Abstract
More than 200 cells were cloned from populations of mammalian cells persistently infected with Japanese encephalitis virus. Only four cloned cultures contained cells that had viral antigen measurable by immunofluorescence and that released infectious virus, yet all clones harbored virus-specific RNA. Superinfection of cloned cells with wild-type Japanese encephalitis virus did not produce cytopathic effects, but resulted in production of viral antigen and infectious virus in formerly nonproducing clones. Cocultivation of nonproducer clone cells with normally permissive cells did not induce virus production, nor did treatment of nonproducer clones with various inhibitors of DNA, RNA, or protein synthesis. It is suggested that the cloning procedure may have selected for a particular subpopulation of cells and that defective virus is also involved in establishment and maintenance of persistent infection.
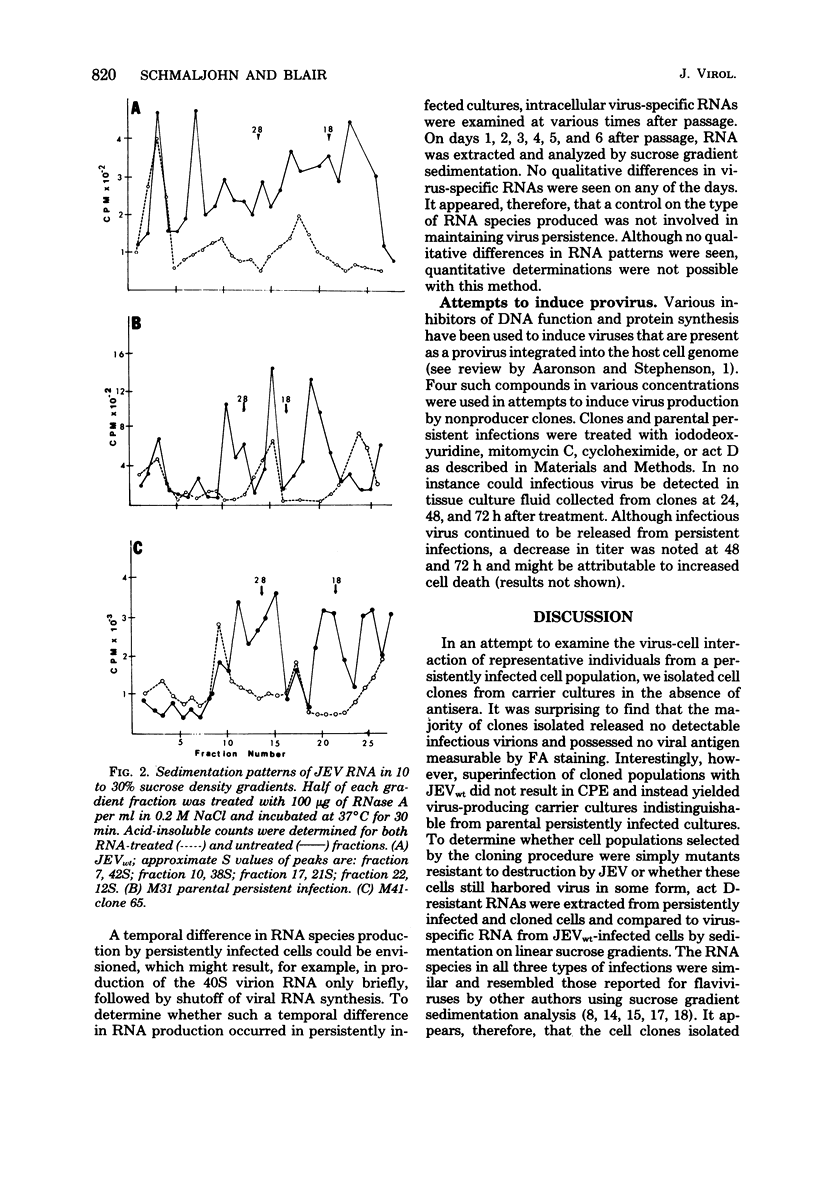
Full text
PDF

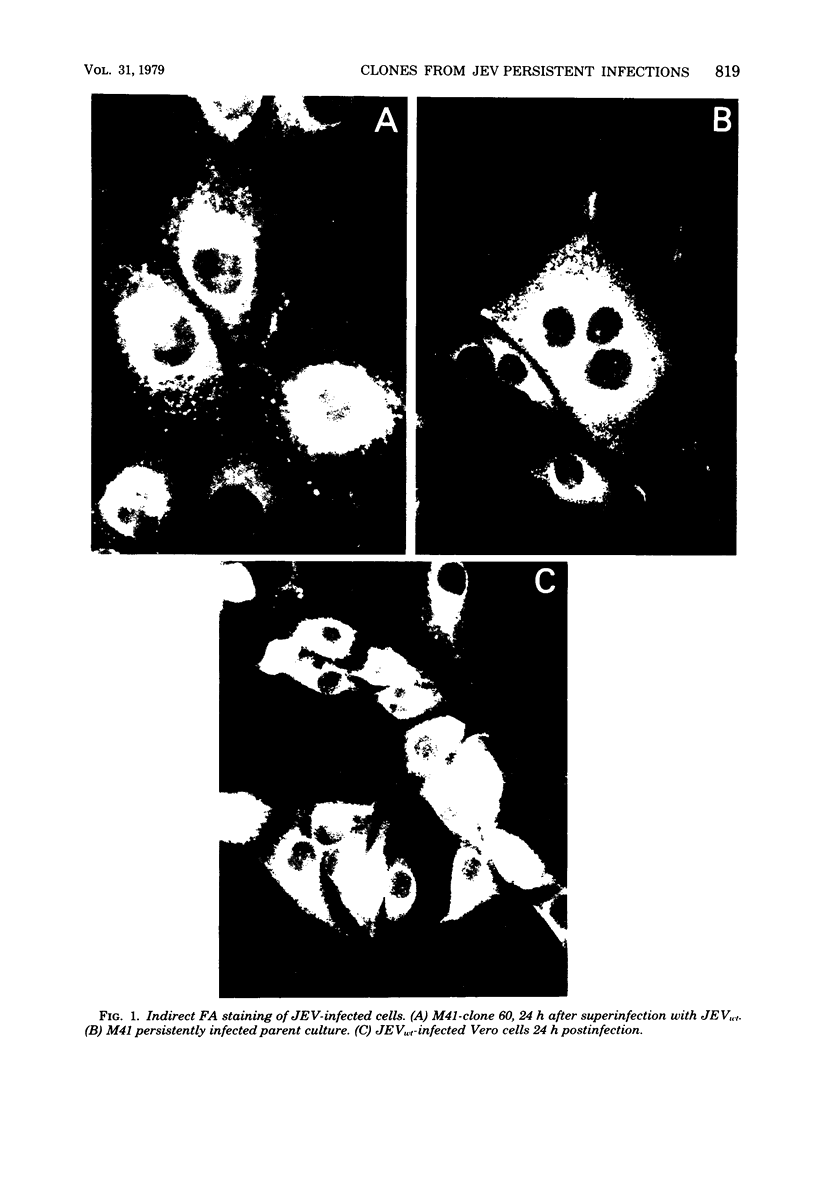


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aaronson S. A., Stephenson J. R. Endogenous type-C RNA viruses of mammalian cells. Biochim Biophys Acta. 1976 Dec 23;458(4):323–354. doi: 10.1016/0304-419x(76)90006-8. [DOI] [PubMed] [Google Scholar]
- Carreño G., Esparza J. Induction of Venezuelan equine encephalitis (Mucambo) virus by iododeoxyuridine in chronically infected 'cured' cultured mosquito cells. Intervirology. 1977;8(4):193–203. doi: 10.1159/000148895. [DOI] [PubMed] [Google Scholar]
- Darnell M. B., Koprowski H. Genetically determined resistance to infection with group B arboviruses. II. Increased production of interfering particles in cell cultures from resistant mice. J Infect Dis. 1974 Mar;129(3):248–256. doi: 10.1093/infdis/129.3.248. [DOI] [PubMed] [Google Scholar]
- Darnell M. B., Koprowski H., Lagerspetz K. Genetically determined resistance to infection with group B arboviruses. I. Distribution of the resistance gene among various mouse populations and characteristics of gene expression in vivo. J Infect Dis. 1974 Mar;129(3):240–247. doi: 10.1093/infdis/129.3.240. [DOI] [PubMed] [Google Scholar]
- Igarashi A., Koo R., Stollar V. Evolution and properties of Aedes albopictus cell cultures persistently infected with sindbis virus. Virology. 1977 Oct 1;82(1):69–83. doi: 10.1016/0042-6822(77)90033-2. [DOI] [PubMed] [Google Scholar]
- Minagawa T. Studies on the persistent infection with measles virus in HeLa cells. I. Clonal analysis of cells of carrier cultures. Jpn J Microbiol. 1971 Jul;15(4):325–331. doi: 10.1111/j.1348-0421.1971.tb00588.x. [DOI] [PubMed] [Google Scholar]
- Naeve C. W., Trent D. W. Identification of Saint Louis encephalitis virus mRNA. J Virol. 1978 Feb;25(2):535–545. doi: 10.1128/jvi.25.2.535-545.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northrop R. L. Effect of puromycin and actinomycin D on a persistent mumps virus infection in vitro. J Virol. 1969 Aug;4(2):133–140. doi: 10.1128/jvi.4.2.133-140.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rima R. K., Martin S. J. Persistent infection of tissue culture cells by RNA viruses. Med Microbiol Immunol. 1976 Jun 1;162(2):89–119. doi: 10.1007/BF02121320. [DOI] [PubMed] [Google Scholar]
- Rustigian R. Persistent infection of cells in culture by measles virus. I. Development and characteristics of HeLa sublines persistently infected with complete virus. J Bacteriol. 1966 Dec;92(6):1792–1804. doi: 10.1128/jb.92.6.1792-1804.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmaljohn C., Blair C. D. Persistent infection of cultured mammalian cells by Japanese encephalitis virus. J Virol. 1977 Nov;24(2):580–589. doi: 10.1128/jvi.24.2.580-589.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda H., Oya A., Hashimoto K., Yamada M. Intracellular distribution of virus-specific RNA in chick embryo cells infected with Japanese encephalitis virus. J Gen Virol. 1977 Jan;34(1):201–205. doi: 10.1099/0022-1317-34-1-201. [DOI] [PubMed] [Google Scholar]
- Trent D. W., Swensen C. C., Qureshi A. A. Synthesis of Saint Louis encephalitis virus ribonucleic acid in BHK-21-13 cells. J Virol. 1969 Apr;3(4):385–394. doi: 10.1128/jvi.3.4.385-394.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WALKER D. L., HINZE H. C. A carrier state of mumps virus in human conjunctiva cells. II. Observations on intercellular transfer of virus and virus release. J Exp Med. 1962 Nov 1;116:751–758. doi: 10.1084/jem.116.5.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong K. T., Robinson W. S., Merigan T. C. Synthesis of viral-specific ribonucleic Acid in rubella virus-infected cells. J Virol. 1969 Dec;4(6):901–903. doi: 10.1128/jvi.4.6.901-903.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zebovitz E., Leong J. K., Doughty S. C. Japanese encephalitis virus replication: a procedure for the selective isolation and characterization of viral RNA species. Arch Gesamte Virusforsch. 1972;38(4):319–327. doi: 10.1007/BF01262822. [DOI] [PubMed] [Google Scholar]
- Zhdanov V. M., Bogomolova N. N., Gavrilov V. I., Andzhaparidze O. G., Deryabin P. G., Astakhova A. N. Infectious DNA of tick-borne encephalitis virus. Arch Gesamte Virusforsch. 1974;45(3):215–224. doi: 10.1007/BF01249684. [DOI] [PubMed] [Google Scholar]