Abstract
Phenethyl isothiocyanate (PEITC) is a naturally occurring electrophile which depletes intracellular glutathione (GSH) levels and triggers accumulation of reactive oxygen species (ROS). PEITC is of considerable interest as a potential chemopreventive/chemotherapeutic agent, and in this work, we have investigated the effects of PEITC on human breast cancer cell lines. Whereas PEITC readily induced apoptosis in MDA-MB-231 cells (associated with rapid activation of caspases 9 and 3, and decreased expression of BAX), MCF7 cells were relatively resistant to the apoptosis promoting effects of PEITC. The relative resistance of MCF7 cells was associated with high basal expression of NRF2, a transcription factor that coordinates cellular protective responses to oxidants and electrophiles and raised intracellular levels of GSH. This raised basal expression of NRF2 appeared to be a response to on-going production of ROS, since treatment with the antioxidant and GSH precursor N-acetylcysteine (NAC) reduced NRF2 expression. Moreover, pre-treatment of MDA-MB-231 cells with NAC rendered these cells relatively resistant to PEITC-induced apoptosis. In summary, our data confirm that PEITC may be an effective chemopreventive/therapeutic agents for breast cancer. However, differences in the basal expression of NRF2 and resultant changes in GSH levels may be an important determinant of sensitivity to PEITC-induced apoptosis.
Keywords: Phenethyl isothiocyante, Breast cancer, NRF2, Glutathione, Reactive oxygen species
Introduction
Phenethyl isothiocyanate (PEITC) is a naturally occurring electrophilic compound that readily undergoes thiocarbamoylation reactions with cellular thiols. Following uptake into cells, the predominant initial reaction of PEITC is with glutathione (GSH) the major intracellular antioxidant (Zhang 2000, 2001). PEITC conjugates are then effluxed from the cell but breakdown of extracellular PEITC conjugates results in liberation of PEITC which is free to re-enter the cell. The net outcome is a rapid depletion of intracellular GSH and accumulation of intracellular PEITC. GSH depletion results in increased accumulation of reactive oxygen species (ROS). The production of intracellular ROS may also increase due to PEITC-mediated inhibition of mitochondrial oxidative phosphorylation (Trachootham et al. 2006; Xiao et al. 2010). Free intracellular PEITC then reacts with cysteinyl thiols of cellular proteins, potentially leading to altered protein function (Mi et al. 2007; Xu and Thornalley 2001).
PEITC has received considerable attention due to its potential chemopreventive and chemotherapeutic activities (Cavell et al. 2011; Cheung and Kong 2010; Hayes et al. 2008). PEITC decreases carcinogen-induced cancer development in vivo and interferes with the activation of carcinogens via inhibition of phase I metabolism and activation of phase II metabolism. PEITC also exerts direct anti-cancer effects against established cancer cells in vitro, including inhibition of cell cycle progression, induction of apoptosis, decreased migration/invasion and/or suppression of angiogenesis. Consistent with this, PEITC exerts therapeutic effects in genetically induced and xenograft tumour models in vivo. PEITC and other related dietary isothiocyanates are thought to play an important role in mediating potential anti-cancer effects associated with diets with a high content of cruciferous vegetables (Kim and Park 2009; Higdon et al. 2007). The chemopreventive/therapeutic effects of PEITC are currently being explored in clinical studies in low grade B-cell lymphoma and lung cancer (NCT00968461, NCT00691132; http://clinicaltrials.gov/).
It is likely that both increased ROS levels and protein thiocarbamoylation contribute to the biological effects of PEITC in cancer cells. For example, thiocarbamoylation of KEAP1 is thought to be important for the induction of the NRF2 transcription factor, a master regulator of antioxidant/electrophile responsive genes (Cheung and Kong 2010; Hayes and McMahon 2009). In normal cells, KEAP1 associates with NRF2 and targets it for degradation via the proteasome. Treatment of cells with isothiocyantes triggers the release of NRF2 from KEAP1 resulting in stabilisation of NRF2. Once NRF2 accumulates in the nucleus it activates a battery of downstream target genes involved in antioxidant defences, including γ-glutamylcysteine synthetase, the rate-limiting enzyme for the biosynthesis of GSH, GSH reductase and GSH peroxidase. Thus, pro-oxidant/electrophilic stress activates a NRF2-dependent feed-back loop resulting in increased expression oxidant/electrophile protective proteins.
Breast cancer is the most common malignancy amongst women in the Western world, responsible for over 450,000 deaths worldwide in 2008 (Ferlay et al. 2008). Breast cancer can be divided into various molecular subtypes (Perou et al. 2000), and these each have prognostic and therapeutic implications. A major subset of tumours is characterised by the expression of oestrogen receptor (ER), and in these patients, tamoxifen reduces the risk of recurrence of breast cancer by approximately one half, and reduces the risk of death from breast cancer by one quarter. A second subset of tumours express the HER2 receptor, and in these patients, the anti-HER2 antibody trastuzumab reduces risk of death from breast cancer by approximately half and the risk of recurrence by about one third (Smith et al. 2007). In contrast triple negative breast cancer, characterised by lack of expression of ER, progesterone receptor, and HER2 constitute ~15% of women (Foulkes et al. 2010), are associated with adverse prognosis, and currently have no biological therapies available.
Several studies have suggested that PEITC may be an effective preventive/therapeutic agent in breast cancer. PEITC has been shown to promote apoptosis in breast cancer cells (Hahm and Singh 2011; Lee and Cho 2008; Tseng et al. 2004) and to decrease ER expression (Kang and Wang 2010). Induction of apoptosis has been associated with increased expression of the pro-apoptotic BAX and BIM proteins (Hahm and Singh 2011; Lee and Cho 2008; Tseng et al. 2004). PEITC can also interfere with pro-angiogenic pathways via down-modulation of the HIF1α transcription factor (Wang et al. 2009).
In this work, we have investigated the effects of PEITC on human breast cancer cell lines. In particular, we have investigated potential mechanisms that mediate differential sensitivity of these cells to PEITC. Our work suggests that differences in basal levels of NRF2 influence the apoptotic response to PEITC.
Materials and methods
Cell culture and chemicals
MCF7, BT549, MDA-MB-231, ZR-75-1, SKBR3, and T47D human breast cancer cells were obtained from American Type Culture Collection (Manassas, VA, USA). Cell lines were maintained in Dulbecco's Modified Eagle's medium (DMEM; Lonza group Ltd, Basel, Switzerland) supplemented with 10% (v/v) fetal calf serum (PAA Laboratories, Yeovil, UK), 1 mM l-glutamine and penicillin/streptomycin (Lonza group Ltd). PEITC, indol-3-carbinol, quercetin, buthionine sulfoximine (BSO), staurosporine, and N-acetylcysteine (NAC) were from Sigma Chemicals (Poole, UK). Dimethylsulfoxide (DMSO) was used as a solvent control and was added at a dilution equivalent to the highest concentration of PEITC tested in each assay.
Growth inhibition
Cells were plated at a density of 20,000 cells per well of a 96-well plate in 50 μl complete growth media. The following day cells were treated with PEITC or DMSO as a solvent control or were left untreated. After 6 days, relative cell number was determined using the CellTiter 96® AQueous One Solution Reagent (Promega, Southampton, UK) according to the manufacturer's instructions. Relative cell number was calculated as a percentage of untreated cells. IC50 values were determined by linear regression using GraphPad Prism (GraphPad Software Inc., La Jolla, CA, USA).
Apoptosis and cell cycle
To determine the proportion of cells in different phases of the cell cycle, drug-treated cells were collected by centrifugation and fixed in 70% (v/v) ice cold ethanol and stored at 4°C. On the day of analysis, cells were collected by centrifugation and resuspended in 300 μl phosphate buffered saline containing 100 μg ml−1 RNAse and 8.3 μg ml−1 propidium iodide (Sigma Chemicals) for 15 min. Cell fluorescence was analysed using a FACS Canto (Becton Dickinson, Oxford, UK). The proportion of cells in G1, S, or G2/M phases of the cell cycle was calculated as a proportion of all cells in cycle, and the proportion of cells with <G1 content was calculated as a proportion of all cells. Apoptosis was analysed by annexin V/propidium iodide staining (Pickering et al. 2007).
Immunoblotting
Immunoblots were performed as previously described (Brimmell et al. 1999) using equal amounts of protein lysates (quantified using the BioRad assay) and antibodies specific for caspase 9, caspase 7, cleaved caspase 9, cleaved caspase 3, (all Cell Signalling Technology, Beverly, MA, USA), BAX, BCL2, NRF2 (all Santa Cruz Biotechnology, Santa Cruz, CA, USA) and a rabbit anti-β-actin antibody (Sigma Chemicals). Horseradish peroxidase conjugated secondary antibodies were from GE Healthcare UK (Amersham, UK) and bound immunocomplexes were detected using SuperSignal West Pico Chemiluminescent reagents (Perbio Science UK Ltd, Northumberland, UK). Immunoblot signals were quantified using Quantity One image analysis software (BioRad, Hemel Hempstead, UK).
GSH assays
GSH/GSSG assays were performed using kits from Cambridge Bioscience (Cambridge, UK) Company. The concentration of GSH and GSSG were determined by the end-point method.
Results
PEITC-induced growth inhibition in human breast cancer cell lines
We first investigated the effects of PEITC on the growth of a panel of human breast cancer cell lines using the MTS assay. All cell lines were sensitive to PEITC but there was a ~4-fold variation in their sensitivity (Table 1). The most sensitive cell line was MDA-MB-231, whereas the least sensitive was ZR-75-1. Overall, there was no clear correlation between breast cancer “sub-type” and sensitivity, at least in this small panel of cell lines. MCF7 and MDA-MB-231 cells were selected for more detailed studies since these lines are widely used as models in breast cancer research and showed a significant difference in sensitivity to PEITC (mean (±SD) IC50 values from up to 9 determinations; 7.2 ± 1.4 μM and 10.6 ± 1.4 μM for MDA-MB-231 and MCF7 cells, respectively; p = 0.0003 Student's t test).
Table 1.
Summary of results for PEITC-induced growth inhibition
| Cell line | Status | IC50 (μM)c | ||
|---|---|---|---|---|
| ERa | PRb | HER2 | ||
| MDA-MB-231 | −ve | −ve | Normal | 7.2 ± 1.4 |
| T47D | +ve | +ve | Normal | 9.2 ± 3.8 |
| BT549 | −ve | −ve | Normal | 11.9 ± 7.3 |
| MCF7 | +ve | +ve | Normal | 10.6 ± 1.4 |
| SKBR3 | −ve | −ve | Amplified | 26.4 ± 2.1 |
| ZR-75-1 | +ve | −ve | Normal | 40.4 ± 4.8 |
aEstrogen receptor
bProgesterone receptor
cPEITC IC50, mean ± SD derived from a minimum of three independent experiments
We also investigated the response of MDA-MB-231 and MCF7 cells to other phytochemicals, indol-3-carbinol and quercetin. Whereas MDA-MB-231 and MCF7 were approximately equally sensitive to indol-3-carbinol (IC50s of 77 ± 5.1 μM and 89 ± 8.1 μM, respectively), MCF7 cells were more sensitive to quercetin-mediated growth inhibition compared to MDA-MB-231 cells (IC50s of 74 ± 8.0 μM and 111 ± 8.2 μM, respectively). Thus, the relative sensitivity of MDA-MB-231 cells to PEITC does not reflect a general increase in sensitivity to growth inhibition.
Effect of PEITC on cell cycle arrest and apoptosis
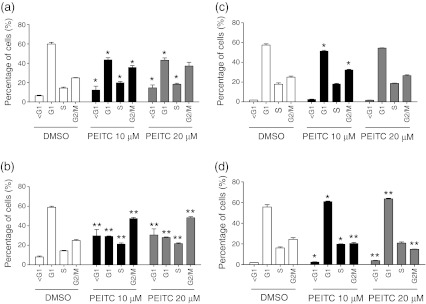
To investigate in more detail the differential responses of MDA-MB-231 and MCF7 cells, we first analysed the effects of PEITC (10 or 20 μM) on cell cycle parameters at 24 and 48 h after treatment (Fig. 1). In MDA-MB-231 cells, PEITC predominantly induced a G2/M phase arrest associated with an increased proportion of cells in G2/M and a reduction of cells in G1 phase. There was also an increase in the proportion of cells in S phase, and cells with sub-G1 DNA content, indicative of cell death, especially after 48 h. PEITC also induced a G2/M phase arrest at 24 h in MCF7 cells, although to a lesser extent than in MDA-MB-231 cells, whereas at 48 h, there was a tendency towards a modest increase in the proportion of cells in the G1/S phases. Overall, there was only a very modest increase in the proportion of cell with sub-G1 DNA content in PEITC-treated MCF7 cells.
Fig. 1.
Cell cycle analysis in PEITC-treated cells. a, b MDA-MB-231 cells or c, d MCF7 cells were treated with PEITC (10 or 20 μM) or DMSO as a control for a, c 24 or b, d 48 h. The proportion of cells in different cell cycle phases was determined using propidium iodide staining and flow cytometry. Data shown are means ± SD derived from three independent experiments. Statistically significant differences between DMSO and PEITC treated cells are indicated (*p < 0.05; **p < 0.005)
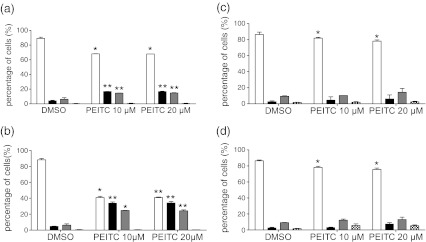
We also directly investigated the effects of PEITC on apoptosis (Fig. 2). In MDA-MB-231, PEITC induced relatively high levels of apoptosis, especially at 48 h after treatment (Fig. 2a). By contrast, in MCF7 cells, PEITC induced only low levels of apoptosis (Fig. 2b).
Fig. 2.
Apoptosis analysis in PEITC-treated cells. a, b MDA-MB-231 cells or c, d MCF7 cells were treated with PEITC (10 or 20 μM) or DMSO as a control for a, c 24 or b, d 48 h. Induction of apoptosis was analysed using annexin V (An)/propidium iodide (PI) staining (open bars, An−/PI−; closed bars, An+/PI−; grey bars, An+/PI+; hatched bars, An−/PI−). Data shown are means ± SD derived from three independent experiments. Statistically significant differences between DMSO and PEITC treated cells are indicated (*p < 0.05; **p < 0.005)
Therefore, MCF7 and MDA-MB-231 cells show different biological responses to PEITC. PEITC readily induces apoptosis in MDA-MB-231 cells but not in MCF7 cells. PEITC induces cell cycle arrest in both lines, although the specific phase of arrest may be distinct.
Effect of PEITC on caspases, BAX, and BCL2
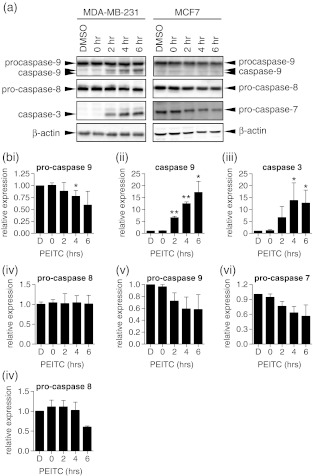
The major difference between MDA-MB-231 and MCF7 in terms of response to PEITC was their differential sensitivity to PEITC-induced apoptosis. To confirm this, we investigated the expression of caspases in PEITC treated cells. In MDA-MB-231 cells, we analysed activation of the initiator caspases for the intrinsic and extrinsic pathways, caspase 9 and caspase 8, respectively, using antibodies that detected both the pro-caspase and activated (cleaved) forms of these caspases. We also analysed activation of the executioner caspase, caspase 3, using an antibody specific for the activated form of caspase 3 (Fig. 3). Similar experiments were performed in MCF7 cells, except we used an antibody that detected pro-caspase and activated (cleaved) forms of the executioner caspase, caspase 7, since these cells do not express caspase 3.
Fig. 3.
Caspase expression in PEITC-treated cells. MDA-MB-231 or MCF7 cells were treated with PEITC (20 μM) for the indicated times or DMSO for 6 h as a control. Expression of caspase 9, caspase 8, caspase 3 (MDA-MB-231 cells) and caspase 7 (MCF7 cells) was analysed by immunoblotting. β-actin was analysed as a loading control. a Representative immunoblots. The positions of migration of pro- and cleaved forms of caspases are indicated. b Quantitation. Data shown are means ± SD derived from two independent experiments for pro-caspase 9, caspase 9, caspase 3 and pro-caspase 8 in MDA-MB-231 cells (i–iv, respectively) and pro-caspase 9, pro-caspase 7 and pro-caspase 8 in MCF7 cells (v–vii, respectively). Expression values were normalized to that of β-actin. Statistically significant differences between DMSO and PEITC treated cells are indicated (*p < 0.05; **p < 0.005). Active caspase 9 was not detected in MCF7 cells
In MDA-MB-231 cells, PEITC treatment resulted in clear processing of caspase 9 and accumulation of active caspase 3. Caspase activation was detected at 2 h and was further increased at 4 and 6 h after addition of PEITC. By contrast, there was no evidence for processing of caspase 8. In MCF7 cells, there was a general decrease in the expression of the pro-caspase forms of caspases 9, 8 and 7, but no evidence for accumulation of active forms (Fig. 3b). These results confirm the differential induction of apoptosis in MDA-MB-231 and MCF7 cells and indicate that PEITC induces apoptosis via the intrinsic pathway.
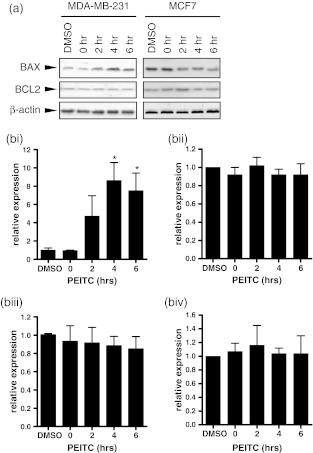
We analysed the effects of PEITC on expression of BAX, a pro-apoptotic BCL2 family protein which has previously been demonstrated to be increased in PEITC-treated in human ovarian and breast cancer cell lines (Hahm and Singh 2011; Lee and Cho 2008; Satyan et al. 2006). An increase in BAX expression was observed at 2 h after addition of PEITC in MDA-MB-231 cells, and BAX levels further increased at 4 and 6 h (Fig. 4). By contrast, PEITC treatment did not alter BAX expression in MCF7 cells. PEITC also did not alter expression of BCL2 in either cell line.
Fig. 4.
BCL2 and BAX expression in PEITC-treated cells. MDA-MB-231 or MCF7 cells were treated with PEITC (20 μM) for the indicated times or DMSO for 6 h as a control. Expression of BAX and BCL2 was analysed by immunoblotting. β-actin was analysed as a loading control. a Representative immunoblots. b Quantitation. (i) BAX MDA-MB-231 cells, (ii) BAX MCF7 cells, (iii) BCL2 MDA-MB-231 cells, (iv) BCL2 MCF7 cells. Data shown are means ± SD derived from three independent experiments. Expression values were normalized to that of β-actin. Statistically significant differences between DMSO and PEITC treated cells are indicated (*p < 0.05; **p < 0.005)
Effect of PEITC on NRF2
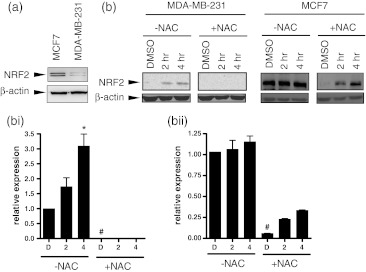
PEITC treatment can elicit increases in intracellular ROS and one possible explanation for the differential response of MCF7 and MDA-MB-231 cells was that these cells differed in their basal levels of ROS or in their ability to tolerate increased ROS (Trachootham et al. 2006). We therefore went on to analyse the expression of NRF2 as a surrogate marker of oxidative stress since NRF2 is induced in cells with high levels of oxidative or electrophilic stress and plays a key role in induction of downstream antioxidant defences. Immunoblot analysis demonstrated that basal (i.e., uninduced) levels of NRF2 were 60 ± 17% lower in MDA-MB-231 cells compared to MCF7 cells (Fig. 5a; mean of 3 determinations, Student's t test p = 0.025). PEITC increased NRF2 expression by ~3-fold in MDA-MB-231 cells at 4 h after treatment with PEITC. By contrast, NRF2 expression in MCF7 cells was not effected by PEITC (Fig. 5).
Fig. 5.
NRF2 expression in PEITC-treated cells. a NRF2 expression in untreated MDA-MB-231 or MCF7 cells was analysed by immunoblotting. b, c MDA-MB-231 or MCF7 cells were treated with PEITC (20 μM) for the indicated times or DMSO for 6 h as a control. Expression of NRF2 and β-actin (loading control) was analysed by immunoblotting. b Representative immunoblots. c Quantitation. (i) MDA-MB-231 cells, (ii) MCF7 cells. Data shown are means ± SD derived from three independent experiments. Expression values were normalized to that of β-actin. Statistically significant differences between DMSO and PEITC treated cells in the absence of NAC are indicated (*p<0.05). Statistically significant differences between NAC treated and untreated cells are indicated (#p<0.05)
Effect of PEITC on total GSH and GSSG
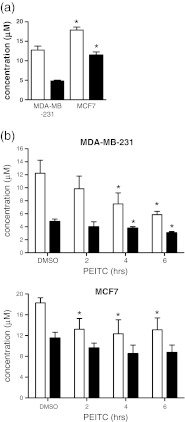
The association between basal NRF2 expression and sensitivity to PEITC suggested that differences in GSH content might influence responses of MDA-MB-231 and MCF7 cells. We therefore analysed the levels of total GSH and oxidised GSH (GSSG) in MDA-MB-231 and MCF7 cells, before and following addition of PEITC (Fig. 6).
Fig. 6.
GSH levels in MCF7 and MDA-MB-231 cells. a Total GSH and GSSG concentration in untreated MDA-MB-231 and MCF7 cells. Data are means ± SD derived from three independent experiments. Statistically significant differences between MDA-MB-231 and MCF7 cells are indicated (*p < 0.05). b Total GSH (open bars) and GSSG (closed bars) concentrations in (i) MDA-MB-231 and (ii) MCF7 cells treated with PEITC (20 μM) for the indicated times or DMSO (6 h) as a control. Data are means ± SD derived from three independent experiments. Statistically significant differences between DMSO and PEITC treated cells are indicated (*p < 0.05)
In untreated cells, the levels of both total GSH and GSSG were higher in MCF7 cells, compared to MDA-MB-231 cells. GSH levels were ~50% higher in MCF7 cells. The ratio of GSSG to total GSH was also higher in MCF7 cells compared to MDA-MB-231 cells.
Total GSH and GSSG levels were reduced in MCF7 cells at 2 h after treatment with PEITC, but then remained at this level for the remainder of the time course. Overall, the GSH/GSSG ratio was not substantially altered and even after 6 h treatment the concentration of total GSH was approximately equivalent to that of untreated MDA-MB-231 cells (~12 μM). By contrast, in MDA-MB-231 cells, total GSH levels decreased up to 6 h and were reduced by ~50% at this time. There was also an increase in the GSSG/GSH ratio, indicative of increasing oxidative stress.
Effects of NAC on NRF2 expression and PEITC-induced apoptosis and cell cycle arrest
We next determined whether the relatively high levels of basal NRF2 expression in MCF7 cells was influenced by on-going oxidative stress in these cells. MCF7 cells were pretreated with the antioxidant and GSH precursor NAC, or left untreated as a control, and then treated with PEITC for various times. Treatment with NAC alone reduced basal NRF2 expression in MCF7 cells by ~90% (Fig. 5). Whereas NRF2 was not induced by PEITC in the absence of NAC, treatment with PEITC after NAC pre-treatment resulted in a ~4-fold induction in NRF2 expression from this reduced basal level. In MDA-MB-231 cells, pretreatment with NAC alone further reduced the already low levels of basal NRF2 expression in MDA-MB-231 cells and NRF2 was no longer detectable in these cells. Addition of PEITC to NAC-pretreated cells did not detectably increase NRF2 expression.
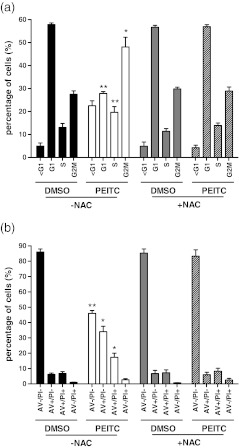
To investigate the role of GSH in determining responses to PEITC, we analysed the effect of NAC on PEITC-induced growth inhibition (MTS assay) in MCF7 and MDA-MB-231 cells. Pre-treatment with NAC increased the IC50 of MDA-MB-231 cells by ~5-fold (i.e., to 45 ± 4.0 μM, Student's t test versus no NAC pre-treatment, p = 0.004). By contrast, the IC50 for PEITC in MCF7 cells was not significantly affected by NAC pre-treatment (11.4 ± 4.7 μM versus 18.3 ± 1.4 μM, Student's t test, p = 0.267). Pre-treatment with NAC effectively prevented both the PEITC-induced G2/M arrest and induction of apoptosis in MDA-MB-231 cells (Fig. 7).
Fig. 7.
Effect of NAC on PEITC-induced cell cycle arrest and apoptosis in MDA-MB-231 cells. MDA-MB-231 cells were treated with PEITC (20 μM) or DMSO as a control, in the presence or absence of NAC. In a, the proportion of cells in different cell cycle phases was determined using propidium iodide staining and flow cytometry. In b, induction of apoptosis was analysed using annexin V (An)/propidium iodide (PI) staining (open bars, An−/PI−; closed bars, An+/PI−; grey bars, An+/PI+; hatched bars, An−/PI−). Data shown are means ± SD derived from three independent experiments. Statistically significant differences between DMSO and PEITC treated cells are indicated (*p < 0.05; **p < 0.005)
Discussion
PEITC is a potential chemopreventive/chemotherapeutic agent, and, with other related phytochemicals, is thought to contribute to the potential protective effects of diets rich in cruciferous vegetables. It is therefore important to understand the mechanisms by which this electrophilic compound exerts its anti-cancer effects and what determines differential responses. Here, we show that individual breast cancer cell lines differ in response to PEITC and that the basal levels of NRF2/GSH may be an important determinant of sensitivity to PEITC-induced apoptosis.
Several previous studies have investigated effects of PEITC in breast cancer cells, demonstrating that PEITC induces apoptosis of human and mouse breast cancer cell lines in vitro (Hahm and Singh 2011; Lee and Cho 2008; Tseng et al. 2004) and decreases breast cancer growth in vivo in a mouse model (McCune et al. 2010). PEITC has also been shown to decrease expression and function of ER (Kang and Wang 2010). Here, we have extended this work demonstrating that PEITC, dependent on the cell line tested, has the potential to induce both cell cycle arrest and apoptosis, associated with activation of the intrinsic cell death pathway. The key observation from this work is that breast cancer cells demonstrate a variable response to PEITC, with overall sensitivity assessed using the MTS assay varying up to 4-fold between individual cell lines. There was no clear correlation between PEITC responsiveness and the breast cancer subtype represented by these lines. However, the number of cell lines studied was relatively small and further studies are required to determine whether distinct breast cancer subtypes are differentially sensitive to PEITC.
Our mechanistic studies focused on MCF7 and MDA-MB-231 cells as two very well characterised cell line models. The relative sensitivity of MDA-MB-231 cells did not reflect a general increased sensitivity of these cells to phytochemicals, since these lines showed very similar response to indol-3-carbinol, and MCF7 cells were more sensitive to quercetin compared to MDA-MB-231 cells. The major determinant of differential response appeared to be sensitivity to PEITC-induced apoptosis. MDA-MB-231 cells were relatively sensitive to PEITC-induced apoptosis, whereas PEITC did not induce significant levels of apoptosis in MCF7 cells. It is important to recognise that the differential induction of apoptosis in MDA-MB-231 and MCF7 cells is relative and not absolute. For example, PEITC has been shown to induce apoptosis in MCF7 cells in previous studies (Lee and Cho 2008; Hahm and Singh 2011). However, our study has focused on relatively early time points to probe some of the more immediate changes in PEITC-treated cells. For example, we studied caspase activation at up to 6 h, whereas other studies which have demonstrated PEITC-induced caspase activation in MCF7 cells have studied caspase activation and BCL2 family protein expression at 24 h (Lee and Cho 2008). PEITC-induced apoptosis in MDA-MB-231 cells appeared to be mediated via the intrinsic pathway since it was associated with increased expression of BAX, and activation of caspase 9, but not caspase 8. In contrast to apoptosis, PEITC induced cell cycle arrest in both cell lines, although there were some differences in the specific phase of arrest between the two lines.
Our work suggests that NRF2 status may influence response to PEITC. NRF2 is a transcription factor that is induced by oxidative/electrophilic stress and regulates a set of 100–200 genes encoding proteins involved in cytoprotection, including many components of the GSH system (Hayes and McMahon 2009). NRF2 was constitutively expressed at ~2.5-fold higher levels in MCF7 cells compared to MDA-MB-231 cells in the absence of PEITC. Raised NRF2 expression appears to be functionally relevant since MCF7 cells also contained higher GSH levels than MDA-MB-231 cells. NRF2 expression is regulated at multiple levels, including transcription, degradation, and phosphorylation (Cheung and Kong 2010; Hayes and McMahon 2009), and the mechanisms that mediate increased NRF2 expression in MCF7 cells may be complex. Basal NRF2 expression appeared to be dependent on on-going ROS production in both cell lines since treatment with NAC effectively reduced NRF2 expression. Elevated ROS may release NRF2 from KEAP1 mediated negative control, or may cause activation via enhanced phosphorylation, perhaps associated with ROS-mediated inhibition of phosphatase activity. We speculate that MCF7 cells may be protected from the pro-apoptotic effects of PEITC relative to MDA-MB-231 cells by enhanced expression of NRF2 and resultant increases in levels of GSH. Notably, NRF2 expression was induced in MDA-MB-231 cells following treatment with PEITC. However, maximal induction of NRF2 was relatively delayed compared to activation of capases in these cells. Thus, it seems likely that although MDA-MB-231 can mount a NRF2-mediated antioxidant response to PEITC, the kinetics are too effectively counter the rapid induction of apoptosis in these cells, at least at the concentrations of PEITC tested here.
The mechanisms leading to increased ROS in MCF7 cells are unclear. However, similar differences between MDA-MB-231 and MCF7 cells in response to pro-oxidants have been observed in previous studies. For example, the pro-oxidant (tert-butyl-2(4,5-dihydrogen-4,4,5,5-tetramethyl-3-O-1H-imidazole-3-cationic-1-oxyl-2-pyrrolidine-1-carboxylic ester) L-NNP induced higher levels of ROS and apoptosis in MDA-MB-231 cells compared to MCF7 cells (Zhang et al. 2011) and 2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD) induces greater levels of GSH depletion and increased ROS in MDA-MB-231 cells compared to MCF7 cells (Lin et al. 2007). Oncogenic transformation has been shown to elevate intracellular ROS (Trachootham et al. 2006) or to promote NRF2-dependent ROS detoxification (DeNicola et al. 2011), possibly dependent on the level of oncogene expression and or cell type. Regardless, differences in the underlying oncogenic drives in MCF7 and MDA-MB-231 cells may account for differences in ROS production and sensitivity to PEITC.
In summary, our data confirm that PEITC may be an effective chemopreventive/therapeutic agents for breast cancer. However, it will be important to investigate in more detail the molecules that influence PEITC responses in individual tumour types. Cells that have adapted to increased transformation-associated ROS production via upregulation of antioxidant defence may be less sensitive to PEITC-induced apoptosis.
Acknowledgements
We thank Ramsey Cutress for his advice on clinical breast cancer. This work was funded by the Biochemical and Biophysical Sciences Research Council, Vitacress Salad Leaves, Universiti Putra Malaysia, the Gerald Kerkut Trust, the Kay Kendall Leukaemia Fund, Cancer Research UK and the University of Southampton. The funders had no involvement in the design, performance or reporting of this work.
References
- Brimmell M, Burns JS, Munson P, McDonald L, O'Hare MJ, Lakhani SR, Packham G. High level expression of differentially localized BAG-1 isoforms in some oestrogen receptor-positive human breast cancers. Br J Cancer. 1999;81:1042–1051. doi: 10.1038/sj.bjc.6690805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavell BE, Syed Alwi SS, Donlevy A, Packham G. Anti-angiogenic effects of dietary isothiocyanates: mechanisms of action and implications for human health. Biochem Pharmacol. 2011;81:327–336. doi: 10.1016/j.bcp.2010.10.005. [DOI] [PubMed] [Google Scholar]
- Cheung KL, Kong AN. Molecular targets of dietary phenethyl isothiocyanate and sulforaphane for cancer chemoprevention. AAPS J. 2010;12:87–97. doi: 10.1208/s12248-009-9162-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeNicola GM, Karreth FA, Humpton TJ, Gopinathan A, Wei C, Frese K, Mangal D, Yu KH, Yeo CJ, Calhoun ES, Scrimieri F, Winter JM, Hruban RH, Iacobuzio-Donahue C, Kern SE, Blair IA, Tuveson DA. Oncogene-induced Nrf2 transcription promotes ROS detoxification and tumorigenesis. Nature. 2011;475:106–109. doi: 10.1038/nature10189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 127:2893–2917 [DOI] [PubMed]
- Foulkes WD, Smith IE, Reis-Filho JS. Triple-negative breast cancer. N Engl J Med. 2010;363:1938–1948. doi: 10.1056/NEJMra1001389. [DOI] [PubMed] [Google Scholar]
- Hahm ER, Singh SV (2011) Bim contributes to phenethyl isothiocyanate-induced apoptosis in breast cancer cells. Mol Carcinog. doi:10.1002/mc.20811 [Epub ahead of print] [DOI] [PMC free article] [PubMed]
- Hayes JD, Kelleher MO, Eggleston IM. The cancer chemopreventive actions of phytochemicals derived from glucosinolates. Eur J Nutr. 2008;47(Suppl 2):73–88. doi: 10.1007/s00394-008-2009-8. [DOI] [PubMed] [Google Scholar]
- Hayes JD, McMahon M. NRF2 and KEAP1 mutations: permanent activation of an adaptive response in cancer. Trends Biochem Sci. 2009;34:176–188. doi: 10.1016/j.tibs.2008.12.008. [DOI] [PubMed] [Google Scholar]
- Higdon JV, Delage B, Williams DE, Dashwood RH. Cruciferous vegetables and human cancer risk: epidemiologic evidence and mechanistic basis. Pharmacol Res. 2007;55:224–236. doi: 10.1016/j.phrs.2007.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang L, Wang ZY. Breast cancer cell growth inhibition by phenethyl isothiocyanate is associated with down-regulation of oestrogen receptor-alpha36. J Cell Mol Med. 2010;14:1485–1493. doi: 10.1111/j.1582-4934.2009.00877.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MK, Park JH. Conference on “Multidisciplinary approaches to nutritional problems”. Symposium on “Nutrition and health”. Cruciferous vegetable intake and the risk of human cancer: epidemiological evidence. Proc Nutr Soc. 2009;68:103–110. doi: 10.1017/S0029665108008884. [DOI] [PubMed] [Google Scholar]
- Lee JW, Cho MK. Phenethyl isothiocyanate induced apoptosis via down regulation of Bcl-2/XIAP and triggering of the mitochondrial pathway in MCF-7 cells. Arch Pharm Res. 2008;31:1604–1612. doi: 10.1007/s12272-001-2158-2. [DOI] [PubMed] [Google Scholar]
- Lin PH, Lin CH, Huang CC, Chuang MC, Lin P. 2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD) induces oxidative stress, DNA strand breaks, and poly(ADP-ribose) polymerase-1 activation in human breast carcinoma cell lines. Toxicol Lett. 2007;172:146–158. doi: 10.1016/j.toxlet.2007.06.003. [DOI] [PubMed] [Google Scholar]
- McCune K, Mehta R, Thorat MA, Badve S, Nakshatri H. Loss of ERalpha and FOXA1 expression in a progression model of luminal type breast cancer: insights from PyMT transgenic mouse model. Oncol Rep. 2010;24:1233–1239. doi: 10.3892/or_00000977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mi L, Wang X, Govind S, Hood BL, Veenstra TD, Conrads TP, Saha DT, Goldman R, Chung FL. The role of protein binding in induction of apoptosis by phenethyl isothiocyanate and sulforaphane in human non-small lung cancer cells. Cancer Res. 2007;67:6409–6416. doi: 10.1158/0008-5472.CAN-07-0340. [DOI] [PubMed] [Google Scholar]
- Perou CM, Sorlie T, Eisen MB, Rijn M, Jeffrey SS, Rees CA, Pollack JR, Ross DT, Johnsen H, Akslen LA, Fluge O, Pergamenschikov A, Williams C, Zhu SX, Lonning PE, Borresen-Dale AL, Brown PO, Botstein D. Molecular portraits of human breast tumours. Nature. 2000;406:747–752. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- Pickering BM, Mel S, Lee M, Howell M, Habens F, Dallman CL, Neville LA, Potter KN, Mann J, Mann DA, Johnson PW, Stevenson FK, Packham G. Pharmacological inhibitors of NF-kappaB accelerate apoptosis in chronic lymphocytic leukaemia cells. Oncogene. 2007;26:1166–1177. doi: 10.1038/sj.onc.1209897. [DOI] [PubMed] [Google Scholar]
- Satyan KS, Swamy N, Dizon DS, Singh R, Granai CO, Brard L. Phenethyl isothiocyanate (PEITC) inhibits growth of ovarian cancer cells by inducing apoptosis: role of caspase and MAPK activation. Gynecol Oncol. 2006;103:261–270. doi: 10.1016/j.ygyno.2006.03.002. [DOI] [PubMed] [Google Scholar]
- Smith I, Procter M, Gelber RD, Guillaume S, Feyereislova A, Dowsett M, Goldhirsch A, Untch M, Mariani G, Baselga J, Kaufmann M, Cameron D, Bell R, Bergh J, Coleman R, Wardley A, Harbeck N, Lopez RI, Mallmann P, Gelmon K, Wilcken N, Wist E, Sanchez Rovira P, Piccart-Gebhart MJ. 2-year follow-up of trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer: a randomised controlled trial. Lancet. 2007;369:29–36. doi: 10.1016/S0140-6736(07)60028-2. [DOI] [PubMed] [Google Scholar]
- Trachootham D, Zhou Y, Zhang H, Demizu Y, Chen Z, Pelicano H, Chiao PJ, Achanta G, Arlinghaus RB, Liu J, Huang P. Selective killing of oncogenically transformed cells through a ROS-mediated mechanism by beta-phenylethyl isothiocyanate. Cancer Cell. 2006;10:241–252. doi: 10.1016/j.ccr.2006.08.009. [DOI] [PubMed] [Google Scholar]
- Tseng E, Scott-Ramsay EA, Morris ME. Dietary organic isothiocyanates are cytotoxic in human breast cancer MCF-7 and mammary epithelial MCF-12A cell lines. Exp Biol Med (Maywood) 2004;229:835–842. doi: 10.1177/153537020422900817. [DOI] [PubMed] [Google Scholar]
- Wang XH, Cavell BE, Syed Alwi SS, Packham G. Inhibition of hypoxia inducible factor by phenethyl isothiocyanate. Biochem Pharmacol. 2009;78:261–272. doi: 10.1016/j.bcp.2009.04.010. [DOI] [PubMed] [Google Scholar]
- Xiao D, Powolny AA, Moura MB, Kelley EE, Bommareddy A, Kim SH, Hahm ER, Normolle D, Houten B, Singh SV. Phenethyl isothiocyanate inhibits oxidative phosphorylation to trigger reactive oxygen species-mediated death of human prostate cancer cells. J Biol Chem. 2010;285:26558–26569. doi: 10.1074/jbc.M109.063255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu K, Thornalley PJ. Involvement of glutathione metabolism in the cytotoxicity of the phenethyl isothiocyanate and its cysteine conjugate to human leukaemia cells in vitro. Biochem Pharmacol. 2001;61:165–177. doi: 10.1016/S0006-2952(00)00526-8. [DOI] [PubMed] [Google Scholar]
- Zhang Y. Role of glutathione in the accumulation of anticarcinogenic isothiocyanates and their glutathione conjugates by murine hepatoma cells. Carcinogenesis. 2000;21:1175–1182. doi: 10.1093/carcin/21.6.1175. [DOI] [PubMed] [Google Scholar]
- Zhang Y. Molecular mechanism of rapid cellular accumulation of anticarcinogenic isothiocyanates. Carcinogenesis. 2001;22:425–431. doi: 10.1093/carcin/22.3.425. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Guo J, Zeng L, Zhang J, Hui Y, Liu J, Qing X, Sun X, Guo G. Tert-butyl-2(4,5-dihydrogen-4,4,5,5-tetramethyl-3-O-1H-imidazole-3-cationi c-1-oxyl-2-pyrrolidine-1-carboxylic ester displays novel cytotoxicity through reactive oxygen species-mediated oxidative damage in MCF-7 and MDA-MB-231 cells. Chem Biol Interact. 2011;192:287–297. doi: 10.1016/j.cbi.2011.04.006. [DOI] [PubMed] [Google Scholar]