Abstract
The treatment of septicemia caused by antibiotic-resistant bacteria is a great challenge in the clinic. Because traditional antibiotics inevitably induce bacterial resistance, which is responsible for many treatment failures, there is an urgent need to develop novel antibiotic drugs. Amino-terminated Poly(amidoamine) dendrimers (PAMAM-NH2) are reported to have antibacterial activities. However, previous studies focused on high generations of PAMAM-NH2, which have been found to exhibit high toxicities. The present study aimed to clarify whether low generations of PAMAM-NH2 could be used as novel antibacterial agents. We found that generation 2 (G2.0) PAMAM-NH2 showed significant antibacterial effects against antibiotic-sensitive and antibiotic-resistant strains but exhibited little toxicity to human gastric epithelial cells and did not induce antibiotic resistance in bacteria. Scanning and transmission electron microscopy analyses suggested that G2.0 PAMAM-NH2 might inhibit the growth of bacteria by destroying their cell membranes. The administration of G2.0 PAMAM-NH2 dose-dependently improved the animal survival rate of mice infected with extended-spectrum beta lactamase-producing Escherichia coli (ESBL-EC) and of animals infected with a combination of ESBL-EC and methicillin-resistant Staphylococcus aureus. A treatment regimen of 10 mg/kg of G2.0 PAMAM-NH2 starting 12 h before inoculation followed by 10 mg/kg at 0.5 h after inoculation rescued 100% of singly infected mice and 60% of multiply infected mice. The protective effects were associated with the reduction of the bacterial titers in the blood and with the morphological amelioration of infected tissues. These findings demonstrate that the G2.0 PAMAM-NH2 is a potential broad-spectrum and nonresistance-inducing antibiotic agent with relatively low toxicity.
Electronic supplementary material
The online version of this article (doi:10.1208/s12248-012-9416-8) contains supplementary material, which is available to authorized users.
Key words: antibacterial activity, antibiotic resistance, extended-spectrum beta lactamase-producing Escherichia coli, methicillin-resistant Staphylococcus aureus, PAMAM dendrimers
INTRODUCTION
Septicemia caused by bacterial infection is one of the most important causes of mortality in hospitals. The failure of treatments for septicemia is primarily due to the emergence of antibiotic-resistant strains, which is a worldwide healthcare concern (1). Among these resistant strains, methicillin-resistant Staphylococcus aureus (MRSA) and extended spectrum β-lactamase-producing Escherichia coli (ESBL-EC) account for most nosocomial infections (2,3). One of the most intractable problems with traditional antibiotics is that they inevitably induce bacterial resistance. In addition, most new antibiotics are chemical modifications of the few classes of known antibiotics originally discovered half a century ago, and few major classes of antibiotics were introduced between 1962 and 2007 (4). It takes approximately 10 years to develop a new antibiotic, whereas bacterial resistance can arise in 1 to 2 years or even just several months. Therefore, the discovery and development of novel antibacterial agents, especially those with structures and mechanisms of action that differ from those of traditional antibiotics and a low potential to induce antibiotic resistance, are needed more than ever.
Cationic dendrimers have emerged as promising novel antibiotic agents in recent years (5,6). Dendrimers are a new class of hyperbranched and nanoscale macromolecules that possess an array of distinctive features including a well-defined globular architecture, narrow polydispersity and tunable surface functionalities. A typical dendrimer consists of a core molecule, monomeric branches called dendrons and surface functional groups that are able to react with other compounds (7–10). Poly(amidoamine) (PAMAM) dendrimers are the most widely studied dendrimers. Among the PAMAM dendrimers harboring a variety of functional groups, amino-terminated PAMAM dendrimers (PAMAM-NH2) show the strongest antibacterial activity. However, the previous studies primarily investigated the antibacterial activity of generation 3 or higher PAMAM-NH2 dendrimers on limited bacterial strains in vitro (11,12). However, the higher-generation PAMAM-NH2 dendrimers often show stronger cytotoxicity, which hinders further exploration of these compounds as antibacterial agents in vivo (13–15). Chemical modifications of PAMAM-NH2 dendrimers were demonstrated to reduce their toxicities, but these modifications simultaneously reduced the antibacterial activities of the PAMAM-NH2 dendrimers (11,16). To date, antibacterial research on PAMAM-NH2 dendrimers has been limited to topical (17–19) and drug carrier applications (20–22). Insufficient data are available to determine whether PAMAM-NH2 dendrimers could be used systemically as antibacterial agents.
To determine the in vivo therapeutic potential of PAMAM-NH2 dendrimers, several critical issues should be addressed. Can lower generations of PAMAM-NH2 serve as novel antimicrobial agents with low toxicity? Will PAMAM-NH2 dendrimers induce bacterial resistance like traditional antibiotics do? What are the potential mechanisms underlying the different responses of bacterial and eukaryotic cells to PAMAM-NH2 dendrimers? Can PAMAM-NH2 dendrimers be used to treat severe infections caused by antibacterial-resistant strains with favorable compatibility in vivo?
Here, we demonstrated that a low-generation PAMAM-NH2 dendrimer (G2.0 PAMAM-NH2) has broad-spectrum antimicrobial activities, excellent therapeutic efficacy for rescuing mice with septicemia induced by ESBL-EC alone or ESBL-EC and MRSA together, and a relatively low cytotoxicity. In addition, this dendrimer does not induce bacterial resistance. Our current results reveal new features of the antibiotic effects of G2.0 PAMAM-NH2, which may be a novel antimicrobial agent that does not induce drug resistance.
MATERIALS AND METHODS
Materials
Amino-terminated generation 1 PAMAM (G1.0 PAMAM-NH2, 9.98% (w/w) in H2O), generation 2 PAMAM (G2.0 PAMAM-NH2, 10.04% (w/w) in H2O), generation 3 PAMAM (G3.0 PAMAM-NH2, 10.01% (w/w) in H2O), and generation 4 PAMAM (G4.0 PAMAM-NH2, 9.76% (w/w) in H2O) were purchased from Dendritech (Midland, MI). All antibiotics used were purchased from the National Institute for the Control of Pharmaceutical and Biological Products (Beijing, China). All other chemicals and solvents were of analytical grade.
Bacterial Strains
E. coli MG1655 was obtained from Prof. Liping Zhao (Shanghai Jiaotong University). Clinical isolates of MRSA (XJ75302), ESBL-Klebsiella pneumoniae (ESBL-KP, XJ75297) and multidrug-resistant E. coli (MDR-EC, XJ74283) were obtained from the Clinical Laboratory of Xijing Hospital (Xi’an, China). Multidrug-resistant Salmonella typhimurium (MDR-S. typhimurium), multidrug-resistant Shigella flexneri (MDR-S. flexneri), and multidrug-resistant Acinetobacter baumannii (MDR-A. baumannii were obtained from Dr. Jun Li (Tangdu Affiliated Hospital, Fourth Military Medical University, Xi’an, China). E. coli (ATCC 25922), MRSA (WHO-2), vancomycin intermediate-resistant S. aureus (VIR-S. aureus Mu50), S. aureus (ATCC 29213), Pseudomonas aeruginosa (ATCC 27853), Salmonella enterica (ATCC 9150), K. pneumoniae subsp. pneumoniae (ATCC13883), Staphylococcus epidermidis (ATCC 14990), ESBL-EC (ATCC 35218), and entero-hemorrhagic E. coli O157:H7 were used as references and were obtained from the Chinese National Center for Surveillance of Antimicrobial Resistance.
Bacterial Susceptibility Testing and Growth Assay
The minimum inhibitory concentration (MIC) values were determined by a microdilution assay in sterile 96-well polypropylene microtiter plates according to the broth microdilution guidelines of the Clinical and Laboratory Standards Institute (23). The minimum bactericidal concentration (MBC) values were determined by plating 10 μL samples from the wells with no visible turbidity onto Mueller–Hinton agar plates. The lowest concentration without visible growth on the agar subculture was taken as the MBC value. All experiments were performed independently three times, using duplicate samples each time.
The growth curves were determined as follows: PAMAM-NH2 and ampicillin were added to cell cultures containing E. coli MG1655 or VIR-S. aureus Mu50 to a final concentration of 12, 60, or 65 μM. An equal volume of diluent was used as a control. Each culture was monitored based on the optical absorbance at 620 nm at 1- to 2-h intervals.
To construct kill curves for ESBL-EC and MRSA (XJ75302), PAMAM-NH2 and ampicillin were added to ESBL-EC cultures or MRSA (XJ75302) cultures to a final concentration of 12, 60, or 65 μM. An equal volume of diluent was used as a control. Aliquots of each culture were collected at 0.5, 1, 2, 4, and 6 h and were diluted and inoculated onto solid agar. The number of colony-forming units (CFU) was calculated from the number of colonies growing on the plates. The statistical data for each experiment were obtained from at least two independent assays performed in duplicate.
Therapeutic Effects of G2.0 PAMAM-NH2 in Infected Animals
Male BALB/c mice, 8–10 weeks of age and weighing 18–22 g, were used in this study. The first animal model of infection was prepared by the intraperitoneal administration of 7.1 × 105 CFU ESBL-EC in 0.4 mL of Mueller–Hinton broth. The second animal model of infection was prepared by the intraperitoneal administration of a mixed bacterial suspension containing 4.0 × 105 CFU ESBL-EC and 6.6 × 108 CFU MRSA (XJ75302) in 0.4 mL of Mueller–Hinton broth. Then, the infected mice were randomly treated with a control solution (0.2% bovine serum albumin containing 0.01% acetic acid), 20 mg/kg ampicillin, 10 mg/kg G2.0 PAMAM-NH2, 20 mg/kg G2.0 PAMAM-NH2, or 10 + 10 mg/kg G2.0 PAMAM-NH2 (10 mg/kg was administered 12 h before the bacterial challenge and another 10 mg/kg was administered 0.5 h after bacterial challenge). To assess bacterial clearance, blood samples from six mice in each group were obtained from the tail vein by aseptic percutaneous puncture 12 h after bacterial challenge. The blood samples were serially diluted, and a 100-μL volume of each dilution was spread on Mueller–Hinton agar plates. The plates were incubated at 37°C for 24 h, and the CFU were enumerated by visual inspection. Parts of the lungs and livers were harvested and fixed in 10% neutral buffered formalin for 48 h, and then their morphologies were observed using hematoxylin and eosin staining. The PAMAMs used in the animal experiments were dissolved in 0.2% bovine serum albumin containing 0.01% acetic acid. The survival of the 12 mice in each group was monitored every 1 h for 7 days after infection, and the cumulative percent survival was determined.
Toxicity Assays
-
MTT assay
The cytotoxicity of G1.0, G2.0, G3.0, and G4.0 PAMAM-NH2 to the human gastric epithelial cell line GES-1 was determined by a standard MTT assay (24,25). Briefly, cells were maintained in DMEM media with 10% fetal bovine serum (FBS) and 30 μg/mL gentamicin. Cells (5.0 × 103/well) were grown for 24 h at 37 C to confluence in 96-well plates. The media with FBS was removed, and the cells were washed with 1 × phosphate-buffered saline (PBS) and serum-starved for 4 h by incubation in DMEM media alone at 37°C. The medium in the plate was replaced with 100 μL of a prepared PAMAM dendrimer solution. Then, the plate was incubated at 37°C for 48 h. The absorbance was read at 490 nm.
-
Median lethal dose (LD50)
Male and female BALB/c mice, 8–10 weeks of age and weighing approximately 20 g, were used in this study. Animals were maintained in a humidity- and temperature-controlled facility with a 12-h light/dark cycle. The LD50 was determined by the Spearman–Karber method (13,26,27). Briefly, the animals were intraperitoneally injected with G2.0 PAMAM-NH2 dissolved in 0.01 M PBS, pH 7.4, at doses of 30, 47, 73.5, 115, and 180 mg/kg. Control animals received the vehicle alone. The animals were observed for a period of 4 h after injection for behavioral abnormalities such as changes in horizontal or vertical motion, level of activity, and eating and drinking behavior. The percent survival of each concentration group was observed for 7 days and recorded to calculate the LD50.
Induction of Resistance
After the MIC values were determined for S. aureus (ATCC 29213) and ESBL-EC (ATCC 35218) as described above, the MIC values were determined anew daily as follows (28,29). For each compound tested, a bacterial suspension was obtained from the well corresponding to one half of the MIC determined in the previous MIC assay, and the concentration of this suspension was adjusted to the 0.5 McFarland Standard in Mueller–Hinton broth. Then, the suspension was diluted 1:100 to the concentration of 5.0 × 105 cells/mL in Mueller–Hinton broth. The MIC was determined again as described for the initial MIC assay. The relative MIC value was determined by calculating the ratio of the MIC obtained for the 15th subculture to the MIC obtained for the first culture.
Scanning and Transmission Electron Microscopy Detection
Bacteria (1.0×108 CFU/mL) were cultured with G2.0 PAMAM-NH2 (12 μM) at 120 rpm for 60, 120, and 300 min; harvested; and washed. The specimens were observed with a scanning electron microscope (Hitachi S-3400N) or a transmission electron microscope (JEM-2000EX; JOEL), and images were recorded.
Statistical Analysis
Results are expressed as the mean values (±SD). Student’s t test, one-way ANOVA, and Kaplan–Meier survival analysis were used to evaluate statistical significance as appropriate. A probability value of p < 0.05 was considered indicative of statistical significance.
RESULTS
Broad-Spectrum Antibacterial Activities of G1.0, G2.0, G3.0, and G4.0-PAMAM-NH2
Seventeen bacterial strains were used to systematically test the antibacterial activities and spectrum of G1.0–4.0 PAMAM-NH2 dendrimers in vitro. As shown in Table I, G2.0–4.0 PAMAM-NH2 exhibited potent antibacterial effects against almost all Gram-positive and Gram-negative bacterial strains tested (15 of 17 strains, all except ESBL-KP and MDR-S. typhimurium), with most MIC values ranging from 0.78 to 25 μg/mL. In the control assays, the MIC values of oxacillin, ceftazidime, and levofloxacin for susceptible strains were lower (0.12–8 μg/mL), but the MIC values for these antibiotics resistant strains were much higher. The antibacterial activity of G1.0 PAMAM-NH2 was much lower than the activities of the other three generations of PAMAM-NH2. Interestingly, G2.0 PAMAM-NH2 showed the greatest antibacterial activity against almost all bacterial strains (Table I). This dendrimer had 2-fold higher MBC/MIC values against S. epidermidis (ATCC 14990), ESBL-EC (ATCC 35218), and MDR-S. flexneri and 4-fold higher values against E. coli (ATCC 25922), S. aureus (ATCC 29213), MRSA (XJ75302), K. pneumoniae (ATCC13883), and S. enteric (ATCC 9150).
Table I.
Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC) of Amino-Terminated Poly(amidoamine) in Mueller–Hinton Broth Culture
| Strain | MIC (μg/mL) | MBC (fold increase) | ||||||
|---|---|---|---|---|---|---|---|---|
| PAMAM | OXA | CAZ | LEV | PAMAM G2.0 | ||||
| G1.0 | G2.0 | G3.0 | G4.0 | |||||
| Escherichia coli | ||||||||
| ESBL-ATCC 35218 | 50 | 6.25 | 12.5 | 12.5 | >256 | 64 | 16 | 2 |
| MDR-XJ74283 | >100 | 12.5 | 50 | >100 | >256 | 64 | 256 | ND |
| EHEC O157:H7 | 50 | 6.25 | 12.5 | 12.5 | ND | ND | 1 | ND |
| MG1655 | >100 | 6.25 | 12.5 | 12.5 | 0.5 | 0.5 | 8 | ND |
| ATCC 25922 | 50 | 6.25 | 12.5 | 12.5 | 0.5 | 0.5 | 0.5 | 4 |
| Staphylococcus aureus | ||||||||
| ATCC 29213 | 50 | 6.25 | 6.25 | 6.25 | 0.25 | 2 | 0.5 | 4 |
| MRSA XJ75302 | 50 | 6.25 | 6.25 | 6.25 | >256 | 256 | 16 | 4 |
| MRSA WHO-2 | 50 | 3.12 | 6.25 | 6.25 | >256 | ND | 8 | ND |
| VIR Mu50 | 50 | 6.25 | 6.25 | 12.5 | 8 | ND | 8 | ND |
| Staphylococcus epidermidis ATCC 14990 | 25 | 0.78 | 1.56 | 3.12 | 0.12 | ND | 0.12 | 2 |
| ESBL-KP XJ75297 | >100 | 100 | >100 | >100 | >256 | >128 | 2 | ND |
| Salmonella enterica ATCC 9150 | 50 | 3.12 | 12.5 | 12.5 | 0.5 | 0.25 | 4 | 4 |
| Pseudomonas aeruginosa ATCC 27853 | 50 | 6.25 | 12.5 | 12.5 | 2 | 2 | 0.25 | ND |
| Klebsiella pneumoniae ATCC13883 | 50 | 6.25 | 12.5 | 12.5 | ND | ND | 1 | 4 |
| MDR- Shigella flexneri | 50 | 6.25 | 6.25 | 12.5 | ND | ND | 2 | 2 |
| MDR- Salmonella typhimurium | >100 | 100 | 100 | 100 | ND | ND | 64 | ND |
| MDR- Acinetobacter baumannii | >100 | 25 | 25 | 50 | ND | ND | 32 | ND |
OXA oxacillin, CAZ ceftazidime, LEV levofloxacin, MDR multi-drug resistance, ESBL extended-spectrum beta-lactamase, KP K. pneumoniae, MRSA methicillin-resistant S. aureus, VIR vancomycin intermediate-resistant, EHEC entero-hemorrhagic E. coli O157:H7, ND test not done
Additional experiments were performed to determine the growth rate of E. coli MG1655 in liquid medium containing G1.0–4.0 PAMAM-NH2 dendrimers (Fig. 1 in the Electronic Supplementary Material (ESM)). The results showed that the growth of E. coli MG1655 was apparently inhibited by G1.0–4.0 PAMAM-NH2 dendrimers in a concentration-dependent manner. The representative results shown in Fig. 1a, b revealed that 60 μM G1.0 PAMAM-NH2 had the least inhibitory efficacy on the growth of E. coli MG1655 and VIR-S. aureus Mu50, and 12 μM G2.0 PAMAM-NH2 exhibited the greatest inhibitory effect among all generations of PAMAM-NH2.
Fig. 1.
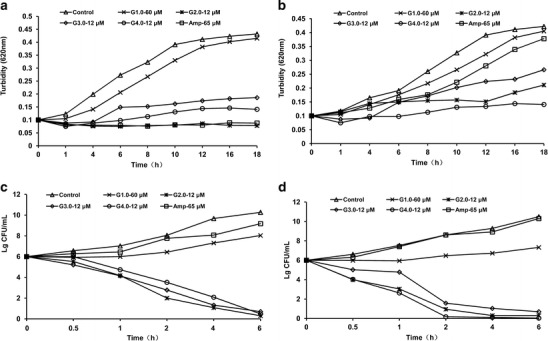
In vitro growth inhibition and bactericidal effects of G1.0–4.0 PAMAM-NH2 dendrimers. E. coli MG1655 (a) and VIR-S. aureus Mu50 (b) were cultured in liquid culture medium and treated with different concentrations of PAMAM-NH2 dendrimers. Aliquots of each culture were removed at 1- to 2-h intervals and evaluated spectrophotometrically at 620 nm. ESBL-EC (c) and MRSA (d) cultures were established in Mueller–Hinton broth at 106 CFU/mL and treated with PAMAM dendrimers at 12 or 60 μM. Aliquots of each culture were collected at 0.5, 1, 2, 4, and 6 h and were diluted and inoculated onto solid agar. The number of CFU was calculated from the number of colonies growing on the plates
The bactericidal effects of the G1.0–4.0 PAMAM-NH2 dendrimers were evaluated using cell viability assays. G2.0–4.0 PAMAM-NH2 dendrimers, but not G1.0 PAMAM-NH2, exerted time-dependent bactericidal effects on the tested pathogens. The CFU values of ESBL-EC and MRSA decreased appreciably from a starting value of 106 CFU/mL, and less than 50 CFU/mL was detected 6 h after treatment with G2.0–4.0 PAMAM-NH2 dendrimers at 12 μM. The results of the cell viability assays using G1.0–4.0 PAMAM-NH2 dendrimers were in accordance with the results of the cell growth assays described above. Compared with the control group, ampicillin and G1.0 PAMAM-NH2 had no bactericidal effects against ESBL-EC or MRSA at the tested concentrations (Fig. 1c, d). These results demonstrate that G2.0–4.0 PAMAM-NH2 dendrimers exerted broad-spectrum antibacterial activity in a bactericidal manner.
In Vitro Toxicity of PAMAM-NH2
To further explore the selectivity of PAMAM-NH2 dendrimers, we investigated their cytotoxicities to the human gastric epithelial cell line GES-1 in vitro. The cell viability determined using the MTT assay vs. the concentration of the dendrimers is plotted in Fig. 2. The results indicate that the cytotoxicity of PAMAM-NH2 was related to the generation of the dendrimers, the cell type tested and the concentration of the PAMAM-NH2. The cytotoxicities increased significantly with increasing PAMAM-NH2 generation and concentration (Fig. 2). Among all of the PAMAM-NH2 dendrimers tested, G1.0 and G2.0 PAMAM-NH2 showed the least toxicity to GES-1 cells, exhibiting only slight toxicity to GES-1 cells even at the highest concentration of 1,024 μg/mL (Fig. 2). In contrast, a significant decrease in the GES-1 cell viability was observed upon treatment with G3.0 PAMAM-NH2 at concentrations higher than 102.4 μg/mL and upon treatment with G4.0 at concentrations higher than 0.51 μg/mL (Fig. 2). These results imply that G1.0 and G2.0 PAMAM-NH2 have minimal toxicity to cells at concentrations lower than 102.4 μg/mL. Given that G2.0 PAMAM-NH2 has antibacterial activity at concentrations from 0.78 to 25 μg/mL, the G2.0 PAMAM-NH2 dendrimer has a relatively wide range of safe usage for potential therapeutic applications.
Fig. 2.
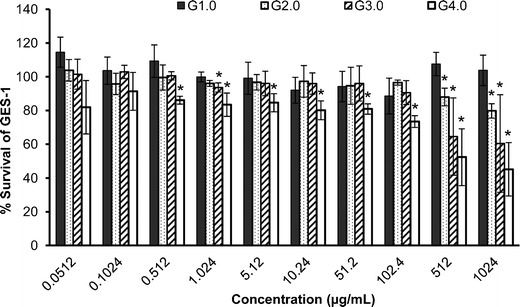
Percent survival of GES-1 cells after incubation with various concentrations of G1.0–4.0 PAMAM-NH2 dendrimers for 48 h. Untreated GES-1 cells were used as the control. Each plot was obtained from a representative experiment, and the data points are the means of four replicates ± standard deviation. The values were compared with the value for the untreated control at *p < 0.05 using Tukey’s test
In Vivo Antibacterial Activity
After we found that G2.0 PAMAM-NH2 exhibited stronger antibacterial activity and lower cytotoxicity than higher generation PAMAM-NH2 dendrimers in vitro, we next investigated whether G2.0 PAMAM-NH2 could improve the survival of infected animals in vivo. The challenge experiments were performed using ESBL-EC alone or ESBL-EC and MRSA together. All of the mice infected with ESBL-EC in the control group or in the 20 mg/kg ampicillin-treated group died within 24 h. Intraperitoneal administration of 10 and 20 mg/kg G2.0 PAMAM-NH2 dose-dependently improved the animal survival rate to 25.0% and 41.7%, respectively, compared with the 0% survival rate in the control group and ampicillin-treated group (Fig. 3). In addition, G2.0 PAMAM-NH2 treatment with the regimen of 10 + 10 mg/kg rescued 100% infected mice (Fig. 3). This increased rescue was associated with a reduction in the bacterial titer in the blood of mice inoculated with ESBL-EC. The bacterial titer in the blood was 3.0×107 CFU/mL in the control group and was reduced to 7.5 × 106, 1.2 × 106, and 6.2 × 104 after treatment with 10, 20, and 10 + 10 mg/kg G2.0 PAMAM-NH2, respectively. In contrast, no reduction in the bacterial titer was found in the group that received 20 mg/kg ampicillin (Fig. 3b). To further investigate the protective effect of G2.0 PAMAM-NH2 against sepsis in mice, we performed an in vivo assay in a multiple infection animal model, in which the mice were infected with both ESBL-EC and MRSA. All multiply infected mice that did not receive treatment died within 24 h, whereas 10% of the ampicillin-treated mice survived. Treatment with 10, 20, and the regimen of 10 + 10 mg/kg G2.0 PAMAM-NH2 significantly improved the survival rate of infected animals to 30%, 40%, and 60%, respectively (Fig. 3c, d).
Fig. 3.
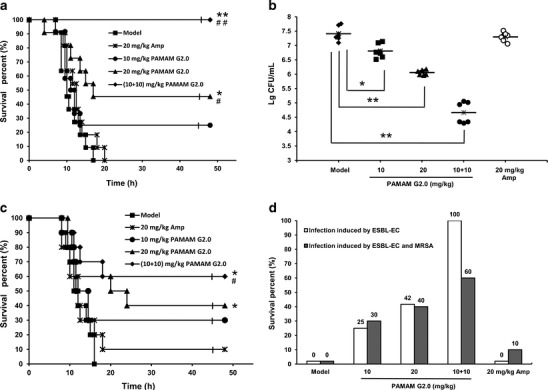
In vivo antibacterial effects of G2.0 PAMAM-NH2 in mice infected with ESBL-EC alone or ESBL-EC and MRSA. a Survival of BALB/c mice (n = 12) inoculated by intraperitoneal injection with ESBL-EC (7.1 × 105 CFU) and treated with G2.0 PAMAM-NH2 at 10 mg/kg (circles), 20 mg/kg (triangles), or 10 + 10 mg/kg (diamonds; 10 mg/kg was administered 12 h before the bacterial challenge, and another 10 mg/kg was administered after the bacterial challenge); a single dose of ampicillin at 20 mg/kg (*) and diluent (■, infected group) were administered as controls. b ESBL-EC in the blood cultures of G2.0 PAMAM-NH2 dendrimer- and ampicillin-treated BALB/c mice. c Survival of BALB/c mice (n = 10) inoculated by intraperitoneal injection with 8.0 × 105 CFU ESBL-EC and 6. 6 × 108 CFU MRSA and treated with different concentrations of PAMAM-NH2 dendrimer. d Survival rates of BALB/c mice on the 7th day after infection and treatment. *p < 0.05; **p < 0.01 vs. the infected group; #p < 0.05; ##p < 0.01 vs. the ampicillin-treated group
Pathological observations and the analysis of the mouse tissues indicated that the morphological structure of the lung tissue samples from the control ESBL-EC-infected mice was very similar to that of the samples from the ampicillin-treated group (Fig. 4b, c). In these two groups, the infiltration and accumulation of neutrophils and the disappearance of the alveolar structure were observed (Fig. 4a). Alveolar fusion, alveolar septum thickening, and neutrophil infiltration were significantly reduced in the groups treated with 10, 20, and 10 + 10 mg/kg of G2.0 PAMAM-NH2 (Fig. 4d–f). Hematoxylin and eosin staining of BALB/c mouse liver tissue showed similar results (Fig. 2 in the ESM).
Fig. 4.
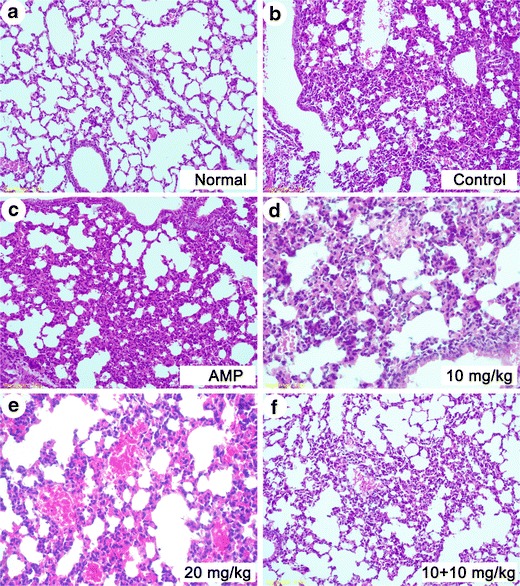
Morphology of the lungs examined with hematoxylin and eosin staining in BALB/c mice (original magnification, ×200). a Normal; b control; c 20 mg/kg ampicillin treatment; d 10 mg/kg G2.0 PAMAM-NH2 treatment; e 20 mg/kg G2.0 PAMAM-NH2 treatment; and f G2.0 PAMAM-NH2 10 + 10 mg/kg regimen treatment (10 mg/kg was administered 12 h before the bacterial challenge, and another 10 mg/kg was administered after the bacterial challenge)
In Vivo Toxicity of PAMAM-NH2
The in vivo toxicity was analyzed by determining the LD50 of G2.0 PAMAM-NH2 in BALB/c mice to evaluate its safety. All of the mice that received 180 mg/kg displayed symptoms of toxicity including twitching, stiffness and urinary and fecal incontinence 30 min after administration and died within 3 h after administration (Table I in the ESM). No symptoms of toxicity were observed in the 30 and 47 mg/kg groups during the first 2-h period following administration, and one mouse in the 47 mg/kg group died approximately 72 h after treatment. In the 115 mg/kg group, one, two, and seven mice died by 3, 60, and 120 h, respectively, after administration. The LD50 with a 95% confidence limit of G2.0 PAMAM-NH2 was 80 ± 20 mg/kg.
Induction of Resistance
After 15 successive subcultures, the relative MIC values of G2.0 PAMAM-NH2 for S. aureus (ATCC 29213) and ESBL-EC (ATCC 35218) were unchanged (Fig. 5a, b), indicating a lack of induction of antibiotic resistance. In contrast, the MIC values of classical antibiotics, including ceftazidime, ampicillin, levofloxacin, oxacillin, and erythromycin, increased by 8- to 64-fold after 15 successive subcultures, reflecting the emergence of resistant strains (Fig. 5a, b).
Fig. 5.
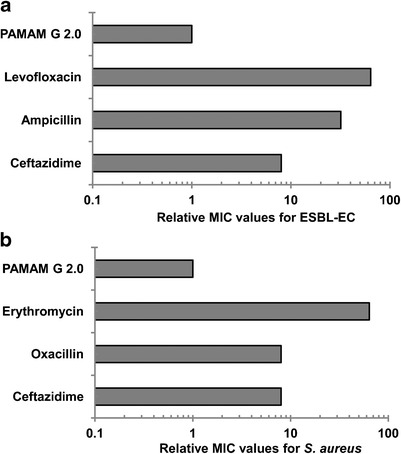
Induction of resistance to G2.0 PAMAM-NH2. Emergence of resistance in ESBL-EC (a) and S. aureus ATCC 29213 (b) after 15 serial passages in the presence of antimicrobials. The relative MIC is the normalized ratio of the MIC obtained for the fifteenth subculture to the MIC obtained for the first culture
Scanning Electron Microscopy and Transmission Electron Microscopy
Scanning electron microscopy (SEM) images revealed a marked change in bacterial morphology after treatment with G2.0 PAMAM-NH2. The bacteria shrank and became shorter, and cracks or holes formed on their surfaces (Fig. 6b–d). The untreated E. coli MG1655 bacteria had a distinct rod shape with intact cell walls and a relatively uniform length (Fig. 6a). The longer the incubation time with G2.0 PAMAM-NH2, the greater the damage to the bacterial cells observed (Fig. 6b–d). Morphological damage to the VIR-S. aureus Mu50 strain was also observed after G2.0 PAMAM-NH2 treatment (Fig. 6e, f).
Fig. 6.
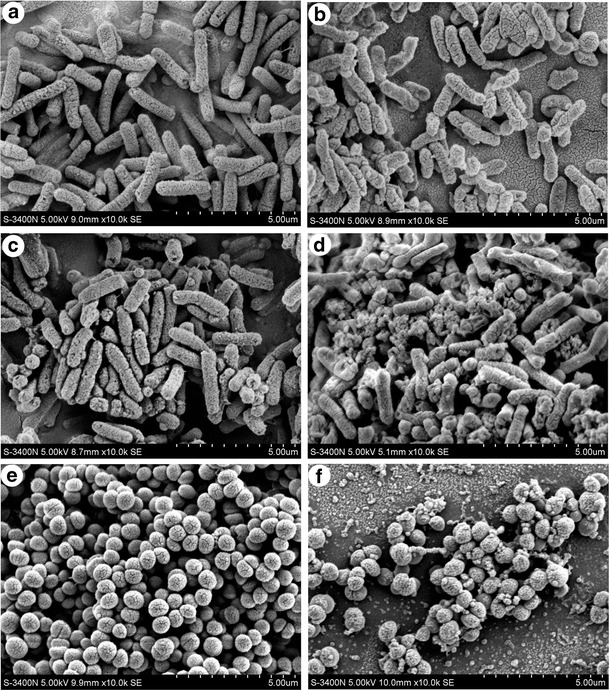
The morphologies of ESBL-EC and VIR-S. aureus Mu50 were investigated by scanning electron microscopy at 0, 30, 120 and 300 min after treatment with 12 μM G2.0 PAMAM-NH2. a Control ESBL-EC cells; b ESBL-EC treated with G2.0 PAMAM-NH2 for 30 min; c ESBL-EC treated with G2.0 PAMAM-NH2 for 120 min; and d ESBL-EC treated with G2.0 PAMAM-NH2 for 300 min. e Control VIR-S. aureus Mu50 cells, and f Mu50 treated with G2.0 PAMAM-NH2 for 120 min
Transmission electron microscopy (TEM) images demonstrated that E. coli MG1655 cells treated with G2.0 PAMAM-NH2 for 2 h were seriously damaged (Fig. 7b–d). Pyknosis and necrosis were observed in some of the damaged cells (Fig. 7b, arrow). The cytoplasm of some cells had shrunk dramatically, with noticeable bleb-like gaps between the cell membrane and the cytoplasm (Fig. 7c, arrow). The membrane integrity of the cells was seriously damaged, leading to the leakage of intracellular contents (Fig. 7d, arrow). The dramatic morphological changes in the bacteria, including the significant disruption of the cell wall and the erosion of the cell membrane, are consistent with the antibacterial activity observed in the previous assays, implying that PAMAM-NH2 dendrimers may kill bacteria by disrupting the cell wall or the permeability of the cell membrane.
Fig. 7.
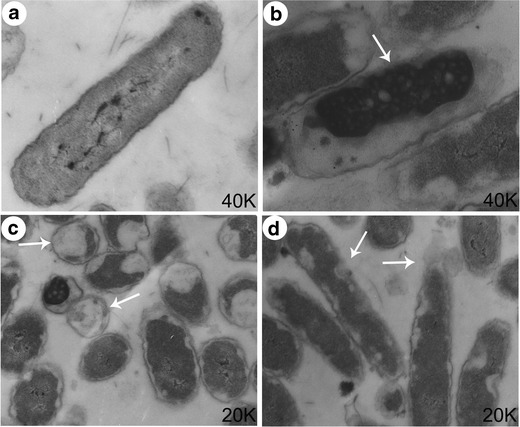
The morphology of E. coli MG1655 was investigated by transmission electron microscopy after treatment with 12 μM G2.0 PAMAM-NH2 for 120 min. a Control; b–d E. coli MG1655 treated with 12 μM G2.0 PAMAM-NH2 for 120 min
DISCUSSION
The antibacterial mechanism of PAMAM-NH2 has been an open question for a long time. It has been reported that the antibacterial activity is related to the generation of the PAMAM-NH2 (11,12). Our current results showed that G2.0–4.0 PAMAM-NH2 had greater antibacterial activity than G1.0 PAMAM-NH2, whereas G2.0 PAMAM-NH2 exhibited comparable or higher antibiotic activities than G3.0 and G4.0 PAMAM-NH2. Although it has been reported that PAMAM-NH2 have better antibiotic activity against Gram-negative than against Gram-positive bacteria, our systematic in vitro observations did not reveal greater antibacterial activity against Gram-negative strains for G2.0–4.0 PAMAM-NH2. The toxicity of PAMAM-NH2 consistently increases with the generation of the molecule, as demonstrated by both the present study (Fig. 2) and previous reports (30). The broad-spectrum antibacterial activity and toxicity to eukaryotic cells imply that PAMAM-NH2 may disrupt cells irrespective of their species, and it has been suggested that PAMAM-NH2 may damage cells through nonspecific physical mechanisms rather than by targeting specific molecules (19). The G2.0 PAMAM-NH2 dendrimer is a polycation that may replace the divalent ions binding to the polyanions present in the cell membrane. This binding could lead to insoluble polyanion–polycation complexes and the efflux of calcium ions, both of which would contribute to major structural changes in the cell membrane, resulting in bacterial cell death (31). Our SEM and TEM results also support the hypothesis that PAMAM-NH2 damages bacteria by disrupting the cell membrane. Therefore, unlike traditional antibiotics, which have specific molecular targets, PAMAM-NH2 may exert antibacterial activity by directly disrupting the bacterial cell wall and membrane permeability. The antibacterial mechanism of PAMAM-NH2 dendrimers may also contribute to the most unique feature of G2.0 PAMAM-NH2—its apparent lack of induction of resistance (Fig. 5).
In this study, we found that G2.0 PAMAM-NH2 displayed significant protective effects against septicemia in mice induced by inoculation with ESBL-EC alone or ESBL-EC and MRSA together in a dose-dependent manner. The 10 + 10 mg/kg regimen showed the strongest therapeutic effect and rescued 100% of singly infected mice and 60% of multiply infected mice. It is not clear why the 10 + 10 mg/kg regimen had the best protective effects in infected animals. In our control experiment, treatment with 10 mg/kg G2.0 PAMAM-NH2 12 h before inoculation without further treatment after inoculation also exhibited a limited protective effect (e.g., 30% survival vs. 10% survival in the untreated control group, data not shown). It is interesting to note that PAMAM-NH2 dendrimer show the highest accumulation in liver and kidney tissue more than 48 h after administration (13,32). The biodistribution and long-term accumulation of PAMAM-NH2 in these tissues might explain the protective effects of G2.0 PAMAM-NH2 in infected animals. However, the exact mechanisms remain to be determined. The combination of the prophylactic and therapeutic effects of G2.0 PAMAM-NH2 may contribute to the high potency of the 10 + 10 mg/kg regimen.
The acute toxicity assay revealed that the LD50 of G2.0 PAMAM-NH2 for BALB/c mice was 80 mg/kg. It seems that G2.0 PAMAM-NH2 falls in the range of significant antibacterial activity but tolerable toxicity (Fig. 8a). However, G3.0 and G4.0 PAMAM-NH2 exhibited similar antibacterial activities against most of the strains but greater toxicity, making them unsuitable for use as antibacterial agents because the therapeutic dosages of these high-generation PAMAM-NH2 dendrimers may cause severe toxicity.
Fig. 8.
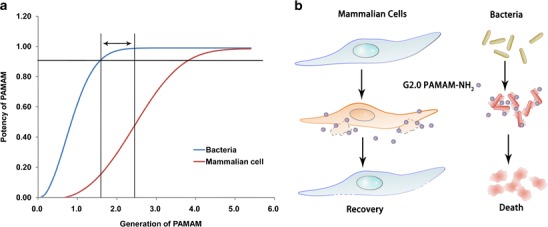
The scheme of the proposed mechanisms underlying the selective killing effect of G2.0 PAMAM-NH2 in bacteria. a PAMAM-NH2 exhibited generation-dependent killing abilities in bacteria and mammalian cells. Lower-generation PAMAM-NH2 dendrimers (e.g., G2.0) seem to destroy bacterial cells more easily than mammalian cells. Therefore, lower-generation PAMAM-NH2 dendrimers display a relatively wide safety range compared with the higher generations of PAMAM-NH2. b The different selectivities of G2.0 PAMAM-NH2 against bacteria and mammalian cells are most likely attributable to the differences in the cell size or charge density of PAMAM-NH2 on the cell membranes. Because bacterial cells are much smaller than mammalian cells, the same concentration of PAMAM-NH2 will result in each single small bacterial cell having more PAMAM-NH2 molecules attached relative to the larger mammalian cells and will therefore be more easily destroyed by PAMAM-NH2
The unique structure of PAMAM-NH2 dendrimers and the association between the generation and the antibacterial activity imply that their antibacterial effects are closely related to the density of the terminal groups. For example, G2.0 and higher generation PAMAM-NH2 dendrimer, but not G1.0 PAMAM-NH2, exhibited significant antibacterial activity, suggesting there is a threshold amount of terminated groups required for such activity. PAMAM-NH2 dendrimers with a density of terminal groups exceeding the threshold level may exhibit antibacterial activity. Similarly, the toxicity of PAMAM-NH2 in mammalian cells showed a similar pattern. At higher concentrations (>102.4 μg/mL), G3.0 and higher generations of PAMAM-NH2 showed significant toxicity to mammalian cells compared with G2.0 and G1.0 PAMAM-NH2. It seems that higher generations (greater density of terminal groups) are needed to damage the more complex cells. These results indicate that G2.0 or higher generation PAMAM-NH2 can significantly damage bacteria, whereas G3.0 or highe-generation PAMAM-NH2 acquire the potential to damage mammalian cells. Because PAMAM-NH2 may damage cells through nonspecific physical mechanisms, the ability of different generation PAMAM-NH2 to damage bacterial and mammalian cells may be attributed to the size of the cell or the relative charge density on the cell membrane (Fig. 8b). Therefore, we propose that there exists a range of generations of PAMAM-NH2 between these two thresholds that may have significant antibacterial activity but tolerable toxicities (Fig. 8a). This scenario also partially explains previous studies that showed that G3.0 or higher generation PAMAM-NH2 dendrimers exhibited excellent antibacterial activities but intolerable toxicities (11).
SUMMARY
This study demonstrated that G2.0 PAMAM-NH2 is highly effective against Gram-positive and Gram-negative bacteria, including both sensitive and resistant strains, without inducing drug-resistance or exhibiting toxicity. The favorable in vivo therapeutic effects suggested that G2.0 PAMAM-NH2 could provide a novel strategy for treating infections induced by ESBL-EC and MRSA. Further clarification of the antimicrobial and toxic mechanisms of PAMAM-NH2 may suggest approaches to improve their antibacterial activity and decrease their toxicity. Deciphering the differences in the general biology and PAMAM-NH2 responses between bacterial and mammalian cells would allow the creation of more sophisticated antibiotics based on G2.0 PAMAM-NH2. Our findings also indicate ways to determine whether other types of dendrimers may have better antibacterial activity and lower toxicity.
Electronic supplementary materials
Below is the link to the electronic supplementary material.
(DOCX 5198 kb)
Acknowledgments
This research was supported by grants from the National Fund for Natural Science, China (No. 30973666). The authors would like to thank Prof. Liping Zhao (Shang Hai Jiao Tong University, Shanghai, China) for the bacterial strain E. coli MG1655 and Na Chai, Ph.D., (Xijing Affiliated Hospital, Fourth Military Medical University, Xi’an, China) for the endothelial cell line.
Disclosure Statement
The authors declare that they have no conflicts of interest to disclose.
Ethical approval
The animal experiments were approved by the Ethics Committee of the Fourth Military Medical University.
Footnotes
Xiaoyan Xue, Xiaoqing Chen, and Xinggang Mao contributed equally to this work.
References
- 1.Gyssens IC. Antibiotic policy. Int J Antimicrob Agents. 2011;38(Suppl):11–20. doi: 10.1016/j.ijantimicag.2011.09.002. [DOI] [PubMed] [Google Scholar]
- 2.Jean SS, Hsueh PR. High burden of antimicrobial resistance in Asia. Int J Antimicrob Agents. 2011;37:291–295. doi: 10.1016/j.ijantimicag.2011.01.009. [DOI] [PubMed] [Google Scholar]
- 3.Wang JL, Wang JT, Chen SY, Chen YC, Chang SC. Distribution of staphylococcal cassette chromosome mec types and correlation with comorbidity and infection type in patients with MRSA bacteremia. PLoS One. 2010;5:e9489. doi: 10.1371/journal.pone.0009489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fischbach MA, Walsh CT. Antibiotics for emerging pathogens. Science. 2009;325:1089–1093. doi: 10.1126/science.1176667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Castonguay A, Ladd E, van de Ven TGM, Kakkar A. Dendrimers as bactericides. New J Chem. 2012;36:199–204. doi: 10.1039/c1nj20481e. [DOI] [Google Scholar]
- 6.Mintzer MA, Dane EL, O’Toole GA, Grinstaff MW. Exploiting dendrimer multivalency to combat emerging and re-emerging infectious diseases. Mol Pharm. 2012;9:342–354. doi: 10.1021/mp2005033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Han L, Huang R, Li J, Liu S, Huang S, Jiang C. Plasmid pORF-hTRAIL and doxorubicin co-delivery targeting to tumor using peptide-conjugated polyamidoamine dendrimer. Biomaterials. 2011;32:1242–1252. doi: 10.1016/j.biomaterials.2010.09.070. [DOI] [PubMed] [Google Scholar]
- 8.Beg S, Samad A, Alam MI, Nazish I. Dendrimers as novel systems for delivery of neuropharmaceuticals to the brain. CNS Neurol Disord Drug Targets. 2011;10:576–588. doi: 10.2174/187152711796235023. [DOI] [PubMed] [Google Scholar]
- 9.Gajbhiye V, Palanirajan VK, Tekade RK, Jain NK. Dendrimers as therapeutic agents: a systematic review. J Pharm Pharmacol. 2009;61:989–1003. doi: 10.1211/jpp.61.08.0002. [DOI] [PubMed] [Google Scholar]
- 10.Nanjwade BK, Bechra HM, Derkar GK, Manvi FV, Nanjwade VK. Dendrimers: emerging polymers for drug-delivery systems. Eur J Pharm Sci. 2009;38:185–196. doi: 10.1016/j.ejps.2009.07.008. [DOI] [PubMed] [Google Scholar]
- 11.Lopez AI, Reins RY, McDermott AM, Trautner BW, Cai C. Antibacterial activity and cytotoxicity of PEGylated poly(amidoamine) dendrimers. Mol Biosyst. 2009;5:1148–1156. doi: 10.1039/b904746h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Calabretta MK, Kumar A, McDermott AM, Cai C. Antibacterial activities of poly(amidoamine) dendrimers terminated with amino and poly(ethylene glycol) groups. Biomacromolecules. 2007;8:1807–1811. doi: 10.1021/bm0701088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roberts JC, Bhalgat MK, Zera RT. Preliminary biological evaluation of polyamidoamine (PAMAM) Starburst dendrimers. J Biomed Mater Res. 1996;30:53–65. doi: 10.1002/(SICI)1097-4636(199601)30:1<53::AID-JBM8>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 14.Malik N, Wiwattanapatapee R, Klopsch R, Lorenz K, Frey H, Weener JW, et al. Dendrimers: relationship between structure and biocompatibility in vitro, and preliminary studies on the biodistribution of 125I-labelled polyamidoamine dendrimers in vivo. J Control Release. 2000;65:133–148. doi: 10.1016/S0168-3659(99)00246-1. [DOI] [PubMed] [Google Scholar]
- 15.McNerny DQ, Leroueil PR, Baker JR. Understanding specific and nonspecific toxicities: a requirement for the development of dendrimer-based pharmaceuticals. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2010;2:249–259. doi: 10.1002/wnan.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ortega P, Copa-Patino JL, Munoz-Fernandez MA, Soliveri J, Gomez R, de la Mata FJ. Amine and ammonium functionalization of chloromethylsilane-ended dendrimers. Antimicrobial activity studies. Org Biomol Chem. 2008;6:3264–3269. doi: 10.1039/b809569h. [DOI] [PubMed] [Google Scholar]
- 17.Eichler M, Katzur V, Scheideler L, Haupt M, Geis-Gerstorfer J, Schmalz G, et al. The impact of dendrimer-grafted modifications to model silicon surfaces on protein adsorption and bacterial adhesion. Biomaterials. 2011;32:9168–9179. doi: 10.1016/j.biomaterials.2011.08.063. [DOI] [PubMed] [Google Scholar]
- 18.Wang L, Erasquin UJ, Zhao M, Ren L, Zhang MY, Cheng GJ, et al. Stability, antimicrobial activity, and cytotoxicity of poly(amidoamine) dendrimers on titanium substrates. ACS Appl Mater Interfaces. 2011;3:2885–2894. doi: 10.1021/am2004398. [DOI] [PubMed] [Google Scholar]
- 19.Wang B, Navath RS, Menjoge AR, Balakrishnan B, Bellair R, Dai H, et al. Inhibition of bacterial growth and intramniotic infection in a guinea pig model of chorioamnionitis using PAMAM dendrimers. Int J Pharm. 2010;395:298–308. doi: 10.1016/j.ijpharm.2010.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cheng Y, Qu H, Ma M, Xu Z, Xu P, Fang Y, et al. Polyamidoamine (PAMAM) dendrimers as biocompatible carriers of quinolone antimicrobials: an in vitro study. Eur J Med Chem. 2007;42:1032–1038. doi: 10.1016/j.ejmech.2006.12.035. [DOI] [PubMed] [Google Scholar]
- 21.Ghosh S, Ghosh D, Bag PK, Bhattacharya SC, Saha A. Aqueous synthesis of ZnTe/dendrimer nanocomposites and their antimicrobial activity: implications in therapeutics. Nanoscale. 2011;3:1139–1148. doi: 10.1039/c0nr00610f. [DOI] [PubMed] [Google Scholar]
- 22.Neelgund GM, Oki A. Deposition of silver nanoparticles on dendrimer functionalized multiwalled carbon nanotubes: synthesis, characterization and antimicrobial activity. J Nanosci Nanotechnol. 2011;11:3621–3629. doi: 10.1166/jnn.2011.3756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reynolds R, Shackcloth J, Felmingham D, MacGowan A. Comparison of BSAC agar dilution and NCCLS broth microdilution MIC methods for in vitro susceptibility testing of Streptococcus pneumoniae, Haemophilus influenzae and Moraxella catarrhalis: the BSAC Respiratory Resistance Surveillance Programme. J Antimicrob Chemother. 2003;52:925–930. doi: 10.1093/jac/dkg462. [DOI] [PubMed] [Google Scholar]
- 24.Kumar A, Yellepeddi VK, Vangara KK, Strychar KB, Palakurthi S. Mechanism of gene transfection by polyamidoamine (PAMAM) dendrimers modified with ornithine residues. J Drug Target. 2011;19:770–780. doi: 10.3109/1061186X.2011.568061. [DOI] [PubMed] [Google Scholar]
- 25.Xue XY, Zhou Y, Chen YY, Meng JR, Jia M, Hou Z, et al. Promoting effects of chemical permeation enhancers on insulin permeation across TR146 cell model of buccal epithelium in vitro. Drug Chem Toxicol. 2011;35 :199–207. doi: 10.3109/01480545.2011.589848. [DOI] [PubMed] [Google Scholar]
- 26.Gilles HJ. Calculation of the index of acute toxicity by the method of linear regression. Comparison with the method of “Karber and Behrens”. Eur J Toxicol Environ Hyg. 1974;7:77–84. [PubMed] [Google Scholar]
- 27.Ulrich R, Miller J. Threshold estimation in two-alternative forced-choice (2AFC) tasks: the Spearman–Karber method. Percept Psychophys. 2004;66:517–533. doi: 10.3758/BF03194898. [DOI] [PubMed] [Google Scholar]
- 28.Radzishevsky IS, Rotem S, Bourdetsky D, Navon-Venezia S, Carmeli Y, Mor A. Improved antimicrobial peptides based on acyl-lysine oligomers. Nat Biotechnol. 2007;25:657–659. doi: 10.1038/nbt1309. [DOI] [PubMed] [Google Scholar]
- 29.Rozgonyi F, Szabo D, Kocsis B, Ostorhazi E, Abbadessa G, Cassone M, et al. The antibacterial effect of a proline-rich antibacterial peptide A3-APO. Curr Med Chem. 2009;16:3996–4002. doi: 10.2174/092986709789352295. [DOI] [PubMed] [Google Scholar]
- 30.Sadekar S, Ghandehari H. Transepithelial transport and toxicity of PAMAM dendrimers: implications for oral drug delivery. Adv Drug Deliv Rev. 2011;64:571–588. doi: 10.1016/j.addr.2011.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cao W, Zhou J, Mann A, Wang Y, Zhu L. Folate-functionalized unimolecular micelles based on a degradable amphiphilic dendrimer-like star polymer for cancer cell-targeted drug delivery. Biomacromolecules. 2011;12:2697–2707. doi: 10.1021/bm200487h. [DOI] [PubMed] [Google Scholar]
- 32.Samad A, Alam MI, Saxena K. Dendrimers: a class of polymers in the nanotechnology for the delivery of active pharmaceuticals. Curr Pharm Des. 2009;15:2958–2969. doi: 10.2174/138161209789058200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 5198 kb)


