various treatments for chondral lesions of the hip; (2) define the techniques for the treatment of cartilage defects in the hip that have been published; (3) provide treatment recommendations based on the best currently available evidence; (4) highlight new and innovative techniques in recent case studies; and (5) highlight gaps in the literature that require further research.
Methods
Literature search
We searched PubMed (1948 to March Week 1 2012) using the following key words: (hip OR acetabulum OR femoral) AND (cartilage OR osteochondral OR articular). Search terms were broad as to encompass all possibilities for applicable studies. All review articles were then manually cross-referenced to make certain no relevant studies were missed.
Inclusion criteria were studies that reported on clinical outcomes or techniques following non-arthroplasty treatment for the spectrum of chondral lesions of the hip including focal and diffuse articular disease on the femur and/or acetabulum. We excluded review articles.
Data abstraction
The data from each study that met the inclusion criteria was abstracted by one reviewer (M.J.) and verified by another (G.V.). Study data, which was determined to be of interest a priori, included the type of treatment, year of publication, study period, type of clinical study, inclusion/exclusion criteria, number of patients enrolled, number of patients available for follow-up, age, minimum follow-up, length of follow-up, gender, concomitant procedures, classification of pre-operative arthritis, post-operative rehabilitation, and statistical analysis used. Preoperative and postoperative data of interest was patient satisfaction, clinical outcome scores, and the amount of people that ultimately failed treatment (requiring resurfacing or arthroplasty) was also recorded.
Results
We obtained 2794 articles from PubMed. Of these articles, we screened the articles by article title relevance and were left with 25 studies. These articles were then further screened to remove review papers. The full manuscripts of 15 studies were evaluated for this systematic review, including case studies, case series, and clinical studies. Due to the low number of reported cases, the majority of studies were case reports or small case series. Thus, pooling of data was impossible, and a discussion of the relevant studies was completed. The data are summarized in Tables 1 and 2.
Table 1.
Patient demographics
| Author | Age [years (range)] | Number of patients and gender [n(%male)] | Concomitant procedures[% of patients] | Open vs arthroscopic | Postop rehab |
|---|---|---|---|---|---|
| Microfracture | |||||
| Horisberger [16] | 47.3 (22–65) | 20 (80 %) | 100 % | Arthroscopic | Partial weight-bearing at 4–6 weeks, low impact sports after 6 weeks, high impact sports after 3 months |
| *Non-microfracture patients had full weight-bearing as tolerated postoperatively | |||||
| Phillipon [18] | 37.2 (21–47) | 9 (55.6 %) | Yes, but % not specified | Arthroscopic | Toe-touch weight-bearing until 8 weeks with concurrent CPM, return to sport at 4–6 months |
| Microfracture vs debridement/radiofrequency ablation | |||||
| Haviv [15] | 37 (14–78) | 166 (79.5 %) | 100 % | Arthroscopic | Weight-bearing as tolerated from day 1, jogging allowed at 4–6 weeks |
| Mosaicplasty /OATS | |||||
| Girard [4••] | 18 (15–21) | 10 (70 %) | Yes, but % not specified | Open | CPM for 1 week, non-weight-bearing for 6 weeks, then full weight-bearing as tolerated |
| Hart [21] | 28 | 1 (male) | 0 % | Open | CPM early, partial weight-bearing at 6 weeks, full weight-bearing at 10 months |
| Nam [17•] | 18 (15 and 21) | 2 (50 %) | 100 % | Open | Non-weight-bearing for 6 weeks, increased as tolerated thereafter |
| Rittmeister [29] | n/a | 5 (n/a) | n/a | Open | n/a |
| Sotereanos [22] | 36 | 1 (male) | 0 % | Open | Toe-touch weight-bearing at 8 weeks, then full weight-bearing as tolerated |
| Osteochondral allograft transplantation | |||||
| Krych [23•] | 28 (24 and 32) | 2 (50 %) | 100 % | Open | 8 Weeks of CPM with protected weight-bearing, high impact activity at 6 months |
| Meyers [5] | n/a | 21 (71.4 %) | n/a | Open | n/a |
| Autologous chondrocyte implantation | |||||
| Akimau [20] | 31 | 1 (male) | 0 % | Open | CPM for 48 hours, partial- weight-bearing for 8 weeks |
| Autologous chondrocyte transplantation vs debridement | |||||
| Fontana [14••] | 41.5 (20–53) | 30 (40 %) | 0 % | Arthroscopic | Partial-weight-bearing at 2 weeks in debridement group and at 4 weeks in the ACT group. Passive and active physiotherapy for the first 4 weeks |
| Fibrin adhesive | |||||
| Tzaveas [26] | 36 (18–57) | 19 (73.7 %) | 100 % | Arthroscopic | Toe-touch weight-bearing for 4 weeks, high impact exercise allowed at 3 months |
| Synthetic osteochondral plugs | |||||
| Field [28•] | 48.5 (31.6–63.3) | 4 (25 %) | 75 % | Open | 50 % Weight-bearing for 6 weeks, full weight-bearing by 8 weeks |
| Absorbable sutures combined with microfracture | |||||
| Sekiya [19] | 17 | 1 (male) | 100 % | Arthroscopic | 30 % Weight-bearing for 6 weeks, full weight-bearing at 8 weeks, and progression of resistive weight-bearing exercises thereafter |
| Acetabular rim resection | |||||
| Anderson [30] | 19.8 (15–29) | 4 (75 %) | 100 % | Arthroscopic with pelvic tunnel | No comment other than ambulating with crutches at 6 weeks |
| Partial resurfacing prosthesis with high varus osteotomy | |||||
| Van Stralen [24] | 16 | 1 (male) | 100 % | Open | n/a |
CPM, continuous passive motion; ACT, autologous chondrocyte transplantation
Table 2.
Outcomes following various treatment modalities for cartilage lesions
| Author | No. of patients | Duration of follow-up [months (range)] | Outcomes measure(s) | Preop value [mean (range)] * = 95 % CI | Postop value [mean (range)] * = 95 % CI | Resurfacing/ arthroplasty [n (%)] |
|---|---|---|---|---|---|---|
| Microfracture | ||||||
| Horisberger [16] | 20 | 3.0 (1.5–4.1) | NAHS | 47.15 (23.75–66.25) | 78.3 (63.75–95.0) | 10 (50 %) |
| Phillipon [18] | 9 | 20 (9–36) | % fill of defect | n/a | 91 % | 2 (22.2 %) |
| Microfracture vs debridement/radiofrequency ablation | ||||||
| Haviv [15] | 166 (19 in Microfx subgroup) | 22 (n/a) | MHHS and NAHS | MHHS (all groups): 70.8 | MHHS (all groups): 86.1 (79.7–92.5)* | 2 (1.2 %) |
| NAHS (all groups): 69.8 | NAHS (all groups): 84.8 (79.2–90.4)* | |||||
| NAHS improvement (microfracture vs debridement): 20.2 vs 12.6 (significant) | ||||||
| Mosaicplasty/OATS | ||||||
| Girard [4••] | 10 | 29.2 (20-39) | HHS and Postel Merle d’Aubigne score | HHS: 52.8 (35-74) | HHS: 79.5 (65–93) | 0 |
| PMdAS: 10.5 (8–13) | PMdAS: 15.5 (12–17) | |||||
| Hart [21] | 1 | 6 | HHS | 69 | 100 | 0 |
| Nam [17•] | 2 | Case 1: 12 | Pain and mechanics | Case 1: femoral head osteochondral fracture with full-thickness cartilage loss | Case 1: ambulating without pain at 12 weeks, no pain at last follow-up | 0 |
| Case 2: 60 | Case 2: same | Case 2: ambulating without pain or assistance at 12 weeks, at last follow-up no pain or difficulties | ||||
| Rittmeister [29] | 5 | 57 (n/a) | Progression to THA | n/a | n/a | 4 (80 %) |
| Sotereanos [22] | 1 | 66 | HHS and pain | HHS: 45 (retrospective) | HHS: 100 | 0 |
| Pain: 90/100 | Pain: 0/100 (at 17 months) | |||||
| Osteochondral allograft transplantation | ||||||
| Krych [23•] | 2 | Case 1: 24 | HHS | Case 1: 75 | Case 1: 79 | 0 |
| Case 2: 36 | Case 2: 97 | Case 2: 100 | ||||
| Meyers[5] | 21 | n/a (9–63) | Failure rate (failure = mod/severe pain or THA) | n/a | 38 % failure | 5 (23 %) |
| Autologous chondrocyte implantation | ||||||
| Akimau [20] | 1 | 18 | HHS | 52 | 76 | 0 |
| Autologous chondrocyte transplantation vs debridement | ||||||
| Fontana [14••] | 30 (15 in each subgroup) | ACT: 73.8 (72–76) | HHS and Pain Score (0 = max pain, 44 = no pain) | ACT HHS: 48.3 (45.4–51.2)* | ACT HHS (5-year): 87.7 (84.5-90.3)* | n/a |
| Debridement: 74.3 (72–76) | Debridement HHS: 46.4 (43.5– 9.3)* | Debridement HHS (5-year): 56.3 (53.4–59.1)* | ||||
| ACT Pain: 20 (18.7–21.38)* | ACT pain: 40 (38.6 – 41.4)* | |||||
| Debridement pain: 20 (18.9 – 21.1)* | Debridement pain: 35 (33.2–36.8)* | |||||
| Fibrin adhesive | ||||||
| Tzaveas [26] | 19 | 12 | MHHS (×1.1) ➔ | 53.3 | 80.3 | 1 (5.3 %) |
| Pain Score ➔ | 15.7 | 28.9 | ||||
| Function Score ➔ | 37.2 | 44.1 | ||||
| Synthetic osteochondral plugs | ||||||
| Field [28•] | 4 | 10 (8-11) | NAHS (only Obtained in 3 out of 4 patients) | 53.8 (43.8–70.0) | 84.6 (range, 78.8–87.5) -obtained at 6 months | 0 |
| Absorbable sutures combined with microfracture | ||||||
| Sekiya [19] | 1 | 27 | MHHS (postop) Pain | Pain and decreased ROM | MHHS: 97 Pain-free 90 % of time, 2/10 at worst | 0 |
| Acetabular rim resection | ||||||
| Anderson [30] | 4 | 38 (34-42) | HHS | 67.5 (58–74), obtained retrospectively | 98 (95-100) | 0 |
| Partial resurfacing prosthesis with high varus osteotomy | ||||||
| Van Stralen[24] | 1 | 24 | ROM and Pain | Flexion/extension: 110-0-10 | Flexion/extension: 110-0-50° | 0 |
| Abduction/adduction: 40-0-15° | Abduction/Adduction: 35-0-20° | |||||
| External/internal rotation: 25-0-0° | Extension/internal rotation: 60-0-20° | |||||
| Significant pain | Pain free | |||||
PMdAS, Postel Merle d’Aubigne score; HHS, Harris Hip Score; MHHS, Modified Harris Hip Score; NAHS, Non-Arthritic Hip Score; ROM, range of motion; THA, total hip arthroplasty
Operative procedures
Autologous Chondrocyte Implantation (ACI)
ACI has been used extensively in the knee with good literature support. The technique includes the harvest of chondrocytes with growth and expansion at an off-site facility. These cells are then re-implanted into the affected area. There are only limited reports of the use of ACI in the hip. This process is most likely complicated by the difficulty with harvest in the hip or the need to complete a surgical procedure on an unaffected joint (knee). Regardless, Akimau et al [20] were the first to describe the use of autologous chondrocyte implantation (ACI) in the hip. They reported a case in a 31-year-old male with osteonecrosis of the femoral head following open reduction internal fixation for a traumatic fracture dislocation. Initial Harris Hip Score (HHS) before ACI was 52 and the patient required a crutch. Femoral head defects were filled in with a greater trochanter bone graft, and chondrocytes were obtained from the femoral trochlea and transplanted under a collagen patch after culture expansion. Sixteen-month post-ACI, HHS was 76, and there was no use of any walking aids, ROM was markedly improved, and the patient was able to run on the spot as well as walk more than a mile. A second look biopsy demonstrated 2 mm thick cartilage, predominantly fibrocartilage. CT was performed at 18 months post-ACI, and demonstrated retention of joint space despite cystic sclerotic changes of the previous osteonecrosis.
These limited results have been supported in a larger study by Fontana et al [14••] with a retrospective comparative level III study between ACI and simple debridement. Inclusion criteria included Tönnis grade 2 arthritic changes on radiograph with grade 3 hips excluded. There were a total of 15 patients in the ACI group and 15 patients in the debridement group, all having third or fourth degree (Outerbridge classification) chondral lesions of 2 cm2 or more. ACI was performed first by doing an arthroscopic evaluation, debridement and cartilage biopsy from the pulvinar. These cells were then cultured and re-implanted at a second stage arthroscopy using a 3-dimensional polymer scaffold. Mean age in the ACI and debridement groups was 40.7 and 42.3, respectively. Mean defect size was 2.6 cm2 in both groups. Follow-up period was 73.8 months in the ACI group and 74.3 months in the debridement group. Preoperative Harris Hip Score (HS) was 48.3 for the ACI group and 46 for the debridement group (not statistically significant). At last clinical evaluation (approximately 5 years), HS was 87.6 in the ACI group and 56.3 in the debridement group. This difference was statistically significant (P < 0.001). No postoperative radiographs, MRI, or arthroscopy was done, so it could not be verified whether viable cartilage truly formed. The authors suggest that arthroscopic debridement has limited utility in treating chondral lesions of the hip, especially in lesions >3 cm2 due to the fact that these patients had the worst outcomes.
Microfracture
Microfracture has been a widely accepted technique for the treatment of cartilage lesions in the knee. However, due to both technical difficulty and the relatively new expansion of hip arthroscopy, microfracture has only experienced limited exploration in the hip. Byrd et al [27] were one of the first to describe microfracture in the hip. In 220 arthroscopic cases, they noted that 9 patients had an “inverted” labrum as a potential cause of secondary osteoarthritis. Of these 9 patients, all had grade IV changes on their acetabulum and 3 of them received microfracture for an isolated defect. At final 2-year follow-up, the 3 patients with microfracture were the only patients who returned to an active lifestyle.
Haviv et al [15] subsequently completed a large comparative study with 166 patients who had grade 1–3 chondral damage and underwent hip arthroscopy with a femoral osteochondroplasty. In addition, 29 of 135 patients with grade 2 and 3 lesions were treated with microfracture. Mean follow-up was 22-months. Interestingly, the 29 patients treated by microfracture had a significantly higher NAHS than those with grade 2 and 3 lesions treated by debridement (postop 90.2 vs 80.2, preop 70.0 vs 67.6). However, to be a candidate for microfracture, the lesion had to be <300 mm2, but mean lesion size was not provided for the debridement group. Without this information there is the potential for bias based on the defect size. In another interesting study, Philippon et al [18] followed 9 patients undergoing revision arthroscopy for a variety of conditions after having microfracture of a full-thickness acetabular defect done at the primary arthroscopy. Average chondral lesion size at primary arthroscopy was 163 mm2; all lesions were located in the superior acetabulum. Mean time from index arthroscopy to revision procedure was 20 months. Mean percent fill of the defects was 91 %. One patient had diffuse osteoarthritis at primary arthroscopy but wished to proceed with microfracture to finish out his baseball season, and his percent fill at 10 months was 25 % with grade 4 repair tissue (full-thickness cartilage loss). Eight out of 9 patients had 95 % to 100 % fill of the defect, with grade 1 or 2 repair tissue (normal cartilage or mild fibrillation/discolored/softer-than-normal cartilage, respectively).
Horisberger et al [16], however has shown less than ideal results with microfracture. In this retrospective study, 20 patients with an average age of 47 years were identified that underwent hip arthroscopy with Outerbridge grade II or higher lesions. The mean follow-up was 3 years. Fourteen patients had grade IV lesions in the impingement zone, 6 patients had grade III lesions and 3 patients had grade IV changes on the femoral head. Ten patients went on to require total hip arthroplasty. Fifty percent of the microfracture patients required THA and 2 of the 3 patients with grade IV femoral head changes required THA. The authors concluded that patients with Tonnis grade III osteoarthritis should not undergo hip arthroscopy and if grade IV femoral changes are found in addition to the acetabular arthritis, total hip arthroplasty will most likely be required. Overall, microfracture has shown some guarded promise from both a clinical and biologic standpoint. However, the available literature does not control for external variables and, further well controlled studies are necessary before any definitive conclusions can be drawn.
Synthetic treatment
There has been 1 clinical report from Field et al [28•] describing the use of an osteochondral synthetic plug for the treatment of acetabular cystic cartilage lesions in 4 patients. All 4 patients underwent hip arthroscopy followed by the antegrade insertion of a plug through the ilium until the surface of the plug was flush with the articular surface. At 10-month follow-up, patients reported increased function with an improvement in the non-arthritic hip score from 54 to 84. One patient continued to have moderate pain and a second look arthroscopy with debridement and biopsy showed bone formation of the plug. CT and MRI at 6 months showed incorporation and continued healing of the plug. The authors concluded that this was a viable treatment for cavitary lesions in the acetabulum. Perhaps, the most interesting aspect of this article was the use of an antegrade technique to place the graft flush with the acetabular articular surface. This technique could theoretically be used with a multitude of other grafts.
Mosaicplasty
Mosaicplasty involves the use of multiple small autograft plugs to replace an articular defect. This technique was first described in the knee, but has been modified for use in the hip. Multiple authors have detailed the technique with a variety of options for the origin of the osteochondral graft. Some have described a harvest site from the knee as a separate procedure, whereas others have utilized the inferolateral femoral head of the affected hip (Fig. 1).
Fig. 1.
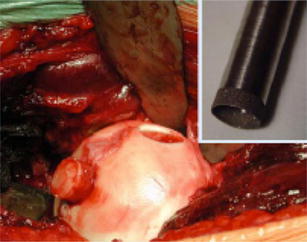
Mosaicplasty – autograft taken from the affected femoral head. (From [29], with permission)
Girard et al [4••] completed a prospective study evaluating the results of mosaicplasty to the femoral head utilizing a surgical dislocation of the hip in 10 patients. Etiology of the lesions included Legg-Calve-Perthes disease, spondylo-epiphyseal dysplasia, and epiphyseal dysplasia. Exclusion criteria included age above 25 years, osteonecrosis, or acetabular chondropathy. Mean patient age was 18 years. Mean femoral head lesion size was 4.8 cm2. Plugs were obtained from the most inferior non-weight-bearing portion the femoral head, and cancellous bone was packed in between the plugs at the recipient site. Mean follow-up was 29.2 months. Mean preop Postel Merle d’Aubigne score was 10.5 and at last follow-up was 15.5. Mean HHS increased from 52.8 to 79.5. There was 1 sciatic nerve palsy that improved spontaneously after 3 months, and no hip arthroplasty was required at final follow-up. CT arthrogram at 6 months revealed excellent autograft incorporation with intact cartilage for all 10 plugs.
Hart et al [21] has also reported a case of mosaicplasty for an osteochondral defect in the femoral head. The defect arose in a 28-year-old patient due to migration of a resorbable screw into the hip joint that was used for open reduction internal fixation of an acetabular fragment following a posterior hip dislocation. A round defect (14 mm diameter, 16 mm depth) was identified on the posterior non-weight-bearing portion of the femoral head. A mosaicplasty with an open approach was undertaken using 4 cylindrical osteochondral grafts from the lateral femoral condyle. HHS before the revision procedure was 69; 6 months postoperatively the score was 100 with full range of motion and absence of hip pain.
In another small case series, Nam et al [17•] reported 2 cases of mosaicplasty for correction of osteochondral injuries to the femoral head sustained after traumatic posterior hip dislocation. In one case, an osteochondral fracture was stabilized with bioabsorbable pins, but there was a full-thickness cartilage defect in the anterior-superior weight-bearing zone of the femoral head. Three osteochondral plugs were transferred from the lateral knee to treat this lesion (Fig. 2). In the other case, a femoral head fracture was stabilized with screws and an osteochondral plug was obtained from the most inferior non-weight-bearing portion of the femoral head and transferred to the full-thickness chondral lesion at the fracture apex. MRI showed incorporation of both grafts and the patients returned to their baseline activity level. However, it should be noted that these patients did not have symptoms prior to their injury and procedure.
Fig. 2.
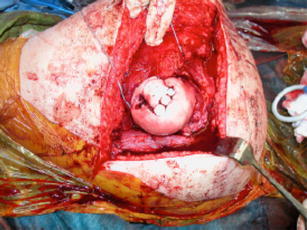
Mosaicplasty – autograft plugs taken from the knee and placed in the femoral head. (From [17•], with permission)
Lastly, Sotereanos et al [22] published a case of a 32-year-old male with bilateral osteonecrosis of the femoral head. He underwent free fibular grafts to both femoral heads, but continued to have significant pain and was scheduled for a total hip arthroplasty. At the time of the arthroplasty his articular cartilage was found to be intact except for one discreet area of softening. A mosaicplasty with grafts from the inferolateral head was done instead of the arthroplasty. At 66 month follow up his pain score has decreased from a preoperative 90 to 9. The case reports of mosaicplasty have been positive, but it should be noted that half of these patients did not have problems before an injury and mosaicplasty was done in anticipation of future issues. Regardless, mosaicplasty clearly represents a technique that can be completed with local osteochondral plugs and provides a useful tool for the treating surgeon.
Osteochondral allograft transplantation
Mosaicplasty has been shown to be a useful technique, but there can be donor site morbidity and the knee experience has also shown that there is a limit to the size of the treatable defect. Allograft transplantation has also been shown to be a successful technique for the treatment of cartilage defects. Meyers et al [5] were one of the first to describe the use of osteochondral allografts in the hip. They treated 21 patients with AVN of the femoral head with a femoral head osteochondral allograft of varying sizes. Failure was defined as moderate to severe pain or collapse of the allograft. The authors found that 50 % of the steroid induced osteonecrosis patients experienced a failure, whereas the success rate in non-steroid induced osteonecrosis was 80 %. They concluded that this is a viable treatment option for the young patient with non-steroid induced osteonecrosis of the femoral head.
Krych et al [23•] has also described the results in 2 patients of an osteochondral allograft for the treatment of large cartilage defects with associated bone loss. One case involved a 24-year-old female with previous failure of a femoral neck osteoplasty with a periacetabular cyst (18 mm × 18 mm) in the superior dome. The other case was a 32-year-old male who was treated with bone cement for fibrous dysplasia of the acetabulum with pain and protruding cement. Both patients underwent an open surgical approach and osteochondral grafting of the defect from either an allograft acetabulum or medial tibial plateau. In the first case, MRI at 12 months demonstrated incorporation of the allograft with joint congruity. Harris Hip Score (HHS) was 75 preoperatively and 97 at 2-year follow-up. In the second case, preoperative and postoperative HHS at 3 year follow-up were 79 and 100, respectively, and MRI at 18 months demonstrated incorporation of the allograft. The authors felt that the medial tibial plateau had better cartilage and congruency in the treatment of an acetabular defect.
These 2 studies have reported very good results and provide a good foundation for future study. However, the indications will need to be continually refined in order to determine the specific etiologies of hip pathology that respond well to allograft transplantation. It should also be noted that this technique does require an open surgical dislocation of the hip with its associated risks and limitations.
Articular cartilage repair
Many of the above mentioned procedures require a large open approach and are not techniques that can be utilized with hip arthroscopy. Thus, Sekiya et al [19] described a case of a 17-year-old male high school wrestler with bilateral CAM lesions as well as a 1 cm delaminated unstable cartilage flap in the anterior-superior acetabulum. The authors arthroscopically performed a microfracture underneath the flap of anterior superior acetabular cartilage and, due to flap instability, completed a suture repair of the cartilage with an absorbable polydiaxanone monofilament. This suture was chosen since it absorbs at 6 weeks, before full weight bearing, and may prevent abrasion of the femoral head. At 2-year follow-up, the patient reported 95 % of normal function for both hips. Modified Harris Hip Score (MHSS) was 96 at last follow-up, Hip Outcome Score Activities of Daily Living subscale was 93, and Hip Outcome Score Sports subscale was 81. This case presents direct cartilage repair as a possible technique to treat large delaminated full-thickness acetabular cartilage repairs and potentially prevent progression.
A larger study using a slightly different technique was completed by Tzaveas et al [26]. This prospective study of 19 patients analyzed the efficacy of using fibrin adhesive for arthroscopic repair of chondral delamination lesions with intact gross cartilage structure. Concurrent pathology was present including 15 labral tears and 18 CAM impingements that were treated simultaneously. The correction of the debrided cartilage involved creating an incision at the periphery of the acetabular labrum and passing an awl underneath to microfracture the subchondral bone. The pocket was then filled with fibrin glue, and the cartilage was pressed down until the adhesive had set (<2 minutes). Five patients underwent revision arthroscopy for various reasons at a later date, and the chondral repair appeared intact in all 5 cases. Mean MMHS was 53.3 preop and 80.3 at 1 year. Mean pain score preop was 15.7 and 28.9 at 1 year. The authors discuss that fibrin seems to have good results in short-term follow-up, and longer follow-up studies will be needed.
Conclusion
The gold standard for treatment of cartilage defects in the hip continues to be hip arthroplasty. However, there are significant risks and limitations associated with arthroplasty in the young patient. Thus, a variety of techniques have been reported for the treatment of these symptomatic chondral lesions. Microfracture, cartilage repair, autologous chondrocyte implantation, mosaicplasty, and osteochondral allografting have all been used in very limited case series. Although good results have been reported, most studies lack both a control group and a large number of patients. In order to build on the current reports and recommendations, further study is required. Nonetheless, the reported results in this paper do provide a good foundation for treatments that can provide relief and potentially delay arthroplasty in its associated morbidities in an inherently difficult young patient population.
Acknowledgments
Disclosure
Each author certifies that he has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article. This study did not receive any financial support. In addition, Institutional Review Board is not required as this study does not involve any human subjects. This study involved literature review only and did not involve any non-published human or animal data. No potential conflicts of interest relevant to this article were reported.
Footnotes
The work for this manuscript was performed at Rush University Medical Center in Chicago, IL.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
- 1.Buckwalter JA. Articular cartilage: injuries and potential for healing. J Orthop Sports Phys Ther. 1998;28:192–202. doi: 10.2519/jospt.1998.28.4.192. [DOI] [PubMed] [Google Scholar]
- 2.Sampson TG. Arthroscopic treatment for chondral lesions of the hip. Clin Sports Med. 2011;30:331–48. doi: 10.1016/j.csm.2010.12.012. [DOI] [PubMed] [Google Scholar]
- 3.Stubbs AJ, Potter HG. Section VII: chondral lesions. J Bone Joint Surg Am. 2009;91(Suppl 1):119. doi: 10.2106/JBJS.H.01452. [DOI] [PubMed] [Google Scholar]
- 4.Girard J, Roumazeille T, Sakr M, Migaud H. Osteochondral mosaicplasty of the femoral head. Hip Int. 2011;21:542–8. doi: 10.5301/HIP.2011.8659. [DOI] [PubMed] [Google Scholar]
- 5.Meyers MH. Resurfacing of the femoral head with fresh osteochondral allografts. Long-term results. Clin Orthop Relat Res. 1985;197:111–4. [PubMed] [Google Scholar]
- 6.Crawford K, Philippon MJ, Sekiya JK, Rodkey WG, Steadman JR. Microfracture of the hip in athletes. Clin Sports Med. 2006;25:327–35. doi: 10.1016/j.csm.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 7.Yen YM, Kocher MS. Chondral lesions of the hip: microfracture and chondroplasty. Sports Med Arthrosc. 2010;18:83–9. doi: 10.1097/JSA.0b013e3181de1189. [DOI] [PubMed] [Google Scholar]
- 8.Ellis HB, Briggs KK, Philippon MJ. Innovation in hip arthroscopy: is hip arthritis preventable in the athlete? Br J Sports Med. 2011;45:253–8. doi: 10.1136/bjsm.2010.082529. [DOI] [PubMed] [Google Scholar]
- 9.McCarthy JC, Lee JA. Arthroscopic intervention in early hip disease. Clin Orthop Relat Res. 2004;429:157–62. doi: 10.1097/01.blo.0000150118.42360.1d. [DOI] [PubMed] [Google Scholar]
- 10.Konan S, Rayan F, Meermans G, Witt J, Haddad FS. Validation of the classification system for acetabular chondral lesions identified at arthroscopy in patients with femoroacetabular impingement. J Bone Joint Surg Br. 2011;93:332–6. doi: 10.2106/JBJS.J.01587. [DOI] [PubMed] [Google Scholar]
- 11.Ilizaliturri VM, Jr, Byrd JW, Sampson TG. A geographic zone method to describe intra-articular pathology in hip arthroscopy: cadaveric study and preliminary report. Arthroscopy. 2008;24:534–9. doi: 10.1016/j.arthro.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 12.Keeney JA, Peelle MW, Jackson J, Rubin D, Maloney WJ, Clohisy JC. Magnetic resonance arthrography vs arthroscopy in the evaluation of articular hip pathology. Clin Orthopaed Relat Res. 2004;429:163–9. doi: 10.1097/01.blo.0000150125.34906.7d. [DOI] [PubMed] [Google Scholar]
- 13.Suzuki C, Harada Y, Mitsuhashi S. Repair of cartilage defects and torn acetabular labrum in hip joints after conventional osteotomy: evaluation by follow-up arthroscopy. J Orthop Sci. 2005;10:127–32. doi: 10.1007/s00776-004-0871-7. [DOI] [PubMed] [Google Scholar]
- 14.•• Fontana A, Bistolfi A, Crova M, Rosso F, Massazza G al. Arthroscopic Treatment of Hip Chondral Defects: autologous chondrocyte transplantation versus simple debridement-A Pilot Study. Arthroscopy. 2012;28(3):322–9. One of the only comparative studies on hip chondral treatment modalities. This study compared ACI with simple debridement. In comparable patients with similar defects, the ACI group had significantly higher clinical outcome scores. [DOI] [PubMed]
- 15.Haviv B, Singh PJ, Takla A, O'Donnell J. Arthroscopic femoral osteochondroplasty for cam lesions with isolated acetabular chondral damage. J Bone Joint Surg Br. 2010;92:629–33. doi: 10.1302/0301-620X.92B5.23667. [DOI] [PubMed] [Google Scholar]
- 16.Horisberger M, Brunner A, Herzog RF. Arthroscopic treatment of femoral acetabular impingement in patients with preoperative generalized degenerative changes. Arthroscopy. 2010;26:623–9. doi: 10.1016/j.arthro.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 17.Nam D, Shindle MK, Buly RL, Kelly BT, Lorich DG. Traumatic osteochondral injury of the femoral head treated by mosaicplasty: a report of two cases. HSS J. 2010;6:228–34. doi: 10.1007/s11420-010-9159-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Philippon MJ, Schenker ML, Briggs KK, Maxwell RB. Can microfracture produce repair tissue in acetabular chondral defects? Arthroscopy. 2008;24:46–50. doi: 10.1016/j.arthro.2007.07.027. [DOI] [PubMed] [Google Scholar]
- 19.Sekiya JK, Martin RL, Lesniak BP. Arthroscopic repair of delaminated acetabular articular cartilage in femoroacetabular impingement. Orthopedics. 2009;32. [DOI] [PubMed]
- 20.Akimau P, Bhosale A, Harrison PE. Autologous chondrocyte implantation with bone grafting for osteochondral defect due to posttraumatic osteonecrosis of the hip–a case report. Acta Orthop. 2006;77:333–6. doi: 10.1080/17453670610046208. [DOI] [PubMed] [Google Scholar]
- 21.Hart R, Janecek M, Visna P, Bucek P, Kocis J. Mosaicplasty for the treatment of femoral head defect after incorrect resorbable screw insertion. Arthroscopy. 2003;19:E1–5. doi: 10.1016/S0749-8063(03)00377-3. [DOI] [PubMed] [Google Scholar]
- 22.Sotereanos NG, DeMeo PJ, Hughes TB, Bargiotas K, Wohlrab D, Bargiotas K, Wohlrab D. Autogenous osteochondral transfer in the femoral head after osteonecrosis. Orthopedics. 2008;31:177. doi: 10.3928/01477447-20080201-33. [DOI] [PubMed] [Google Scholar]
- 23.Krych AJ, Lorich DG, Kelly BT. Treatment of focal osteochondral defects of the acetabulum with osteochondral allograft transplantation. Orthopedics. 2011;34:e307–11. doi: 10.3928/01477447-20110526-24. [DOI] [PubMed] [Google Scholar]
- 24.Stralen RA, Haverkamp D, Bergen CJ, Eijer H. Partial resurfacing with varus osteotomy for an osteochondral defect of the femoral head. Hip Int. 2009;19:67–70. doi: 10.1177/112070000901900113. [DOI] [PubMed] [Google Scholar]
- 25.Stafford GH, Bunn JR, Villar RN. Arthroscopic repair of delaminated acetabular articular cartilage using fibrin adhesive. Results at one to three years. Hip Int. 2011;21:744–50. doi: 10.5301/HIP.2011.8843. [DOI] [PubMed] [Google Scholar]
- 26.Tzaveas AP, Villar RN. Arthroscopic repair of acetabular chondral delamination with fibrin adhesive. Hip Int. 2010;20:115–9. doi: 10.1177/112070001002000117. [DOI] [PubMed] [Google Scholar]
- 27.Byrd JW, Jones KS. Osteoarthritis caused by an inverted acetabular labrum: radiographic diagnosis and arthroscopic treatment. Arthroscopy. 2002;18:741–7. doi: 10.1053/jars.2002.32837. [DOI] [PubMed] [Google Scholar]
- 28.Field RE, Rajakulendran K, Strambi F. Arthroscopic grafting of chondral defects and subchondral cysts of the acetabulum. Hip Int. 2011;21:479–86. doi: 10.5301/HIP.2011.8542. [DOI] [PubMed] [Google Scholar]
- 29.Rittmeister M, Hochmuth K, Kriener S, Richolt J. Five-year results following autogenous osteochondral transplantation to the femoral head. Orthopade. 2005;34(320):322–6. doi: 10.1007/s00132-005-0776-y. [DOI] [PubMed] [Google Scholar]
- 30.Anderson LA, Crofoot CD, Erickson J, Morton DA, Peters CL. Acetabular osteochondroplasty and simultaneous reorientation: background and validation of concept. Orthopedics. 2010;33. [DOI] [PubMed]


