Abstract
Induction of heat shock proteins (Hsp) 72 and 27 can improve insulin signalling in obesity and type 2 diabetes via inhibition of key stress kinases. In metabolic disease, altered insulin signalling, as illustrated by increased serine phosphorylation of insulin receptor substrate (IRS)-1 (Ser312), is not confined to muscle or liver and can also affect other tissues and cell types, potentially impairing their primary biological function. This study specifically investigated insulin-stimulated glucose metabolism in monocytes and examined the impact of HSP induction on insulin signalling. Control (CG, BMI < 25 kg/m2) or obese (OG, BMI > 30 kg/m2) participants were included in the study. Glucose transporter (GLUT)4 expression on monocytes, phosphorylated JNK, IKK-β and IRS-1, as well as Hsp27 and Hsp72, were measured in monocytes under fasting conditions. GLUT4 expression was also measured during an oral glucose tolerance test (OGTT). HSP induction as well as JNK, IKK-β activation and IRS-1 serine phosphorylation was investigated following heat stress. Obese patients showed lower GLUT4 levels on monocytes during the OGTT. pJNK, pIKK-β and pIRS-1 levels were increased in OG with pJNK and pIKK-β levels positively correlated with serine pIRS-1 and negatively with GLUT4 supporting their role in insulin resistance. Heat exposure induced Hsp72 and Hps27, but only in CG for the latter, and decreased pJNK, pIKK-β and pIRS-1. Our results show that induction of Hsp72 and 27 via heat stress is associated with inactivation of stress kinases and reduced serine pIRS-1 in monocytes from obese participants. This indicates that metabolic diseases can also affect monocyte metabolism via cellular stress that can be modulated via HSP induction.
Keywords: HSP, JNK, IKK-β, Insulin signalling, Obesity, Monocytes
Introduction
Heat shock proteins (HSPs) have been shown to play a protective role in the context of insulin resistance (McCarty 2006). In obese animals, upregulation of Hsp72 resulted in decreased c-Jun N terminal kinase (JNK) activation and contributed to the improvement of insulin sensitivity in skeletal muscles (Chung et al. 2008). It has further been suggested that Hsp27 upregulation could improve insulin signalling by regulating IκB kinase β (IKK-β) activation although this is yet to be confirmed. These observations clearly support a detrimental role of both JNK and IKK-β stress kinase activation on insulin signalling. Insulin stimulation in skeletal muscles leads to the autophosphorylation of the insulin receptor substrate-1 (IRS-1) on its tyrosine residues, resulting in the activation of the phosphoinositide 3 kinase (PI3k)/Akt pathway. This allows the translocation of the glucose transporter 4 (GLUT4) to the plasma membrane to support glucose uptake (Bryant et al. 2002). In obesity, both intramuscular lipid accumulation and increased systemic inflammation lead to the activation of key stress kinases involved in the disruption of insulin signalling (Adams et al. 2004; Morino et al. 2006; Shoelson et al. 2006). The activation of JNK and IKK-β has been associated with increased phosphorylation of IRS-1 on its serine residues rendering it a poor substrate for the insulin receptor (Petersen and Shulman 2006; Shoelson et al. 2006; Zick 2005), disrupting insulin signalling and altering insulin-mediated GLUT4 levels (Petersen and Shulman 2006).
Leukocytes also express GLUTs and show insulin-stimulated glucose uptake, suggesting a regulatory role of insulin on their metabolic activity (Dimitriadis et al. 2005). As in muscles, leukocyte GLUTs expression, both at the gene and protein levels, is impaired in obesity or type 2 diabetes (Kipmen-Korgun et al. 2009; Maratou et al. 2007). In a small sample of obese people, a reduced response to the stimulating effect of insulin on GLUT4 expression on monocytes was reported (Maratou et al. 2007). Although those studies seem to support an alteration of GLUT expression on monocytes in metabolic disorders, the responsible mechanisms for these impairments are still unclear.
In the present study, we investigated in monocytes the activation of key stress kinases known to impair insulin signalling and examined their activity following induction of Hsp72 and 27 achieved through heat stress. We hypothesised that, in obese people, JNK and IKK-β over-activation contributes to increased serine phosphorylation of IRS-1, disrupting insulin signalling, but that Hsp27 and Hsp72 induction could restore it.
Material and methods
Subjects
Sixteen sedentary participants (eight men and eight women; mean age, 44) underwent a medical screening and gave their informed consent. They were free of recent illness or infection, pregnancy and diagnosed cardiovascular disease and refrained from hypoglycemic drugs for 72 h. Two groups were formed according to body mass index (BMI): obese group (OG, BMI > 30 kg/m2, n = 8, four men and four women) and control group (CG, BMI < 25 kg/m2, n = 8, four men and four women). Only one of our obese participants was diagnosed with type 2 diabetes, based on the previously published norms: a fasting blood glucose higher than 7 mmol/L or blood glucose levels 2 h post the oral glucose tolerance test (OGTT) higher than 11.1 mmol/L (Expert Committee on the Diagnosis and Classification of Diabetes Mellitus 2003) Both groups were paired for gender, height and age (Table 1). The Ethics Committee of the University of Sydney approved all procedures carried out in this study.
Table 1.
Anthropometry and insulin resistance levels in obese and control groups
| OG (n = 8) | CG (n = 8) | |
|---|---|---|
| Age (years) | 46.4 ± 3.6 | 43.3 ± 3.8 |
| Height (cm) | 169 ± 4 | 168 ± 4 |
| Body weight (kg) | 91.4 ± 6.0 | 60.3 ± 4.2* |
| BMI (kg/m2) | 31.6 ± 1.1 | 21.0 ± 0.5* |
| HOMA-%β | 122 ± 18 | 110 ± 7 |
| HOMA-%S | 60 ± 5 | 107 ± 10* |
| HOMA-IR | 1.8 ± 0.2 | 1.0 ± 0.1* |
*p < 0.05 between OG and CG
Analytical methods
Fasting blood samples were collected before an OGTT (75 g glucose), during which blood samples were collected every 30 min for 2 h. Glucose levels were measured on heparinised blood (EML105 analyser, Radiometer, Denmark) and insulin on serum (enzyme-linked immunosorbent assay: DSl-10-1600, Diagnostic Systems Laboratories, USA). Insulin resistance, sensitivity and β cell function were calculated using the homeostatic model assessment (HOMA)2 (Wallace et al. 2004).
Flow cytometry
Protein expression and phosphorylation levels were measured on monocytes by flow cytometry (Dimitriadis et al. 2005; Simar et al. 2007). Leukocytes were stained with anti-CD14-phycoerythrin conjugated (BD Biosciences Pharmingen, USA) and unconjugated rabbit anti-GLUT4 (Chemicon International, USA) after red blood cell removal. After two washes, anti-GLUT4 antibody was then conjugated using anti-rabbit IgG-fluorescein isothiocyanate (FITC) conjugated (DakoCytomation, Denmark). Cells were washed twice again and fixed with 1 % paraformaldehyde and kept at 4 °C until analysis on a flow cytometer (FACSCalibur, BD Biosciences, USA).
For intra-cellular staining, cells were fixed and permeabilised using a Fix and Perm kit (DakoCytomation, Denmark) after red blood cell removal and prior to staining with the primary [rabbit anti-phospho-SAPK/JNK (Thr183/Tyr185, pJNK), anti-phospho IKK-β (Ser176/180, pIKK-β), anti-phospho IRS-1 (Ser312, pIRS-1), all from Cell Signalling Technology (USA), anti-Hsp72-FITC conjugated or Hsp27-FITC conjugated (Stressgen Bioreagents, USA)] and secondary antibodies (anti-rabbit IgG-FITC conjugated, DakoCytomation, Denmark). HSP induction was achieved by incubation of fasting blood sample for 2 h at 42 °C, and Hsp72, Hsp27, pJNK, pIKK-β, pIRS-1 as well as GLUT4 levels (only in a subsample from each group for the latter) were then measured as described above. Data were analysed using FlowJo (Tree Star, USA) and Cytobank softwares (Cytobank Inc, USA). Mean fluorescence intensity was normalised using corresponding isotype controls. Expression of total JNK, IKK-β and IRS-1 (Cell Signalling Technology, USA) was assessed in a subpopulation of each group, and no significant effect of heat stress was observed (p > 0.05, n = 8, data not shown).
Statistical analysis
Data are presented as mean±SE. The normality of the distribution was tested using the skewness and kurtosis tests. Difference between the two groups for anthropometric data and HOMA indexes was analysed using Student's t test. ANOVA for repeated measures was used to test time and group effects on insulin, glucose and GLUT4 levels during the OGTT (post hoc analysis using Fisher's least significant difference (LSD) test). Factorial ANOVA (post hoc analysis using Fisher's LSD test) was used to monitor the effect of heat exposure on protein levels in both groups. Correlations were assessed using Pearson's test. A significance level of p < 0.05 was used. Statistical analysis was run using SPSS Statistics version 18 (IBM Corporation, USA).
Results
As expected, our obese group showed significantly lower HOMA-%S (60 ± 5 vs. 107 ± 10, p = 0.001) and higher HOMA-IR (1.8 ± 0.2 vs. 1.0 ± 0.1, p = 0.001) supporting impaired insulin sensitivity in this group (Table 1). No significant difference was observed between the two groups in terms of β cell function (p > 0.05, Table 1).
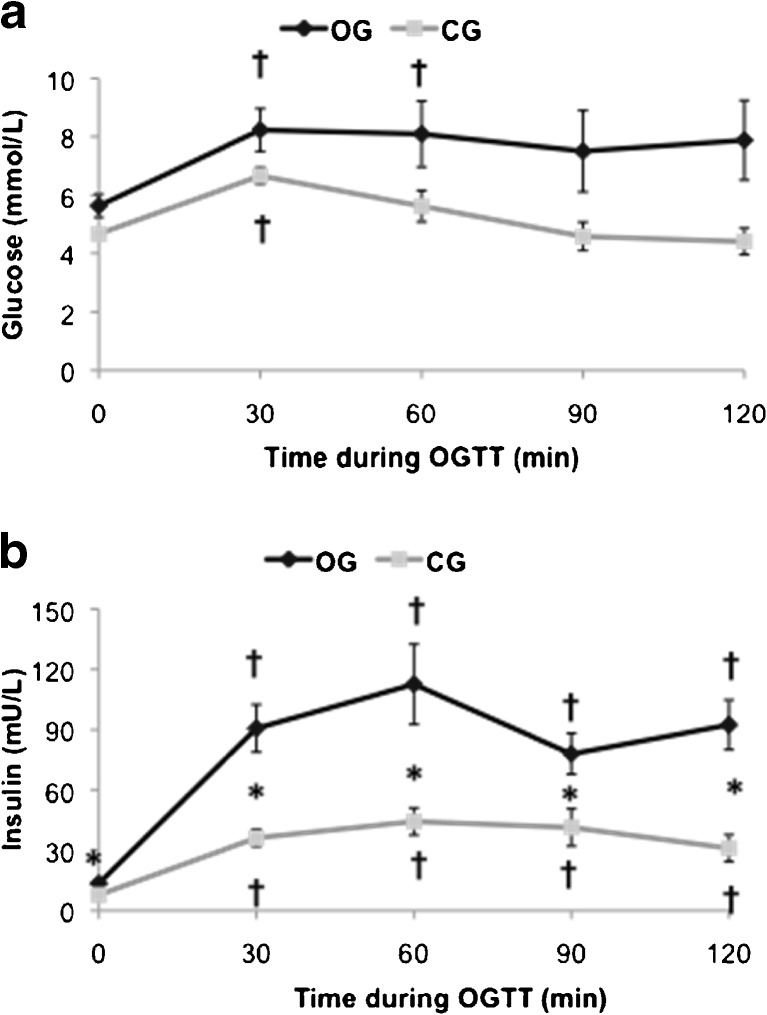
In response to the OGTT, both groups showed increased levels of insulin and glucose (time effect, p < 0.05, Fig. 1a, b), but OG showed higher insulin levels (group effect, p < 0.05, Fig. 1b). GLUT4 levels significantly increased during the OGTT but only in CG (group and time effects, p < 0.05, Fig. 2a, b), supporting a negative impact of obesity on glucose transport in monocytes. This was further supported by negative correlations between GLUT4 and BMI or insulin resistance (r = 0.57, p = 0.02, n = 16 and r = 0.50, p = 0.04, n = 16, respectively) and a positive correlation observed between GLUT4 levels and HOMA-%S (r = 0.72, p = 0.002, n = 16).
Fig. 1.
Glucose and insulin levels during the oral glucose tolerance test. a Glucose levels during the OGTT. b Insulin levels during the OGTT. Error bars showing SE. *p < 0.05 between OG and CG, †p < 0.05 with baseline time point
Fig. 2.
Glucose transporter 4 expression on monocytes during the oral glucose tolerance test. a Representative histograms of GLUT4 levels on monocytes in both groups during the OGTT. bBar graphs representing means±SE. *p < 0.05 between OG and CG, †p < 0.05 with baseline time point
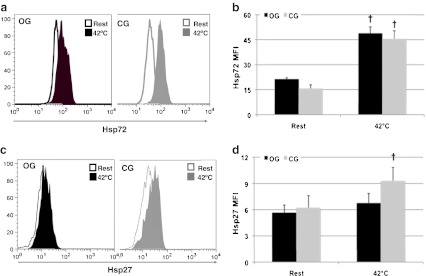
Monocytes collected in fasting condition in OG showed higher levels of pJNK and pIKK-β supporting increased activation (p < 0.05; Fig. 3b, d). In the same group, serine pIRS-1 levels were also significantly increased compared to CG (p < 0.05, Fig. 3e), and both pJNK and pIKK-β were positively correlated with pIRS-1 (r = 0.78, p < 0.001, n = 16 and r = 0.79, p < 0.001, n = 16, respectively) supporting a role for these two stress kinases in impaired insulin signalling. Fasted (non-heated condition) monocytes from OG and CG presented similar levels of Hsp72 and Hsp27 expression (p > 0.05; Fig. 4b, d, respectively).
Fig. 3.
Stress kinase activation and insulin signalling in response to heat shock. a Representative histograms of pJNK levels in monocytes from both groups at rest and after 2 h incubation at 42 °C. bBar graphs representing means±SE. c Representative histograms of pIKK-β levels in monocytes from both groups at rest and after 2 h incubation at 42 °C. dBar graphs representing means±SE. e Representative histograms of pIRS-1 levels in monocytes from both groups at rest and after 2 h incubation at 42 °C. fBar graphs representing means±SE. *p < 0.05 between OG and CG, †p < 0.05 with baseline time point
Fig. 4.
Heat shock protein induction in response to heat shock. a Representative histograms of Hsp72 levels in monocytes from both groups at rest and after 2 h incubation at 42 °C. bBar graphs representing means±SE. c Representative histograms of Hsp27 levels in monocytes from both groups at rest and after 2 h incubation at 42 °C. dBar graphs representing means±SE †p < 0.05 with baseline time point
Heat exposure induced a significant twofold increase in Hsp72 expression in both groups (p < 0.05, Fig. 4b), although Hsp27 levels increased only in CG (p < 0.05, Fig. 4d). HSP induction was associated with a significant decrease of pJNK, pIKK-β (only in the OG group) and pIRS-1 levels in both groups (p < 0.05; Fig. 3b, d, e, respectively). This was particularly obvious in monocytes from OG where activation of stress kinases and serine phosphorylation of IRS-1 were severely decreased.
Discussion
The present study shows that monocytes from obese subjects have increased activation of JNK and IKK-β, suggesting increased metabolic or inflammatory stress. This is associated with increased serine phosphorylation of IRS-1 and reduced GLUT4 levels in response to insulin stimulation, in line with insulin resistance in those cells. Induction of chaperone proteins through heat exposure, particularly Hsp72, was associated with reduced stress kinases activation and improved insulin signalling, as illustrated by reduced serine phosphorylation of IRS-1. Our results also support impaired HSP inducibility in monocytes from obese subjects.
As expected, participants from the OG group showed increased levels of both insulin and glucose at all time points during the OGTT test, increased HOMA-IR and impaired HOMA-%S indexes, indicating increased insulin resistance and impaired insulin sensitivity (Wallace et al. 2004). In addition, we show for the first time that GLUT4 upregulation in monocytes is impaired in response to physiological in vivo stimulation, i.e. during an OGTT. In a different model of obesity, in vitro stimulation using high insulin concentration failed to upregulate GLUT4 on monocytes (Maratou et al. 2007). Here, in vivo physiological stimulation of GLUT4 was totally blunted, and GLUT4 levels were correlated with the level of insulin resistance, confirming that monocytes share common alterations with major insulin-sensitive tissues in metabolic disorders (Dimitriadis et al. 2005).
As previously reported in skeletal muscles or adipose tissues (Beeson et al. 2003; Gao et al. 2002), but for the first time in monocytes, we found increased serine phosphorylation of IRS-1 in obese subjects. In addition, serine pIRS-1 was inversely correlated with GLUT4 expression in monocytes from obese subjects, suggesting impaired insulin signalling. Both increased fatty acids and pro-inflammatory cytokines can contribute to increased serine pIRS-1 in skeletal muscles (Adams et al. 2004). IRS-1 can be a substrate for a wide range of different serine kinases including JNK or IKK-β (Gao et al. 2002) and ceramides, and pro-inflammatory cytokines are well-known activators of JNK or IKK-β (Shoelson et al. 2003; Strle et al. 2006). In the present study, higher levels of pJNK and pIKK-β were also observed in obese subjects and were associated with increased serine pIRS-1. In monocytes and macrophages, members of the toll-like receptor (TLR) family, mainly TLR4 and 2, mediate the activation of both JNK and IKK-β by fatty acids or pro-inflammatory cytokines (Akira 2003). Hyperglycaemia, fatty acid accumulation or systemic inflammation, which are highly prevalent in obesity, can increase TLR4 and TLR2 expression in monocytes (Dasu and Jialal 2010; Devaraj et al. 2008), potentially contributing to the over-activation of JNK and IKK-β, and thus mimicking metabolic alterations previously reported in major metabolically active organs.
Chronic whole-body hyperthermia has previously been shown to improve obesity-induced insulin resistance (Kokura et al. 2007). In this report, db/db mice treated using whole-body hyperthermia showed increased skeletal muscle GLUT4 expression levels and improved whole-body insulin sensitivity. Although the clear mechanism at play was not identified, it was suggested that increased blood flow or intra-cellular Ca2+ could play a significant role. Another well-described adaptation to hyperthermia is the induction of HSPs which can then play an anti-inflammatory role by directly regulating key signalling pathways (McCarty 2006). Hsp27 has been shown to avidly bind to IKK-β, preventing the activation of NF kappa B pathway (Park et al. 2003), whereas Hsp72 can regulate JNK activation (Park et al. 2001). This suggests that heat-induced HSP upregulation could reduce JNK and IKK-β activation. Both chronic and acute heat treatments have been shown to improve insulin resistance in skeletal muscles induced by ageing or high-fat diet consumption (Gupte et al. 2009, 2011). In those reports, heat treatment induced an upregulation of Hsp72 and Hsp27, which was associated with a downregulation of JNK and IKK-β, contributing to the improvement of insulin-stimulated glucose uptake and insulin signalling. Interestingly, the heat stress-mediated inhibition of JNK was blunted by the pharmacological inhibition of Hsp72 (Gupte et al. 2009, 2011), in agreement with a previous study showing that Hsp72 contributes to the restoration of insulin signalling in obese animals through inhibition of JNK (Chung et al. 2008). Here, we observed a significant induction of both Hsp72 and Hsp27 in response to heat stress in vitro. Hsp72 and Hsp27 induction was associated with reduced activation of both IKK-β and JNK, as well as reduced serine pIRS-1, suggesting an improvement in insulin signalling. A protective role for Hsp27 in the context of insulin resistance has previously been suggested (McCarty 2006), which could be mediated by its inhibitory effect on IKK-β (Park et al. 2003). Surprisingly, no significant induction of Hsp27 was observed in the obese group, but this was nonetheless associated with a reduced activation of IKK-β. Not only Hsp27 but also Hsp72 have been shown to have a regulatory effect on IKK-β activation (Kohn et al. 2002; Yoo et al. 2000), and the preserved induction of Hsp72 in obese subjects might have contributed to the decrease in pIKK-β levels. This could suggest a stronger role for Hsp72 compared to Hsp27 in regulating JNK and IKK-β activation and in preserving or restoring insulin signalling, though additional studies would be required to confirm this point. Nonetheless, it must also be acknowledged that additional mechanisms could have played a role here in the heat-induced reduction in JNK and IKK-β activation including the induction of other HSPs not measured here. It should be noted that HSP induction in monocytes was weaker in obese subjects, an observation consistent with earlier reports showing altered HSP expression in metabolic disorders (Bruce et al. 2003). In the context of insulin resistance, it is suspected that impaired cellular metabolism could negatively impact on HSP induction (Hooper and Hooper 2009). Although we observed a reduction in JNK and IKK-β activation and a reduction in pIRS-1 upon HSP induction, this was not associated with increased GLUT4 expression. This could be explained by the fact that HSP induction was performed on fasting samples and so in the absence of insulin stimulation.
We have here identified some of the potential mechanisms responsible for the impaired GLUT4 expression in monocytes. As observed in muscles or adipocytes, monocytes from obese patients are characterised by increased JNK and IKK-β activation leading to increased serine pIRS-1. This in turn results in decreased GLUT4 levels upon physiological stimulation. Induction of HSPs is associated with inhibition of JNK and IKK-β and decreased serine pIRS-1, suggesting improved insulin signalling, but this beneficial adaptation is impaired in obese subjects. These results suggest that monocytes share similar metabolic alterations with major insulin-sensitive tissues in the context of obesity and thus provide a novel cellular model to investigate the development of insulin resistance. As previously suggested in animal models, modulating stress kinases activation through heat stress and HSP induction could provide a novel strategy to restore insulin sensitivity.
Acknowledgments
This work was supported by the University of New South Wales (starting funds to Dr. David Simar). The authors would like to thank the participants for their time and patience, as well as Dr. Pat Ruell and Ms. Ashley Armstrong for technical assistance.
References
- Adams JM, Pratipanawatr T, Berria R, Wang E, DeFronzo RA, Sullards MC, Mandarino LJ. Ceramide content is increased in skeletal muscle from obese insulin-resistant humans. Diabetes. 2004;53:25–31. doi: 10.2337/diabetes.53.1.25. [DOI] [PubMed] [Google Scholar]
- Akira S. Toll-like receptor signaling. J Biol Chem. 2003;278:38105–38108. doi: 10.1074/jbc.R300028200. [DOI] [PubMed] [Google Scholar]
- Beeson M, Sajan MP, Dizon M, Grebenev D, Gomez-Daspet J, Miura A, Kanoh Y, Powe J, Bandyopadhyay G, Standaert ML, Farese RV. Activation of protein kinase C-zeta by insulin and phosphatidylinositol-3,4,5-(PO4)3 is defective in muscle in type 2 diabetes and impaired glucose tolerance: amelioration by rosiglitazone and exercise. Diabetes. 2003;52:1926–1934. doi: 10.2337/diabetes.52.8.1926. [DOI] [PubMed] [Google Scholar]
- Bruce CR, Carey AL, Hawley JA, Febbraio MA. Intramuscular heat shock protein 72 and heme oxygenase-1 mRNA are reduced in patients with type 2 diabetes. Diabetes. 2003;52:2338. doi: 10.2337/diabetes.52.9.2338. [DOI] [PubMed] [Google Scholar]
- Bryant NJ, Govers R, James DE. Regulated transport of the glucose transporter GLUT4. Nat Rev Mol Cell Biol. 2002;3:267–277. doi: 10.1038/nrm782. [DOI] [PubMed] [Google Scholar]
- Chung J, Nguyen A-K, Henstridge DC, et al. HSP72 protects against obesity-induced insulin resistance. Proc Natl Acad Sci USA. 2008;105:1739–1744. doi: 10.1073/pnas.0705799105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasu MR, Jialal I. Free fatty acids in the presence of high glucose amplify monocyte inflammation via toll-like receptors. Am J Physiol Endocrinol Metab. 2010;300:E145–E154. doi: 10.1152/ajpendo.00490.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devaraj S, Dasu MR, Rockwood J, Winter W, Griffen SC, Jialal I. Increased toll-like receptor (TLR) 2 and TLR4 expression in monocytes from patients with type 1 diabetes: further evidence of a proinflammatory state. J Clin Endocrinol Metab. 2008;93:578. doi: 10.1210/jc.2007-2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimitriadis G, Maratou E, Boutati E, Psarra K, Papasteriades C, Raptis SA. Evaluation of glucose transport and its regulation by insulin in human monocytes using flow cytometry. Cytometry A. 2005;64:27–33. doi: 10.1002/cyto.a.20108. [DOI] [PubMed] [Google Scholar]
- Expert Committee on the Diagnosis and Classification of Diabetes Mellitus Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 2003;26(Suppl 1):S5–S20. doi: 10.2337/diacare.26.2007.s5. [DOI] [PubMed] [Google Scholar]
- Gao Z, Hwang D, Bataille F, Lefevre M, York D, Quon MJ, Ye J. Serine phosphorylation of insulin receptor substrate 1 by inhibitor kappa B kinase complex. J Biol Chem. 2002;277:48115–48121. doi: 10.1074/jbc.M209459200. [DOI] [PubMed] [Google Scholar]
- Gupte AA, Bomhoff GL, Swerdlow RH, Geiger PC. Heat treatment improves glucose tolerance and prevents skeletal muscle insulin resistance in rats fed a high-fat diet. Diabetes. 2009;58:567–578. doi: 10.2337/db08-1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupte AA, Bomhoff GL, Touchberry CD, Geiger PC. Acute heat treatment improves insulin-stimulated glucose uptake in aged skeletal muscle. J Appl Physiol. 2011;110:451–457. doi: 10.1152/japplphysiol.00849.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper PL, Hooper PL. Inflammation, heat shock proteins, and type 2 diabetes. Cell Stress Chaperones. 2009;14:113–115. doi: 10.1007/s12192-008-0073-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kipmen-Korgun D, Bilmen-Sarikcioglu S, Altunbas H, Demir R, Korgun ET. Type-2 diabetes down-regulates glucose transporter proteins and genes of the human blood leukocytes. Scand J Clin Lab Investig. 2009;69:350–358. doi: 10.1080/00365510802632163. [DOI] [PubMed] [Google Scholar]
- Kohn G, Wong HR, Bshesh K, Zhao B, Vasi N, Denenberg A, Morris C, Stark J, Shanley TP. Heat shock inhibits tnf-induced ICAM-1 expression in human endothelial cells via I kappa kinase inhibition. Shock. 2002;17:91–97. doi: 10.1097/00024382-200202000-00002. [DOI] [PubMed] [Google Scholar]
- Kokura S, Adachi S, Manabe E, Mizushima K, Hattori T, Okuda T, Nakabe N, Handa O, Takagi T, Naito Y, Yoshida N, Yoshikawa T. Whole body hyperthermia improves obesity-induced insulin resistance in diabetic mice. Int J Hyperthermia. 2007;23:259–265. doi: 10.1080/02656730601176824. [DOI] [PubMed] [Google Scholar]
- Maratou E, Dimitriadis G, Kollias A, Boutati E, Lambadiari V, Mitrou P, Raptis SA. Glucose transporter expression on the plasma membrane of resting and activated white blood cells. Eur J Clin Invest. 2007;37:282–290. doi: 10.1111/j.1365-2362.2007.01786.x. [DOI] [PubMed] [Google Scholar]
- McCarty MF. Induction of heat shock proteins may combat insulin resistance. Med Hypotheses. 2006;66:527–534. doi: 10.1016/j.mehy.2004.08.033. [DOI] [PubMed] [Google Scholar]
- Morino K, Petersen KF, Shulman GI. Molecular mechanisms of insulin resistance in humans and their potential links with mitochondrial dysfunction. Diabetes. 2006;55(Suppl 2):S9–S15. doi: 10.2337/db06-S002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park HS, Lee JS, Huh SH, Seo JS, Choi EJ. Hsp72 functions as a natural inhibitory protein of c-Jun N-terminal kinase. EMBO J. 2001;20:446–456. doi: 10.1093/emboj/20.3.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park K-J, Gaynor RB, Kwak YT. Heat shock protein 27 association with the I kappa B kinase complex regulates tumor necrosis factor alpha-induced NF-kappa B activation. J Biol Chem. 2003;278:35272–35278. doi: 10.1074/jbc.M305095200. [DOI] [PubMed] [Google Scholar]
- Petersen KF, Shulman GI. Etiology of insulin resistance. Am J Med. 2006;119:S10–S16. doi: 10.1016/j.amjmed.2006.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoelson SE, Lee J, Yuan M. Inflammation and the IKK beta/I kappa B/NF-kappa B axis in obesity- and diet-induced insulin resistance. Int J Obes Relat Metab Disord. 2003;27(Suppl 3):S49–S52. doi: 10.1038/sj.ijo.0802501. [DOI] [PubMed] [Google Scholar]
- Shoelson SE, Lee J, Goldfine AB. Inflammation and insulin resistance. J Clin Invest. 2006;116:1793–1801. doi: 10.1172/JCI29069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simar D, Malatesta D, Badiou S, Dupuy AM, Caillaud C. Physical activity modulates heat shock protein-72 expression and limits oxidative damage accumulation in a healthy elderly population aged 60 90 years. J Gerontol A Biol Sci Med Sci. 2007;62:1413–1419. doi: 10.1093/gerona/62.12.1413. [DOI] [PubMed] [Google Scholar]
- Strle K, Broussard SR, McCusker RH, Shen W-H, LeCleir JM, Johnson RW, Freund GG, Dantzer R, Kelley KW. C-jun N-terminal kinase mediates tumor necrosis factor-alpha suppression of differentiation in myoblasts. Endocrinology. 2006;147:4363–4373. doi: 10.1210/en.2005-1541. [DOI] [PubMed] [Google Scholar]
- Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care. 2004;27:1487–1495. doi: 10.2337/diacare.27.6.1487. [DOI] [PubMed] [Google Scholar]
- Yoo CG, Lee S, Lee CT, Kim YW, Han SK, Shim YS. Anti-inflammatory effect of heat shock protein induction is related to stabilization of IκBα through preventing IκB kinase activation in respiratory epithelial cells. J Immunol. 2000;164:5416–5423. doi: 10.4049/jimmunol.164.10.5416. [DOI] [PubMed] [Google Scholar]
- Zick Y (2005) Ser/Thr phosphorylation of IRS proteins: a molecular basis for insulin resistance. Science's STKE 2005: pe4 [DOI] [PubMed]