Abstract
The mammalian RNA-binding protein (RBP) HuR associates with numerous mRNAs encoding proteins with roles in cell division, cell survival, immune response, and differentiation. HuR was known to stabilize many of these mRNAs and/or modulated their translation, but the molecular processes by which HuR affected the fate of target mRNAs was largely unknown. Evidence accumulated over the past five years has revealed that the influence of HuR on many bound transcripts depends on HuR's interplay with microRNAs which associate with the same mRNAs. Here, we review the interactions of HuR and microRNAs – both competitive and cooperative – that govern expression of shared target mRNAs. Competition between HuR and microRNAs typically results in enhanced gene expression if the HuR-mRNA interaction prevails, and in repression if the microRNA remains associated. Cooperation between HuR and microRNAs leads to lower expression of the shared mRNA. We also describe the regulation of HuR levels by microRNAs as well as the regulation of microRNA levels by HuR. Finally, we discuss transcriptome-wide analyses of HuR-bound mRNAs with neighboring microRNA sites, and review the emerging mechanisms whereby microRNAs confer versatility and strength to the post-transcriptional outcomes of HuR targets.
1.0. INTRODUCTION
Post-transcriptional gene expression is controlled at multiple levels, including pre-mRNA splicing and maturation, as well as mRNA transport, editing, storage, stability, and translation [1, 2]. The control of mRNA turnover and translation in the cytoplasm is particularly effective in eliciting rapid changes in the patterns of expressed proteins following environmental perturbations. Noncoding RNAs (particularly microRNAs) and turnover and translation regulatory RNA-binding proteins (TTR-RBPs) are major trans-binding factors that associate with specific cis elements in mRNAs and modulate their stability and translation [3-5].
1.1. MicroRNAs
MicroRNAs (miRNAs) are short (~22-nucleotides) noncoding RNAs that potently influence gene expression patterns [6, 7]. They are synthesized from long primary miRNA (pri-miRNA) transcripts which are processed in the nucleus by ribonucleases (Drosha and DiGeorge critical region 8) to generate precursor (pre-miRNA) transcripts. Following export to the cytoplasm by exportin 5, the pre-miRNA is further processed by the ribonuclease Dicer, yielding ~22-nt duplex RNAs; one strand of the duplex is loaded into the miRNA-containing ribonucleoprotein inhibitory complex (RISC), which contains Argonaute proteins [8, 9]. The miRNA-RISC then targets a specific mRNA, typically at the 3’-untranslated region (UTR). The miRNA and mRNA form a partial hybrid that often requires the presence of a ‘seed’ region (nucleotides 2 to 7 of the miRNA) of strong complementarity with the mRNA. The association of miRNA-RISC with an mRNA lowers the stability of the mRNA, suppresses its translation, or has both of these effects [6, 7]. However, under specific circumstances, like during cellular quiescence, microRNAs can also promote translation [10].
1.1.1. Combinatorial actions of microRNAs
As discussed by Serva and coworkers [11], a single microRNA usually has only partial effect in reducing the stability or translation of a target mRNA; thus, microRNAs often work in concert to inhibit the expression of a shared target mRNA [12]. In addition, given that a single microRNA can target multiple mRNAs, it can simultaneously influence the production of different proteins. In some instances, these coordinate target proteins are related functionally [11].
1.2. HuR
The mammalian Hu/elav (embryonic lethal abnormal vision in Drosophila) family of TTR-RBPs comprises the ubiquitous HuR (HuA) and the primarily neuronal proteins HuB, HuC, and HuD [13]. Since its discovery fifteen years ago [14], HuR has been found to interact with numerous mRNAs [15-18]. It was originally described as a stabilizing TTR-RBP [19], but was later found to affect the translation of target mRNAs, generally promoting translation, but sometimes inhibiting it (reviewed in [13, 18, 20]). In keeping with its abundant nuclear presence, HuR can also interact with pre-mRNA and may affect splicing, as revealed in recent high-throughput studies using PAR-CLIP (photoactivatable-ribonucleoside-enhanced crosslinking and immunoprecipitation [15, 16]). However, in this review, we will focus on the actions of HuR in the cytoplasm, where it stabilizes target mRNAs and modulates their translation.
1.2.1. Influence of HuR on target mRNAs
Through its three RNA recognition motifs (RRMs), HuR interacts with target mRNA, many of which bear U- and AU-rich elements (AREs) in their 3’UTRs. However, HuR can also bind to sequences in the 5’UTR of some target mRNAs [21-23] and, as mentioned above, also with pre-mRNAs near splice sites [15, 16]. HuR target mRNAs encode proteins implicated in cellular proliferation, angiogenesis, inflammation, differentiation, signaling, genotoxic and oxidative damage, nutrient deprivation, and hypoxia (reviewed in [18]).
HuR stabilizes many of its bound mRNAs [18-20]. The exact mechanisms of stabilization have not been fully elucidated, but binding of HuR to a target mRNA was widely believed to block the association of other TTR-RBPs capable of recruiting the mRNA to sites of decay like the exosome or processing bodies (PBs). However, as we discuss here, HuR also appears to protect mRNAs from degradation through competition with microRNAs (section 2.1.) with affinity for the same mRNAs.
HuR also modulates the translation of several target mRNAs [13, 18]. In some cases, HuR promotes the translation of some target mRNA through its association with 5’UTR internal ribosome entry site (IRESs) [24], although competition of HuR with microRNAs also represents an effective way to enhance translation (section 2.1.) [25]. HuR also inhibits the translation of a small subset of target mRNAs; in some instances, this effect was attributed to the disruption of 5’UTR IRESs [21-23], but for some targets, reduced translation was linked to HuR's recruitment of miRNA-RISC complexes to their 3’UTR [26] (section 2.2.).
1.2.2. Regulation of HuR function
Initial studies identified HuR cytoplasmic export as a critical way to control expression of HuR target mRNAs, but recent work has shown that HuR abundance and post-translational modifications also potently influence HuR function. These processes are only covered briefly here, since they are reviewed in detail in an excellent piece by Eberhardt and coworkers in this volume [27].
HuR localization
The transport of HuR across the nuclear envelope requires a specific HuR domain (the HuR nucleocytoplasmic shuttling domain or HNS) and several transport machinery components, including transportins 1 and 2, the chromosome region maintenance 1 (CRM1), and importin-1α [28-31]. HuR nucleocytoplasmic transport is also influenced by kinases [cyclin-dependent kinase 1 (Cdk1), AMP-activated protein kinase (AMPK), protein kinase C (PKC), and the mitogen-activated protein kinase (MAPK) p38] which phosphorylate HuR and proteins that transport HuR [32-36]. In general, modification of residues within or near the HNS alter HuR subcellular localization [27].
HuR abundance
The steady-state levels of HuR protein are regulated transcriptionally by the nuclear factor (NF)-κB and by Smads [37, 38]. At the post-transcriptional level, HuR binds the HuR mRNA, stabilizes it, and enhances its cytoplasmic export [39]. A number of microRNAs negatively regulate HuR production (section 3.0.). The stability of HuR is further controlled by ubiquitination [in response to heat stress] and by cleavage via caspases [40, 41].
HuR binding to target mRNAs
Generally speaking, the phosphorylation of residues within the RRMs affects HuR binding to target mRNAs. Phosphorylation by the checkpoint kinase Chk2 at HuR residues Ser-88, Ser-100, and Thr-118 (located between and within RRM1 and RRM2) modulates HuR binding to several target mRNAs [42, 43]. Oxidative stress and γ irradiation activated Chk2, which in turn phosphorylated HuR and dissociated it from many mRNAs [42, 43]. Phosphorylation by PKCα at HuR Ser-158 and Ser-221 in response to ATP treatment, and phosphorylation by PKCδ of Ser-221 and Ser-318 in response to angiotensin II (AngII) promoted the binding activity of HuR [32, 33, 44, 45]. In cells exposed to γ irradiation, p38 phosphorylated HuR at Thr-118, increasing its association with p21 mRNA [46]. Finally, HuR methylation at Asp-271 by CARM1 (coactivator-associated arginine methyltransferase 1) in response to lipopolysaccharide stimulation promoted HuR binding to and stabilization of TNFα mRNA [47].
2.0. HuR and microRNAs regulate shared target mRNAs
MicroRNAs and HuR regulate each other's function in many ways, including by mutually influencing their expression (sections 3.0. and 4.0.), and by jointly affecting the expression of shared target mRNAs (below). This combinatorial regulation is supported by the observations that HuR and Argonaute share many interacting mRNAs [48, 49], and that HuR and microRNAs can function competitively (section 2.1.) and cooperatively (section 2.2.). In this section, we concentrate on the joint regulation of shared mRNAs by HuR and microRNAs (Table 1) [50,51].
Table 1. Coordiante influence of microRNAs and HuR on shared target mRNAs.
The table lists the reported examples of microRNAs (column 1) that interact with HuR target mRNAs (column 2) and either compete (top set) or cooperate (bottom set) with HuR in controlling expression (stability and/or translation) of the shared target mRNA.
| MicroRNA | Target shared with HuR | Effect of miRNA | Effect of HuR | Type of Interaction | References |
|---|---|---|---|---|---|
| miR-122 | CAT1 mRNA | Repression | Induction | Competitive | [25] |
| miR-548c | TOP2A mRNA | Repression | Induction | Competitive | [52] |
| miR-494 | NCL mRNA | Repression | Induction | Competitive | [53] |
| miR-16 | COX2 mRNA | Repression | Induction | Competitive | [55] |
| miR-331 | ERBB2 mRNA | Repression | Induction | Competitive | [56] |
| let-7 | MYC mRNA | Repression | Repression | Cooperative | [26] |
| miR-19 | RhoB mRNA | Repression | Repression | Cooperative | [58] |
| (RISC) | p16 mRNA | Repression | Repression | Cooperative | [59] |
2.1. Competitive regulation of mRNAs by HuR and miRNAs
Since HuR functions primarily as a positive regulator of mRNA stability and/or translation, whereas microRNAs are typically negative regulators of the same processes, it is easy to envision how these two regulators might antagonize each other's function. Accordingly, their association might result in opposing effects on the localization of mRNAs, either in translationally active polysomes or in repressive cytoplasmic complexes (e.g., PBs) [25, 52, 53].
CAT1 mRNA
In the very first report of functional competition between HuR and microRNA, Bhattacharyya and colleagues found that HuR translocation from nucleus to cytoplasm under stress conditions (amino acid deprivation) relieved the miR-122-elicited repression of the mRNA encoding the cationic amino acid transporter 1 (CAT1 mRNA) [25]. The authors showed that in human liver cancer cells, the stress-induced de-repression of CAT1 mRNA was due to relocation of the transcripts from PBs to translationally engaged polysomes.
TOP2A mRNA
Up- or downregulation HuR levels and microRNA levels during normal cellular processes like cell division or differentiation can also change their relative influence on shared transcripts. For example, the increase expression of topoisomerase II alpha (TOP2A, an enzyme that relieves tension from supercoiled DNA) by HuR was highest during G2/M phase, when HuR was most abundant [54]. Using a novel strategy based on the tagging of a transcript with MS2 RNA hairpins to identify associated microRNAs, miR-548c was found to interact with the TOP2A mRNA 3’UTR [52]. Accordingly, HuR downregulation or miR-548c overexpression increased the interaction of TOP2A mRNA with miR-548c-RISC and the recruitment of TOP2A transcripts to PBs. In turn, HuR overexpression decreased miR-548c-TOP2A mRNA interaction and reversed the miR-548c-mediated repression. Moreover, as miR-548c levels were most abundant during the G1 phase, miR-548c lowered TOP2A mRNA translation in G1 [52].
NCL mRNA
A similar competitive regulation was recently found to control the expression of the nucleolar RNA-binding and DNA-binding protein nucleolin (NCL). NCL expression was promoted by HuR and repressed by miR-494 [53]. As seen with TOP2A, HuR increase lowered NCL mRNA interaction with miR-494-RISC, reduced the recruitment of NCL transcripts to PBs and elevated NCL production. Conversely, miR-494 upregulation increased NCL mRNA interaction with RISC, diminished the interaction of HuR with NCL mRNA, and decreased NCL levels.
COX2 mRNA
Very recently, Young and coworkers reported that in colorectal cancer (CRC) and in cultured CRC lines, expression of the proinflammatory enxyme cyclooxygenase-2 (Cox-2) was controlled by the antagonistic interaction of HuR and miR-16. Cox-2 expression was negatively regulated by miR-16, and this inhibition was reversed when HuR was overexpressed in CRC cells [55] (more details in section 3.3.).
ERBB2 mRNA
ERBB-2 promotes resistance to therapy and malignant progression in prostate cancer. miR-331-3p associates with the ERBB2 3’UTR at two different sites and repressed ERBB2 biosynthesis. A recent study [56] showed that HuR binds to a U-rich element in the ERBB2 mRNA and competed for association of miR-331-3p, thereby preventing the inhibitory action of miR-331-3p and enhancing ERBB2 biosynthesis.
Transcriptome-wide analysis of antagonism between HuR and microRNAs
Two groups recently studied transcriptome-wide HuR-mRNA interactions using PAR-CLIP and compared their results with previously known miRNA-mRNA interactions to understand the prevalence of this combinatorial regulation [15, 16]. In one of these studies [16], Lebedeva and colleagues found that microRNA binding sites were preferentially located toward the boundaries of 3’UTR as previously reported [50, 57] whereas HuR binding sites distributed uniformly along the 3’UTR except for regions surrounding the stop codon and the polyadenylation site. Bioinformatic predictions revealed that instead of overlapping with HuR sites, most of the miRNA sites were found in the immediate vicinity of HuR binding sites. With overlapping binding sites, direct competition between microRNA and HuR should be possible, whereas in the context of nonoverlapping sites, competition could occur by steric hindrance or by non-steric hindrance involving changes in the secondary structure of the RNA. In the second HuR PAR-CLIP study [15], Mukherjee and coworkers broadly confirmed these results and further reported differences in transcript abundance after HuR knockdown or microRNA knockdown. The authors proposed that where microRNA and HuR binding sites overlapped, the transcripts were preferentially regulated by HuR, but when the binding sites are non-overlapping the transcripts were mainly regulated by the microRNA.
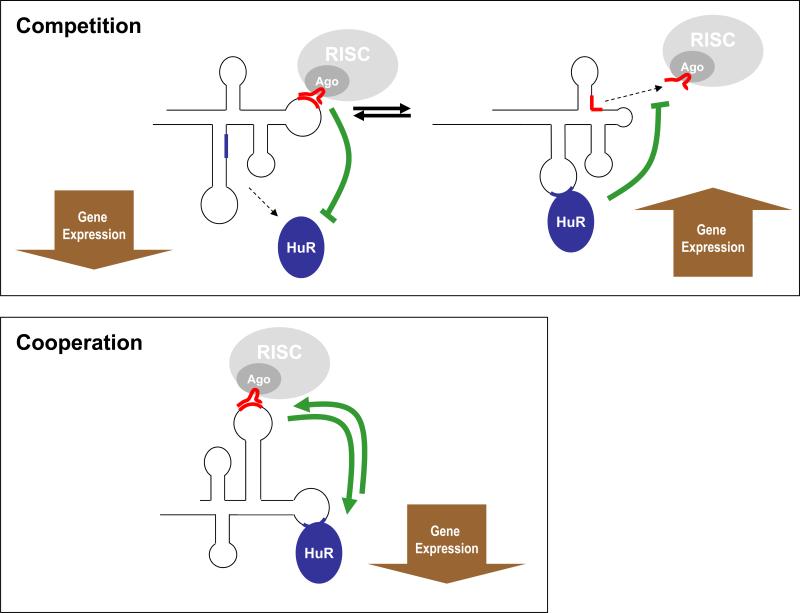
Our understanding of the competition between HuR and microRNAs is still preliminary. Given the results with CAT1, TOP2A, and NCL mRNAs, where HuR binds at far distances from microRNAs, it is generally believed that conformational changes of the RNA (which remain challenging to study) could favor HuR-mRNA interactions and exclude miRNA-mRNA interactions, and vice versa (Fig. 1). Competition at distant sites could also result from physical recruitment of the mRNA to different parts of the cell; for example, microRNAs could recruit mRNAs to PBs, while HuR could recruit mRNAs to polysomes or even to the nucleus. Little experimental evidence is available as-yet in support of these models.
Fig. 1. Schematic of competitive and cooperative interactions of HuR and microRNAs on shared target mRNAs.
Top, under the ‘competition’ model, binding of miRNA-RISC may trigger a conformational change that hides the site of HuR binding to the mRNA, in turn lowering expression of the mRNA (left); conversely, HuR binding may trigger changes in RNA structure that conceal the site of interaction with miRNA-RISC, causing increased expression of the mRNA (right). Bottom, in the ‘cooperation’ model, binding of HuR may trigger conformational changes that allow binding of miRNA-RISC or vice versa, resulting in repression of the mRNA.
2.2. Cooperative regulation of mRNAs by HuR and miRNAs
Although HuR typically stabilizes and/or increases target mRNA translation, there is a handful of examples of HuR's repressive effect on some target mRNAs [21, 22, 26]. In these instances, miRNAs could work co-operatively with HuR to downregulate gene expression. Here, we review the limited examples reported so far of HuR cooperating with microRNAs to repress target mRNAs via destabilization or inhibition of translation.
MYC mRNA
Kim et al. [26] reported that HuR associated with the 3’UTR of the MYC mRNA and enhanced production of the proto-oncogene c-Myc. HuR facilitated the interaction of let-7-RISC with the MYC 3’UTR, leading to its destabilization and translational repression. As with competition examples, the HuR and let-7 binding sites on the MYC 3’UTR were over 100 nt apart, so HuR binding was proposed to trigger a conformational change in the MYC 3’UTR which promoted the interaction of let-7-RISC [26].
RhoB mRNA
More recently, Glorian et al [58] reported that the HuR-dependent recruitment of miR-19 to the RhoB mRNA was responsible for repressing the production of RhoB (Ras homolog B, a GTP-binding protein) after exposure to ultraviolet light. Accordingly, loss of interaction with HuR and miR-19 led to increased RhoB production and triggered apoptosis. In a manner reminiscent of the HuR/let-7 and MYC mRNA, HuR binding to RhoB mRNA facilitated the loading of miR-19-RISC at a distal region of RhoB mRNA.
p16 mRNA
Chang et al [59] similarly observed that RISC components were recruited to the mRNA encoding the cdk inhibitor, tumor suppressor, and senescence-associated protein p16. This interaction led to the destabilization of p16 mRNA in a manner that was dependent on HuR and another RBP (AUF1), although the specific miRNAs involved were not identified.
2.3. General aspects of the combinatorial gene regulation by HuR and miRNAs
While individual mRNA studies have uncovered different ways in which HuR and microRNAs can compete or cooperate, and transcriptome-wide analyses of HuR and Ago2 sites support the existence of these regulatory mechanisms, our understanding of this combinatorial regulation is still rudimentary. To comprehend this process better, further analyses of individual mRNAs is needed, including the specific HuR and microRNA sites, the distance between them, and how binding of each factor affects the local secondary structure of the RNA (using technologies like SHAPE (hydroxyl acylation analyzed by primer extension [60]). Second, it is necessary to understand in better molecular detail how HuR and microRNAs modulate the translation and stability of its targets. Third, while HuR and microRNAs preferentially interact with AU-rich elements [50, 57] in the 3’UTR of target mRNAs, high-throughput analyses did not reveal more widespread examples of competitive regulation of target mRNA decay by HuR and microRNAs. Perhaps different experimental designs to identify Ago2 sites in the presence and absence of HuR or after stress or mitogenic signals will be informative. In this regard, perhaps testing mRNA levels is insufficient, since both HuR and microRNAs can also modulate translation; instead, high-throughput changes in translation should be studied using assays like pulsed SILAC, nascent translation assays, and polysome profiling. Finally, care must be taken to differentiate this combinatorial regulation from the direct regulation of HuR abundance by microRNAs (section 3.0.) or the regulation of microRNAs levels by HuR (section 4.0.).
3.0. microRNAs that regulate HuR expression
MicroRNAs are among the few identified regulators of HuR expression levels. Indeed, some of the effects attributed to these microRNAs could in fact be indirect, through their regulation of HuR production. In this section, we will review the microRNAs that modulate HuR levels.
3.1. miR-519
HuR expression was potently lowered by the microRNA miR-519 in several human cancer cell lines, including HeLa (cervical), HCT116 and RKO (colon), and A2780 (ovarian) [61]. This reduction was primarily caused by the repression of HuR translation, not by decreased HuR mRNA stability. Interestingly, of the two putative sites of miR-519 interaction on the HuR mRNA, one residing in its coding region (CR) and one in its 3’UTR, the CR site repressed HuR translation more potently. In a replicative senescence model, miR-519 levels correlated inversely with HuR, as miR-519 levels rose and HuR levels and cell proliferation declined in senescent cells [62]. The levels of miR-519 and HuR also showed inverse correlation in tissue pairs from cancer patients: cancer tissues displayed high HuR and low miR-519 levels, normal tissues had high miR-519 and low HuR levels. Since HuR promotes cell proliferation and tumorigenesis [61, 63], overexpression of miR-519 inhibited DNA replication and cell division and reduced tumorigenesis, supporting the view that miR-519 could function as a tumor suppressor microRNA [63].
3.2. miR-125a
Although miR-125a and miR-125b differ only by a central diuridine insertion and a U-to-C change, only miR-125a (not miR-125b) suppressed HuR expression by interacting with the 3’UTR of HuR mRNA, as shown in a breast cancer model [64]. As with miR-519, an inverse correlation between HuR of miR-125a was reported in breast cancer cells, where miR-125a levels were low and HuR levels were high [64].
3.3. miR-16
In the human breast carcinoma cell line MDA-MB-231, low miR-16 and high HuR was linked to the interaction of miR-16 to the HuR 3’UTR and its negative influence HuR translation [65]. As miR-16 inhibited the expression of cancer-associated proteins Cox-2, TNFα, Bcl-2, Mcl-1, and Cyclin D1 [66, 67], and these mRNAs are also targets of stabilization and/or translational upregulation by HuR [18], the authors proposed that miR-16 reduced the expression of these target mRNAs both by directly inhibiting them and by lowering the levels of their positive regulator, HuR. Through an unexpected negative feedback mechanism, HuR protein also associated with miR-16 and this interaction was linked to a reduction in miR-16 levels (section 4.1., [55]).
3.4. miR-34a
A direct transcriptional target of p53, miR-34a triggered apoptosis in HCT116 colon cancer cells and in chronic lymphocytic leukemia cells [68, 69], caused G1 arrest and lowered colony formation in the osteosarcoma cell line U2OS [70], and diminished proliferation and accelerated senescence in the fetal lung cell line IMR90 [71]. Accordingly, miR-34a levels decline in many cancers [68, 72] and is a key mediator of tumor suppression by directly and indirectly controlling the expression of many proteins [73]. Recently, miR-34a levels were found to be low, while HuR levels were high, in paclitaxel-resistant cells generated from hormone refractory prostate cancer cells (PC3PR) [74]. Although it was not shown whether miR-34a directly associated with the HuR mRNA, miR-34a overexpression lowered HuR production in PC3PR cells. Therefore, Kojima and colleagues proposed that miR-34a not only directly lowered the levels of cancer-related genes (e.g., Sirt1, Bcl-2) by directly associating with their 3’UTRs and triggering repression, but miR-34a also indirectly reduced their levels by suppressing the production of HuR, a positive regulator of SIRT1 and Bcl-2 mRNAs. Together, HuR, Sirt1, and Bcl2 played important roles in the development of paclitaxel resistance in PC3PR cells [74].
4.0. Regulation of microRNA expression by HuR
HuR also influences the expression of certain microRNAs, although only a limited number of examples has been reported and the mechanisms are poorly understood at present.
4.1. miR-16
HuR was recently found to interact with miR-16 in colon cancer cells [65]. In this model system miR-16 associated with COX2 3’UTR and reduced Cox-2 abundance, as mentioned above (section 3.3). HuR competed for binding to this mRNA and thus promoted COX2 mRNA stability and Cox-2 expression. Additionally, however, the accumulation of HuR in the cytoplasm led to HuR binding to miR-16 and this interaction was linked to a rapid decline in the steady-state levels of miR-16 [55]. The molecular details of this reduction are unknown, but these results make the provocative case that HuR could promote Cox-2 expression both by direct binding to COX2 3’UTR, and by indirectly reducing the abundance of the key COX2 mRNA repressor miR-16.
4.2. miR-7
The levels of miR-7 increased dramatically after silencing HuR [16]. As miR-7 is contained within the last intron of the HNRNPK gene and hnRNP K is a highly expressed housekeeping protein, the authors propose that HuR strongly suppresses the biogenesis of mature miR-7 at the level of processing, perhaps by affecting the fate of the excised intron that contains the miR-7 precursor. The striking rise in miR-7 abundance after silencing HuR prompted the authors to suggest that a number of HuR-elicited actions be the consequence of HuR-mediated lowering of miR-7 production [16].
4.3. Other microRNAs
PAR-CLIP analysis revealed the interaction of HuR with microRNA precursor transcripts (pri-microRNAs) [15, 16]. In addition, this global analysis also found a number of mature microRNAs which are direct targets of HuR, including the oncogenic microRNAs miR-21 and miR-221, and several microRNAs with functional links to HuR activity (e.g., miR-125a and various members of the let-7 family). It is presently unknown whether HuR affects the expression of bound microRNAs by enhancing their degradation (as described for miR-16) or other aspects of their expression or function.
5.0. Conclusions
HuR is a prominent regulator of the cytoplasmic fate of its target mRNAs, but the mechanism of HuR actions were only partly explained by its functional links to TTR-RBPs which promoted decay and repressed translation. Studies over the past half-decade have revealed that many HuR actions on target mRNAs may be due to HuR's physical and functional interactions with microRNAs. HuR competes with several microRNAs (Section 2.1.), thereby antagonizing their repressive influence on target mRNAs, while HuR can also cooperate (Section 2.2.) with other microRNAs in repressing protein production. While some of these influences may be direct (HuR and microRNAs compete for the very same mRNA site), it is likely that both cooperation and competition occur even when HuR and microRNA-RISC interact with distant sites on the shared target mRNA. According to a widespread view, binding of HuR can trigger a conformational change in the local RNA that hides or reveals sites for binding the microRNA-RISC (Fig. 1). Conversely, binding of the microRNA-RISC can cover or uncover a site of HuR interaction on the mRNA. We anticipate that detailed RNA conformation analyses will provide experimental backing to this model in the near future.
MicroRNAs were also found to influence HuR production, while HuR affected the levels of certain microRNAs. In this regard, it was intriguing to find that HuR bound miR-16 and yet it reduced miR-16 abundance. It will be interesting to elucidate the mechanisms behind this loss of a target microRNA. In addition, the fact that HuR binds to multiple microRNAs suggests that it might function as a ‘sponge’ (a ‘sequestering’ factor) of microRNAs to make them unavailable for binding to Ago/RISC. Perhaps HuR may also or alternatively preserve a pool of microRNAs in ‘storage’ or simply protect it from decay. These are interesting possibilities that warrant future study.
Finally, although HuR is one of the best-known proteins that affect target mRNA stability and translation, it is easy to envision how the function of other TTR-RBPs may be regulated by similar interplays with microRNAs [75]. It is increasingly apparent that the coordinated actions of TTR-RBPs and microRNAs add a dynamic layer of complexity to gene regulatory mechanisms that ensure proper development, differentiation and adaptive responses to stimuli.
Table 2. Influence of microRNAs on HuR expression and vice versa.
The table lists the microRNAs that associate with the HuR mRNA (at the CR or 3’UTR) and repress HuR production (top set), and the association of HuR with some mature or primary microRNAs reported to lower their expression levels (bottom set).
ACKNOWLEDGEMENTS
This work was supported in its entirety by the National Institute on Aging-Intramural Research Program, National Institutes of Health.
BIBLIOGRAPHY
- 1.Orphanides G, Reinberg D. A unified theory of gene expression. Cell. 2002;108:439–451. doi: 10.1016/s0092-8674(02)00655-4. [DOI] [PubMed] [Google Scholar]
- 2.Mitchell P, Tollervey D. mRNA stability in eukaryotes. Curr. Opin. Genet. Dev. 2000;10:193–198. doi: 10.1016/s0959-437x(00)00063-0. [DOI] [PubMed] [Google Scholar]
- 3.Pullmann R, Jr., Kim HH, Abdelmohsen K, Lal A, Martindale JL, Yang X, Gorospe M. Analysis of stability and translation regulatory RBP expression through binding to cognate mRNAs. Mol. Cell. Biol. 2007;27:6265–6278. doi: 10.1128/MCB.00500-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Keene JD. RNA regulons: coordination of post-transcriptional events. Nat. Rev. Genet. 2007;8:533–543. doi: 10.1038/nrg2111. [DOI] [PubMed] [Google Scholar]
- 5.Valencia-Sanchez MA, Liu J, Hannon GJ, Parker R. Control of translation and mRNA degradation by miRNAs and siRNAs. Genes Dev. 2006;20:515–524. doi: 10.1101/gad.1399806. [DOI] [PubMed] [Google Scholar]
- 6.Bartel DP. MicroRNAs: Target Recognition and Regulatory Functions. Cell. 2009;136:215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fabian MR, Sonenberg N, Filipowicz W. Regulation of mRNAtranslation and stability by microRNAs. Annu. Rev. Biochem. 2010;79:351–379. doi: 10.1146/annurev-biochem-060308-103103. [DOI] [PubMed] [Google Scholar]
- 8.Kim VN, Han J, Siomi MC. Biogenesis of small RNAs in animals. Nat. Rev. Mol. Cell. Biol. 2009;10:126–139. doi: 10.1038/nrm2632. [DOI] [PubMed] [Google Scholar]
- 9.Newman MA, Hammond SM. Emerging paradigms of regulated microRNA processing. Genes Dev. 2010;24:1086–1092. doi: 10.1101/gad.1919710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vasudevan S, Tong Y, Steitz JA. Switching from repression to activation: microRNAs can up-regulate translation. Science. 2007;318:1931–1934. doi: 10.1126/science.1149460. [DOI] [PubMed] [Google Scholar]
- 11.Serva A, Claas C, Starkuviene VA. Potential of microRNAs for High-Content Screening. J. Nucleic Ac. 2011 doi: 10.4061/2011/870903. (ID 870903) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marasa BS, Srikantan S, Masuda K, Abdelmohsen K, Kuwano Y, Yang X, Martindale JL, Rinker-Schaeffer CW, Gorospe M. Increased MKK4 abundance with replicative senescence is linked to the joint reduction of multiple microRNAs. Sci. Signal. 2009;2(94):ra69. doi: 10.1126/scisignal.2000442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hinman MN, Lou H. Diverse molecular functions of Hu proteins. Cell Mol. Life Sci. 2008;65:3168–3181. doi: 10.1007/s00018-008-8252-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ma WJ, Cheng S, Campbell C, Wright A, Furneaux H. Cloning and characterization of HuR, a ubiquitously expressed Elav-like protein. J. Biol. Chem. 1996;271:8144–8151. doi: 10.1074/jbc.271.14.8144. [DOI] [PubMed] [Google Scholar]
- 15.Mukherjee N, Corcoran DL, Nusbaum JD, Reid DW, Georgiev S, Hafner M, Ascano M, Jr., Tuschl T, Ohler U, Keene JD. Integrative regulatory mapping indicates that the RNA-binding protein HuR couples pre-mRNA processing and mRNA stability. Mol. Cell. 2011;43:327–339. doi: 10.1016/j.molcel.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lebedeva S, Jens M, Theil K, Schwanhäusser B, Selbach M, Landthaler M, Rajewsky N. Transcriptome-wide analysis of regulatory interactions of the RNA-binding protein HuR. Mol. Cell. 2011;43:340–352. doi: 10.1016/j.molcel.2011.06.008. [DOI] [PubMed] [Google Scholar]
- 17.López de Silanes I, Zhan M, Lal A, Yang X, Gorospe M. Identification of a target RNA motif for RNA-binding protein HuR. Proc. Natl. Acad. Sci. USA. 2004;101:2987–2992. doi: 10.1073/pnas.0306453101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abdelmohsen K, Kuwano Y, Kim HH, Gorospe M. Posttranscriptional gene regulation by RNA-binding proteins during oxidative stress: implications for cellular senescence. Biol. Chem. 2008;389:243–255. doi: 10.1515/BC.2008.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brennan CM, Steitz JA. HuR and mRNA stability. Cell. Mol. Life Sci. 2001;58:266–277. doi: 10.1007/PL00000854. (2001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abdelmohsen K, Gorospe M. Posttranscriptional regulation of cancer traits by HuR. Wiley Interdiscip Rev. RNA. 2010;1:214–229. doi: 10.1002/wrna.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kullmann M, Gopfert U, Siewe B, Hengst L. ELAV/Hu proteins inhibit p27 translation via an IRES element in the p27 5’UTR. Genes Dev. 2002;16:3087–3099. doi: 10.1101/gad.248902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meng Z, Jackson NL, Choi H, King PH, Emanuel PD, Blume SW. Alterations in RNA-binding activities of IRES-regulatory proteins as a mechanism for physiological variability and pathological dysregulation of IGF-IR translational control in human breast tumor cells. J. Cell Physiol. 2008;217:172–183. doi: 10.1002/jcp.21486. [DOI] [PubMed] [Google Scholar]
- 23.Yeh CH, Hung LY, Hsu C, Le SY, Lee PT, Liao WL, Lin YT, Chang WC, Tseng JT. RNA-binding protein HuR interacts with thrombomodulin 5'untranslated region and represses internal ribosome entry site-mediated translation under IL-1 beta treatment. Mol. Biol. Cell. 2008;19:3812–3822. doi: 10.1091/mbc.E07-09-0962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Durie D, Lewis SM, Liwak U, Kisilewicz M, Gorospe M, Holcik M. RNA-binding protein HuR mediates cytoprotection through stimulation of XIAP translation. Oncogene. 2010;30:1460–1469. doi: 10.1038/onc.2010.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bhattacharyya SN, Habermacher R, Martine U, Closs EI, Filipowicz W. Relief of microRNA-mediated translational repression in human cells subjected to stress. Cell. 2006;125:1111–1124. doi: 10.1016/j.cell.2006.04.031. [DOI] [PubMed] [Google Scholar]
- 26.Kim HH, Kuwano Y, Srikantan S, Lee EK, Martindale JL, Gorospe M. HuR recruits let-7/RISC to repress c-Myc expression. Genes Dev. 2009;23:1743–1748. doi: 10.1101/gad.1812509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eberhardt W, Doller A, Pfeilschifter J. Regulation of the mRNA-binding protein HuR by posttranslational modification: spotlight on phosphorylation. This volume. [DOI] [PubMed]
- 28.Gallouzi IE, Steitz JA. Delineation of mRNA export pathways by the use of cell-permeable peptides. Science. 2001;294:1895–1901. doi: 10.1126/science.1064693. [DOI] [PubMed] [Google Scholar]
- 29.Fan XC, Steitz JA. HNS, a nuclear-cytoplasmic shuttling sequence in HuR. Proc. Natl. Acad. Sci. USA. 1998;95:15293–15298. doi: 10.1073/pnas.95.26.15293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Güttinger S, Mühlhäusser P, Koller-Eichhorn R, Brennecke J, Kutay U. Transportin2 functions as importin and mediates nuclear import of HuR. Proc. Natl. Acad. Sci. USA. 2004;101:2918–2923. doi: 10.1073/pnas.0400342101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rebane A, Aab A, Steitz JA. Transportins 1 and 2 are redundant nuclear import factors for hnRNP A1 and HuR. RNA. 2004;10:590–599. doi: 10.1261/rna.5224304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Doller A, Akool el-S., Huwiler A, Müller R, Radeke HH, Pfeilschifter J, Eberhardt W. Posttranslational modification of the AU-rich element binding protein HuR by protein kinase Cδ elicits angiotensin II-induced stabilization and nuclear export of cyclooxygenase 2 mRNA. Mol. Cell. Biol. 2008;28:2608–2625. doi: 10.1128/MCB.01530-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Doller A, Huwiler A, Müller R, Radeke HH, Pfeilschifter J, Eberhardt W. Protein kinase C alpha-dependent phosphorylation of the mRNA-stabilizing factor HuR: implications for posttranscriptional regulation of cyclooxygenase-2. Mol. Biol. Cell. 2007;18:2137–2148. doi: 10.1091/mbc.E06-09-0850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang W, Fan J, Yang X, Fürer-Galban S, López de Silanes I, von Kobbe C, Guo J, Georas SN, Foufelle F, Hardie DG, Carling D, Gorospe M. AMP-activated kinase regulates cytoplasmic HuR. Mol. Cell. Biol. 2002;22:3425–3436. doi: 10.1128/MCB.22.10.3425-3436.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim HH, Yang X, Kuwano Y, Gorospe M. Modification at HuR(S242) alters HuR localization and proliferative influence. Cell Cycle. 2008;7:3371–3377. doi: 10.4161/cc.7.21.6895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim HH, Gorospe M. Phosphorylated HuR shuttles in cycles. Cell Cycle. 2008;7:3124–3126. doi: 10.4161/cc.7.20.6884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kang MJ, Ryu BK, Lee MG, Han J, Lee JH, Ha TK, Byun DS, Chae KS, Lee BH, Chun HS, Lee KY, Kim HJ, Chi SG. NF-κB activates transcription of the RNA-binding factor HuR, via PI3K-AKT signaling, to promote gastric tumorigenesis. Gastroenterology. 2008;135:2030–2042. doi: 10.1053/j.gastro.2008.08.009. [DOI] [PubMed] [Google Scholar]
- 38.Jeyaraj SC, Singh M, Ayupova DA, Govindaraju S, Lee BS. Transcriptional control of human antigen R by bone morphogenetic protein. J. Biol. Chem. 2010;285:4432–4440. doi: 10.1074/jbc.M109.062216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yi J, Chang N, Liu X, Guo G, Xue L, Tong T, Gorospe M, Wang W. Reduced nuclear export of HuR mRNA by HuR is linked to the loss of HuR in replicative senescence. Nucleic Acids Res. 2010;38:1547–1558. doi: 10.1093/nar/gkp1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Abdelmohsen K, Srikantan S, Yang X, Lal A, Kim HH, Kuwano Y, Galban S, Becker KG, Kamara D, de Cabo R, Gorospe M. Ubiquitin-mediated proteolysis of HuR by heat shock. EMBO J. 2009;28:1271–1282. doi: 10.1038/emboj.2009.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mazroui R, Di Marco S, Clair E, von Roretz C, Tenenbaum SA, Keene JD, Saleh M, Gallouzi IE. Caspase-mediated cleavage of HuR in the cytoplasm contributes to pp32/PHAP-I regulation of apoptosis. J. Cell Biol. 2008;180:113–127. doi: 10.1083/jcb.200709030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Masuda K, Abdelmohsen K, Kim MM, Srikantan S, Lee EK, Tominaga K, Selimyan R, Martindale JL, Yang X, Lehrmann E, Zhang Y, Becker KG, Wang JY, Kim HH, Gorospe M. Global dissociation of HuR-mRNA complexes promotes cell survival after ionizing radiation. EMBO J. 2011;30:1040–1053. doi: 10.1038/emboj.2011.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Abdelmohsen K, Pullmann R, Jr., Lal A, Kim HH, Galban S, Yang X, Blethrow JD, Walker M, Shubert J, Gillespie DA, Furneaux H, Gorospe M. Phosphorylation of HuR by Chk2 regulates SIRT1 expression. Mol. Cell. 2007;25:543–557. doi: 10.1016/j.molcel.2007.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Doller A, Schlepckow K, Schwalbe H, Pfeilschifter J, Eberhardt W. Tandem phosphorylation of serines 221 and 318 by protein kinase Cδ coordinates mRNA binding and nucleocytoplasmic shuttling of HuR. Mol. Cell. Biol. 2010;30:1397–1410. doi: 10.1128/MCB.01373-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Doller A, Pfeilschifter J, Eberhardt W. Signalling pathways regulating nucleo-cytoplasmic shuttling of the mRNA-binding protein HuR. Cell Signal. 2008;20:2165–2173. doi: 10.1016/j.cellsig.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 46.Lafarga V, Cuadrado A, López de Silanes I, Bengoechea R, Fernandez-Capetillo O, Nebreda AR. p38 Mitogen-activated protein kinase- and HuR-dependent stabilization of p21(Cip1) mRNA mediates the G(1)/S checkpoint. Mol. Cell. Biol. 2009;29:4341–4351. doi: 10.1128/MCB.00210-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li H, Park S, Kilburn B, Jelinek MA, Henschen-Edman A, Aswad DW, Stallcup MR, Laird-Offringa IA. Lipopolysaccharide-induced methylation of HuR, an mRNA-stabilizing protein, by CARM1. Coactivator-associated arginine methyltransferase. J. Biol. Chem. 2002;277:44623–44630. doi: 10.1074/jbc.M206187200. [DOI] [PubMed] [Google Scholar]
- 48.Kedde M, Agami R. Interplay between microRNAs and RNA-binding proteins determines developmental processes. Cell Cycle. 2008;7:899–903. doi: 10.4161/cc.7.7.5644. [DOI] [PubMed] [Google Scholar]
- 49.Höck J, Weinmann L, Ender C, Rüdel S, Kremmer E, Raabe M, Urlaub H, Meister G. Proteomic and functional analysis of Argonaute-containing mRNA–protein complexes in human cells. EMBO Rep. 2007;8:1052–1060. doi: 10.1038/sj.embor.7401088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Grimson A, Farh KK, Johnston WK, Garrett-Engele P, Lim LP, Bartel DP. MicroRNA targeting specificity in mammals: determinants beyond seed pairing. Mol. Cell. 2007;27:91–105. doi: 10.1016/j.molcel.2007.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Meisner NC, Filipowicz W. Properties of the Regulatory RNA-Binding Protein HuR and its Role in Controlling miRNA Repression. Adv. Exp. Med. Biol. 2011;700:106–23. doi: 10.1007/978-1-4419-7823-3_10. [DOI] [PubMed] [Google Scholar]
- 52.Srikantan S, Abdelmohsen K, Lee EK, Tominaga K, Subaran SS, Kuwano Y, Kulshrestha R, Panchakshari R, Kim HH, Yang X, Martindale JL, Marasa B, Kim MM, Wersto RP, Indig FE, Chowdhury D, Gorospe M. Translational control of Top2A influences doxorubicin efficacy. Mol. Cell. Biol. 2011;31:3790–801. doi: 10.1128/MCB.05639-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tominaga K, Srikantan S, Lee EK, Subaran SS, Martindale JL, Abdelmohsen K, Gorospe M. Competitive regulation of Nucleolin expression by HuR and miR-494. Mol. Cell. Biol. 2011;31:4219–4231. doi: 10.1128/MCB.05955-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang W, Caldwell MC, Lin S, Furneaux H, Gorospe M. HuR regulates cyclin A and cyclin B1 mRNA stability during cell proliferation. EMBO J. 2000;19:2340–2350. doi: 10.1093/emboj/19.10.2340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Young LE, Moore AE, Sokol L, Meisner-Kober N, Dixon DA. The mRNA Stability Factor HuR Inhibits MicroRNA-16 Targeting of Cyclooxygenase-2. Mol. Cancer Res. 2011 doi: 10.1158/1541-7786.MCR-11-0337. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Epis MR, Barker A, Giles KM, Beveridge DJ, Leedman PJ. The RNA-binding protein HuR opposes the repression of ERBB-2 expression by miR-331-3p in prostate cancer cells. J. Biol. Chem. 2011 doi: 10.1074/jbc.M111.301481. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nielsen CB, Shomron N, Sandberg R, Hornstein E, Kitzman J, Burge CB. Determinants of targeting by endogenous and exogenous microRNAs and siRNAs. RNA. 2007;13:1894–1910. doi: 10.1261/rna.768207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Glorian V, Maillot G, Polès S, Iacovoni JS, Favre G, Vagner S. HuR-dependent loading of miRNA RISC to the mRNA encoding the Ras-related small GTPase RhoB controls its translation during UV-induced apoptosis. Cell Death Differ. 2011;18:1692–1701. doi: 10.1038/cdd.2011.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chang N, Yi J, Guo G, Liu X, Shang Y, Tong T, Cui Q, Zhan M, Gorospe M, Wang W. HuR uses AUF1 as a cofactor to promote p16INK4 mRNA decay. Mol. Cell Biol. 2010;30:3875–3886. doi: 10.1128/MCB.00169-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Merino EJ, Wilkinson KA, Coughlan JL, Weeks KM. RNA structure analysis at single nucleotide resolution by selective 2'-hydroxyl acylation and primer extension (SHAPE). J. Am. Chem. Soc. 2005;127:4223–4231. doi: 10.1021/ja043822v. [DOI] [PubMed] [Google Scholar]
- 61.Abdelmohsen K, Srikantan S, Kuwano Y, Gorospe M. miR-519 reduces cell proliferation by lowering RNA-binding protein HuR levels. Proc. Natl. Acad. Sci. USA. 2008;105:20297–20302. doi: 10.1073/pnas.0809376106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Marasa BS, Srikantan S, Martindale JL, Kim MM, Lee EK, Gorospe M, Abdelmohsen K. MicroRNA profiling in human diploid fibroblasts uncovers miR-519 role in replicative senescence. Aging. 2010;2:333–343. doi: 10.18632/aging.100159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Abdelmohsen K, Kim MM, Srikantan S, Mercken EM, Brennan SE, Wilson GM, de Cabo R, Gorospe M. miR-519 suppresses tumor growth by reducing HuR levels. Cell Cycle. 2010;9:1354–1359. doi: 10.4161/cc.9.7.11164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Guo X, Wu Y, Hartley RS. MicroRNA-125a represses cell growth by targeting HuR in breast cancer. RNA Biol. 2009;6:575–583. doi: 10.4161/rna.6.5.10079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Xu F, Zhang X, Lei Y, Liu X, Liu Z, Tong T, Wang W. Loss of repression of HuR translation by miR-16 may be responsible for the elevation of HuR in human breast carcinoma. J. Cell. Biochem. 2010;111:727–734. doi: 10.1002/jcb.22762. [DOI] [PubMed] [Google Scholar]
- 66.Jing Q, Huang S, Guth S, Zarubin T, Motoyama A, Chen J, Di Padova F, Lin SC, Gram H, Han J. Involvement of microRNA in AU-rich elementmediated mRNA instability. Cell. 2005;120:623–634. doi: 10.1016/j.cell.2004.12.038. [DOI] [PubMed] [Google Scholar]
- 67.Cimmino A, Calin GA, Fabbri M, Iorio MV, Ferracin M, Shimizu M, Wojcik SE, Aqeilan RI, Zupo S, Dono M, Rassenti L, Alder H, Volinia S, Liu CG, Kipps TJ, Negrini M, Croce CM. miR-15 and miR-16 induce apoptosis by targeting BCL2. Proc. Natl. Acad. Sci. USA. 2006;102:13944–13949. doi: 10.1073/pnas.0506654102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chang TC, Wentzel EA, Kent OA, Ramachandran K, Mullendore M, Lee KH, Feldmann G, Yamakuchi M, Ferlito M, Lowenstein CJ, Arking DE, Beer MA, Maitra A, Mendell JT. Transactivation of miR-34a by p53 broadly influences gene expression and promotes apoptosis. Mol. Cell. 2007;26:745–752. doi: 10.1016/j.molcel.2007.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Merkel O, Asslaber D, Piñón JD, Egle A, Greil R. Interdependent regulation of p53 and miR-34a in chronic lymphocytic leukemia. Cell Cycle. 2010;9:2764–2768. [PubMed] [Google Scholar]
- 70.Tarasov V, Jung P, Verdoodt B, Lodygin D, Epanchintsev A, Menssen A, Meister G, Hermeking H. Differential regulation of microRNAs by p53 revealed by massively parallel sequencing: miR-34a is a p53 target that induces apoptosis and G1-arrest. Cell Cycle. 2007;6:1586–1593. doi: 10.4161/cc.6.13.4436. [DOI] [PubMed] [Google Scholar]
- 71.He L, He X, Lim LP, de Stanchina E, Xuan Z, Liang Y, Xue W, Zender L, Magnus J, Ridzon D, Jackson AL, Linsley PS, Chen C, Lowe SW, Cleary MA, Hannon GJ. A microRNA component of the p53 tumour suppressor network. Nature. 2007;447:1130–1134. doi: 10.1038/nature05939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Welch C, Chen Y, Stallings RL. MicroRNA-34a functions as a potential tumor suppressor by inducing apoptosis in neuroblastoma cells. Oncogene. 2007;26:5017–5022. doi: 10.1038/sj.onc.1210293. [DOI] [PubMed] [Google Scholar]
- 73.Kaller M, Liffers ST, Oeljeklaus S, Kuhlmann K, Röh S, Hoffmann R, Warscheid B, Hermeking H. Genome-wide characterization of miR-34a induced changes in protein and mRNA expression by a combined pulsed SILAC and microarray analysis. Mol. Cell. Proteomics. 2011;10:M111.010462. doi: 10.1074/mcp.M111.010462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kojima K, Fujita Y, Nozawa Y, Deguchi T, Ito M. MiR-34a attenuates paclitaxel-resistance of hormone-refractory prostate cancer PC3 cells through direct and indirect mechanisms. Prostate. 2010;70:1501–1512. doi: 10.1002/pros.21185. [DOI] [PubMed] [Google Scholar]
- 75.van Kouwenhove M, Kedde M, Agami R. MicroRNA regulation by RNA-binding proteins and its implications for cancer. Nat. Rev. Cancer. 2011;11:644–656. doi: 10.1038/nrc3107. [DOI] [PubMed] [Google Scholar]