Abstract
The most prominent capabilities of mesenchymal stem cells (MCSs) which make them promising for therapeutic applications are their capacity to endure and implant in the target tissue. However, the therapeutic applications of these cells are limited due to their early death within the first few days following transplantation. Therefore, to improve cell therapy efficacy, it is necessary to manipulate MSCs to resist severe stresses imposed by microenvironment. In this study, we manipulated MSCs to express a cytoprotective factor, nuclear factor erythroid-2 related factor 2 (Nrf2) to address this issue. Full-length human Nrf2 cDNA was isolated and TOPO cloned into TOPO cloning vector and then transferred to gateway adapted adenovirus expression vector by LR recombination reaction. Afterwards, the Nrf2 bearing recombinant virus was prepared in appropriate mammalian cell line and used to infect MSCs. The viability and apoptosis of the Nrf2 expressing MSCs were evaluated following hypoxic and oxidative stress conditions. Transient expression of Nrf2 by MSCs protected them against cell death and the apoptosis triggered by hypoxic and oxidative stress conditions. Nrf2 also enhanced the activity of SOD and HO-1. These findings could be used as a strategy for prevention of graft cell death in MSC-based cell therapy. It also indicates that management of cellular stress responses can be used for practical applications.
Keywords: Mesenchymal stem cells, Nrf2, Adenoviral vector, Oxidative stress, Apoptosis
Introduction
Mesenchymal stem cells (MSCs) maintain self renewality and are capable to differentiate into osteoblasts, chondrocytes, astrocytes, neurons, skeletal muscle cells, and cardiomyocytes (Liu et al. 2009; Makino et al. 1999; Pereira et al. 1995; Toma et al. 2002). Multilineage potential, ability to evade from host immune system, immunomodulatory capacities, and being easy to expand in vitro, make MSCs promising for cell therapy applications. Transplantation of MSCs is an effective treatment in tissue injuries including myocardial infarction, hind limb ischemia, acute renal failure, and liver transplantation (Zhang et al. 2009; Kinnaird et al. 2004; Iwase et al. 2005). In spite of several advantages of MSCs, they did not achieve satisfactory effects in some investigations, mostly due to poor survival after transplantation (Toma et al. 2002; Kolossov et al. 2006; LIU et al. 2008; Lunde et al. 2006; Zhang et al. 2001). For example, more than 99% of transplanted MSCs have died 1 day after transplantation. The reasons of low survival rate of MSCs are not fully understood. However, it is noteworthy that during isolation and processing of the MSCs from their natural niche, they are inevitably exposed to stress conditions such as, serum deprivation, hypoxia, and oxidative stresses (Zhu et al. 2006b). On the other hand, due to inflammation, chemotherapy, radiotherapy, and expression of pro-apoptotic factors, the microenvironment of damaged tissue in patients who receive MSCs as treatment does not favor the survival of MSCs. Hence, it is necessary to reinforce the MSCs to withstand the rigors of the stresses to develop an effective therapeutic modality.
Novel strategies are being developed to modify biological and functional properties of MSCs, such as preparation of the cells in special bioscaffolds, preconditioning of the cells in culture, and genetic alterations (Zhang et al. 2011).
The latter strategy usually implies introduction of a cytoprotective factor into MSCs before transplantation which could result in their enhanced survival, better engraftment, and improved reconstruction.
The nuclear factor E2-related factor 2 (Nrf2) is a transcription factor critical for protection against electrophilic and oxidative stresses (Surh et al. 2008; Zhu et al. 2006a). When challenged by the stresses, Nrf2 induces transcription of diverse antioxidants and detoxification enzymes that eventually play an important role to ameliorate oxidative stress-induced injuries in the cells (Jin et al. 2009; Element 2004; Levonen et al. 2007; Osburn et al. 2006).
In this study, we employed these properties of Nrf2 to equip MSCs with this cytoprotective factor to withstand the cytotoxic conditions.
In this regard, we transiently over-expressed the human Nrf2 gene in MSCs using adenoviral expression system and examined whether Nrf2 producing MSCs show improved anti-oxidative and anti-apoptotic capabilities over unmodified MSCs in vitro. Application of Nrf2 transcription factor, which itself induces several antioxidants, may be a better approach than transferring the gene of individual antioxidants. Our results can provide important clues to increase the stability of MSCs for improving the efficacy of mesenchymal stem cell therapy.
Methods and materials
Isolation, cultivation, and identification of MSCs
Human bone marrow (BM) aspirate was obtained from Hematopoietic Stem Cell Transplantation Facility of the Shariati hospital under an institutional protocol and consent procedure. MSCs were isolated from bone marrow of healthy human volunteers and expanded according to Song et al. (2005) based on their adherence to tissue culture surfaces. Whole marrow cells were cultured at a density of 1 × 106 cells/cm2 in Dulbecco's modified Eagle's medium containing 1 g glucose/L (DMEM-LG) (Gibco, USA), 10% fetal bovine serum (FBS; Invitrogen, USA), 100 U penicillin/ml, and 100 mg streptomycin/ml (Sigma, USA). Non-adherent cells were removed by changing the medium after 72 h and every 4 days thereafter. Then the monolayer, expected to be MSCs, was expanded by two passages. The characteristics of MSCs were tested by immunophenotyping and differentiation capacities. To confirm the identity of the cultured MSCs, they were harvested, washed with PBS, and labeled with the following fluorescin isothiocyanate (FITC) or phycoerythrin (PE) (Becton-Dickinson) conjugated antibodies: CD14, CD105, CD73, CD166, CD45, CD34, and CD90. Mouse-IgG1-FITC and IgG1-PE were used as isotype controls. The labeled cells were assayed by flow cytometry and analyzed with FloMax Software. MSCs were also functionally characterized by in vitro differentiation assays.
Adenovirus-mediated expression of Nrf2
Recombinant adenoviruses were produced using Gateway pAd/CMV/V5-DEST vector and ViraPower Adenoviral Expression System (Invitrogen), according to the manufacturer's instructions. The bone marrow-isolated MSCs were used as a source of Nrf2 gene. The cells were cultured in 25 cm2 cell culture flasks and were grown in DMEM-LG (Gibco, USA) supplemented with 10% fetal bovine serum (FBS; Invitrogen, USA) 100 U penicillin/ml and 100 mg streptomycin/ml (Sigma, USA)in a humidified atmosphere of 5% CO2 and 95% air at 37°C. In order to induce expression of Nrf2, the MSCs were UV irradiated for 1 h, and then subjected to RNA extraction by a commercial kit according to the manufacturer's protocols (Invitrogen, Carlsbad, CA, USA). Full-length cDNAs of Nrf2 were amplified through RT-PCR using specific forward 5′-CACCATGGGAATGGACTTGGAGCTGCC-3′and reverse 5′-CTAGTTTTTCTTAACATCTGGCTTCTTAC-3′ primers. The amplified cDNA fragment was cloned into the pENTER/TEV/D-TOPO vector using the pENTER Directional TOPO Cloning kit (Invitrogen). The fidelity of Nrf2 coding sequence in the pENTER/TEV/D-TOPO vector was confirmed by DNA sequencing. Finally, the Nrf2 expression cassette was transferred from the pENTR gateway vector to the adenovirus expression plasmid pAd/CMV/V5-DEST (Invitrogen Life Technologies) through homologous recombination by using LR clonase II.
To obtain virus particles, the pAd/CMV plasmids carrying the desired gene were linearized by PacI restriction endonuclease and mixed with Lipofectamine 2000 (Invitrogen) in Opti-MEM medium (Invitrogen) and transfected into sub-confluent 293A cells (a subclone of the 293 cell line). Then, the 293A cells were cultured in DMEM-HG (Gibco, USA) medium containing 10% fetal calf serum, and the medium was replaced every 2 days until 80% of the cells detached from plates. The viral particles were harvested with three cycles of freeze/thawing followed by centrifugation at 1,750×g for 15 min. To amplify viral titer, the cell lysates were added to fresh 293A cells, and cultured for several days until all cells were detached. After three consecutive amplification rounds, the collected adenovirus-containing media were used as virus stock. The number of viral particles (VP) was determined by end-point dilution assay as described previously (Weng et al. 2009). Multiplicity of infection was defined as the ratio of total number of plaque-forming units to the total number of cells that were infected. As a control, the pAd/CMV/V5-GW/lacZ vector (Invitrogen) was digested with PacI and transfected into 293A cells to produce lacZ-bearing adenoviruses. Aliquots of the adenovirus-containing medium were added to the cells for subsequent analyses.
RT-PCR and Western blot analyses
RT-PCR was used for isolation of the Nrf2 cDNA and also to evaluate the expression of Nrf2, SOD-1, SOD-2, and HO-1.
To assess the expression of Nrf2 by RT-PCR, total cell RNA was extracted and used at concentration of 500 ng/μl to construct cDNA using First Strand cDNA Synthesis Superscript kit (Invitrogen) according to the manufacturer's protocol. The sequence of primer pairs used for each reaction are shown in Table 1. PCR condition included a primary denaturation step of 5 min at 94°C, followed by 33 cycles of 30 s at 94°C, 30 s at appropriate annealing temperature, and 45 s at 72°C, and a final extension step of 5 min at 72°C. Finally, 7 μl of each PCR product were analyzed by 2% agarose gel electrophoresis. β-actin expression was also evaluated for normalization.
Table 1.
Sequences of the primers used in RT-PCR studies
| Sequence | Primer name | No. |
|---|---|---|
| 5′-AGGGCATCATCAATTTCGAGC-3′ | Forward SOD1 | 1 |
| 5′-ACATTGCCCAAGTCTCCAAC-3′ | Reverse SOD1 | 2 |
| 5′-GGAAGCCATCAAACGTGACT-3′ | Forward SOD2 | 3 |
| 5′-CCTTGCAGTGGATCCTGATT-3′ | Reverse SOD2 | 4 |
| 5′-CACCATGGAGCGTCCGCAACCCGAC-3′ | Forward HO-1 | 5 |
| 5′-TTGTTGCGCTCAATCTCCTCCTCC-3′ | Reverse HO-1 | 6 |
Western blot analysis was performed to detect the expression of Nrf2 protein. Total cell proteins were released using Complete Lysis M reagent (Roche, Germany) according to the manufacturer's instruction. Then, the samples were boiled in loading buffer containing 4% sodium dodecyl sulfate (SDS), 20% glycerol, and bromophenol blue for 5 min. Then, the proteins were resolved on 12% SDS-PAGE and transblotted onto polyvinylidene fluoride (PVDF) membrane (Roche, Germany) followed by blocking and overnight incubation at 4°C with specific primary antibodies, i.e., β-actin (Sigma, USA) or polyclonal anti-Nrf2 antibodies (Santa Cruz biotechnology, Santa Cruz, CA, USA). Afterwards, the membranes were washed with tris-buffered saline (TBS) containing 0.1% Tween 20 and incubated with secondary HRP (horse raddish peroxidase) conjugated antibodies (Sigma). Finally, the membranes were developed by DAB solution (Sigma).
Differentiation studies
The basic differentiation potential of V-Nrf2-MSCs and V-MSCs into three mesenchymal lineages of osteogenic, adipogenic, and chondrogenic was evaluated. In this regard, MSCs were expanded in Dulbecco's Modified Eagle Medium containing 10% FBS and seeded into StemPro® osteogenesis, adipogenesis, or chondrogenesis differentiation media (Invitrogen) in 24-well tissue culture plates according to the manufacturer's protocol. Cultures were refed every 2 to 3 days. After 1–3 weeks, the cultures were monitored for differentiation using lineage-specific biologic stains. MSCs expanded in standard growth medium (DMEM + 10% FBS) was used as negative control.
For assessment of adipogenic differentiation, 8 days after incubation of the MSCs under differentiating condition, they were stained with HCS LipidTOX™ Green, washed with Dulbecco's phosphate-buffered saline (DPBS) and visualized under fluorescent microscope. For evaluation of chondrogenic differentiation, 15 days after incubation of the cells under differentiating conditions, micromass pellet cultures were stained with 1% Alcian Blue solution prepared in 0.1 N HCl. Then, the cells were washed with 0.1 N HCl and visualized under light microscope. To assess osteogenic differentiation, after 3 weeks of culturing under differentiating condition, the cells were stained with 2% Alizarin Red S solution and visualized. The positive areas of staining were (at least 50 areas that were obtained by three independent experiments performed in triplicate) quantified using Image J software (version 1.42, National Institutes of Health, Bethesda, Maryland) as previously described (Kamiya et al. 2006).
Measurement of SOD and HO-1 activities
The bilirubin concentration of the culture media was quantified as reported by Tsai et al. (2006) and Turcanu et al. (1998)) to determine HO activity. Briefly, porphyrins were added to the cells and after incubation in dark, culture supernatant was mixed with barium chloride (250 mg) and benzene (750 μl) followed by vigorous vortexing leading to formation of a relatively stable milky-white emulsion and centrifuged at 13,000×g for 30 min. The upper benzene layer containing bilirubin was collected, and the produced bilirubin was quantified spectrophotometrically. The HO activity was measured as picomoles of bilirubin formed per milligram of cell protein. The SOD activity was determined by measuring sample-mediated inhibition of xanthine oxidase-dependent O−2 (superoxide radical) production using Superoxide Dismutase Assay II kit as instructed by the manufacturer (Calbiochem). Briefly, after harvesting 1 × 106 cells by scraping and centrifugation at 1,000×g for 10 min, they were subjected to protein extraction by 1 mL of SOD lysis buffer (20 mM HEPES, 1 mM ethylene glycol tetra acetic acid (EGTA), 210 mM mannitol, 70 mM sucrose, and 0.1% (v/v) Triton X-100; pH 7.2). Then, the lysate was centrifuged at 1,500×g and 4°C for 5 min. The supernatant was then added to chromogenic tetrazolium salt (free radical detector), and xanthine oxidase was supplemented as an enzymatic source of O−2 (superoxide radical). Following 20 min of incubation at room temperature, absorbance was read at 450 nm. One unit of SOD is defined as the amount of enzyme needed to exhibit 50% dismutation of the superoxide radical.
Cell survival assay following in vitro treatment of MSCs with different stresses
For oxidative stress experiments, 2 × 104 cells/well were seeded in a 96-well plate and 12 h later, the cells were exposed to 1–10 mM hydrogen peroxide (H2O2) (Sigma, Dusseldorf, Germany) for various time lengths. For serum deprivation (SD) stress, the MSCs were washed three times with serum-free medium and maintained at this condition for various time lengths. Hypoxia was achieved by placing the cells in Galaxy 48 R Incubator (New Brunswick, Germany). These cells were incubated at 37°C, in the presence of 5% CO2 and 1% O2 in N2 (hypoxia) for different time lengths. Each test was performed in triplicate and at appropriate time points, the cytotoxic effects of H2O2, hypoxia, or serum deprivation were determined by trypan blue dye exclusion and MTT assays. To perform MTT assay, 3-(4,5-dimethlthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT, Sigma, Dusseldorf, Germany) was added to the cells at 0.5 mg/ml final concentration and incubated at 37°C in a 5% CO2 atmosphere for 4 h. Finally, the reaction was stopped by addition of 10% SDS and 0.01 M HCl. When the insoluble crystals were dissolved completely, absorbance of the mixture was read at 570 nm.
Assessment of cell apoptosis by annexin-V/propidium iodide staining and caspase 3 evaluation
Induction of apoptosis in V-Nrf2-MSCs by different stresses was evaluated using Annexin-V-FLUOS staining kit (Roche Diagnostics, Germany) according to the manufacturer's instructions. In brief, approximately, 2 × 105 cells were harvested, washed twice with PBS, and resuspended in incubation buffer containing annexin-V-FITC and propidium iodide. The mixture was incubated for 15 min at room temperature and analyzed using a flow cytometer (Partec) and FloMax software.
Caspase-3 activity was measured by a colorimetric caspase-3 assay kit according to the manufacturer's protocol (PharMingen, Germany). The cells were seeded in 24-well plates at a density of 3 × 105 cells/well and incubated at 37°C in a 5% CO2 atmosphere. Following treatment of the cells with different stresses, they were washed with PBS, trypsinized, and subjected to caspase-3 measurement according to the manufacturer's protocol.
Statistical analysis
The statistical significance of the results was evaluated using analysis of variance, ANOVA, and Student's t test. In all tests, p < 0.05 was considered significant.
Results
Characterization of MSCs
MSCs were isolated from BM cells by their adhesion to tissue culture surfaces and consisted of a heterogeneous cell population with a predominant spindle-shaped morphology and were able to form fibroblast-like colonies. For further evaluation of the human MSCs phenotype, cell-surface antigens were analyzed by flow cytometry which confirmed expression of characteristic markers for MSCs, i.e., CD166, CD105, CD73, and CD90, while they were negative for hematopoietic antigens like CD45, CD34, and CD14 (Hamedi-Asl et al. 2011) (Fig. 1).
Fig. 1.
Flow cytometric analysis indicates that MSCs are positive for the antigens CD166, CD105, CD90, and CD73 but negative for CD45, CD34, and CD14
Adenoviral-mediated gene delivery to MSCs
The MSCs were UV irradiated for 1 h to induce expression of Nrf2. Amplification of Nrf2 cDNA is then performed using specific primers and total cell RNA as template which resulted in a single band of about 1,825 bp on agarose gel electrophoresis (Fig. 2a). The PCR product is directionally cloned into pENTR/D-TOPO which is a donor vector for Gateway recombination cloning. PCR analysis of the extracted recombinant plasmid confirmed the cloning of the Nrf2 coding sequence into the pENTR/D-TOPO vector (Fig. 2b). Then, the fidelity of the cloned sequence was confirmed by sequencing (GenBank accession number HM446346). Finally, the LR recombination reaction was performed and proper recombination was confirmed by PCR analysis using gene specific primers for Nrf2 (data not shown).
Fig. 2.
a Isolation of Nrf2 cDNA. Two different clones of the mesenchymal stem cells were UV irradiated for 1 h to induce the Nrf2 gene expression. Then, total RNA was extracted and cDNA was synthesized by RT-PCR. There was no difference between these two clones. Following exposure of the cells to UV irradiation, the induction of Nrf2 expression was detected (1 and 2). M 1,000 bp DNA marker (upper image). Expression of beta-actin was used for normalization. M 100 bp DNA marker (lower image). b Cloning of the Nrf2 cDNA into pENTR/D-TOPO plasmid. The blunt-end PCR product was TOPO-cloned into the pENTR TOPO/D vector and used to transform competent E. coli cells. Positive clones were selected on LB-agar medium containing appropriate antibiotic. The recombinant plasmids were extracted from resistant colonies and analyzed by PCR using the Nrf2 specific primers. Two clones (1 and 3) were positive for Nrf2. M 1,000 bp DNA marker
Transient expression of Nrf2 in MSCs using adenoviral vector
Fourth passage of the MSCs was infected with appropriate multiplicity of infection (MOI) of the recombinant adenovirus vector harboring the Nrf2 (Ad-hNrf2) coding sequence or the control viral vector (Ad-V). RT-PCR was used to compare changes in the expression of Nrf2 at different time points (1, 2, 4, 6, 8, 10, 12, and 14 days post-infection). Initial expression of Nrf2 was detected on day 2 and increased to the highest expression level at day 6, then it decreased thereafter and no expression was detected on day 14 (Fig. 3a). Accordingly, the highest expression level of Nrf2 protein was also detected by Western blotting at day 6 (Fig. 3b). Many attempts failed to detect the expressed Nrf2 protein in other days by western blot analysis using DAB staining method. These results indicated the transient overexpression of Nrf2. Most of the transduced MSCs continuously adhered to the tissue culture dishes, and no distinct morphological changes were observed.
Fig. 3.
Transient expression of Nrf2 by the MSCs infected with the recombinant adenovirus. (aupper image) RT-PCR analysis of the Nrf2 expression levels up to 14 days after infection of the MSCs with the recombinant adenoviruses showed the highest expression level on day 6. No detectable expression was observed 2 weeks after infection. M 100 bp DNA marker. (alower image) Expression of beta-actin was used for normalization. M 100 bp DNA marker. b Western blot analysis of Nrf2 expression on day 6. Control MSCs revealed no detectable Nrf2 protein expression. Lower figure represents the assessment of beta-actin expression as a control for both group
Nrf2 modified MSCs maintained their multi-differentiation capacity
Osteogenic, adipogenic, and chonderogenic differentiations are considered the functional standards for MSC precursors. Therefore, to evaluate the possible effects of Nrf2 expression on the differentiation capacity of the Nrf2-MSCs, they were induced toward adipogenic, chondrogenic, and osteogenic differentiation 5 days post-infection.
After 7 days of induction toward the adipogenic lineage, considerable morphological changes with lipid vacuole accumulation were observed (Fig. 4b). Following 21 days of osteogenic differentiation, positive staining for Alizarin Red S confirmed the ability of Nrf2-MSCs to differentiate into osteocytes (Fig. 4c). After 15 days of chondrogenic induction, synthesis of proteoglycans by chondrocytes was detected following Alcian blue staining (Fig. 4d). Positive area analysis with Image J software used to obtain quantitative results.
Fig. 4.
Differentiation capacity of the Nrf2-MSCs. MSCs infected with Ad-Nrf2 were cultured in osteogenic, adipogenic, and chondrogenic differentiation media. The Nrf2-MSCs maintained their multi-differentiation capacity. a Control MSCs cultivated in normal medium (DMEM low glucose), b adipogenic differentiation, c osteogenic differentiation, and d chondrogenic differentiation. e Measurements of percent of positive area showed no significant difference between Nrf2-MSCs and Ad-MSCs differentiation capacities
As shown in Fig. 4e, multilineage differentiation capability of Nrf2-MSCs was slightly decreased, but there was no significant difference compared to the control cells (Ad-MSC). Taken together, these results indicated that the Nrf2-MSCs retained their normal ability to differentiate into adipogenic, chondrogenic, and osteogenic lineages.
Nrf2 induces SOD-1, SOD-2, and HO-1 in MSCs
Induction of antioxidants is one of the well-known functions of Nrf2. Superoxide dismutases (SODs) and heme oxygenase 1 (HO-1) are two important antioxidants induced by Nrf2. Therefore, to determine whether Nrf2 would also induce expression of these antioxidants in MSCs, we evaluated the expression of SOD-1, SOD-2, and HO-1 by RT-PCR in both Nrf2-MCSs and V-MSCs. As shown in Fig. 5a, b, Nrf2 upregulates the expression of SOD-1, SOD-2, and HO-1. The activity of SODs and HO-1 was also measured by commercially available kits. As shown in Fig. 5c, Nrf2 also enhances the activity of SODs and HO-1. These findings suggest that the cytoprotective effects of Nrf2 may be attributed to the induction of theses enzymes.
Fig. 5.
a RT-PCR analysis of the SOD-1, SOD-2, and HO-1 expression by the Nrf2-MSCs. Expression of these genes was up-regulated in two Nrf2 overexpressing clones. M 100 bp DNA marker. b Quantification of the bands was analyzed by UVIdoc Gel Documentation System (Avebury House 36a Union Lane Cambridge CB4 1QB-uk). c Total SOD and HO-1 activities were compared between test (V-Nrf2) and control (V) groups. Results showed significant enhancement of activities of these proteins following Nrf2 overexpression (mean±SD; ***p < 0.001; three independent experiments were carried out)
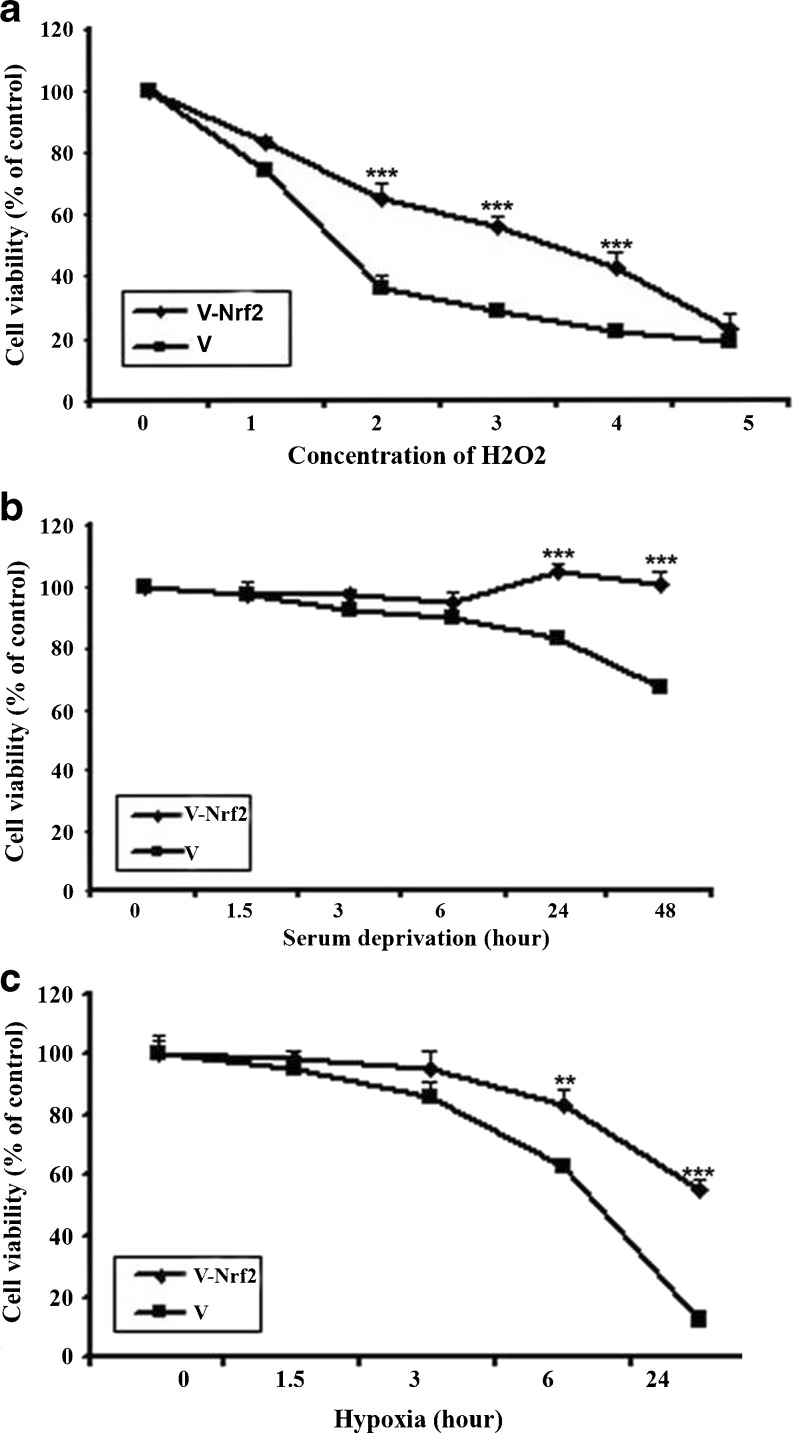
Nrf2 protects MSCs against H2O2, hypoxia, and serum deprivation induced-toxicities
To determine whether Nrf2 can protect human bone marrow-derived MSCs against the stresses which MSCs will inevitably face, the Nrf2 expressing cells were exposed to hydrogen peroxide, hypoxia, and serum deprivation for different time lengths and then subjected to cytotoxicity and proliferation assays. Exposure of the cells to 1 mM hydrogen peroxide for 2 h did not significantly change the viabilities of both Nrf2-MSCs and V-MSCs. Nevertheless, hydrogen peroxide concentrations higher than 4 mM caused a severe decrease in viabilities of both Nrf2-MSCs and V-MSCs (Fig. 6a). However, following exposure of the cells to hydrogen peroxide concentrations of 2–4 mM, the Nrf2-MSCs were more resistant to cell death than the V-MSCs confirming the cytoprotective effect of the Nrf2 overexpression against hydrogen peroxide cytotoxicity (Fig. 6a). Both Nrf2-MSCs and V-MSCs were also exposed to serum deprivation or hypoxia conditions for different time lengths followed by cell viability assay (Fig. 6b, c). The higher number of surviving Nrf2-MSCs comparing to the number of viable V-MSCs indicated the protective effects of Nrf2 on MSCs against hypoxia/serum deprivation induced toxicities.
Fig. 6.
a Cytotoxic effects of different H2O2 concentrations on the MSCs infected with the recombinant construct or empty vector were determined by MTT assay after 2 h of exposure. The viability of the MSCs infected with the Ad-Nrf2 was higher than the cells infected with empty virus (Ad-) at hydrogen peroxide concentrations of 2, 3, and 4 mM, indicating that Nrf2 protects the cells against hydrogen peroxide toxicity. b Cytotoxic effects of serum deprivation (SD) on MSCs infected with the recombinant construct or empty vector at different time points determined by MTT assay. Twenty-four and 48 h following incubation of the cells under SD, the number of viable cells in Nrf2-MSCs was higher than those of MSCs infected with empty virus. This showed that Nrf2-MSCs approximately expanded independent of serum. c The effect of incubation under hypoxia condition on MSCs infected with the recombinant construct or empty vector was determined by MTT assay at different time points. Following 6 and 24 h of incubation of the cells under hypoxia condition, Nrf2 protected the MSCs (mean±SD; **p < 0.01 and ***p < 0.001; three independent experiments were carried out)
Nrf2 expression protects MSCs against apoptosis in vitro
To study the effect of Nrf2 on apoptosis, which is one of the well-known causes of MSCs death, the Nrf2-MSCs and V-MSCs were exposed to hypoxia, serum deprivation, or hydrogen peroxide for various time lengths. Subsequently, induction of apoptosis was analyzed by Anexin V staining and caspase assay kit. Following treatment of MSCs with 400 μM hydrogen peroxide for 24 h, the number of apoptotic Nrf2-MSCs was significantly lower than the V-MSCs (Fig. 7a). In case of exposure of the cells to hypoxia and serum deprivation conditions, the number of apoptotic Nrf2-MCSs was again lower than the number of apoptotic V-MCSs (Fig. 7a).
Fig. 7.
Apoptotic effects of the H2O2, serum deprivation (SD), and hypoxia treatment on the MSCs. a Evaluation of apoptosis using annexin-V kit. After treatment of the cells with 400 μM H2O2, 24 h of hypoxia or 3 days of serum deprivation, the number of apoptotic cells was higher in cells transfected with the empty virus (V) comparing to those infected with the Ad-Nrf2 virus. b Evaluation of apoptosis by assessment of the activated caspase-3. Under the conditions described above, the lower level of the activated caspase 3 was observed in Nrf2-MSCs comparing to the control cells (mean±SD of three independent experiments; ***p < 0.001)
Induction of apoptosis following exposure to the oxidative conditions was also evaluated by assessment of the activated caspase 3. As Fig. 7b represents, higher levels of the activated caspase 3 was detected in V-MSCs comparing to the Nrf2-MCSs. Taken together, these findings indicated that the cytosolic overexpression of the Nrf2 protein protects MSCs against the H2O2, serum deprivation, and hypoxia-induced apoptosis.
Discussion
Despite the prominent promise of MSCs in regenerative medicine by repopulating the damaged tissues and restoring their function (Zhu et al. 2006b), their poor survival following tissue implantation limited their therapeutic efficacy (Li et al. 2007). The exact cellular mechanisms resulting in loss of the MSCs are unclear, and very little is known about the procedures that may mediate this process (Zhu et al. 2006b).
On the other hand, bone marrow is a common target of numerous toxic agents, cancer chemotherapeutic drugs, and environmental chemicals. Increasing evidences indicate the role of oxidative and electrophilic stresses in bone marrow depression and toxicity under various pathophysiological conditions. This makes it important to determine pathways controlling detoxification of these reactive intermediates in bone marrow cells including MSCs.
Because of continuous exposure of cells to damaging oxidizing agents, the oxygen-dependent organisms have developed a highly evolutionarily conserved mechanism for preventing oxidative stresses with Nrf2 as a key protein in the cellular stress response (Braun et al. 2002).
It is noteworthy that hypoxia, serum deprivation, and oxidative stresses are the well-known causes of MSCs death (Zhu et al. 2006b). Interestingly, the expression of Nrf2 is up-regulated under these conditions. Nrf2 also controls bone marrow stromal cell susceptibility to oxidative and electrophilic stresses (Kim et al. 2007b; Yu 2007; Umemura et al. 2008). These observations led us to the hypothesis that if MSCs are equipped with this cytoprotective factor, they would withstand the cytotoxic conditions.
In this study, we expressed Nrf2 by using an adenoviral expression system. This system has several advantages such as transient and high expression levels and the simplicity of infection conditions (Conget and Minguell 2000).
It should be noted that Nrf2 is induced in response to oxidative stresses in most cell types and rapidly returns to its basal levels and its continuous expression could be harmful to cells (Kim et al. 2007b; Padmanabhan et al. 2006; Singh et al. 2006; Kim et al. 2007a; Itoh et al. 1997; Rushmore and Tony 2002; Nguyen et al. 2004). Therefore, it would be reasonable to use the adenoviral expression system to take advantage of transient expression of Nrf2 in MSCs in order to prevent the majority of MSCs of being died a few days after transplantations.
Here, we showed that the differentiation capability of the MSCs is not affected by their transduction with the Nrf2 harboring adenoviral vector, which suggests the potential application of Nrf2 engineered MSCs for further in vivo studies. Furthermore, efficient gene delivery with adenoviral vectors in addition to the use of gateway technology adds to the advantages of our study. In order to test the survival rate of the MSCs, they were exposed to H2O2, serum deprivation, and hypoxia conditions. The results revealed that overexpression of Nrf2 in the MSCs enhance their survival and resistance to oxidative stresses. Several studies have demonstrated that Nrf2 is up-regulated by hydrogen peroxide, hypoxia, and serum deprivation stresses, and this is in favor of the cell viability (Kim et al. 2007b; Yu 2007; Umemura et al. 2008).
Supporting our study, in an in vivo study, Abdel-Mageed et al. (2009) showed that intravenous administration of MSCs following their genetic modification with extracellular superoxide dismutase improves survival in irradiated mice. Additionally, Tang et al. (2005) demonstrated that modification of MSCs by a hypoxia-regulatable HO-1 expressing vector increases the tolerance of engrafted MSCs to hypoxia-re-oxygen injury in vitro and improves their viability in ischemic hearts. More recently, in an in vitro study, we showed that adenovirus-mediated expression of the HO-1 protein within MSCs decreased cytotoxicity of oxidative stresses and inhibited the apoptosis induced by them (Hamedi-Asl et al. 2011). Wang et al. (2009) also expressed HSP20 in MSCs using adenoviral expression system, and an improved heart function was observed in an in vivo study. Our present study also further supports the previous findings of Li et al. (2007) that showed inhibition of apoptosis in bcl-2 engineered MSCs and improvement of heart function.
Apoptosis is a well-known mechanism of cell death affecting the viability of MSCs. On the other hand, one of the well-known functions of Nrf2 is its anti-apoptotic effect. Taking this into consideration, we proposed that the expression of Nrf2 by the MSCs might inhibit the activation of programmed cell death within the sensitized cells. In support of this hypothesis, our results revealed lower numbers of apoptotic Nrf2-MSCs comparing to the control cells. There are two probable mechanisms for resistance of Nrf2-MSCs to different stresses. First, Nrf2 signaling may up-regulate certain anti-apoptotic genes (Levonen et al. 2007; Ho et al. 2005; Calkins et al. 2009; Nakaso et al. 2003). Another cytoprotective mechanism of Nrf2 may involve the paracrine effects by secretion of growth factors (Imberti et al. 2007; Morigi et al. 2008). In the present study, upregulation of SOD-1, SOD-2, and HO-1 expression were observed in Nrf2-MSCs comparing to the control V-MCS.
Various studies have shown that Nrf2 induces antioxidants with the two important examples of superoxide dismutases (SODs) and heme oxygenases-1 (HO-1). Zhu et al. (2006a) demonstrated a significant reduction in the constitutive expression of antioxidants and phase 2 enzymes, including SOD, in Nrf2−/− stromal cells as compared to wild-type cells. Using oligonucleotide microarray analysis, Lee et al. (2003) showed that SODs, HO-1, and many other antioxidant genes are induced by Nrf2 transcription factor (Zhu et al. 2006a; Surh et al. 2008; de Vries et al. 2008; Copple et al. 2008; Lee et al. 2003).
Another study has shown that adenoviral-mediated Nrf2 overexpression or Nrf2-inducing drugs may have therapeutic applications in vascular diseases in which inflammation and oxidative stress play an important role. Also, it has been stated that Nrf2 exert its beneficial effects via secretion of growth factors and cytokines such as HO-1(Levonen et al. 2007).
In conclusion, the observed improvement in the resistance of the MSCs to the oxidative and apoptotic stimuli following transient overexpression of Nrf2 by adenoviral vectors could be used as a graft cell death prevention strategy in transplantation and may emerge as an alternative plan for stem cell therapy. Equipping the MSCs with the Nrf2 transcription factor which in turn induces several antioxidants may be a more effective strategy than transferring individual antioxidant gene.
However, a possible disadvantage of Nrf2 overexpression is the risk of tumor progression (Kim et al. 2007b; Padmanabhan et al. 2006; Singh et al. 2006; Kim et al. 2007a; Itoh et al. 1997; Rushmore and Tony 2002; Nguyen et al. 2004). It has been demonstrated that a hypoxic tumor microenvironment increases transactivation of Nrf2 (Kim et al. 2007a). Considering the function of Nrf2 in regulating a battery of genes that act to detoxify cancer drugs and/or attenuate drug-induced oxidative stress, the Nrf2 overexpression may play a role in increasing the resistance to treatment (Kim et al. 2007b; Itoh et al. 1997; Rushmore and Tony 2002; Nguyen et al. 2004).
Nevertheless, in case of short-term therapeutic or diagnostic gene therapies, the transient adenovirus-mediated expression of the Nrf2 gene would be advantageous. Thus, the adenoviral-mediated overexpression of the Nrf2 protein within the MSCs is proposed for further studies to evaluate the safety and efficacy of the Nrf2-MSCs in vivo.
Acknowledgments
We thank Professor Reinhard Dietrich Henschler (Head, Research and Development, German Red Cross Blood Donor Service Baden-Württemberg—Hessen) for his scientific support and providing some materials.
References
- Abdel-Mageed AS, Senagore AJ, Pietryga DW, Connors RH, Giambernardi TA, Hay RV, Deng W. Intravenous administration of mesenchymal stem cells genetically modified with extracellular superoxide dismutase improves survival in irradiated mice. Blood. 2009;113(5):1201. doi: 10.1182/blood-2008-07-170936. [DOI] [PubMed] [Google Scholar]
- Braun S, Hanselmann C, Gassmann MG. Nrf2 transcription factor, a novel target of keratinocyte growth factor action which regulates gene expression and inflammation in the healing skin wound. Mol Cell Biol. 2002;22(15):5492. doi: 10.1128/MCB.22.15.5492-5505.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calkins MJ, Johnson DA, Townsend JA, Vargas MR, Dowell JA, Williamson TP, Kraft AD, Lee JM, Li J, Johnson JA. The Nrf2/ARE pathway as a potential therapeutic target in neurodegenerative disease. Antioxid Redox Signal. 2009;11(3):497–508. doi: 10.1089/ars.2008.2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conget PA, Minguell JJ. Adenoviral-mediated gene transfer into ex vivo expanded human bone marrow mesenchymal progenitor cells. Exp Hematol. 2000;28(4):382–390. doi: 10.1016/S0301-472X(00)00134-X. [DOI] [PubMed] [Google Scholar]
- Copple IM, Goldring CE, Kitteringham NR, Park BK. The Nrf2-Keap1 defence pathway: role in protection against drug-induced toxicity. Toxicology. 2008;246(1):24–33. doi: 10.1016/j.tox.2007.10.029. [DOI] [PubMed] [Google Scholar]
- Vries HE, Witte M, Hondius D, Rozemuller AJM, Drukarch B, Hoozemans J, Horssen J. Nrf2-induced antioxidant protection: a promising target to counteract ROS-mediated damage in neurodegenerative disease? Free Radic Biol Med. 2008;45(10):1375–1383. doi: 10.1016/j.freeradbiomed.2008.09.001. [DOI] [PubMed] [Google Scholar]
- Element AR. An important role of Nrf2-ARE pathway in the cellular defense mechanism. J Biochem Mol Biol. 2004;37(2):139–143. doi: 10.5483/BMBRep.2004.37.2.139. [DOI] [PubMed] [Google Scholar]
- Hamedi-Asl P, Halabian R, Bahmani P, Mohammadipour M, Mohammadzadeh M, Roushandeh AM, Jahanian-Najafabadi A, Kuwahara Y, Roudkenar MH (2011) Adenovirus-mediated expression of the HO-1 protein within MSCs decreased cytotoxicity and inhibited apoptosis induced by oxidative stresses. Cell Stress Chaperon 17(2):181–190 [DOI] [PMC free article] [PubMed]
- Ho HK, White CC, Fernandez C, Fausto N, Kavanagh TJ, Nelson SD, Bruschi SA. Nrf2 activation involves an oxidative-stress independent pathway in tetrafluoroethylcysteine-induced cytotoxicity. Toxicol Sci. 2005;86(2):354. doi: 10.1093/toxsci/kfi205. [DOI] [PubMed] [Google Scholar]
- Imberti B, Morigi M, Tomasoni S, Rota C, Corna D, Longaretti L, Rottoli D, Valsecchi F, Benigni A, Wang J. Insulin-like growth factor-1 sustains stem cell-mediated renal repair. J Am Soc Nephrol. 2007;18(11):2921. doi: 10.1681/ASN.2006121318. [DOI] [PubMed] [Google Scholar]
- Itoh K, Chiba T, Takahashi S, Ishii T, Igarashi K, Katoh Y, Oyake T, Hayashi N, Satoh K, Hatayama I. An Nrf2/small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochem Biophys Res Commun. 1997;236(2):313–322. doi: 10.1006/bbrc.1997.6943. [DOI] [PubMed] [Google Scholar]
- Iwase T, Nagaya N, Fujii T, Itoh T, Murakami S, Matsumoto T, Kangawa K, Kitamura S. Comparison of angiogenic potency between mesenchymal stem cells and mononuclear cells in a rat model of hindlimb ischemia. Cardiovasc Res. 2005;66(3):543. doi: 10.1016/j.cardiores.2005.02.006. [DOI] [PubMed] [Google Scholar]
- Jin W, Wang H, Ji Y, Zhu L, Yan W, Qiao L, Yin H. Genetic ablation of Nrf2 enhances susceptibility to acute lung injury after traumatic brain injury in mice. Exp Biol Med. 2009;234(2):181–189. doi: 10.3181/0807-RM-232. [DOI] [PubMed] [Google Scholar]
- Kamiya N, Watanabe H, Habuchi H, Takagi H, Shinomura T, Shimizu K, Kimata K. Versican/PG-M regulates chondrogenesis as an extracellular matrix molecule crucial for mesenchymal condensation. J Biol Chem. 2006;281(4):2390–2400. doi: 10.1074/jbc.M509341200. [DOI] [PubMed] [Google Scholar]
- Kim YJ, Ahn JY, Liang P, Ip C, Zhang Y, Park YM. Human prx1 gene is a target of Nrf2 and is up-regulated by hypoxia/reoxygenation: implication to tumor biology. Cancer Res. 2007;67(2):546. doi: 10.1158/0008-5472.CAN-06-2401. [DOI] [PubMed] [Google Scholar]
- Kim YJ, Baek SH, Bogner PN, Ip C, Rustum YM, Fakih MG, Ramnath N, Park YM. Targeting the Nrf2-Prx1 pathway with selenium to enhance the efficacy and selectivity of cancer therapy. J Canc Mol. 2007;3(2):37–43. [Google Scholar]
- Kinnaird T, Stabile E, Burnett M, Shou M, Lee C, Barr S, Fuchs S, Epstein S. Local delivery of marrow-derived stromal cells augments collateral perfusion through paracrine mechanisms. Circulation. 2004;109(12):1543–1549. doi: 10.1161/01.CIR.0000124062.31102.57. [DOI] [PubMed] [Google Scholar]
- Kolossov E, Bostani T, Roell W, Breitbach M, Pillekamp F, Nygren JM, Sasse P, Rubenchik O, Fries JWU, Wenzel D, Geisen C, Xia Y, Lu Z, Duan Y, Kettenhofen R, Jovinge S, Bloch W, Bohlen H, Welz A, Hescheler J, Jacobsen SE, Fleischmann BK. Engraftment of engineered ES cellâ derived cardiomyocytes but not BM cells restores contractile function to the infarcted myocardium. J Exp Med. 2006;203(10):2315–2327. doi: 10.1084/jem.20061469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JM, Calkins MJ, Chan K, Kan YW, Johnson JA. Identification of the NF-E2-related factor-2-dependent genes conferring protection against oxidative stress in primary cortical astrocytes using oligonucleotide microarray analysis. J Biol Chem. 2003;278(14):12029. doi: 10.1074/jbc.M211558200. [DOI] [PubMed] [Google Scholar]
- Levonen AL, Inkala M, Heikura T, Jauhiainen S, Jyrkkänen HK, Kansanen E, Määttä K, Romppanen E, Turunen P, Rutanen J. Nrf2 gene transfer induces antioxidant enzymes and suppresses smooth muscle cell growth in vitro and reduces oxidative stress in rabbit aorta in vivo. Arterioscler Thromb Vasc Biol. 2007;27(4):741–747. doi: 10.1161/01.ATV.0000258868.80079.4d. [DOI] [PubMed] [Google Scholar]
- Li W, Ma N, Ong LL, Nesselmann C, Klopsch C, Ladilov Y, Furlani D, Piechaczek C, Moebius JM, Lützow K. Bcl 2 engineered MSCs inhibited apoptosis and improved heart function. Stem Cells. 2007;25(8):2118–2127. doi: 10.1634/stemcells.2006-0771. [DOI] [PubMed] [Google Scholar]
- Liu X, Jiang J, Gui C, Hu X, Xiang M, Wang J. Angiopoietin–1 protects mesenchymal stem cells against serum deprivation and hypoxia–induced apoptosis through the PI3K/Akt pathway1. Acta Pharmacol Sin. 2008;29(7):815–822. doi: 10.1111/j.1745-7254.2008.00811.x. [DOI] [PubMed] [Google Scholar]
- Liu X, Hou J, Shi L, Chen J, Sang J, Hu S, Cong X, Chen X. Lysophosphatidic acid protects mesenchymal stem cells against ischemia-induced apoptosis in vivo. Stem Cells Dev. 2009;18(7):947–954. doi: 10.1089/scd.2008.0352. [DOI] [PubMed] [Google Scholar]
- Lunde K, Solheim S, Aakhus S, Arnesen H, Abdelnoor M, Egeland T, Endresen K, Ilebekk A, Mangschau A, Fjeld JG. Intracoronary injection of mononuclear bone marrow cells in acute myocardial infarction. N Engl J Med. 2006;355(12):1199–1209. doi: 10.1056/NEJMoa055706. [DOI] [PubMed] [Google Scholar]
- Makino S, Fukuda K, Miyoshi S, Konishi F, Kodama H, Pan J, Sano M, Takahashi T, Hori S, Abe H. Cardiomyocytes can be generated from marrow stromal cells in vitro. J Clin Invest. 1999;103:697–705. doi: 10.1172/JCI5298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morigi M, Introna M, Imberti B, Corna D, Abbate M, Rota C, Rottoli D, Benigni A, Perico N, Zoja C. Human bone marrow mesenchymal stem cells accelerate recovery of acute renal injury and prolong survival in mice. Stem Cells. 2008;26(8):2075–2082. doi: 10.1634/stemcells.2007-0795. [DOI] [PubMed] [Google Scholar]
- Nakaso K, Yano H, Fukuhara Y, Takeshima T, Wada-Isoe K, Nakashima K. PI3K is a key molecule in the Nrf2-mediated regulation of antioxidative proteins by hemin in human neuroblastoma cells. FEBS Lett. 2003;546(2–3):181–184. doi: 10.1016/S0014-5793(03)00517-9. [DOI] [PubMed] [Google Scholar]
- Nguyen T, Yang CS, Pickett CB. The pathways and molecular mechanisms regulating Nrf2 activation in response to chemical stress* 1. Free Radic Biol Med. 2004;37(4):433–441. doi: 10.1016/j.freeradbiomed.2004.04.033. [DOI] [PubMed] [Google Scholar]
- Osburn WO, Wakabayashi N, Misra V, Nilles T, Biswal S, Trush MA, Kensler TW. Nrf2 regulates an adaptive response protecting against oxidative damage following diquat-mediated formation of superoxide anion. Arch Biochem Biophys. 2006;454(1):7–15. doi: 10.1016/j.abb.2006.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padmanabhan B, Tong KI, Ohta T, Nakamura Y, Scharlock M, Ohtsuji M, Kang MI, Kobayashi A, Yokoyama S, Yamamoto M. Structural basis for defects of Keap1 activity provoked by its point mutations in lung cancer. Mol Cell. 2006;21(5):689–700. doi: 10.1016/j.molcel.2006.01.013. [DOI] [PubMed] [Google Scholar]
- Pereira R, Halford K, O'hara M, Leeper D, Sokolov B, Pollard M, Bagasra O, Prockop D. Cultured adherent cells from marrow can serve as long-lasting precursor cells for bone, cartilage, and lung in irradiated mice. Proc Natl Acad Sci. 1995;92(11):4857. doi: 10.1073/pnas.92.11.4857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rushmore TH, Tony KA. Pharmacogenomics, regulation and signaling pathways of phase I and II drug metabolizing enzymes. Curr Drug Metab. 2002;3(5):481–490. doi: 10.2174/1389200023337171. [DOI] [PubMed] [Google Scholar]
- Singh A, Misra V, Thimmulappa RK, Lee H, Ames S, Hoque MO, Herman JG, Baylin SB, Sidransky D, Gabrielson E. Dysfunctional KEAP1–NRF2 interaction in non-small-cell lung cancer. PLoS Med. 2006;3(10):e420. doi: 10.1371/journal.pmed.0030420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song H, Kwon K, Lim S, Kang SM, Ko YG, Xu Z, Chung JH, Kim BS, Lee H, Joung B. Transfection of mesenchymal stem cells with the FGF-2 gene improves their survival under hypoxic conditions. Mol Cells. 2005;19(3):402. [PubMed] [Google Scholar]
- Surh YJ, Kundu JK, Na HK. Nrf2 as a master redox switch in turning on the cellular signaling involved in the induction of cytoprotective genes by some chemopreventive phytochemicals. Planta Med. 2008;74(13):1526–1539. doi: 10.1055/s-0028-1088302. [DOI] [PubMed] [Google Scholar]
- Tang YL, Tang Y, Zhang YC, Qian K, Shen L, Phillips MI. Improved graft mesenchymal stem cell survival in ischemic heart with a hypoxia-regulated heme oxygenase-1 vector. J Am Coll Cardiol. 2005;46(7):1339–1350. doi: 10.1016/j.jacc.2005.05.079. [DOI] [PubMed] [Google Scholar]
- Toma C, Pittenger MF, Cahill KS, Byrne BJ, Kessler PD. Human mesenchymal stem cells differentiate to a cardiomyocyte phenotype in the adult murine heart. Circulation. 2002;105(1):93–98. doi: 10.1161/hc0102.101442. [DOI] [PubMed] [Google Scholar]
- Tsai PS, Chen CC, Yang LC, Huang WY, Huang CJ. Heme oxygenase 1, nuclear factor E2-related factor 2, and nuclear factor κB are involved in hemin inhibition of type 2 cationic amino acid transporter expression and l-arginine transport in stimulated macrophages. Anesthesiology. 2006;105(6):1201. doi: 10.1097/00000542-200612000-00020. [DOI] [PubMed] [Google Scholar]
- Turcanu V, Dhouib M, Poindron P. Determination of heme oxygenase activity in murine macrophages for studying oxidative stress inhibitors. Anal Biochem. 1998;263(2):251–253. doi: 10.1006/abio.1998.2806. [DOI] [PubMed] [Google Scholar]
- Umemura K, Itoh T, Hamada N, Fujita Y, Akao Y, Nozawa Y, Matsuura N, Iinuma M, Ito M. Preconditioning by sesquiterpene lactone enhances H2O2-induced Nrf2/ARE activation. Biochem Biophys Res Commun. 2008;368(4):948–954. doi: 10.1016/j.bbrc.2008.02.018. [DOI] [PubMed] [Google Scholar]
- Wang X, Zhao T, Huang W, Wang T, Qian J, Xu M, Kranias EG, Wang Y, Fan GC. Hsp20–engineered mesenchymal stem cells are resistant to oxidative stress via enhanced activation of Akt and increased secretion of growth factors. Stem Cells. 2009;27(12):3021–3031. doi: 10.1002/stem.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng Q, Yang K, Xiao W, Yuan M, Zhang W, Pang Y. Establishment of an insect cell clone that harbours a partial baculoviral genome and is resistant to homologous virus infection. J Gen Virol. 2009;90(12):2871. doi: 10.1099/vir.0.013334-0. [DOI] [PubMed] [Google Scholar]
- Yu X (2007) Role of alkenal/one oxidoreductase (AOR) and serum deprivation response factor (Sdr)-related gene product that binds to c-kinase (SRBC) in chemoprevention of cancer. Dissertation, The Johns Hopkins University
- Zhang M, Methot D, Poppa V, Fujio Y, Walsh K, Murry CE. Cardiomyocyte grafting for cardiac repair: graft cell death and anti-death strategies. J Mol Cell Cardiol. 2001;33(5):907–921. doi: 10.1006/jmcc.2001.1367. [DOI] [PubMed] [Google Scholar]
- Zhang W, Su X, Gao Y, Sun B, Yu Y, Wang X, Zhang F. Berberine protects mesenchymal stem cells against hypoxia-induced apoptosis in vitro. Biol Pharm Bull. 2009;32(8):1335. doi: 10.1248/bpb.32.1335. [DOI] [PubMed] [Google Scholar]
- Zhang D, Li Y, Zhu T, Zhang F, Yang Z, Miao D. Zinc supplementation results in improved therapeutic potential of bone marrow-derived mesenchymal stromal cells in a mouse ischemic limb model. Cytotherapy. 2011;13(2):156–164. doi: 10.3109/14653249.2010.512633. [DOI] [PubMed] [Google Scholar]
- Zhu H, Zhang L, Itoh K, Yamamoto M, Ross D, Trush MA, Zweier JL, Li Y. Nrf2 controls bone marrow stromal cell susceptibility to oxidative and electrophilic stress. Free Radic Biol Med. 2006;41(1):132–143. doi: 10.1016/j.freeradbiomed.2006.03.020. [DOI] [PubMed] [Google Scholar]
- Zhu W, Chen J, Cong X, Hu S, Chen X. Hypoxia and serum deprivation–induced apoptosis in mesenchymal stem cells. Stem Cells. 2006;24(2):416–425. doi: 10.1634/stemcells.2005-0121. [DOI] [PubMed] [Google Scholar]