Abstract
The modification of proteins by ubiquitination and deubiquitination plays an important role in various cellular processes. BRCA1-associated protein-1 (BAP1) is a deubiquitinating enzyme whose function in the control of the cell cycle requires both its deubiquitinating activity and nuclear localization. In the present study, a ubiquitin carboxyl-terminal hydrolase belonging to the BAP1 family was identified and characterized from Artemia parthenogenetica, a member of a family of brine shrimp that, under certain conditions, produce and release diapause embryos in which cell division and turnover of macromolecules are arrested. Western blot analysis and in vitro enzyme activity assay revealed ArBAP1 to be a cytoplasmic protein with typical ubiquitin hydrolase activity. Northern blot analysis revealed that ArBAP1 was abundant in the abdomen of Artemia producing diapause-destined embryos. Furthermore, by in situ hybridization, ArBAP1 was located exclusively in the embryos. In vivo knockdown of ArBAP1 by RNA interference resulted in the formation of embryos with split shells and abortive nauplii. The present findings suggest that ArBAP1, the first reported cytoplasmic BAP1, participates in the formation of diapause embryos and plays an important role in the control of cell cycle arrest in these encysted embryos.
Keywords: Artemia, BAP1, Deubiquitinate, Diapause, Formation, Maintenance
Introduction
The role of ubiquitin and ubiquitin-mediated pathways in the control of the cellular process has been studied in detail. The process of ubiquitination involves the formation of a peptide bond between the C-terminal Gly of ubiquitin and the ε-amino residue of a Lys in the target protein (Kim et al. 2003). The addition of a single ubiquitin moiety or a chain of ubiquitin moieties can either target a protein for degradation or the ubiquitinated protein can function in the regulation of numerous pathways (Spence et al. 2000; Galan and Haguenauer-Tsapis 1997; Fisk and Yaffe 1999). Like many other post-translational modifications, ubiquitination is reversible through a process catalyzed by a large family of proteins known as deubiquitinating enzymes (DUBs). These enzymes are highly specialized and are involved in various cellular processes such as cell cycle regulation, gene expression, DNA repair, signal transduction, and protein trafficking (Nijman et al. 2005).
Ubiquitin C-terminal hydrolases (UCHs) are a small class of DUBs that catalyze the removal of adducts from the C terminus of ubiquitin. UCHs have a conserved catalytic domain (about 230 amino acids) formed by three positionally conserved Cys, His, and Asp residues (Larsen et al. 1996). In mammals, four UCHs have been identified to date, namely UCH-L1, UCH-L3, UCH37, and BRCA1-associated protein-1 (BAP1). UCH-L1 and UCH-L3 are responsible for the maintenance of the monoubiquitin pool within the cell through ubiquitin recycling and are also implicated in certain diseases (Kurihara et al. 2001; Osaka et al. 2003). UCH37 is associated with the 26S proteasome in the ubiquitin degradation pathway and regulates its function (Lam et al. 1997a, b).
BAP1, which was first discovered through its interaction with the RING finger domain of tumor suppressor BRCA1, is a nuclear-located DUB that enhances the growth suppressive effect of BRCA1 (Jensen et al. 1998). However, BAP1 also possesses a BRCA1-independent activity, as demonstrated by its overexpression in cells lacking BRCA1, where it inhibits cell proliferation and tumor growth (Ventii et al. 2008). Mutations in BAP1 have been found in several lung carcinoma and breast cancer cell lines (Jensen et al. 1998; Wood et al. 2007). Restored BAP1 suppressed the growth of non-small-cell lung carcinoma NCI-H226 cells (BAP1−/−) in culture and in solid tumors in athymic nude mice (Ventii et al. 2008). Inhibition of BAP1 expression by short hairpin RNA in HeLa cells resulted in the hypersensitivity of cells to ionizing irradiation and retardation of S-phase progression (Nishikawa et al. 2009). It was suggested that this inhibition of cell growth by BAP1 could be attributed to its assembly into high molecular weight multiprotein complexes containing transcription factors and cofactors, which might have an effect on the expression of specific genes involved in the regulation of the cell cycle (Machida et al. 2009; Yu et al. 2010; Eletr and Wilkinson 2011).
Artemia, a primitive crustacean known as the brine shrimp, possesses a reproductive system designed to withstand harsh habitats such as hypersalinity, anoxia, and severe temperature changes (Abatzopoulos et al. 2002). Under favorable environmental conditions, Artemia commonly produces nauplius larvae. However, under unfavorable conditions, it is common for the female to release encysted embryos in diapause (Sleger 1991). Diapause is an obligatory dormant state that is often induced in a variety of organisms in response to unfavorable conditions (MacRae 2010). Encysted embryos, which are composed of about 4,000 cells, are arrested at the gastrulae stage and are characterized by an extremely low metabolic rate and the absence of DNA replication, transcription, and translation, all without the loss of viability (Drinkwater and Clegg 1991). After termination of diapause by certain environmental cues, the embryos resume development, given suitable environmental conditions (Abatzopoulos et al. 2002). Although several molecules associated with diapause have been described, the identification of factors involved in diapause initiation is still in its infancy (Sun et al. 2004; Viner and Clegg 2001; Sharon et al. 2009). Recently, flow cytometry analysis revealed that the cells of encysted embryos are arrested at the G1/S stage (data not shown), which indicated a relationship between the induction of diapause and control of the cell cycle.
The present study describes the identification of a BAP1 family known as DUB in Artemia parthenogenetica. This cytoplasmic ArBAP1 contains the conserved UCH domain but lacks two NLS signals found in other BAP1s. By Northern blot analysis, ArBAP1 was found to be abundant in the abdomen of adult Artemia. Furthermore by in situ hybridization, ArBAP1 was found mainly in embryos in the abdominal part of Artemia containing them. Down-regulation of ArBAP1 by double-stranded RNA resulted in the production of embryos with split shells and defective nauplii instead of normal encysted embryos. These findings indicated that ArBAP1 might function in the formation and maintenance of encysted embryos. To date, this is the first reported cytoplasmic member of the BAP1 family. Its identification and characterization could provide new insights into the functions of the deubiquitinating enzymes.
Materials and methods
Animals
A. parthenogenetica from Gahai Lake, China, was a kind gift from Feng-Qi Liu of Nankai University, Tianjin, China. Animals were cultured at room temperature in an environment consisting of 8% artificial seawater and a photoperiod of 5 h light and 19 h dark. Animals were fed once every 2 days with Chlorella powder. Under these conditions, diapause cysts were commonly produced by the oviparous pathway. Examination of Artemia shell gland morphology was used to differentiate between oviparity and ovoviviparity as described by Liang and MacRae (1999). Based on these criteria and those established by Dai et al. (2010), the developmental stages in the reproductive tract were defined as follows: stage 1 is the pre-vitellogenic stage; stage 2 is the early vitellogenic stage, where oocytes reside in ovaries; stage 3 is the late vitellogenic stage, during which the oocytes enter the oviducts; and stages 4 to 6 are embryo developmental stages achieved after the eggs enter the uterus: stage 4 after 1 day, stage 5 after 2 days, and stage 6 after 4 days. After about 4 days of development, the release of encysted embryos or nauplius larvae takes place, depending on the reproductive mode.
Molecular cloning of ArBAP1 encoding cDNA
Adult Artemia carrying different embryonic stages were snap-frozen in liquid nitrogen and homogenized in Trizol Reagent (Invitrogen, Grand Island, NY, USA). Total RNA was prepared according to the manufacturer’s instructions and was quantified on a Genova UV/visible spectrophotometer at 260 nm.
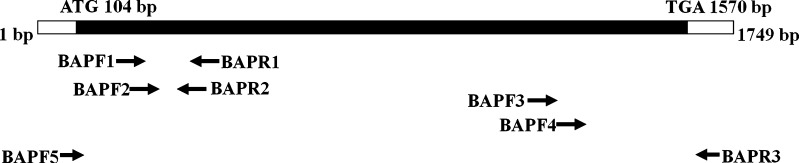
First-strand cDNA was synthesized from 2 μg of total RNA using oligo(dT) and M-MLV Reverse Transcriptase (Promega, Madison, WI, USA) in a 20-μl reaction system. For each sample, an aliquot of 0.5 μl of first-strand cDNA was used as a template for the following PCR amplification. Two primers (BAPF1 and BAPR1) (Table 1) derived from an EST sequence (GenBank number DW678176, Qiu et al. 2007) were used to amplify a fragment of ArBAP1, using the following program: 94°C for 4 min, followed by 30 cycles at 94°C for 30 s, 54°C for 30 s, and 72°C for 30 s, with a final step at 72°C for 10 min. A 231-bp fragment was amplified and showed high identity with the former EST sequence.
Table 1.
Nucleotide sequences and positions of primers used in polymerase chain reactions
| Primer | Length (bp) | Positiona | Direction | Sequence (5′–3′) |
|---|---|---|---|---|
| BAPF1 | 23 | 180–202 | F | TGGAAGATTTTGGAGTATCAGGC |
| BAPR1 | 23 | 389–411 | R | AAAGCATGGGTAGCACAGCTATT |
| BAPF2 | 23 | 184–206 | F | AGATTTTGGAGTATCAGGCGTGC |
| BAPR2 | 23 | 370–392 | R | TATTTGGAACAACTTGATGAGCA |
| BAPF3 | 23 | 1,135–1,157 | F | TGAACTGACCATAGAATCCAAAG |
| BAPF4 | 23 | 1,141–1,163 | F | GACCATAGAATCCAAAGCAGAAG |
| BAPF5 | 25 | 71–95 | F | CAAACACGTTATTTAAGATTCAGCA |
| BAPR3 | 24 | 1,578–1,602 | R | GGCAAATTTTACAAAACATTAGAGC |
| PETF | 33 | 107–126 | F | GGAATTCCATATGCCAGTAGATATAAATAATTT |
| PETR | 32 | 1,548–1,570 | R | CCGCTCGAGCTATCCTTTTCCTGTTGGTTTAG |
| T7F | 46 | 281–300 | F | GATCACTAATACGACTCACTATAGGGTGGAAAGAAGAGAGAAGAGC |
| SP6R | 41 | 693–712 | R | CATTTAGGTGACACTATAGAGCATTGGGTAGGGTTTGAGTC |
| RiF | 28 | 281–300 | F | GCTCTAGATGGAAAGAAGAGAGAAGAGC |
| RiR | 27 | 693–712 | R | GGAATTCCATTGGGTAGGGTTTGAGTC |
| GFPF | 28 | F | GCTCTAGAGCCATTCTTTGGTTTGTCTC | |
| GFPR | 30 | R | GGAATTCAACTTACCCTTAATTTTATTTGC | |
| 18SF | 22 | F | GAAGCACTCTCTACCCTTCCTG | |
| 18SR | 25 | R | ATTCACACGTAGAAAATATACATCG |
The underlined regions represent the adscititious recognition sequences of restriction endonucleases
F forward direction, R reverse direction
aPosition reflect nucleic acid locations in Fig. 2
In the following step, 3′ and 5′ rapid amplification of cDNA ends (5′ and 3′ RACE) was performed with gene-specific primers (BAPF2 and BAPR2) (Table 1) to achieve the full-length cDNA using the FirstChoiceTM RLM-RACE kit (Ambion, Grand Island, NY, USA) according to the manufacturer’s protocol. In 5′ RACE, one 392-bp fragment was achieved and sequenced. Because the full length 3′ end was not obtained with the 3′ RACE, another two gene-specific primers (BAPF3 and BAPF4) (Table 1) were used to screen a cDNA library constructed as previously reported (Dai et al. 2010), which resulted in the amplification of a 610-bp fragment. To verify that those fragments were synthesized from one identical molecule, two primers (BAPF5 and BAPR3 in Table 1) were designed, and amplification was conducted as follows: 35 cycles at 94°C for 30 s (4 min only for the first cycle), 54°C for 30 s, and 72°C for 2 min (10 min only for the last cycle). PCR products were subcloned into the pUCm-T vector (Sangon, Shanghai, China) for sequencing analysis.
Northern blot analysis
The RNA probe used for Northern blot analysis and in situ hybridization was generated by PCR amplification. Based on the ArBAP1 cDNA sequence, two gene-specific primers (T7F, SP6R) (Table 1) with T7 and Sp6 promoter sequences, respectively, were designed. Using purified plasmid containing the full sequence of ArBAP1, the PCR product was gel-purified and labeled with DIG RNA labeling mix (Roche, Indianapolis, IN, USA) using RiboMAXTM Large Scale RNA Production System T7 and SP6 (Promega, Madison, WI, USA).
The body of the adult female Artemia is divided into two parts: cephalothorax and abdomen. The abdomen contains the anterior genital segment, which comprises the female reproductive system (lateral ovaries, oviduct, and ovisac, also known as the uterus) and seven other segments (Abatzopoulos et al. 2002). Total RNA (10 μg) from the cephalothorax and abdomen of animals carrying specific developmental stages as described above was electrophoresed on a 1.0% agarose gel and transferred to a nylon membrane (Millipore, Billerica, MA, USA), followed by pre-hybridization at 42°C for 1 h and hybridization at 55°C overnight with a DIG-labeled antisense probe. After hybridization, the membrane was washed twice in 2× saline sodium citrate (SSC), 0.1% sodium dodecyl sulfate (SDS) at room temperature for 5 min, and twice in 0.5× SSC, 0.1% SDS at 65°C for 15 min. Hybridized probes were visualized with an Ap-conjugated anti-DIG antibody (1:10,000) (Roche, Indianapolis, IN, USA) and the CDP-Star chemiluminescent detection system (Roche, Indianapolis, IN, USA). The hybridized membrane was exposed to Kodak X-ray film for 30 min to 4 h. Ribosomal RNA was used as a control and visualized by goldview staining.
In situ hybridization
Artemia in late embryonic stages were anesthetized on ice, snap-frozen in liquid nitrogen, and embedded in Tissue Tek™ (Sakura, Japan). The embedded samples were cut into 8-μm-thick frozen sections using a frozen ultramicrotome. Dry sections were fixed in paraformaldehyde, digested with proteinase K, and hybridized with the RNA probe mentioned above at 42°C overnight. Then, the slices were washed at 52°C and blocked with blocking solution (Roche, Indianapolis, IN, USA). After blocking, the samples were treated with an anti-DIG-AP conjugate (Roche, 1:500) and visualized with nitroblue tetrazolium/4-bromo-4-chloro-30-indolylphosphate (Promega, Madison, WI, USA), according to the manufacturer’s instructions. Finally, photographs were taken on an inverted microscope (Nikon, Japan).
Localization of ArBAP1
The open reading frame of the ArBAP1 gene was amplified by PCR using the primers PETF and PETR (Table 1). The amplified fragment was subcloned into the pET-28 vector (Novagen, USA) to generate a recombinant His6-ArBAP1 protein. In vitro expression of the fusion protein was induced in Escherichia coli by treatment with isopropyl β-d-1-thiogalactopyranoside, and the proteins were purified using the Ni-NTA resin of QIAexpressionist (Qiagen, Valencia, CA, USA). The purified protein was used as an immunogen to generate a polyclonal antibody against ArBAP1 (HuaAn, Hangzhou, China).
Cell fractionation was performed as described previously (Ausubel 1995). Briefly, decapsulated encysted embryos were homogenized with a pellet pestle® cordless motor (Fisher Scientific, USA) in 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) buffer (10 mM HEPES, 4 mM MgCl2, 1 mM phenylmethylsulfonyl fluoride (PMSF), pH 7.5) for 10 min on ice. Homogenates were centrifuged at 3,300×g for 15 min at 4°C to obtain supernatant and pellet fractions. The pellets (nuclei, yolk platelets, and shell fragments) were resuspended and homogenized in fractionation buffer (10 mM Tris, 10 mM NaCl, 10 mM EDTA, 0.5 mM EGTA, 4 mM MgCl2, pH 7.4) using Dounce homogenizers. Preparations were then loaded on 45% (w/v) sucrose buffer and centrifuged at 12,000×g for 15 min at 4°C. Pellets were washed once with washing buffer (50 mM Tris, 120 mM MgCl2, 1 mM EDTA, 0.5% NP-40, 1 mM PMSF, pH 7.5) and restored to the initial volumes. Proteins were electrophoresed in 10% SDS–polyacrylamide gel electrophoresis (PAGE) and transferred to polyvinylidene fluoride (PVDF) membranes (Millipore, Billerica, MA, USA). The membranes were incubated with anti-ArBAP1, anti-α-tubulin (Sigma, St. Louis, MO, USA) and anti-histone H3 (Epitomics, Burlingame, CA, USA) at 4°C overnight and detection was performed using BM Chemiluminescence western blotting kits (Roche, Indianapolis, IN, USA).
In vitro enzyme activity assays
The ubiquitin carboxy-terminal hydrolase activity of ArBAP1 was assayed using the Ub-AMC substrate (ubiquitin C-terminal 7-amido-4-methylcoumarin) (Boston Biochem, Cambridge, MA, USA) as described previously (Masson et al. 2004). Briefly, purified recombinant His-ArBAP1 was dialyzed against the dialysis buffer (50 mM Tris–HCl, pH 7.5, 0.5 mM EDTA) and then the activity assay. Ub-AMC was diluted to a final concentration of 0.5 μM in 100 μl of assay buffer (50 mM HEPES, 0.5 mM EDTA, 1 mM dithiothreitol, pH 7.5). Samples were incubated for 30 min at 37°C. The levels of hydrolyzed AMC were measured by excitation at 380 nm and emission at 460 nm in an Infinite 200 PRO (Tecan, Mannedorf, Switzerland). UCH-L3 (Boston Biochem, Cambridge, MA, USA) at a concentration of 1 nM was used as a positive control, and bovine serum albumin (Takara, Japan) at 0.15 μM was used as a negative control. In each assay, 19 nmol of recombinant ArBAP1 was used. All samples were analyzed in triplicate.
Double-stranded RNA preparation and RNA interference
For RNAi, a double-stranded RNA (dsRNA) fragment targeting ArBAP1 was generated using the primers RiF and RiR (Table 1). A 431-bp cDNA was amplified and then subcloned into pET-T7 digested with XbaI and EcoRI. As a negative control, a 359-bp cDNA fragment of the GFP gene was amplified using the primers GFPF and GFPR (Table 1). The GFP fragment was then subcloned into pET-T7 in a similar manner as ArBAP1. Recombinant plasmids were transformed into E. coli HT115, and dsRNAs were purified as described by Yodmuang et al. (2006).
A dose gradient (20, 40, 60, 80, and100 ng) of ArBAP1 dsRNA and 100 ng of control GFP dsRNA were injected into the body cavity of Artemia just before ovarian development, with 100 individuals injected for each preparation. An UltraMicroPump II equipped with the Micro4™ MicroSyringe Pump Controller was used for the microinjection. The RNAi-injected and control Artemia were cultured in 8% artificial seawater under the conditions of L5/D19. Cysts from RNAi-treated and control animals were collected and observed by light microscopy.
Real-time quantitative PCR
Total RNAs were extracted from ovisacs and lateral pouches filled with oocytes or embryos 3 weeks after injection, from both RNAi-treated and control groups. After reverse transcription, real-time PCR reactions were performed on the Bio-Rad IQ5TM Gradient Real-Time PCR System using the SYBR® Premix Ex TaqTM (TaKaRa, Shiga, Japan) and 200 nM BAPF1 and BAPR1 (Table 1). Cycling parameters were 40 cycles of 10 s at 95°C (30 s only for the first cycle), 10 s at 56°C, and 10 s at 72°C (5 min only for the last cycle). Dissociation curves were analyzed at the end of each run to determine the purity of the product and the specificity of amplification. Relative transcript levels are presented as fold-changes calculated using the comparative CT method as described by Livak and Schmittgen (2001) and Schmittgen and Livak (2008) using 18S cDNA (GeneBankTM accession number DQ201283, amplified by the primers 18S F/18S R in Table 1) as the internal reference. All data are given as means ± SD of independent experiments from three separate RNA pools. All statistical analyses were performed using one-way analysis of variance, and differences were considered significant at p < 0.01.
Results
Characterization of ArBAP1 encoding cDNA
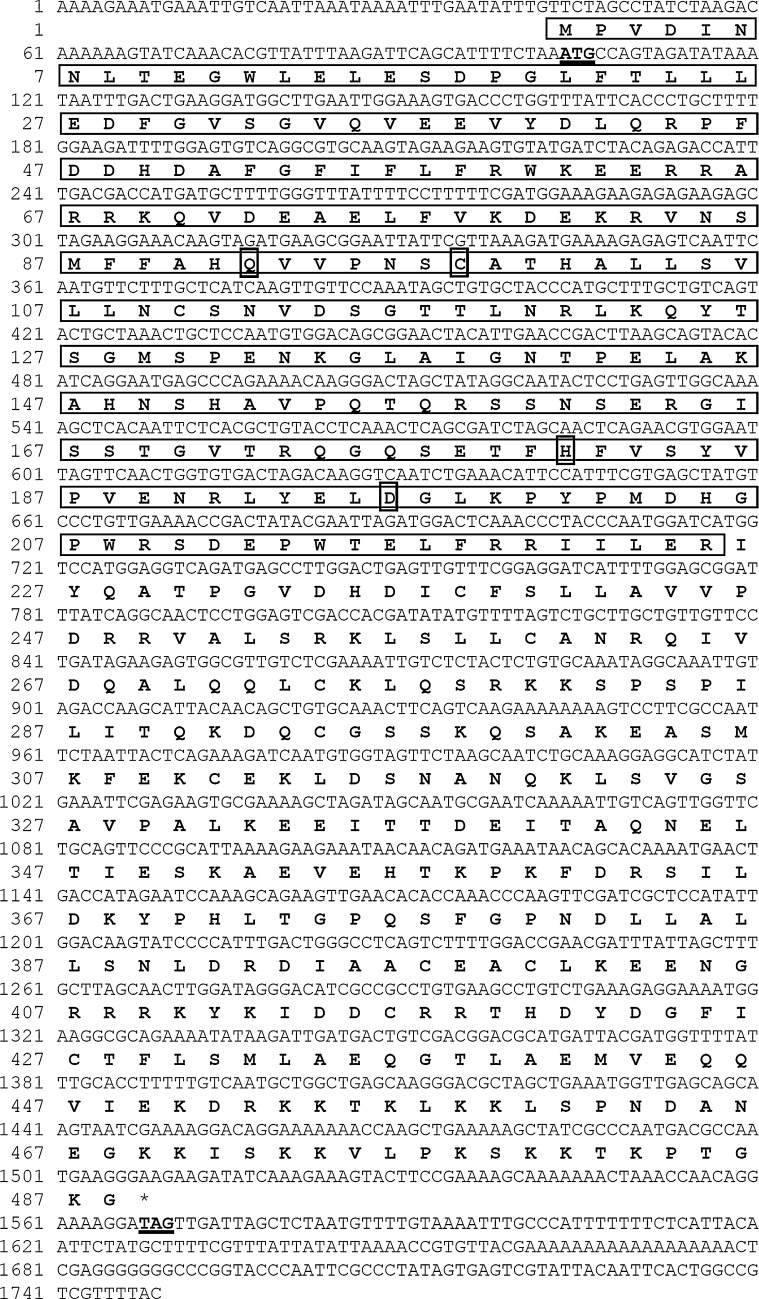
The full-length cDNA sequence of ArBAP1 was constructed by overlapping of several fragments and aligned with the whole length product, amplified by BAPF5 and BAPR3 (Fig. 1). The product contained 1,749 nucleotides with a 1467 open reading frame (ORF), a 103-bp 5′ untranslated region (5′ UTR) and a 179-bp 3′ untranslated region (3′ UTR). The ORF of ArBAP1 was conceptually translated into a 489-amino acid protein with a calculated molecular mass of approximately 55.1 kDa (GenBank accession number: HM853988.1) (Fig. 2). A BLAST search using the deduced amino acid sequence of this protein against the NCBI database revealed that this protein belongs to the BAP1 family of UCH, and the 225 amino acid residues near the N terminus were identified as the conserved UCH domain.
Fig. 1.
Cloning strategy and schematic view of ArBAP1. The open reading frame is represented by the thick black line, and the positions of the start codon (ATG) and the stop codon (TGA) are indicated. The sequences and binding sites of the primers are listed in Table 1
Fig. 2.
Nucleotide sequences and deduced amino acid sequences of ArBAP1.The start and stop codons are in bold and are underlined. The deduced ubiquitin C-terminal hydrolase domain in the N terminus of ArBAP1 is boxed. The conserved catalytic sites in the UCH domain (Q, C, H, D) are identified
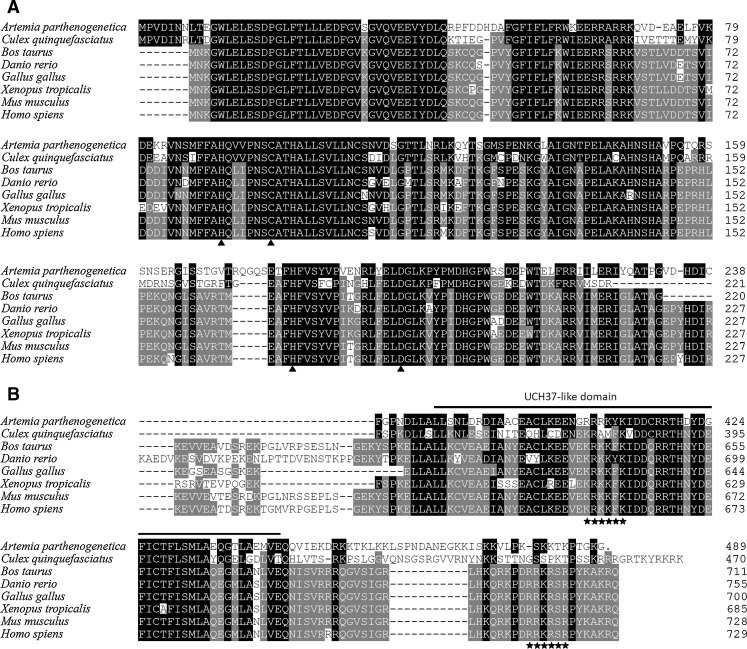
When aligned with other known BAP1s, ArBAP1 shares 45.3% sequence identity with the BAP1 of Culex quinquefasciatus (XM_001842946), 42.9% with the BAP1 of Homo sapiens (NM_004656), and 42.7–41.5% with various other BAP1s. Especially in the N terminus, ArBAP1 shared over 50% identity with other BAP1s, in which the catalytic active residues of UCH (Q92, C98, H181, and D196) are totally conserved (Larsen et al. 1996) (Fig. 3a). In the C terminus, ArBAP1 has a similar UCH37-like domain as other BAP1s but lacks the two putative nuclear localization signals (KRKKFK and RRKRSR) (Jensen et al. 1998) (Fig. 3b).
Fig. 3.
Amino acid sequence alignments of ArBAP1 with other known BAP1s (GenBank accession numbers of the sequences used are as follows: A. parthenogenetica HM853988.1, C. quinquefasciatus XM_001842946, Bos taurus BC133317.1, Danio rerio NM_01163837.1, Gallus gallus NM_001030590.1, Xenopus tropicalis BC080985, Mus musculus NM_02788, H. sapiens NM_004656). a Alignment of the ubiquitin C-terminal hydrolase domain in the N terminus of the BAP1s. Small black triangles indicate the conserved catalytic sites. b Alignment of the C-terminal ends of the BAP1s. Amino acid sequences under the bold line correspond to the UCH37-like domain. Two putative nuclear-localized signals are identified with asterisks
Expression and distribution of ArBAP1
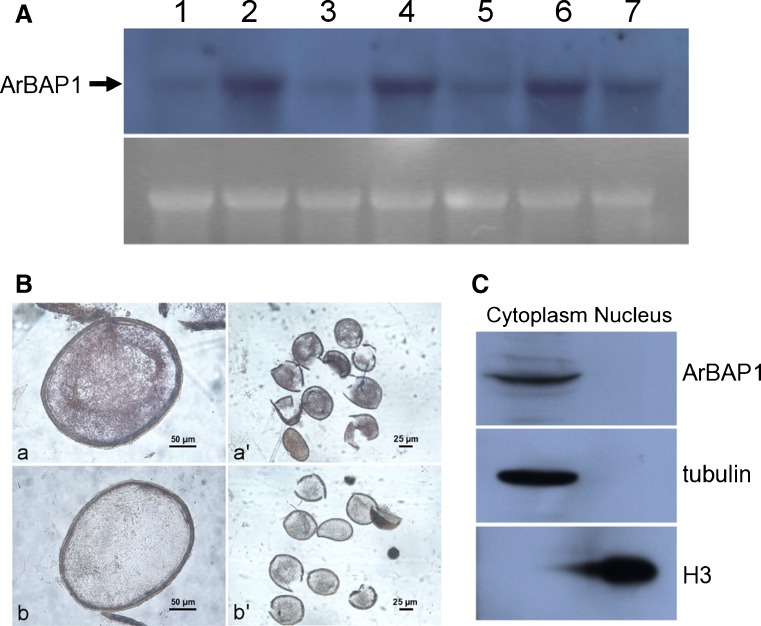
Using an RNA probe and Northern blotting on mature Artemia preparations, it was found that ArBAP1 was abundant and stable in the abdomens, from the early vitellogenic stage to the embryonic stages, although traces could also be detected in the cephalothorax (Fig. 4a). ArBAP1 was also relatively abundant in encysted embryos (Fig. 4a), but no signal was detected in nauplius larvae directly after release from females (data not shown).
Fig. 4.
Expression and distribution of ArBAP1. a Northern blot analysis of Artemia body parts during different developmental stages. Lane 1 cephalothorax from early vitellogenic stage; lane 2 abdomen from early vitellogenic stage; lane 3 cephalothorax from late vitellogenic stage; lane 4 abdomen from late vitellogenic stage; lane 5 cephalothorax from embryonic stage; lane 6 abdomen from embryonic stage; lane 7 encysted embryos. Lower panel rRNA was visualized by goldview staining as a loading control. b In situ hybridization of the abdomen of Artemia in late embryonic stage. a, a′ antisense probes; b, b′ sense probes. c Subcellular localization of ArBAP1. A cell fraction system was used to separate nuclear and cytoplasmic proteins as pellet and supernatant. Proteins were separated by SDS–PAGE and transferred to PVDF membranes. Detections were conducted with anti-ArBAP1 antibody, anti-tubulin antibody, and anti-histone (H3) antibody
To further study the localization of ArBAP1 in the abdomens of Artemia, in situ hybridization with a RNA probe was conducted. Abdomens of Artemia carrying late embryonic stages were examined. ArBAP1 was found to be localized exclusively and widely distributed in the embryos, while no signal was detected in any other part of the abdomen (Fig. 4b).
The subcellular distribution of proteins is usually closely related to their function. BAP1s reported to date, and human BAP1 in particular, were shown to be nuclear-localized. In the present study, cytoplasmic and nuclear proteins were separated by centrifugation of cyst homogenates. The separation was confirmed by western blots using anti-tubulin and anti-histone antibodies (Fig. 4c). ArBAP1 was detected in the supernatant fraction but not in the pellet (Fig. 4c) indicating that, unlike other BAP1s, ArBAP1 is a cytoplasmic protein.
The ubiquitin carboxy-terminal hydrolase activity of ArBAP1
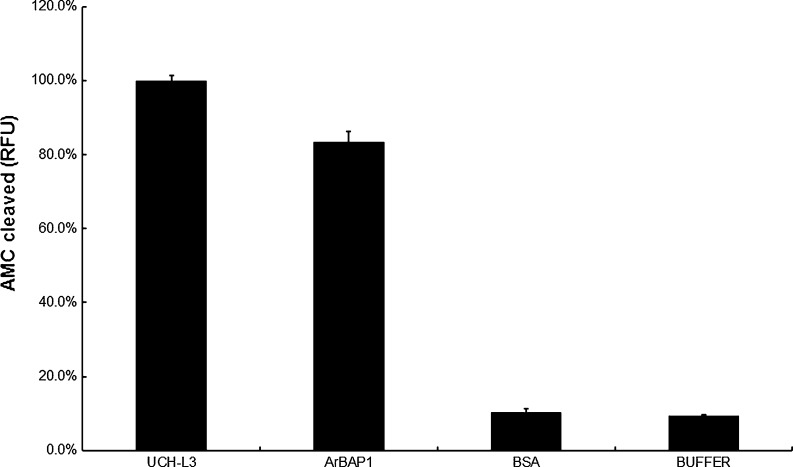
The deubiquitinating activity of recombinant ArBAP1 was measured using a fluorogenic substrate Ub-AMC, which is based on the C terminus derivatization of ubiquitin with 7-amido-4-methylcoumarin (AMC). Ub-AMC is an exquisitely sensitive substrate for UCH-L3 and has been used most frequently for ubiquitin hydrolases assay. As expected, recombinant ArBAP1 hydrolyzed the substrate, releasing AMC and exhibiting similar deubiquitinating activity as UCH-L3, while BSA and buffer control samples produced only background noise (Fig. 5).
Fig. 5.
In vitro enzyme activity assay. Ub-AMC was used as a substrate to assess the ubiquitin carboxy-terminal hydrolase activity of recombinant ArBAP1. The levels of hydrolyzed AMC were measured by excitation at 380 nm and emission at 460 nm. UCHL3 was used as the positive control; BSA and assay buffer were used as the negative controls
ArBAP1 is involved in the formation and development of encysted embryos
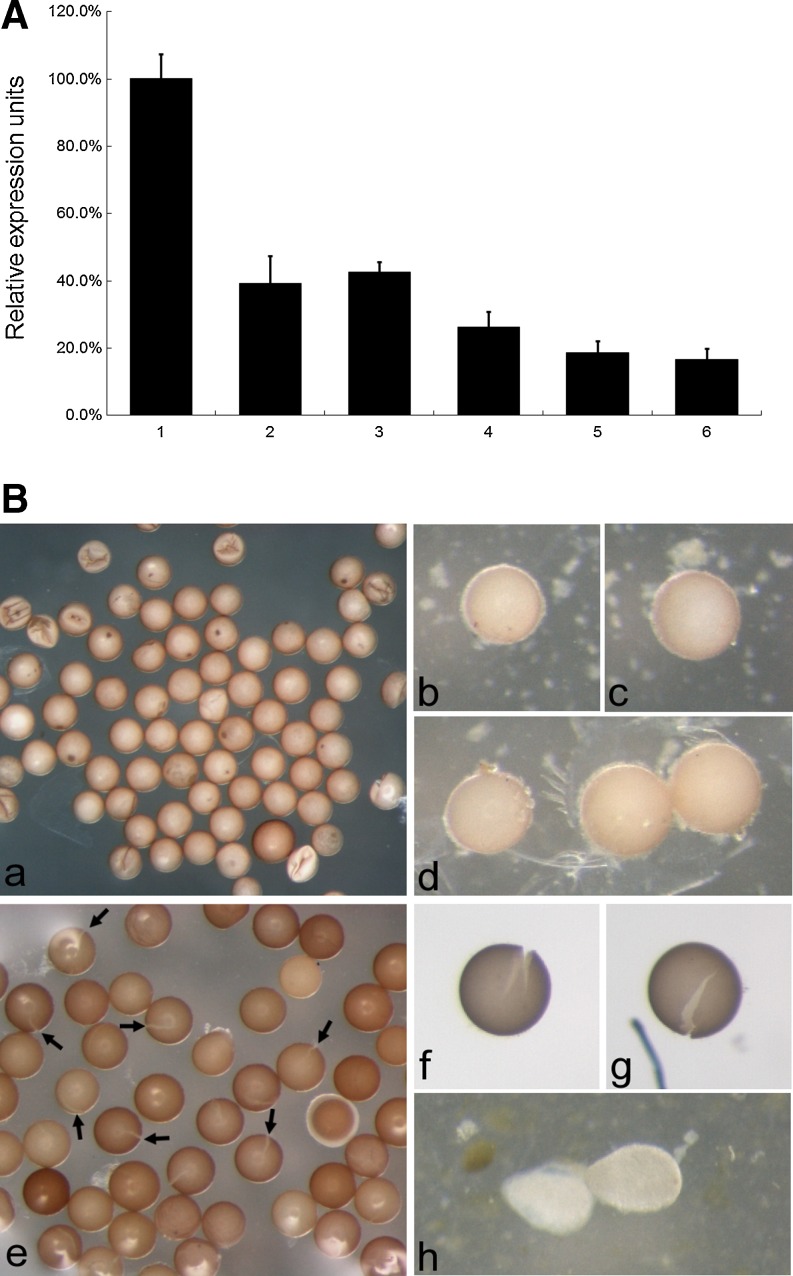
As shown by quantitative RT-PCR, treatment of animals with ArBAP1 dsRNA resulted in a dose-dependence decrease in ArBAP1 levels at 3 weeks after injection. The relative mRNA level of ArBAP1 decreased to a maximum 80% compared with the control (Fig. 6a). In addition, ArBAP1 knockdown produced embryos with a split along the middle of the shell, and these sank to the bottom of the tank (Fig. 6b). Several abortive nauplii were also observed (Fig. 6b). These defective nauplii were released from cysts but could not develop further. GFP dsRNA-injected animals continued to produce and release normal diapause embryos (Fig. 6b).
Fig. 6.
In vivo knockdown of ArBAP1. a The mRNA level of ArBAP1 after RNAi treatment was determined by real-time quantitative PCR. 1 GFP dsRNA (100 ng); 2–6 dsRNA against ArBAP1 (lanes 2, 3, 4, 5, and 6 represent 20, 40, 60, 80, and 100 ng, respectively). The expression level of the GFP dsRNA-injected group was considered to be 100% as a control. The means + SD are plotted. p < 0.001. b Released embryos after ArBAP1 knockdown by dsRNA injection. a–d embryos released by the GFP dsRNA-injected group; e–g embryos released by the ArBAP1 dsRNA-injected group; h abortive nauplius found in the ArBAP1 dsRNA-injected group. In b (e) arrows indicate some cracked cyst shells
Discussion
The UCH family is characterized as a group of small proteins (about 25–30 kDa) localized in the cytoplasm. UCH-L1 and UCH-L3 are both single domain proteins that consist entirely of the UCH domain (Johnston et al. 1999). UCH37 contains a shorter coiled coil C-terminal extension that interacts with the Rpn13 subunit of the proteasome (Hamazaki et al. 2006). However, all BAP1s except those of C. quinquefasciatus have a molecular mass of more than 90 kDa. ArBAP1, which was identified in the present study as a new member of the UCH family, has a molecular mass of 55.1 kDa. It has a conserved domain and catalytic active sites in its N-terminal end, which were able to cleave the ubiquitin isopeptide bond similar to other UCHs (Figs. 2 and 5). In its longer C-terminal extension, ArBAP1 has a UCH37-like domain similar to those found in other BAP1s, but it lacks the shorter KEKE-motif within the ULD of UCH37, necessary for the interaction of UCH37 with Rpn13 (Hamazaki et al. 2006; Misaqhi et al. 2009). These results suggest that, unlike UCH37, ArBAP1 might not associate with the 26S proteasome.
As reported, the majority of BAP1s possess two typical C-terminal nuclear localization signals (Misaqhi et al. 2009). However, analysis of ArBAP1 by the PredictProteinNLS (www.predictprotein.org) and sequence analysis failed to detect any nuclear localization signal in its C terminus (Fig. 3b). Thus, ArBAP1 is the first reported cytoplasmic member of the BAP1 family. Cell fractionation and western blot analysis were used to confirm the cytoplasmic localization of ArBAP1 (Fig. 4c). Recently, human BAP1 was reported to be involved in the regulation of the G1/S transition on the cell cycle. In the study by Ventii et al. (2008), BAP1 in the NCI-H226 cell line (BAP1−/−) resulted in complete growth suppression and an increase in the number of cells in S and sub-G1 phases (Ventii et al. 2008). In MCF10A cells that have endogenous BAP1, overexpression of mutant BAP1-C91S or knockdown of endogenous BAP1 led to reduced cell growth (Machida et al. 2009). The inhibition of cell cycle progression by BAP1 is closely related to the nuclear localization of BAP1, where it might interact with several transcription factors (Ventii et al. 2008; Misaqhi et al. 2009; Machida et al. 2009; Yu et al. 2010). In Artemia, total cell cycle arrest has been reported to occur in the encysted gastrula, mainly at the G2/M phase (Nakanishi et al. 1962; Olson and Clegg 1978; Dai et al. 2007). Recently, based on results from flow cytometry analysis, it was proposed that these cells are arrested at the G1/S transition (data not shown). In the present study, ArBAP1 knockdown resulted in the appearance of embryos with a split across the middle of the shell, which sank to the bottom of the tank, as well as several abortive nauplii. Unlike the normal diapause embryos arrested in the G1/S checkpoint, gene-knockdown embryos continued to develop. However, for unknown reasons, they just stopped and could not reach the normal nauplius stage. It was suggested that ArBAP1, despite its cytoplasmic location, might also play an important role in the regulation of the G1/S transition in a manner similar to the nuclear-localized human BAP1 (Nishikawa et al. 2009; Machida et al. 2009; Yu et al. 2010). The exact role of ArBAP1 in the diapause embryos of Artemia remains to be fully understood.
Spatiotemporal analysis of ArBAP1 expression during Artemia development showed that it was focused in the abdominal region of Artemia, from early vitellogenesis to embryonic stages (Fig. 4a). In adult female Artemia, the abdomen is composed of eight annular segments and contains the female reproductive organs (including ovaries, oviducts, and ovisac) (Abatzopoulos et al. 2002). In the present study, RNA interference experiments showed that in ArBAP1 knockdown, development progressed smoothly from early vitellogenesis to the embryonic stages, but that cysts were released with shells split in the equatorial position (Fig. 6b). These findings suggested that ArBAP1 might not be involved in oogenesis and early development of Artemia but could play a role in the final formation and maintenance of the encysted diapause gastrula.
Artemia releases diapause embryos via the oviparous pathway in part as a survival response to environmental stress. In eukaryotic cells, the ubiquitination of specific proteins not only mediates the selective degradation pathway of proteins but is also an adaptive response to stress (Wilkinson et al. 1992). In yeast, it has been reported that UBI4, a ubiquitin-coding locus, is specifically required for the resistance of cells to stress and ubiquitin is an essential component of the yeast stress response system (Finley et al. 1987). In addition, in the fungus Candida albicans, mutants with the deletion of UBI4, displayed morphological and cell cycle defects and rapidly lost their viability under starvation conditions (Leach et al. 2011). The findings in Artemia, however, are very different. Anchordoguy et al. (1993) investigated the rates of protein turnover in Artemia embryos subjected to anoxia and found that the half life of cytochrome c oxidase was extended by 77-fold (Anchordoguy et al. 1993). Anoxia-induced quiescent embryos of Artemia franciscana showed a block in the ubiquitin-mediated proteolytic pathway leading to a decrease in the levels of ubiquitin-conjugated proteins, from 37% to 7% (Anchordoguy and Hand 1994). The inhibition of protein ubiquitination could be attributed to a reduction in adenylate energy status and the associated intracellular acidosis, but the detailed mechanism is not clear (Anchordoguy and Hand 1994). One amazing characteristic of diapause and anoxic embryos of Artemia is that they undergo full metabolic arrest but still maintain integrity of their cellular machinery for very long periods time. In order to accomplish this, they must severely restrict energy-consuming processes, including macromolecular synthesis, and also tightly control proteolysis. In effect, the embryos must bring turnover of these important macromolecules to a standstill (Abatzopoulos et al. 2002).
Deubiquitinating enzymes catalyze the removal of Ub from Ub-conjugated substrate proteins, and the role of Ub deconjugation in the regulation of several pathways is becoming increasing clear (Anchordoguy and Hand 1994; Wilkinson 1997; Song and Rape 2008). The present results showed that ArBAP1 was abundant both in the abdomen of Artemia producing diapause-destined embryos and in the diapause embryos themselves (Fig. 4a) while no signal was detected in the nauplius larvae directly released by Artemia (data not shown). These findings suggested that ArBAP1 might cleave the Ub moieties from Ub-tagged proteins, leading to the arrest of the ubiquitination-dependent protein degradation pathway in cysts while contributing to the maintenance of diapause embryos.
Acknowledgments
We thank Zhijun Qiu, Stephen C.M. Tsoi, and Thomas H. MacRae for the EST sequence of ArBAP1 from their work on the subtractive hybridization library of A. franciscana. This work was supported by the National Natural Science Foundation of China (30901093 and 40730212).
References
- Abatzopoulos TJ, Beardmore JA, Clegg JS, Sorgeloos P. Artemia: basic and applied biology. the Netherlands: Kluwer Academic; 2002. [Google Scholar]
- Anchordoguy TJ, Hand SC. Acute blockage of the ubiquitin-mediated proteolytic pathway during invertebrate quiescence. Am J Physiol. 1994;267:R895–R900. doi: 10.1152/ajpregu.1994.267.4.R895. [DOI] [PubMed] [Google Scholar]
- Anchordoguy TJ, Hofmann GE, Hand SC. Extension of enzyme half-life during quiescence in Artemia embryos. Am J Physiol. 1993;264:R85–R89. doi: 10.1152/ajpregu.1993.264.1.R85. [DOI] [PubMed] [Google Scholar]
- Ausubel FM (1995) Short protocols in molecular biology, 3rd edn. Wiley, New York
- Dai JQ, Zhu XJ, Liu FQ, Xiang JH, Nagasawa H, Yang WJ. Involvement of p90 ribosomal S6 kinase in termination of cell cycle arrest during development of Artemia-encysted embryos. J Bio Chem. 2007;283:1705–1712. doi: 10.1074/jbc.M707853200. [DOI] [PubMed] [Google Scholar]
- Dai ZM, Li R, Dai L, Yang JS, Chen S, Zeng QG, Yang F, Yang WJ. Determination in oocytes of the reproductive modes for the brine shrimp, Artemia parthenogenetica. Biosci Rep. 2010;31:17–30. doi: 10.1042/BSR20090141. [DOI] [PubMed] [Google Scholar]
- Drinkwater LE, Clegg JS. Experimental biology of cyst diapause. In: Browne RA, Sorgeloos P, Trotman C, editors. Artemia biology. Boca Raton: CRC; 1991. pp. 93–117. [Google Scholar]
- Eletr ZM, Wilkinson KD. An emerging model for BAP1’s role in regulating cell cycle progression. Cell Biochem Biophys. 2011;60:3–11. doi: 10.1007/s12013-011-9184-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finley D, Ozkaynak E, Varshavsky A. The yeast polyubiquitin gene is essential for resistance to high temperature, starvation and other stresses. Cell. 1987;48:1035–1046. doi: 10.1016/0092-8674(87)90711-2. [DOI] [PubMed] [Google Scholar]
- Fisk HA, Yaffe MP. A role for ubiquitination in mitochondrial inheritance in Saccharomyces cerevisiae. J Cell Biol. 1999;145:1199–1208. doi: 10.1083/jcb.145.6.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galan JM, Haguenauer-Tsapis R. Ubiquitin Lys63 is involved in ubiquitination of a yeast plasma membrane protein. EMBO J. 1997;16:5847–5854. doi: 10.1093/emboj/16.19.5847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamazaki J, Lemura S, Natsume T, Yashiroda H, Tanaka K, Murata S. A novel proteasome interacting protein recruits the deubiquitinating enzyme UCH37 to 26S proteasome. EMBO J. 2006;25:4524–4536. doi: 10.1038/sj.emboj.7601338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen DE, Proctor M, Marquis ST, Gardner HP, Ha SI, Chodosh LA, Ishov AM, Tommerup N, Vissing H, Sekido Y, Minna J, Borodovsky A, Schultz DC, Wilkinson KD, Maul GG, Barlev N, Berger SL, Prendergast GC, Rauscher FJ., 3rd BAP1: a novel ubiquitin hydrolase which binds to the BRCA1 ring finger and enhances BRCA1-mediated cell growth suppression. Oncogen. 1998;16:1097–1112. doi: 10.1038/sj.onc.1201861. [DOI] [PubMed] [Google Scholar]
- Johnston SC, Riddle SM, Cohen RE, Hill CP. Structural basis for the specificity of ubiquitin C-terminal hydrolases. EMBO J. 1999;18:3877–3887. doi: 10.1093/emboj/18.14.3877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Park KC, Chung SS, Bang O, Chung CH. Deubiquitinating enzymes as cellular regulators. J Biochem. 2003;134:9–18. doi: 10.1093/jb/mvg107. [DOI] [PubMed] [Google Scholar]
- Kurihara LJ, Kikuchi T, Wada K, Tilghman SM. Loss of Uch-L1 and Uch-L3 leads to neurodegeneration, posterior paralysis and dysphasia. Hum Mol Genet. 2001;10:1963–1970. doi: 10.1093/hmg/10.18.1963. [DOI] [PubMed] [Google Scholar]
- Lam YA, Xu W, DeMartino GN, Cohen RE. Editing of ubiquitin conjugates by an isopeptidase in the 26S proteasomes. Nature. 1997;385:737–740. doi: 10.1038/385737a0. [DOI] [PubMed] [Google Scholar]
- Lam YA, DeMartino GN, Pickart CM, Cohen RE. Specificity of the ubiquitin isopeptidase in the PA700 regulatory complex of 26S proteasomes. J Biol Chem. 1997;272:28438–28446. doi: 10.1074/jbc.272.45.28438. [DOI] [PubMed] [Google Scholar]
- Larsen CN, Price JS, Wilkinson KD. Substrate binding and catalysis by ubiquitin C-terminal hydrolases: identification of two active site residues. Biochemistry. 1996;35:6735–6744. doi: 10.1021/bi960099f. [DOI] [PubMed] [Google Scholar]
- Leach MD, Stead DA, Arqo E, Dm M, Brown AJ. Molecular and proteomic analyses highlight the importance of ubiquitination for the stress resistance, metabolic adaptation, morphogenetic regulation and virulence of Candida albicans. Mol Microbiol. 2011;79:1574–1593. doi: 10.1111/j.1365-2958.2011.07542.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang P, MacRae TH. The synthesis of a small heat shock/alpha-crystallin protein in Artemia and its relationship to stress tolerance during development. Dev Biol. 1999;207:445–456. doi: 10.1006/dbio.1998.9138. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Machida YJ, Machida Y, Vashisht AA, Wohlschleqel JA, Dutta A. The deubiquitinating enzyme BAP1 regulates cell growth via interaction with HCF-1. J Biol Chem. 2009;284:34179–34188. doi: 10.1074/jbc.M109.046755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacRae TH. Gene expression, metabolic regulation and stress tolerance during diapauses. Cell Mol Life Sci. 2010;67:2405–2424. doi: 10.1007/s00018-010-0311-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masson DE, Ek J, Peters EC, Harris JL. Substrate profiling of deubiquitin hydrolases with a positional scanning library and mass spectrometry. Biochemistry. 2004;43:6535–6544. doi: 10.1021/bi049722j. [DOI] [PubMed] [Google Scholar]
- Misaqhi S, Ottosen S, Izrael-Tomasevic A, Arnott D, Lamkanfi M, Lee J, Liu J, O’Rourke DVM, Wilson AC. Association of C-terminal ubiquitin hydrolases BRCA1-associated protein with cell cycle regulator host cell factor 1. Mol Cell Biol. 2009;29:2181–2192. doi: 10.1128/MCB.01517-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakanishi YH, Iwasaki T, Okigaki T, Kano H. Cytological studies of Artemia salina I. Embryonic development without cell multiplication after the blastula stage. Annot Zool Jap. 1962;35:223–228. [Google Scholar]
- Nijman SM, Luna-Vargas MP, Velds A, Brummelkamp TR, Dirac AM, Sixma TK, Bernards R. A genomic and functional inventory of deubiquitinating enzymes. Cell. 2005;123:773–786. doi: 10.1016/j.cell.2005.11.007. [DOI] [PubMed] [Google Scholar]
- Nishikawa H, Wu W, Koike A, Kojima R, Gomi H, Fukuda M, Ohata T. BRCA1-associated protein 1 interferes with BRCA1/BARD1 RING heterodimer activity. Cancer Res. 2009;69:111–119. doi: 10.1158/0008-5472.CAN-08-3355. [DOI] [PubMed] [Google Scholar]
- Olson CS, Clegg JS. Cell division during the development of Artemia salina. Roux’s Arch Dev Biol. 1978;184:1–1. doi: 10.1007/BF00848665. [DOI] [PubMed] [Google Scholar]
- Osaka H, Wang YL, Takada K, Takizawa S, Setsuie R, Li H, Nishikwa K, Sun YJ, Sakurai M, Harada T, Hara Y, Kimura I, Chiba S, Namikawa K, Kiyama H, Noda M, Aoki S, Wada K. Ubiquitin carboxy-terminal hydrolase L1 binds to and stabilizes monoubiquitin in neuron. Hum Mol Genet. 2003;12:1945–1958. doi: 10.1093/hmg/ddg211. [DOI] [PubMed] [Google Scholar]
- Qiu Z, Tsoi SC, MacRae TH. Gene expression in diapause-destined embryos of the crustacean, Artemia franciscana. Mech. Dev. 2007;124:856–867. doi: 10.1016/j.mod.2007.09.001. [DOI] [PubMed] [Google Scholar]
- Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative CT method. Nat Protoc. 2008;3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- Sharon MA, Kozarova A, Clegg JS, Vacratsis PO, Warner AH. Characterization of a group 1 late embryogenesis abundant protein in encysted embryos of the brine shrimp Artemia franciscana. Biochem Cell Biol. 2009;87:415–430. doi: 10.1139/O09-001. [DOI] [PubMed] [Google Scholar]
- Sleger H. Enzyme activities through development: a synthesis of the activity and control of the various enzymes as the embryo matures. In: Browne RA, Sorgeloos P, Trotman C, editors. Artemia biology. Boca Raton: CRC; 1991. pp. 37–73. [Google Scholar]
- Song L, Rape M. Reverse the curse—the role of deubiquitination in cell cycle control. Curr Opin Cell Biol. 2008;20:156–163. doi: 10.1016/j.ceb.2008.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spence J, Gali RR, Dittmar G, Sherman F, Karin M, Finely D. Cell cycle-regulated modification of the ribosome by a variant multiubiquitin chain. Cell. 2000;102:67–76. doi: 10.1016/S0092-8674(00)00011-8. [DOI] [PubMed] [Google Scholar]
- Sun Y, Mansour M, Crack JA, Gass GL, MacRae TH. Oligomerization, chaperone and nuclear localization of p26, a small heat shock protein from Artemia franciscana. J Biol Chem. 2004;29:39999–40006. doi: 10.1074/jbc.M406999200. [DOI] [PubMed] [Google Scholar]
- Ventii KH, Devi NS, Frendrich KL, Chernova TA, Tighiouart M, Meir EG, Wilkinson KD. BRCA1-associated protein-1 is a tumor suppressor that requires deubiquitinating activity and nuclear localization. Cancer Res. 2008;68:6953–6962. doi: 10.1158/0008-5472.CAN-08-0365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viner RI, Clegg JS. Influence of trehalose on the molecular chaperon activity of p26, a small heat shock/alpha-crystallin protein. Cell Sress Chaperones. 2001;6:126–135. doi: 10.1379/1466-1268(2001)006<0126:IOTOTM>2.0.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson KD. Regulation of ubiquitin-dependent processes by deubiquitinating enzymes. FASEB J. 1997;11:1245–1256. doi: 10.1096/fasebj.11.14.9409543. [DOI] [PubMed] [Google Scholar]
- Wilkinson KD, Deshpande S, Larsen CN. Comparisons of neuronal (PGP9.5) and non-neuronal ubiquitin C-terminal hydrolases. Biochem Soc Trans. 1992;20:631–637. doi: 10.1042/bst0200631. [DOI] [PubMed] [Google Scholar]
- Wood LD, Parsons DW, Jones S, Lin J, Sjoblom T, Leary RJ, Shen D, Boca SM, Barber T, Ptak J, Silliman N, Szabo S, Dezso Z, Ustyanksky V, Nikolskaya T, Nikolsky Y, Karchin R, Wilson PA, Kaminker JS, Zhang Z, Croshaw R, Willis J, Dawson D, Shipitsin M, Willson JK, Sukumar S, Polyak K, Park BH, Pethiyagoda CL, Pant PV, Ballinger DG, Sparks AB, Hartigan J, Smith DR, Suh E, Papadopoulos N, Buckhaults P, Markowitz SD, Parmigiani G, Kinzler KW, Velculescu VE, Vogelstein B. The genomic landscapes of human breast and colorectal cancers. Science. 2007;318:1108–1113. doi: 10.1126/science.1145720. [DOI] [PubMed] [Google Scholar]
- Yodmuang S, Tirasophon W, Roshorm Y, Chinnirunvong W, Panyim S. YHV-protease dsRNA inhibits YHV replication in Penaeus monodon and prevents mortality. Biochem Biophys Res Commun. 2006;341:351–356. doi: 10.1016/j.bbrc.2005.12.186. [DOI] [PubMed] [Google Scholar]
- Yu H, Mashtalir N, Daou S, Hammond-Martel I, Ross J, Sui G, Hart GW, Rauscher FJ, 3rd, Drobbetsky E, Milot E, Shi Y, Affarel B. The ubiquitin carboxyl hydrolase BAP1 forms a ternary complex with YY1 and HCF-1 and is a critical regulator of gene expression. Mol Cell Biol. 2010;30:5071–5085. doi: 10.1128/MCB.00396-10. [DOI] [PMC free article] [PubMed] [Google Scholar]