Abstract
Heat shock protein 60 (HSP60) is a highly conserved and multi-functional molecular chaperone that plays an essential role in both cellular metabolism and stress response. Portunus trituberculatus is an important marine fishery and aquaculture species, and water salinity condition influenced its artificial propagations significantly. In order to investigate the function of P. trituberculatus HSP60 against osmotic stress, P. trituberculatus HSP60 gene was firstly cloned. The full-length cDNA of PtHSP60 contains 1,743 nucleotides encoding 577 amino acids with a calculated molecular weight of 61.25 kDa. Multiple alignments indicated that the deduced amino acid sequences of PtHSP60 shared a high level of identity with invertebrate and vertebrate HSP60 sequence including shrimp, fruit fly, zebrafish, and human. The expression profiles of PtHSP60 at mRNA and protein levels under salinity treatment were investigated by semi-quantitative reverse transcriptase-polymerase chain reaction (RT-PCR) and Western blot analysis, respectively. It was found that the mRNA transcripts of PtHSP60 gene varied among different tissues under normal salinity conditions, and the antennal gland showed the highest expression level among the tissues tested. As for low salinity challenge, the mRNA expression of PtHSP60 gene was higher in the gill and appendicular muscle compared with other tissues, and gill and hypodermis represented the higher gene expressions during the hyperosmotic stress, which indicated that those tissues were salinity-sensitive tissues. In addition, salinity challenges significantly altered the expression of PtHSP60 at mRNA and protein level in a salinity- and time-dependent manner in P. trituberculatus gill tissue. The results indicate that PtHSP60 played important roles in mediating the salinity stress in P. trituberculatus.
Keywords: Portunus trituberculatus, HSP60, Expression profile, Salinity stress
Introduction
Heat shock proteins (HSPs) are highly conserved proteins found in all eukaryotes and prokaryotes. These proteins are present in all cells in all forms of life and in a variety of intracellular locations, such as the cytosol of prokaryotes, as well as the nuclei, endoplasmic reticulum (ER), mitochondria, chloroplasts, and cytosol of eukaryotes (Lindquist and Craig 1988).
HSPs gene families consist of stress-inducible and constitutively expressed genes (Parsell and Lindquist 1993). Environmental stresses such as high temperature (Spees et al. 2002a), heavy metals (Pedersen and Lundebye 1996), salinity (Gonzalez and Bradley 1994), and polluting chemical compounds (Werner and Nagel 1997) are all stimuli for the production of HSPs. Several studies demonstrated that HSPs display other essential roles including folding, assembly, intracellular localization and degradation of other proteins and regulation of gene expression (Lindquist and Craig 1988; Morimoto 1998; Terasawa et al. 2005).
Generally, HSPs can be divided into five families according to the molecular weight of their subunits: HSP100, HSP90, HSP70, HSP60, and small HSPs (Georgopoulos and Welch 1993; Nover and Scharf 1997). The HSP60 or chaperonin family is a group of proteins with distinct ring-shaped, or toroid quaternary structures (Quintana and Cohen 2005). An important activity of HSP60s is mediation of the native folding of proteins in an ATP-dependent manner (Ellis and van der Vies 1991).
Up to the present, most studies of HSP60 are focused on mammals and typical model organisms. Lots of studies have indicated its possible role in certain cellular processes, such as germ cell differentiation, reproduction, development, thermo protection, mammalian autoimmune defense, and toxic stress response, and it was even regarded as an potential environment stress marker (Kozlova et al. 1997; Meinhardt et al. 1999; Timakov and Zhang 2001; Choresh et al. 2001; Vabulas et al. 2001; Kammenga et al. 1998; Chen et al. 2008). However, little is known on the response of HSP60 against osmotic exposure. As for HSP60 gene sequence information, relatively little gene information regarding HSP60s of aquatic invertebrates has been obtained, including sea anemone (Anemonia viridis) (Choresh et al. 2001; 2004), zebra mussel (Dreissena polymorpha) (Clayton et al. 2000), and white shrimp (Litopenaeus vannamei) (Zhou et al. 2010; Huang et al. 2011). Yet no sequence of HSP60 is cloned from any crab species to date.
The swimming crab Portunus trituberculatus (Crustacea: Decapoda: Brachyura), also called Japanese blue crab, is widely distributed in the coastal waters of Japan, Korea, China, and Taiwan (Dai et al. 1986). This species is one of the most common edible crabs in China and Korea and supports a large crab fishery and aquaculture in China (Sun 1984).
P. trituberculatus is a euryhaline crab species, surviving in wide-range salinity conditions, but different water salinity condition might influence its distribution and migration route (Dai 1977; Dai et al. 1986; Xue et al. 1997). Water salinity condition is also an important factor for artificial propagation of the swimming crab. Some studies focused on the salinity response in P. trituberculatus found that variable salinity could significantly influence larval development and high level salinity condition would even inhibit zoea change to megalops (Ji 2005; Guo et al. 2003). However, most of these studies concentrated on physiological characterization of P. trituberculatus, there is little information on genomic response of swimming crab exposed to environmental salinity stress.
In our previous study, gill cDNA library of expressed sequence tags (ESTs) were constructed from the swimming crab exposed to two different salinity challenges (10 and 35 ppt) (Xu et al. 2010). To investigate gene expression in the P. trituberculatus exposed to different salinity stresses, 2,426 ESTs from gill cDNA library were selected to spot on a cDNA microarray chip (Xu and Liu 2011). Our cDNA microarray data suggested that there were differences in gene expression patterns of P. trituberculatus for low salinity and high salinity acclimation, and a series of genes including HSPs genes were suggested to be key elements during salinity acclimation process (Xu and Liu 2011).
In this paper, we report the molecular cloning of a full-length cDNA encoding HSP60 from P. trituberculatus and compare the expression patterns at transcription and protein levels of HSP60 by semi-quantitative RT-PCR and Western blot analysis. The results of our study will provide insight for salinity stress-related cellular response in the crustacean and may also be useful for identifying the potential biomarkers of environmental stressors in P. trituberculatus.
Materials and methods
Animal collection and salinity challenge
The specimens of P. trituberculatus were collected from Zhoushan Archipelago of the East China Sea. The salinity challenge experiment was performed as described previously (Xu et al. 2010). Briefly, all the samples were calmed down in the lab breeding conditions (25 ppt, 18°C) with a constant air supply. After acclimation for 3 days, the specimens were divided into two groups and acclimated to two different salinity challenges (10 or 40 ppt) at 18°C.
After salinity challenge for 24 h, three individuals in each salinity group were randomly selected and various tissues, including gill (the 6th pair of gills), gill muscle, ovary, antennal gland, abdominal muscle, hypodermis, heart, and intestine were dissected and then frozen in a −80°C freezer. To determine the expression levels after exposure for different lengths of time to salinity treatment, three crabs from each treatment were sampled at 12, 24, 48, 72, and 120 h, respectively. Since main functions of posterior gills of the swimming crab were osmotic regulation (Jiang and Xu 2011), the 6th and 7th gills of each crab were dissected from each sample at each time point then stored in a −80°C freezer.
RNA extraction
The harvested tissue samples were treated with TRIZOL reagent (Invitrogen) according to the manufacturer’s protocol to extract total RNA. The concentration of RNA was determined spectrophotometrically. All 260/280 ratios were between 1.8 and 2.1. Quality of the RNA was checked by observing intact rRNA on denaturing RNA gels. Extracted RNA was stored in a −80°C freezer for later use.
Cloning and sequencing of PtHSP60 full-length cDNA
In our previous study, gill cDNA library was constructed by using the SMART cDNA Library Construction Kit described as Xu et al. 2010. In total, 4,433 randomly collected clones were sequenced using an ABI Prism 3,730 automated sequencer and the partial HSP60 cDNA sequences (PT0005F02) were obtained from this EST library (Xu et al. 2010).
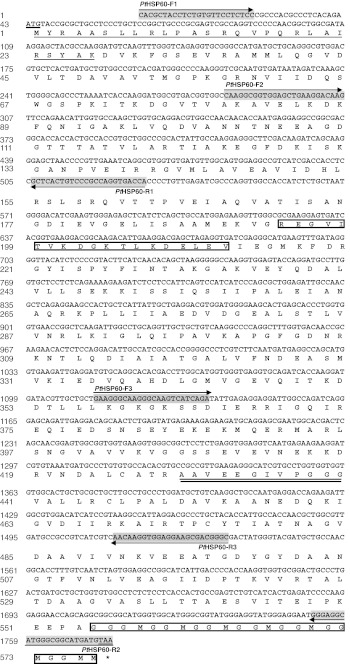
In order to acquire full-length PtHSP60 sequence, we designed three primers: PtHSP60-F1 (5′ CAC GCT ACC TCT GTG TTC CTC TCC 3′, position: 1–24, see Fig. 1), PtHSP60-R1 (5′ TGG TCA CCT GGC GGG ACA GTG AGC 3′, position: 506–529, see Fig. 1) and PtHSP60-F2 (5′CAA GGC GGT GGA GCT GAA GGA CAA G 3′, position: 282–306, see Fig. 1) based on the partial HSP60 EST sequence obtained previously. One degenerate primer PtHSP60-R2 (5′ TTA CAT CAT GCC DCC CAT RCC KCC C 5′, position: 1,752–1,776, see Fig. 1) was designed based on the conserved amino acid sequence of HSP60s in the National Centre for Biotechnology Information (NCBI) database.
Fig. 1.
Nucleotide and deduced amino acid sequences of PtHSP60 (GenBank accession JN628037). The nucleotide sequence is numbered from the 5′end, and the single letter aa code is shown below the corresponding codon. Nucleotide and deduced amino acid residues are numbered on the left. The start code ATG and the termination code are shown underlined. The classical mt-HSP60 signature motifs were double underlined. The 1,734 bp (577 amino acids) sequence contains a presequence of 27 amino acids at the N terminus that is required for import into the mitochondria (underlined). The ATP-binding motif and a typical GGM repeat motif at the C terminus are boxed. The locations of three pairs of primers (PtHSP60-F1/PtHSP60-R1, PtHSP60-F2/PtHSP60-R2, PtHSP60-F3/PtHSP60-R3) designed for PCR used in our study are indicated by arrow and detailed sequences for those primers are indicated by light grey
Samples (2 μg) of total RNA obtained from the 6th gill dissected from three cabs acclimated to two different salinity challenges (10 or 40 ppt). Then, cDNA was generated by reverse transcription with an oligo(dT) primer using Superscript II RNase H-Reverse Transcriptase (Invitrogen). PCR amplification was conducted using a final concentration of 0.5 μM of each primer, 2.5 μM MgCl2, 1 × PCR buffer, 200 μM of each dNTP, 100 μg/ml bovine serum albumin (BSA), 2.5units TaqDNA polymerase (TIANGEN, China), and 80–100 ng cDNA templates. PCRs were conducted on a thermocycler (Mastercycler gradient, Eppendorf) with the samples loaded at 95°C and denatured initially for 5 min. This was followed by 30 cycles of 1 min denaturation at 95°C, 1 min annealing at 55°C, and 2 min extension at 72°C. The 30 cycles were followed by a final extension of 10 min, and cooling to 4°C before the PCR products were removed from the thermocycler.
The DNA fragments encoding full-length PtHSP60 were amplified from P. trituberculatus cDNA by PCR with the PtHSP60-F1/PtHSP60-R1 primer set and PtHSP60-F2/PtHSP60-R2 primer set, respectively. After amplification, the PCR products were cleaned using TIANgel Mini/Midi Purification Kit (TIANGEN, China), integrated into pMD18-T vector (TAKARA) and transformed into competent DH5a, Escherichia coli (TIANGEN). Cells were spread on to agar plates containing LB-ampicillin and incubated overnight at 37°C to promote selective growth of transformed colonies. Positive colonies were identified by white/blue selection and then subject to ABI 3730 DNA sequencing with T3 and T7 universal primers.
Sequence analysis, multiple sequence alignment, and phylogenetic analysis
The full-length cDNA sequence of PtHSP60 was analyzed for similarity with the BLAST programs (Altschul et al. 1997) at the National Center for Biotechnology Information (http://www.ncbi.nlm.nih.gov/BLAST/). The deduced amino acid sequences were obtained using BioEdit software (Hall 1999) and motifs were predicted using ExPASy (http://www.au.expasy.org). The molecular mass and theoretical isoelectric point was predicted using the compute pI/Mw tool (http://www.expasy.org/tools/pi_tool.html). Phylogenetic trees were constructed using the amino acid sequences from various organisms, including vertebrates and invertebrates. The amino acid sequences were aligned using Clustal W software (Thompson et al. 1997). Molecular phylogenetic trees were constructed using the neighbor-joining method from the phylogenetic component of the MEGA3.1 software, and the tree topology was evaluated by 1,000 replications bootstraps.
Tissue expression analysis
The expression of HSP60 mRNA in various tissues was examined by semi- quantitative RT-PCR. Samples (2 μg) of total RNA from gill (the 6th pair of gills), appendicular muscle, intestine, antennal gland, abdominal muscle, hypodermis, heart, and hepatopancreas were reverse-transcribed with an oligo(dT) primer, respectively. The amplification of HSP60 mRNAs was performed using a pairs of specific primers: PtHSP60-F3 (5′- GAA GGG CAA GGG CAA GTC ATC AGA -3′, position: 1,113–1,136, see Fig. 1) and PtHSP60-R3 (5′- GCC CGT CGC TTC CTC CAC CTT GTT -3′, position: 1,513–1,536, see Fig. 1). The amplification of β-actin mRNA (120 bp; GenBank accession number FJ641977), which was used as an internal PCR control, was performed using a pairs of specific primers: actF (5′- TGC TGT CCT TGT ACG CCT CC -3′) and actR (5′- CCA GAC GCA GGA TAG CGT GA -3′). RT-PCR was performed using the following conditions: denaturation at 95°C for 5 min, 30 cycles of 95°C for 30 s, 56°C for 30 s and 72°C for 30 s; extension at 72°C for 10 min. The PCR products were visualized on a UV transilluminator after electrophoresis on a 2.0 % agarose gel containing ethidium bromide (0.5 μg/μl).
The relative expression of PtHSP60 of each tissue for each salinity treatment was quantified by densitometry, measuring the relative intensity of amplified bands, using the software of AlphaView (version 1.2.0.1). Expression index is calculated based on the ratio of band intensity of PtHSP60 and β-actin for each tissue. Statistical analysis was performed with SPSS software (version 11.0). The data were analyzed with a one-way ANOVA followed by the Duncan’s and Tukey’s multiple comparison tests. Differences were considered to be significant at P < 0.05.
PtHSP60 gill mRNA expression profile against salinity challenge
The gill mRNA expression of PtHSP60 at different exposure times under different salinity challenges were examined using semi-quantitative RT-PCR experiments. Briefly, total RNAs were extracted from each group including three crabs and first-strand cDNAs were synthesized using the method described above.
The amplification of gill PtHSP60 mRNAs was performed using a pair of specific primers: PtHSP60-F3/PtHSP60-R3 and RT-PCR was performed described above. In our previous study, ribosomal protein L8 (RpL8) was considered as the stable gene in gill tissue during salinity challenge in swimming crab, and it was therefore selected as an internal PCR control (Xu and Liu 2011). The amplification of RpL8 mRNA (158 bp) was performed using a pairs of specific primers: RpL8F (5′- GCG TAC CAC AAG TAT CGC GT -3′) and RpL8R (5′- AGA CCG ACC TTC CTA CCA GC -3′). RpL8 was an internal control to verify the successful transcription and to calibrate the cDNA template for corresponding samples.
Semi-quantitative RT-PCR was carried out described above. The PCR products were analyzed by electrophoresis on a 2.0 % agarose gel containing ethidium bromide. The relative expression of PtHSP60 was calculated based on the ratio of band intensity of PtHSP60 and RpL8 at each sampling point. Statistical analysis was performed with SPSS software (version 11.0) described as above.
Western blot analysis
The PtHSP60 expressions at protein level of gill tissues at different exposure times under different salinity challenges were examined using Western blot analysis. The gill (the 7th pair of gills) tissues from each group including three crabs were sampled after salinity acclamations were each homogenized in 5 ml of 50 mM Tris–HCl (pH = 7.2) with 50 mM NaCl on ice, and centrifuged at 10,000×g for 20 min at 4°C (5 min each time). The resulting supernatants were collected for protein concentration measurement. Protein concentrations were determined by the method of Bradford (1976) using bovine serum albumin as a standard.
Sixty micrograms of protein were loaded in separate lanes of a 12 % SDS-PAGE gel, and electrophoretically separated. The gels were then washed for 15 min in 20 mM PBS containing 0.1 % Tween-20, and the proteins in the gels were blotted onto a nitrocellulose membrane (Hybond, Amersham Pharmacia). Blotted membranes were incubated in 20 mM PBS containing 3 % BSA at 4°C overnight, and then in anti-Hsp60 antibody (HuaAn. Biotechnology Co. China) diluted 1:800 with 20 mM PBS containing 0.1 % Tween-20 for 1 h. After washing in 20 mM PBS, the membranes were incubated with HRP-conjugated IgG diluted 1:2,000 at 25°C for 1 h, after which the bands were visualized using DAB and 0.03 % H2O2. The expression of β-actin was used as control. Anti-β-actin antibody (HuaAn, Biotechnology Co. China) was diluted 1:1,000, and the same detection methods were used as above. The PtHSP60 levels of each tissue under different salinity treatments were quantified by densitometry, through measuring the relative intensity of each band, using the software of Image-Pro Plus 6.0. The protein expression index is calculated based on the ratio between PtHSP60 and β-actin. The data were expressed as means ± SD from three different Western blot experiments using the same samples. Statistical analysis was performed with SPSS software (version 11.0) described as above.
Results
Identification of PtHSP60 full-length cDNA
Based on partial HSP60 cDNA sequences (PT0005F02) obtained from our EST library (Xu et al. 2010), two pairs of primers, PtHSP60-F1/PtHSP60-R1(529 bp), PtHSP60-F2/PtHSP60-R2(1,495 bp) were designed to clone full-length cDNA sequence of the PtHSP60 gene. The coding sequence of the full-length cDNA of PtHSP60, from the start codon (ATG) to the stop codon (TAA), possessed an open-reading frame (ORF) of 1,734 bp (Fig. 1). The predicted ORF encoded a protein of 577 amino acids with a predicted molecular mass of 61.25 kDa and a theoretical isoelectric point (pI) of 5.28. The 1,734-bp (577 amino acids) sequence contains a presequence of 27 amino acids at the N terminus that is required for import into the mitochondria (Fig. 1). As expected, the mitochondrial presequence is less conserved among the various species, and it contains a majority of nonpolar amino acids (56 % of the sequence), 18 % of basic acids (arginine), and no acidic amino acids (Bedwell et al. 1989; Emanuelsson et al. 2001). The cDNA sequence of the PtHSP60 gene is deposited in the GenBank database with accession number JN628037.
The BLAST (BLASTP) search of the NCBI database with the deduced amino acid sequence for the HSP60 from P. trituberculatus revealed that it has similar, conserved substitutions (positive) with HSP60s/chaperonins from four animal phylums: Arthropoda, Mollusca, Echinodermata, and Chordata. The detailed comparisons were shown in Table 1. A conserved domain search of the NCBI database and application of the program patmatmotifs in EMBOSS identified a conserved ATP-binding/Mg2+-binding site, hinge regions, and stacking interaction sites (Marchler-Bauer et al. 2007).
Table 1.
The related information of HSP60 genes and the similarity to Portunus trituberculatus
| Species | Common name | Class | Accession number | Number of amino acids | Similarity to P. trituberculatus | |
|---|---|---|---|---|---|---|
| Identities (%) | Positives (%) | |||||
| Litopenaeus vannamei | Pacific white shrimp | Arthropoda | ACN30235 | 578Aa | 88 | 95 |
| Drosophila melanogaster | Fruit fly | Arthropoda | NP_511115 | 573Aa | 78 | 90 |
| Polypedilum vanderplanki | African chironomid | Arthropoda | ADM13383 | 569Aa | 77 | 89 |
| Biomphalaria glabrata | Blood fluke planorb | Mollusca | ACL00842 | 571Aa | 77 | 89 |
| Culex quinquefasciatus | Southern house mosquito | Arthropoda | XP_001850501 | 573Aa | 77 | 89 |
| Aedes aegypti | Yellow fever mosquito | Arthropoda | XP_001661764 | 574Aa | 76 | 89 |
| Chilo suppressalis | Asiatic rice borer | Arthropoda | ACT52824 | 572Aa | 75 | 89 |
| Tigriopus japonicus | Intertidal harpacticoid copepod | Arthropoda | ACA03522 | 564Aa | 76 | 88 |
| Paracentrotus lividus | Sea urchin | Echinodermata | CAB56199 | 582Aa | 75 | 86 |
| Danio rerio | Zebrafish | Chordata | NP_851847 | 575Aa | 74 | 87 |
| Ctenopharyngodon idella | Grass carp | Chordata | ADU34083 | 575Aa | 74 | 87 |
| Kryptolebias marmoratus | Mangrove killifish | Chordata | AEM65177 | 575Aa | 74 | 87 |
| Paralichthys olivaceus | Japanese flounder | Chordata | ABB76384 | 575Aa | 72 | 87 |
| Mus musculus | House mouse | Chordata | NP_034607 | 573Aa | 74 | 88 |
| Rattus norvegicus | Norway rat | Chordata | AAC53362 | 573Aa | 74 | 87 |
| Homo sapiens | Human | Chordata | AAA36022 | 573Aa | 74 | 86 |
Phylogenetic analysis of the PtHSP60 protein
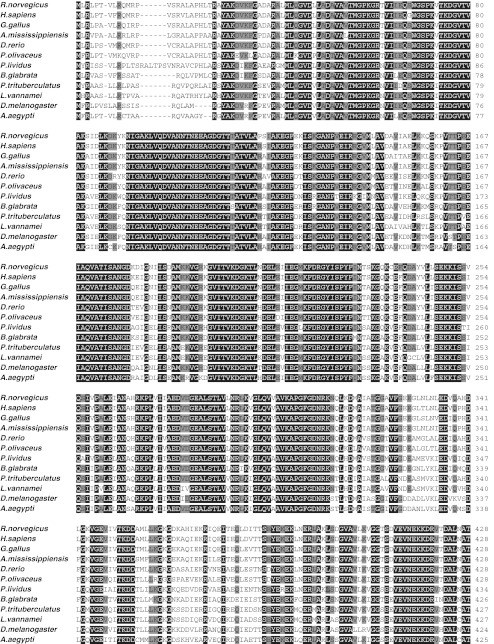
Twelve different HSP60 sequences were aligned and the amino acid similarity was calculated. Multiple alignments of HSP60 full amino sequences were shown in Fig. 2. The deduced amino acid sequence of PtHSP60 showed the highest identity (88 %) with Pacific white shrimp (L. vannamei, ACN30235). It also shared high identities (78, 77, 76, and 75 %) with Drosophila melanogaster HSP60 (NP_511115), Biomphalaria glabrata HSP60 (ACL00842), Aedes aegypti HSP60 (XP_001661764), and Paracentrotus lividus HSP60 (CAB56199) (Fig. 2; Table 1). Furthermore, PtHSP60 also showed significant identity with other invertebrate and vertebrate HSP60 (Fig. 2).
Fig. 2.
Alignment of the PtHSP60 amino acid sequence of known HSP60s. The amino acids are numbered along the right margin. The common names, species names, and GenBank accession numbers are as follows: rat, Rattus norvegicus, AAC53362; human, Homo sapiens, AAA36022; chicken, Gallus gallus, Q5ZL72; American alligator, Alligator mississippiensis, BAF94141; zebrafish, Danio rerio, NP_851847; Japanese flounder, Paralichthys olivaceus, ABB76384; common urchin, P. lividus, CAB56199; blood fluke planorb, B. glabrata, ACL00842; swimming crab, P. trituberculatus, JN628037; Pacific white shrimp, L. vannamei, ACN30235; fruit fly, D. melanogaster, NP_511115; and yellow fever mosquito, A. aegypti, XP_001661764. Residues identical with the threshold of 80 % in all sequences are shaded. Residues in black background indicate 100 % of amino acid similarity
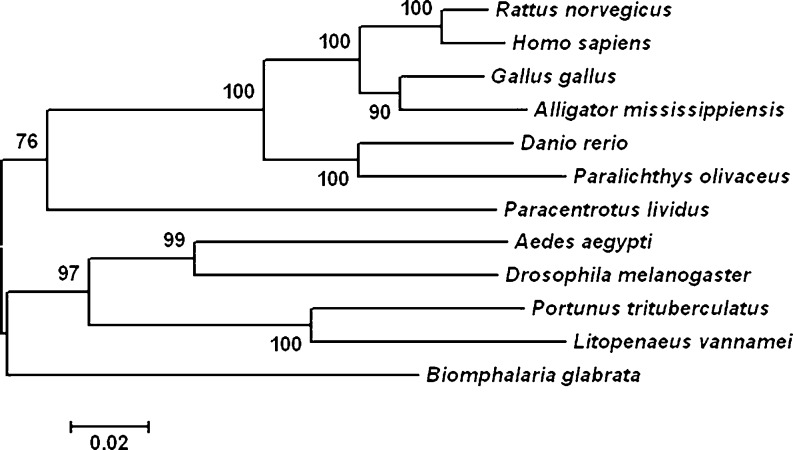
A phylogenetic tree was constructed by analyzing the amino acid sequences of P. trituberculatus HSPs and similar HSPs of other invertebrate and vertebrate species. The result indicated that PtHSP60 belong to the HSPs family. As shown in Fig. 3, P. trituberculatus HSP60 shares greater identity with the Pacific white shrimp (L. vannamei, ACN30235) than it does with insects.
Fig. 3.
The neighbor-joining tree shows the relationship of PtHSP60 with other known HSP60s. Alignment of amino acid sequences are produced by Clustal W, and the bootstrap neighbor-joining phylogeny tree was constructed by MEGA 3.1 (bootstrap = 1,000). The species and accession numbers are the same as shown in Fig. 2. Branch lengths are proportional to estimates of evolutionary change. The number associated with each internal branch is the local bootstrap probability, which is an indicator of confidence
Tissue distribution of PtHSP60 mRNA
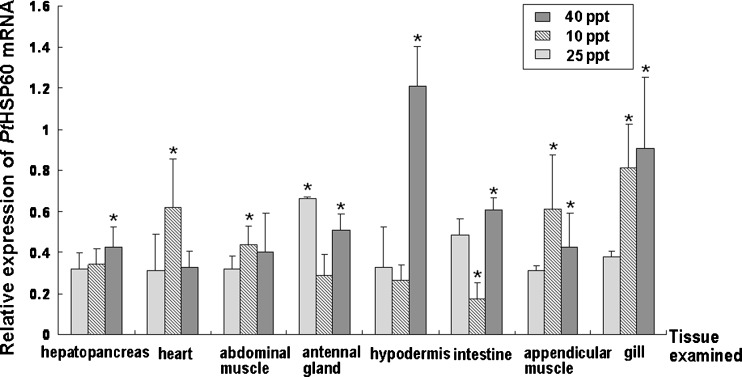
To examine the tissue distribution profile of the PtHSP60 gene, semi-quantitative RT-PCR analysis from several tissues including gill (the 6th pair of gills), appendicular muscle, intestine, antennal gland, abdominal muscle, hypodermis, heart, and hepatopancreas were conducted. A 424-bp fragment of the HSP60 gene was amplified in all tissues examined with primers PtHSP60-F3 and PtHSP60-R3.
As shown in Fig. 4, the mRNA level of P. trituberculatus HSP60 in the antennal gland was comparably higher than it was in other tissues at normal salinity (25 ppt). As for the salinity challenge, the mRNA expression of PtHSP60 showed a salinity-dependent response under salinity stress (Fig. 4). During the low salinity challenge, the mRNA expression of PtHSP60 was higher in the gill and appendicular muscle compared with other tissues, and gill and hypodermis represented the comparably higher gene expressions during the hyperosmotic stress, which indicated that those tissues were salinity sensitive tissue (Fig. 4). Moreover, it was also clear that during low and high salinity challenges (10 and 40 ppt), mRNA level of P. trituberculatus HSP60 in the gill was significantly higher (P < 0.05) than that at the control salinity (25 ppt) (Fig. 4), which was consistent with the suggestion that the gill was the main tissue to respond to environmental salinity stressors.
Fig. 4.
Relative expression of PtHSP60 mRNA in various tissues of P. trituberculatus during normal salinity (25 ppt) and salinity challenges (10 or 40 ppt), as determined by semi-quantitative RT-PCR. The examined tissues of three crabs in each group were collected. The β-actin RNA was used as an internal and the relative expression levels of PtHSP60 were obtained relative to β-actin expression. Values are expressed as means ± SD of the relative variations (fold induction) between each treatment (10 or 40 ppt) and the control sample (25 ppt); asterisks beside the bars indicate statistically significant differences (*P < 0.05)
The PtHSP60 transcription and protein level changes in P. turberculartus gills under salinity challenges
To determine whether PtHSP60 expression was time dependent under salinity challenges, three swimming crabs were sampled at each time point. RNA and protein level expression of PtHSP60 in P. turberculartus gill tissue at different exposure times under different salinity challenges were examined using semi-quantitative RT-PCR experiments and Western blot analysis, respectively.
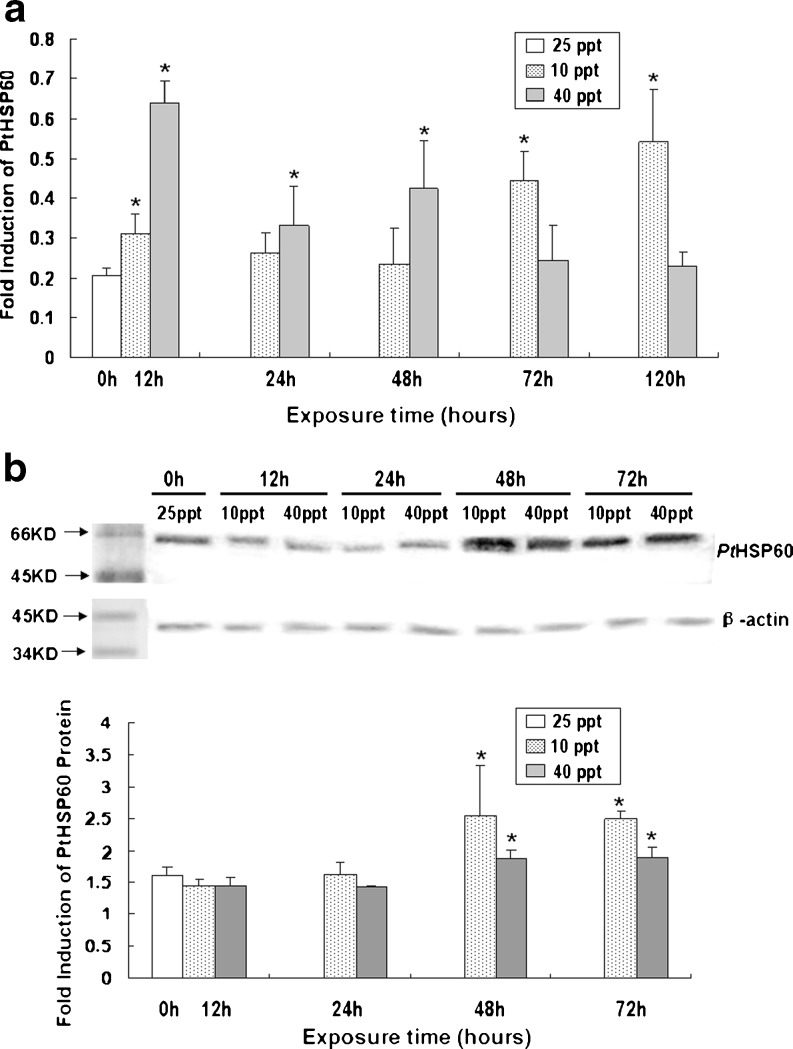
It was found that the mRNA expression of PtHSP60 in P. trituberculatus under salinity stress had a curvilinear trend with time. During high salinity stress, the mRNA level reached a peak at 12 h, decreased a little at 24 h, then increased at 48 h, then decreased gradually to the pretreatment level at 120 h (Fig. 5a). As for the low salinity challenges, the mRNA level increased at 12 h, then gradually decreased to the pretreatment level at 48 h, and then increased sharply and reached a peak at 120 h (Fig. 5a).
Fig. 5.
RNA and protein level expression of PtHSP60 in P. turberculartus gill tissue at different exposure times under different salinity challenges. a Relative PtHSP60 mRNA expression levels in gill tissue at different time points in response to salinity challenges. The 6th pair of gills tissues of three crabs in each group was collected. Transcript levels for all samples were assessed by semi-quantitative RT-PCR, and the relative expression levels of PtHSP60 were obtained relative to RpL8 expression. Values are expressed as means ± SD of the relative variations (fold induction) between each treatment (10 or 40 ppt) and the control sample (25 ppt); asterisks above thebars indicate statistically significant differences (*P < 0.05); 0–120 h, sampling point after salinity challenge. b Western blot analysis of PtHSP60 expression in gill tissue at different time points in response to salinity challenges. The 7th pair of gills tissues of three crabs in each group was collected. Protein expression levels for all samples were assessed by Western blot analysis, and the relative expression levels of PtHSP60 protein were obtained relative to β-actin expression. For Western blot expression analysis, 60 μg of protein were loaded into each lane. Values are expressed as means ± SD of the relative variations (fold induction) between each treatment (10 or 40 ppt) and the control sample (25 ppt); asterisks above the bars indicate statistically significant differences (*P < 0.05); 0–72 h, sampling point after salinity challenge
As shown in Fig. 5a, during high salinity challenge, the PtHSP60 transcript was significantly upregulated (3.2-fold) in the gill at only 12 h (P < 0.05). With prolonged exposure time, the expression level of PtHSP60 mRNA dropped back down to the control level. However, significant differences in the expression levels of PtHSP60 in the gills were observed at 12, 24, and 48 h compared with the control.
As for the low salinity stress, the expression of PtHSP60 transcripts was gradually upregulated to 2.2-fold at 72 h in the gills, and then reached the highest level (2.7-fold) at 120 h of low salinity challenge (Fig. 5a). Moreover, significant differences in the expression levels of PtHSP60 mRNA were observed at 12, 72 and 120 h compared with the control when facing low salinity stress (Fig. 5a).
To further examine the levels of PtHSP60 protein, Western blot analysis was performed, and we found that salinity challenges significantly altered the PtHSP60 levels in a salinity- and time-dependent manner in the P. trituberculatus gill tissue (Fig. 5b). Different from the semi-quantitive RT-PCR results from the gill tissue, PtHSP60 protein was not significantly induced at the early stage (12 and 24 h) (Fig. 5b), but significantly increased at 48 and at 72 h time points comparing with the controls under both hypo- and hypersaline challenges (Fig. 5b).
Discussion
For most aquatic organisms, they are confronted with numerous environmental stressors such as osmotic stress. In response to osmotic stress, a highly conserved set of proteins, termed HSPs are induced. Acting as molecular chaperones, HSPs assist in the refolding of stress-denatured proteins, and prevent those proteins from aggregating in the cell. And HSPs’ expressions are induced as a result of environmental stress (Frydman and Höhfeld 1997; Morimoto 1998; Feder and Hofmann 1999; Hartl 1996; Hasday and Singh 2000; Deane et al. 2002).
Among the different families of HSPs, HSP70, HSP90, and HSP60 were mostly researched proteins. HSP70 is essential in mediating the effects of environmental stresses, maintaining cellular homeostasis (Geething and Sambrook 1992; Parsell and Lindquist 1993; Chen et al. 2008), and playing a critical role in toleration of osmotic stress in various species including lobster (Homarus americanus) (Spees et al. 2002b), sea cucumber (Apostichopus japonicus) (Dong et al. 2008), rainbow trout (Oncorhynchus mykiss) (Niu et al. 2008), euryhaline cilate (Paramecium nephridiatum) (Smurov et al. 2007), intertidal copepod (Tigriopus japonicus) (Rhee et al. 2009), and so on. Moreover, multiple functions have been assigned to HSP90 such as protein folding, cell signaling, and protein degradation, in both normal metabolism and protecting organisms under stressful conditions including osmotic stress condition (Geething and Sambrook 1992; Spees et al. 2002b; Pearl and Prodromou 2006). For example, HSP90 from Pennisetum glaucum were suggested having roles in abiotic stress (including salinity stress) adaptation (Reddy et al. 2011). HSP90 from Crassostrea hongkongensis was also suggested to play an important role in both salinity tolerance and immune defense (Fu et al. 2011). HSP60 is primarily a mitochondrial protein that is important for folding key proteins after import into the mitochondria. An important activity of HSP60s is mediation of the native folding of proteins (Ellis and van der Vies 1991). Thus, the chaperone activity appears to play central roles in defense ability and response to stress in addition to normal cells (Vabulas et al. 2001). However, very few literatures addressed HSP60s function on salinity tolerance so far.
P. trituberculatus is one of the most important aquaculture species and water salinity condition is an important factor for artificial propagation of the swimming crab. In an attempt to better understand the response to salinity stress in this crab species, the PtHSP60 gene was cloned and the expression patterns induced by salinity stress were analyzed. Therefore, the results presented here provide useful insight for investigating stress-related cellular response and for identifying potential biomarkers of environmental stressors in P. trituberculatus.
In this study, the full-length gill cDNAs of the PtHSP60 gene was identified and characterized. The sequence of PtHSP60 contains 1,734 bp and encodes 577 amino acids. Alignment also revealed that The 1,734 bp (577 amino acids) sequence contains a 27-amino acid extension at the N terminus, which may serve as a mitochondrial targeting signal (Fig. 1 underlined) (Emanuelsson et al. 2001). Conserved sequences and characteristic motifs, such as HSP60 family signatures, ATP-binding/Mg2+-binding site (from 194 to 212 amino acid residues) (Marchler-Bauer et al. 2007), and conserved GGM repeats at the C-terminal end (the structure and function of which are unknown (Sanchez et al. 1999)), as well as the major structural and functional domains typically found in HSP60 proteins (Choresh et al. 2004), were found in the deduced PtHSP60 amino acid sequence, which suggested that HSP60 protein of P. trituberculatus presented here was a member of the mitochondrial HSP60 chaperone family.
Sequence similarity searches revealed that the deduced amino acid sequence of PtHSP60 shares high similarity with previously described HSP60 sequences from shrimp, fruit fly, zebrafish, human, etc. (more than 80 % similarity in all matches) (Table 1; Fig. 2). The neighbor-joining method was used to conduct a phylogenetic analysis including HSP60 from vertebrate and invertebrate species (Fig. 3), which indicated that the HSP60 genes have highly conserved sequences and could be used for evolutionary and phylogenetic analysis.
The distribution patterns of PtHSP60 were determined in eight different tissues. Under normal salinity condition, PtHSP60 gene was expressed in all tested tissues of P. trituberculatus, suggesting that these gene products were required to maintain cell homeostasis. Although PtHSP60 gene was expressed in different tissues, the expression level was comparably higher in some tissues such as antennal gland and intestine than in others. The transcription expression profile of PtHSP60 among different tissues revealed that PtHSP60 was significantly up-regulated in the gills after low and high salinity challenges, which suggested that the P. trituberculatus gill was the salinity sensitive tissue and therefore could be used as the target in a time-dependent study. In addition, levels of PtHSP60 transcripts in appendicular muscle and hypodermis were significantly increased during low salinity and high salinity, respectively, which suggested that those two tissues might be involved in osmoregulation process.
As we know, salinity stress impacted on the folding and transformation of proteins and the HSPs played important roles in maintaining biological processes in the organism challenged (Smurov et al. 2007; Rhee et al. 2009). HSP60 contributes to the maintenance of structural integrity (Ellis and van der Vies 1991). The damage to an organism was increased with salinity variations and in order to maintain structural integrity at salinity stress needed more molecular chaperones, so the transcripts of the HSP60 gene were increased.
In crustaceans, gills are respiratory organs that are in direct contact with the external environment and are known the major active site for osmoregulation, detoxification, and defense mechanisms (Henry and Wheatly 1992; Taylor and Taylor 1992; Péqueux 1995). Owing to its function in metabolism, the gill can be sensitive to environmental changes. The time-course expression pattern at mRNA and protein levels of PtHSP60 was determined in the P. trituberculatus gill by semi-quantitative RT-PCR and Western blot analysis, respectively. Our studies suggested the expressions of the gill PtHSP60 at mRNA and protein level were altered with the time and salinity (Fig. 5a, b), a response possibly linked to the repair of misfolding proteins and to maintaining homeostasis of the cellular metabolism in P. trituberculatus under salinity stress. During high salinity challenge (40 ppt), the mRNA expression level of PtHSP60 reached peak at 12 h and remained high at 48 h, whereas the mRNA expression of PtHSP60 reached a minimum at 48 h during low salinity stress (10 ppt) (Fig. 5a). The different expression profiles of PtHSP60 indicated its different functions under different salinity challenges. However, the continued synthesis of HSPs requires a great deal of energy and has an impact on the synthesis of other proteins and on the growth of the organism (Krebs and Feder 1997; Viant et al. 2003). Thus, during the high salinity stress, for the maintenance of biological processes, the transcripts of PtHSP60 gene decreased gradually after 48 h, and returned to the untreated level at 120 h (Fig. 5a).
As for the protein expression of PtHSP60, it was clear that PtHSP60 protein expression were significantly up regulated in gill tissue after 48 h salinity acclimation for both hypo- and hypersaline challenges (Fig. 5b). The elevated protein levels of the PtHSP60 could enhance the salinity tolerance of P. trituberculatus and strengthen the correction of misfolding protein. However, compared with mRNA expression profile of PtHSP60, the time-course expression pattern at protein level of PtHSP60 exhibited apparent disagreement responding to different salinity challenges. It was indicated in higher vertebrate models that the transcription of message and the translation of HSPs are in apparent disagreement and it has been proved that the transcriptional activation of HSPs might not be paralleled by protein synthesis (Hensold et al. 1990; Bruce et al. 1993). Therefore, the disagreement between time-course expression pattern at mRNA and protein levels of PtHSP60 in P. trituberculatus gill tissue against salinity challenges was not unexpected.
In conclusion, we identified the effective chaperone activity of PtHSP60 and described its differential expression patterns in response to environmental salinity stress. Our results support that PtHSP60 possibly participates in crab salinity stress response. This indicates that PtHSP60 regulates the salinity response via an intrinsic pathway. Further research is required to confirm this speculation.
Acknowledgments
This work is supported in part by grants from the National Natural Science Foundation of China (grant no. 30800840), and the Shanghai Young Rising Star of Science and Technology Program (grant no. 09QA1402600) to Qianghua Xu.
References
- Altschul SF, Madden TL, Schäffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedwell DM, Strobel SA, Yun K, Jongeward GD, Emr SD. Sequence and structural requirements of a mitochondrial protein import signal defined by saturation cassette mutagenesis. Mol Cell Biol. 1989;9:1014–1025. doi: 10.1128/mcb.9.3.1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein–dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Bruce JL, Price BD, Coleman N, Calderwood SK. Oxidant injury rapidly activates the heat shock transcription factor but fails to increase levels of heat shock proteins. Cancer Res. 1993;53:12–15. [PubMed] [Google Scholar]
- Chen Z, Christina CC-H, Zhang J, Cao L, Chen L, Zhou L, Jin Y, Ye H, Deng C, Dai Z, Xu Q, Hu P, Sun S, Shen Y, Chen L. Transcriptomic and genomic evolution under constant cold in Antarctic notothenioid fish. Proc Natl Acad Sci USA. 2008;105(35):12944–12949. doi: 10.1073/pnas.0802432105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choresh O, Ron E, Loya Y. The 60-kDa heat shock protein (HSP60) of the sea anemone Anemonia viridis: a potential early warning system for monitoring environmental changes. Mar Biotechnol. 2001;3:501–508. doi: 10.1007/s10126-001-0007-4. [DOI] [PubMed] [Google Scholar]
- Choresh O, Loya Y, Muller WEG, Wiedenmann J, Azem A. The mitochondrial 60-kDa heat shock protein in marine invertebrates: biochemical purification and molecular characterization. Cell Stress Chaperones. 2004;9:38–47. doi: 10.1379/469.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton ME, Steinmann R, Fent K. Different expression patterns of heat shock proteins hsp 60 and hsp 70 in zebra mussels (Dreissena polymorpha) exposed to copper and tributyltin. Aquat Toxicol. 2000;47:213–226. doi: 10.1016/S0166-445X(99)00022-3. [DOI] [Google Scholar]
- Dai AY. Primary investigation on the fishery biology of the Portunus trituberculatus. Mar Fish. 1977;25:136–141. [Google Scholar]
- Dai AY, Yang SL, Song YZ. Marine crabs in China Sea. Beijing: Marine Publishing Company; 1986. pp. 194–196. [Google Scholar]
- Deane E, Kelly S, Luk J, Woo N. Chronic salinity adaptation modulates hepatic heat shock protein and insulin-like growth factor I expression in black sea bream. Mar Biotechnol. 2002;4:193–205. doi: 10.1007/pl00021690. [DOI] [PubMed] [Google Scholar]
- Dong YW, Dong SL, Meng XL. Effects of thermal and osmotic stress on growth, osmoregulation and Hsp70 in sea cucumber (Apostichopus japonicus Selenka) Aquaculture. 2008;276:179–186. doi: 10.1016/j.aquaculture.2008.01.028. [DOI] [Google Scholar]
- Ellis RJ, Vies SM. Molecular chaperones. Annu Rev Biochem. 1991;60:321–347. doi: 10.1146/annurev.bi.60.070191.001541. [DOI] [PubMed] [Google Scholar]
- Emanuelsson O, Heijne G, Schneider G. Analysis and prediction of mitochondrial targeting peptides. Methods Cell Biol. 2001;65:175–187. doi: 10.1016/S0091-679X(01)65011-8. [DOI] [PubMed] [Google Scholar]
- Feder ME, Hofmann GE. Heat-shock proteins, molecular chaperones, and the stress response: evolutionary and ecological physiology. Annu Rev Physiol. 1999;61:243–282. doi: 10.1146/annurev.physiol.61.1.243. [DOI] [PubMed] [Google Scholar]
- Frydman J, Höhfeld J. Chaperones get in touch: the hip-hop connection. Trends Biochem Sci. 1997;22:87–92. doi: 10.1016/S0968-0004(97)01005-0. [DOI] [PubMed] [Google Scholar]
- Fu D, Chen J, Zhang Y, Yu Z. Cloning and expression of a heat shock protein (HSP) 90 gene in the haemocytes of Crassostrea hongkongensis under osmotic stress and bacterial challenge. Fish Shellfish Immun. 2011;31:118–125. doi: 10.1016/j.fsi.2011.04.011. [DOI] [PubMed] [Google Scholar]
- Geething MJ, Sambrook J. Protein folding in the cell. Nature. 1992;355:33–45. doi: 10.1038/355033a0. [DOI] [PubMed] [Google Scholar]
- Georgopoulos C, Welch WJ. Role of the major heat shock proteins as molecular chaperones. Ann Rev Cell Biol. 1993;9:601–634. doi: 10.1146/annurev.cb.09.110193.003125. [DOI] [PubMed] [Google Scholar]
- Gonzalez CRM, Bradley BP. Are there salinity stress proteins? Mar Enivron Res. 1994;35:79–83. [Google Scholar]
- Guo JW, Xu ZW, Yu XJ, Qin SK, Ren PJ. The tactics and reasons of the difficulty of larval abnormality. Sci Fish Cul. 2003;9:30–31. [Google Scholar]
- Hall TA. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98 NT. Nucl Acids Symp Ser. 1999;41:95–98. [Google Scholar]
- Hartl FU. Molecular chaperones in cellular protein folding. Nature. 1996;381:571–580. doi: 10.1038/381571a0. [DOI] [PubMed] [Google Scholar]
- Hasday JD, Singh IS. Fever and the heat shock response: distinct, partially overlapping processes. Cell Stress Chaperones. 2000;5:471–480. doi: 10.1379/1466-1268(2000)005<0471:FATHSR>2.0.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry RP, Wheatly MG. Interaction of respiration, ion regulation, and acid–base balance in the everyday life of aquatic crustaceans. Am Zool. 1992;32:407–416. [Google Scholar]
- Hensold JO, Hunt CR, Calderwood SK, Housman DE, Kingston RE. DNA binding of the heat shock element is insufficient for transcriptional activation in murine erythroleukemia cells. Mol Cell Biol. 1990;10:1600–1608. doi: 10.1128/mcb.10.4.1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang WJ, Leu JH, Tsau MT, Chen JC, Chen LL. Differential expression of LvHSP60 in shrimp in response to environmental stress. Fish Shellfish Immun. 2011;30:576–582. doi: 10.1016/j.fsi.2010.12.001. [DOI] [PubMed] [Google Scholar]
- Ji DS. Techniques of pond-farming of swimming crab, Portunus trituberculatus. Spe Econo Ani Plant. 2005;3:12–13. [Google Scholar]
- Jiang S, Xu Q. The influence of salinity stress on the activity of gill Na+/K+-ATPase in swimming crab, Portunus trituberculatus. J Fishery Science. 2011;35:45–50. [Google Scholar]
- Kammenga JE, Arts MSJ, Oude-Breuil WJM. HSP60 as a potential biomarker of toxic stress in the Nematode Plectus acuminatus. Arch Environ Contam Toxicol. 1998;34:253–258. doi: 10.1007/s002449900314. [DOI] [PubMed] [Google Scholar]
- Kozlova T, Perezgasga T, Reynaud E, Zurita M. The Drosophila melanogaster homologue of the hsp60 gene is encoded by the essential locus 1 (1)10Ac and is differentially expressed during fly development. Dev Genes Evol. 1997;207:253–263. doi: 10.1007/s004270050113. [DOI] [PubMed] [Google Scholar]
- Krebs RA, Feder ME. Deleterious consequences of Hsp70 overexpression in Drosophila melanogaster larvae. Cell Stress Chaperones. 1997;2:60–71. doi: 10.1379/1466-1268(1997)002<0060:DCOHOI>2.3.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindquist S, Craig EA. The heat-shock proteins. Annu Rev Genet. 1988;22:631–677. doi: 10.1146/annurev.ge.22.120188.003215. [DOI] [PubMed] [Google Scholar]
- Marchler-Bauer A, Anderson JB, Derbyshire MK, DeWeese-Scott C, Gonzales NR, Gwadz M, Hao L, He S, Hurwitz DI, Jackson JD, Ke Z, Krylov D, Lanczycki CJ, Liebert CA, Liu C, Lu F, Lu S, Marchler GH, Mullokandov M, Song JS, Thanki N, Yamashita RA, Yin JJ, Zhang D, Bryant SH. CDD: a conserved domain database for interactive domain family analysis. Nucleic Acids Res. 2007;35:D237–D240. doi: 10.1093/nar/gkl951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinhardt A, Wilhem B, Seitz J. Expression of mitochondrial marker proteins during spermatogenesis. Human Reprod Update. 1999;5:108–119. doi: 10.1093/humupd/5.2.108. [DOI] [PubMed] [Google Scholar]
- Morimoto RI. Regulation of the heat shock transcriptional response: cross talk between a family of heat shock factors, molecular chaperones, and negative regulators. Genes Dev. 1998;12:3788–3796. doi: 10.1101/gad.12.24.3788. [DOI] [PubMed] [Google Scholar]
- Niu C, Rummer J, Brauner C, Schulte P. Heat shock protein (HSP 70) induced by mild heat shock inhibits sharp plasma osmolarity increases upon seawater transfer in rainbow trout (Oncorhynchus mykiss) Comp Biochem Physiol. 2008;148 C:460–461. doi: 10.1016/j.cbpc.2008.04.011. [DOI] [PubMed] [Google Scholar]
- Nover L, Scharf KD. Heat stress proteins and transcription factors. Cell Mol Life Sci. 1997;53:80–103. doi: 10.1007/PL00000583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsell DA, Lindquist S. The function of heat-shock proteins in stress tolerance: degradation and reactivation of damaged proteins. Annu Rev Genet. 1993;27:437–496. doi: 10.1146/annurev.ge.27.120193.002253. [DOI] [PubMed] [Google Scholar]
- Pearl LH, Prodromou C. Structure and mechanism of the Hsp90 molecular chaperone machinery. Annu Rev Biochem. 2006;75:271–294. doi: 10.1146/annurev.biochem.75.103004.142738. [DOI] [PubMed] [Google Scholar]
- Pedersen SN, Lundebye AK. Metallothionein and stress protein levels in shore crabs (Carcinus maenas) along a trace metal gradient in the Fal Estuary System (UK) Mar Environ Res. 1996;42:241–246. doi: 10.1016/0141-1136(96)00077-3. [DOI] [Google Scholar]
- Péqueux A. Osmotic regulation in crustaceans. J Crust Biol. 1995;15:1–60. doi: 10.2307/1549010. [DOI] [Google Scholar]
- Quintana FJ, Cohen IR. Heat shock proteins as endogenous adjuvants in sterile and septic inflammation. J Immunol. 2005;175:2777–2782. doi: 10.4049/jimmunol.175.5.2777. [DOI] [PubMed] [Google Scholar]
- Reddy PS, Thirulogachandar V, Vaishnavi CS, Aakrati A, Sopory SK, Reddy MK. Molecular characterization and expression of a gene encoding cytosolic Hsp90 from Pennisetum glaucum and its role in abiotic stress adaptation. Gene. 2011;474:29–38. doi: 10.1016/j.gene.2010.12.004. [DOI] [PubMed] [Google Scholar]
- Rhee JS, Raisuddin S, Lee KW, Seo JS, Ki JS, Kim IC, Park HG, Lee JS. Heat shock protein (Hsp) gene responses of the intertidal copepod Tigriopus japonicus to environmental toxicants. Comp Biochem Physiol Part C. 2009;149:104–112. doi: 10.1016/j.cbpc.2008.07.009. [DOI] [PubMed] [Google Scholar]
- Sanchez GI, Carucci DJ, Sacci JJ, Resau JH, Rogers WO, Kumar N, Hoffman SL. Plasmodium yoelli: cloning and characterization of the gene encoding for the mitochondrial heat shock protein 60. Exp Parasitol. 1999;93:181–190. doi: 10.1006/expr.1999.4455. [DOI] [PubMed] [Google Scholar]
- Smurov A, Podlipaeva Y, Goodkov A. Heat shock protein of the Hsp70 family in the euryhaline cilate Paramecium nephridiatum and its role in adaptation to salinity changes. Cell Tissue Biol. 2007;1:244–247. doi: 10.1134/S1990519X07030066. [DOI] [PubMed] [Google Scholar]
- Spees JL, Chang SA, Snyder MJ, Chang ES. Thermal acclimation and stress in the American lobster, Homarus americanus: equivalent temperature shifts elicit unique gene expression patterns for molecular chaperones and polyunbiquitin. Cell Stress Chaperones. 2002;7:97–106. doi: 10.1379/1466-1268(2002)007<0097:TAASIT>2.0.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spees JL, Chang SA, Snyder MJ, Chang ES. Osmotic induction of stress-responsive gene expression in the lobster Homarus americanus. Biol Bull. 2002;203:331–337. doi: 10.2307/1543575. [DOI] [PubMed] [Google Scholar]
- Sun YM. Larval development of the swimming crab, Portunus trituberculatus. J Fish China. 1984;8:219–226. [Google Scholar]
- Taylor HH, Taylor EW. Gills and lungs: the exchange of gases and ions. In: Harrison FW, Humes AG, editors. Microscopic anatomy of invertebrates. Vol 10. Decapod Crustacea. New York: Wiley; 1992. pp. 203–293. [Google Scholar]
- Terasawa K, Minami M, Minam Y. Constantly updated knowledge of Hsp90. J Biochem. 2005;137:443–447. doi: 10.1093/jb/mvi056. [DOI] [PubMed] [Google Scholar]
- Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG. The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 1997;25:4876–4882. doi: 10.1093/nar/25.24.4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timakov B, Zhang P. The hsp60B gene in Drosophila melanogaster is essential for the spermatid individualization process. Cell Stress Chaperones. 2001;6:71–77. doi: 10.1379/1466-1268(2001)006<0071:THGODM>2.0.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vabulas RM, Ahmad-Nejad P, Costa C, Miethke T, Kirschning CJ, Haucker H, Wagner H. Endocytosed HSP60s use toll-like receptor 2 (TLR2) and TLR4 to activate the toll/interleukin-1 receptor signaling pathway in innate immune cells. J Biol Chem. 2001;276:31332–31339. doi: 10.1074/jbc.M103217200. [DOI] [PubMed] [Google Scholar]
- Viant MR, Werner I, Rosenblum ES. Correlation between heatshock protein induction and reducedmetabolic condition in juvenile steelhead trout (Oncorhynchus mykiss) chronically exposed to elevated temperature. Fish Physiol Biochem. 2003;29:159–171. doi: 10.1023/B:FISH.0000035938.92027.81. [DOI] [Google Scholar]
- Werner I, Nagel R. Stress proteins HSP60 and HSP70 in three species of amphipods exposed to cadmium, diazinon, dieldrin, and fluoranthene. Environ Toxicol Chem. 1997;16:2393–2403. doi: 10.1002/etc.5620161127. [DOI] [Google Scholar]
- Xu Q, Liu Y. Gene expression profiles of the swimming crab Portunus trituberculatus exposed to salinity stress. Mar Biol. 2011;158:2161–2172. doi: 10.1007/s00227-011-1721-8. [DOI] [Google Scholar]
- Xu Q, Liu Y, Liu R. Expressed sequence tags from cDNA library prepared from gills of the swimming crab, Portunus trituberculatus. J Exp Mar Biol Ecol. 2010;394:105–115. doi: 10.1016/j.jembe.2010.08.002. [DOI] [Google Scholar]
- Xue J, Du N, Nai W. The researches on the Portunus trituberculatus in China. Donghai Mar Sci. 1997;15:60–64. [Google Scholar]
- Zhou J, Wang W, He W, Zheng Y, Wang L, Xin Y, Liu Y, Wang A. Expression of HSP60 and HSP70 in white shrimp, Litopenaeus vannamei in response to bacterial challenge. J Invertebr Pathol. 2010;103:170–178. doi: 10.1016/j.jip.2009.12.006. [DOI] [PubMed] [Google Scholar]