Abstract
Background
Interferon-γ (IFN-γ) plays a crucial role in Mycobacterium tuberculosis induced pleural responses. Interleukin (IL)-33 up-regulates the production of IFN-γ. We aimed to identify whether an association between pleural IL-33 levels and tuberculous pleurisy exists and determine its diagnostic value.
Methods
Pleural IL-33, ST2 (a receptor of IL-33), adenosine deaminase (ADA), and IFN-γ, as well as serum IL-33 and ST2 were measured in 220 patients with pleural effusions (PEs). Patients with malignant (MPEs), parapneumonic (PPEs), tuberculous (TPEs), and cardiogenic (CPEs) pleural effusions were included.
Results
Pleural and serum IL-33 levels were highest or tended to be higher in patients with TPEs than in those with other types of PEs. The median pleural fluid-to-serum IL-33 ratio was higher in TPE cases (≥ 0.91) than in other PE cases (≤ 0.56). Pleural IL-33 levels correlated with those of pleural ADA and IFN-γ. However, the diagnostic accuracies of pleural IL-33 (0.74) and pleural fluid-to-serum IL-33 ratio (0.75) were lower than that of ADA (0.95) or IFN-γ (0.97). Pleural ST2 levels in patients with MPEs were higher than in patients with TPEs. Serum ST2 levels did not differ among the groups.
Conclusions
We identified an association between elevated pleural IL-33 levels and tuberculous pleurisy. However, we recommend conventional pleural markers (ADA or IFN-γ) as diagnostic markers of TPE.
Keywords: Interleukin-33, ST2, Tuberculosis, Pleural effusion
INTRODUCTION
Tuberculosis (TB) is the second most important cause of death from infectious disease in the world [1]. Tuberculous pleurisy is one of the most common forms of extrapulmonary TB and may develop as a complication of pulmonary tuberculosis [2, 3]. Interferon-γ (IFN-γ), a Th1 cytokine produced predominantly by natural killer (NK) cells, natural killer T (NKT) cells, and Th1 cells [4-7], plays a pivotal role in pleural granuloma formation and protection against Mycobacterium tuberculosis (M. tuberculosis) [4-11].
Interleukin (IL)-33, an IL-1 family cytokine, is expressed in lung tissue. IL-33 displays pro-Th2 functions and is involved in allergic inflammation and asthma [12-15]. Recent studies found that IL-33 expression is induced by IFN-γ or tumor necrosis factor (TNF) [15-17], which are locally increased in the pleural space of patients with tuberculous pleural effusion (TPE) [18-20]. Conversely, additional studies demonstrated enhanced IFN-γ or TNF production by IL-33 and that the pro-Th1 functions of IL-33 are dependent upon the presence of IL-12 [13, 21-23]. IL-12 is a key player in host defense against M. tuberculosis and a crucial inducer of IFN-γ production [11, 24], which is remarkably elevated in TPE [25]. ST2, a soluble IL-33 receptor, is expressed on Th2 cells and exerts Th2 effector functions upon IL-33 stimulation [12]. In addition, elevated levels of soluble ST2 are found in malignant pleural effusions (MPEs), which have a depressed Th1-mediated immune response [26, 27].
To date, there have been no studies on pleural IL-33 levels in human disease. We aimed to identify: 1) pleural IL-33 levels, their association with circulatory levels, and pleural IL-33 soluble receptor levels in patients with TPE; 2) the associations between pleural IL-33 and the conventional TPE markers, IFN-γ and adenosine deaminase (ADA) [8, 19, 28-30]; and 3) the diagnostic usefulness of IL-33 and its soluble receptor levels for the detection of tuberculous pleurisy.
METHODS
1. Study design and population
This study was a cross-sectional, case-control study. The study protocol was approved by the Institutional Review Board for Human Studies at the Clinical Research Center of Wonkwang University Hospital. All recruited subjects provided written informed consent.
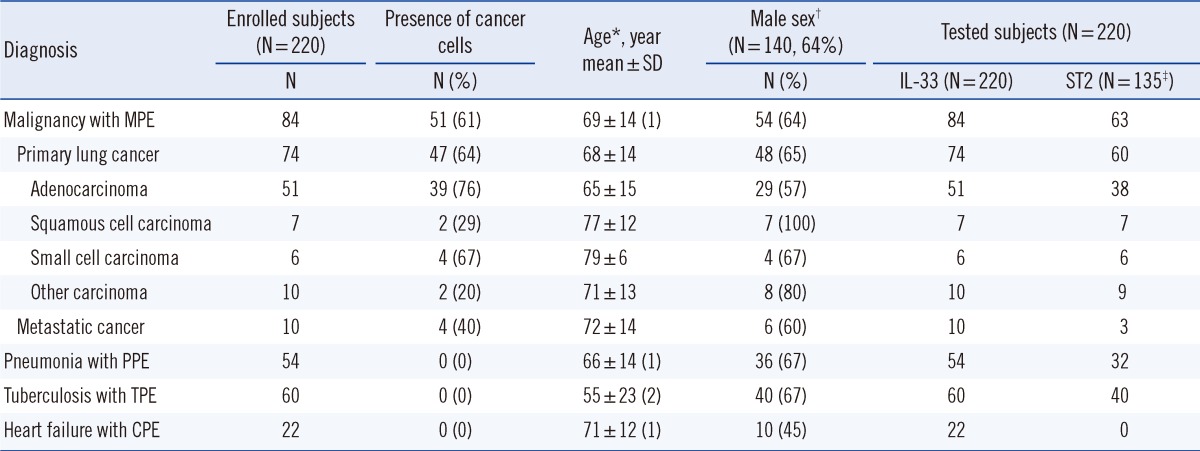
A total of 537 consecutive patients with pleural effusions (PEs) were prospectively recruited upon admission to the Departments of Emergency, Internal Medicine, and Thoracic Surgery at Wonkwang University Hospital between January 2008 and November 2011. Of these, 317 subjects were excluded because the cause of PE did not definitely meet our diagnostic criteria or paired samples of pleural fluid and serum were not obtainable. Finally, 220 patients were enrolled into the study and all patients (N=220) had sufficient sample-volumes for duplicate pleural and serum IL-33 testing (Table 1). Pleural and serum ST2 were analyzed in 135 cases. We did not measure pleural and serum ST2 levels in 85 cases because sample volumes from these cases were insufficient. Pleural levels of ADA, IFN-γ, lactate dehydrogenase (LDH), total protein, and total lymphocytes were measured in all patients (Table 2).
Table 1.
Characteristics of study subjects
*Mean ages of the groups increased significantly (P<0.05) according to the decreasing values in parentheses, and equal numbers in parentheses indicate that there was no significant difference between the respective groups; †Sex ratios were not significantly (P>0.05) different among the 4 groups; ‡Pleural and serum ST2 were analyzed in 135 cases. Of the 220 patients, 85 cases (heart failure 22, malignancy 21, pneumonia 22, tuberculosis 20) were unable to be tested for pleural and serum ST2 because of insufficient sample volume.
Abbreviations: MPE, malignant pleural effusion; PPE, parapneumonic pleural effusion; TPE, tuberculous pleural effusion; CPE, cardiogenic pleural effusion.
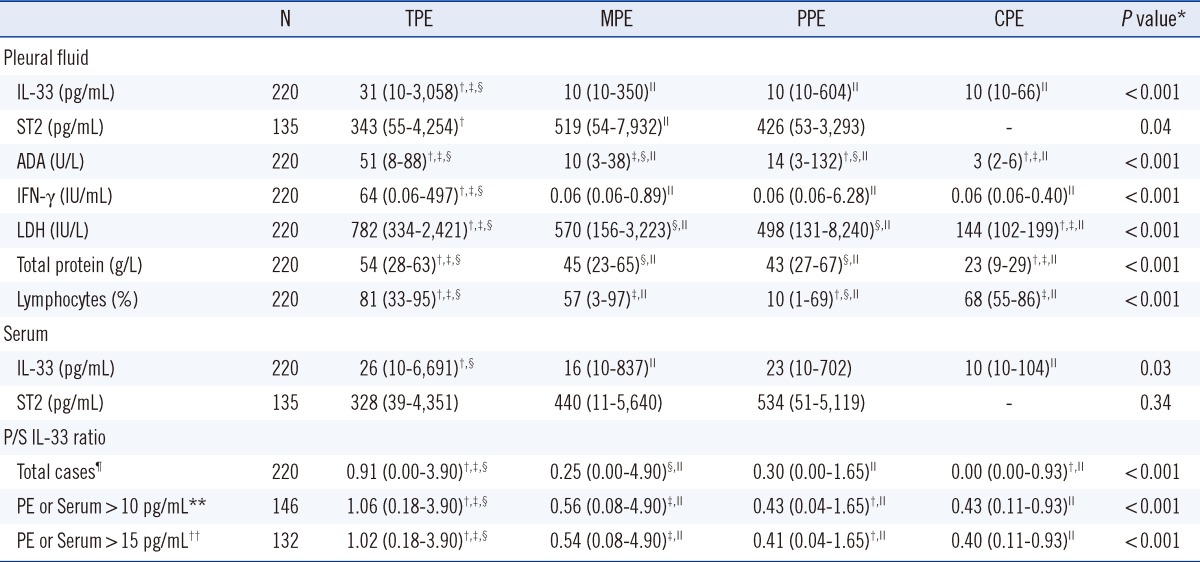
Table 2.
Pleural fluid and serum characteristics of the 220 patients with pleural effusions
Values are expressed as medians (full range).
*By Kruskal-Wallis test; †P<0.05, vs. MPE; ‡P<0.05 vs. PPE; §P<0.05 vs. CPE; ∥P<0.05 vs. TPE; ¶A value of zero was assigned to pleural fluid-to-serum (P/S) ratios of cases with both pleural and serum IL-33 levels≤10 pg/mL (detection limit); **The cases have higher IL-33 levels in either pleural fluid or serum or in both specimens than the detection limit; ††The cases have higher IL-33 levels in either pleural fluid or serum or in both specimens than 1.5 times the detection limit.
Abbreviations: TPE, tuberculous pleural effusion; MPE, malignant pleural effusion; PPE, parapneumonic pleural effusion; CPE, cardiogenic pleural effusion; ADA, adenosine deaminase; IFN-γ, interferon gamma; LDH, lactate dehydrogenase.
2. Sample collection and quantification
Blood and/or pleural fluid samples were collected within 24 hr of admission or on a symptomatic day before treatment. Blood and/or pleural fluid were collected in plain tubes and centrifuged (1,200×g for 10 min) within 60 min of collection. The serum and pleural sample supernatants were stored at -75℃ until IL-33, ST2, and IFN-γ analyses. Stability of IL-33 with repeated freezing-thawing was ensured for up to 10 freeze-thaw cycles. The change in IL-33 levels after 10 freeze-thaw cycles was less than 10% of the estimated pre-freeze concentration (data not shown). IL-33 and ST2 levels were measured using commercial ELISA kits (R&D Systems, Minneapolis, MN, USA). IFN-γ levels were also measured with a commercially available ELISA kit (Cellestis, Carnegie, Australia). Measurements of IL-33, ST2, and IFN-γ were performed in duplicate and the results were averaged. The limits of detection for IL-33, ST2, and IFN-γ were 10 pg/mL, 6 pg/mL, and 0.06 IU/mL, respectively. Values of 10 (IL-33), 6 (ST2) or 0.06 (IFN-γ) were assigned to results that were below a particular assay's limit of detection. The maximum linear responses of IL-33, ST2, and IFN-γ were 1,500 pg/mL, 2,000 pg/mL, and 40 IU/mL, respectively. Samples with values greater than the maximum linear response were diluted 10-fold and reanalyzed. If the 10-fold diluted sample levels were higher than the linear range, 100-fold dilutions were performed. The IL-33 test indicated no significant cross-reactivity with or interference by 50 ng/mL of the following cytokines (ST2, IL-1α, IL-1β, IL-2, IL-6, IL-12, IL-18, IFN-γ, and TNF-α). None of the 3 ELISA kits were validated by the manufacturer for the accuracy of pleural fluid analyses. In the current study, we analyzed pleural fluids using each ELISA kit as an experimental pilot research. Dilution assessment of each sample by using 4 different concentrations of each patient pleural fluid sample indicated that signal level depended on sample dilution levels (90-110% of expected values) for each cytokine. Spike and recovery assessment of pleural fluid sample demonstrated acceptable performances (90-110% of recovery rates) for each cytokine. Levels of ADA (Diazyme Laboratories, San Diego, CA, USA), LDH, and total protein were analyzed by routine clinical laboratory test protocols using an automated chemical analyzer (Modular P800; Roche Diagnostics GmbH, Mannheim, Germany).
3. Diagnostic criteria for PEs
Determination of the etiology of the PEs was provided by 2 pulmonologists. The pulmonologists independently reviewed clinical presentations, relevant diagnostic test results, and each patient's response to treatment. PEs were differentiated by the following criteria: 1) MPE was diagnosed if cancer cells were present in pleural fluid or pleural biopsies, or in patients with disseminated malignancy without any alternative explanation for an exudative PE; 2) Parapneumonic PE (PPE) was diagnosed based on the presence of pulmonary infections associated with acute febrile illness, pulmonary infiltrates, purulent sputum, and response to antibiotic treatment or identification of the organism in the pleural fluid; 3) TPE was diagnosed on the basis of the presence of positive staining or culture for M. tuberculosis in the pleural fluid, sputum or pleural biopsy specimen or typical caseating granulomas on pleural biopsy; and 4) Cardiogenic PE (CPE) was diagnosed on the basis of the presence of heart failure as a cause of transudative PE with the exclusion of any other cause of PE. All exudates were defined as described by Light et al [31].
4. Statistical analyses
Gender ratios and proportions were compared using chi-square tests. Mean ages were compared using a one-way ANOVA followed by Tukey's Honestly Significant Difference (HSD) post-hoc tests. The median levels of sera and pleural fluid markers were compared using nonparametric tests, because the concentrations were not normally distributed. Differences between more than 2 groups were determined using Kruskal-Wallis tests, with subsequent Conover post-hoc tests if the differences were significant. Spearman correlations were used to determine the relationships between the levels of pleural IL-33 and levels of pleural ADA or IFN-γ.
The diagnostic accuracies of pleural markers for discriminating the etiology of PEs were determined through analyses of ROC curves. Statistical differences were analyzed using the DeLong method. Data were analyzed using MedCalc version 11.5 (Med-Calc Software, Mariakerke, Belgium) and SPSS version 15 (SPSS Inc., Chicago, IL, USA). P values<0.05 were considered statistically significant.
RESULTS
1. Demographic characteristics of the study subjects and basic characteristics of pleural fluid samples
The demographic characteristics of the study subjects are presented in Table 1. The mean ages of subjects in the malignancy, pneumonia, and heart failure groups were higher than that of the tuberculosis group. The groups did not significantly differ with regard to subject gender. The basic characteristics of the pleural fluid samples are recorded in Table 2.
2. Levels of IL-33 in PEs and serum
The median level of pleural IL-33 was higher in patients with TPEs (31 pg/mL) than in patients with other types of PEs (MPE 10 pg/mL, P<0.001; PPE 10 pg/mL, P<0.001; and CPE 10 pg/mL, P<0.001) (Table 2). The median levels of pleural IL-33 were not significantly different among the patients with MPEs, PPEs, and CPEs. The median level of serum IL-33 was significantly higher in patients with TPEs (26 pg/mL) than in patients with MPEs (16 pg/mL, P=0.03) or CPEs (10 pg/mL, P=0.01), and tended to be greater in patients with TPEs compared to levels in patients with PPEs (23 pg/mL, P=0.42) (Table 2).
3. Levels of ST2 in PEs and serum
The median level of pleural ST2 was significantly higher in patients with MPEs (519 pg/mL) than in patients with TPEs (343 pg/mL, P=0.01), and tended to be higher in patients with MPEs than in patients with PPEs (426 pg/mL, P=0.30) (Table 2). There were no significant differences in median levels of serum ST2 among the MPE, PPE, and TPE groups (P=0.34) (Table 2).
4. Relationship between pleural/serum (P/S) IL-33 ratio and etiologies of PE
The relationship between pleural/serum (P/S) IL-33 ratio and etiologies of PE are shown in Table 2. The median P/S IL-33 ratio was significantly higher in patients with TPEs (≥0.91) than in patients with MPEs, PPEs, or CPEs (≤0.56, P<0.001). This result held true for all cases and in cases with IL-33 pleural fluid or serum levels above the detection limit (10 pg/mL) or 1.5 times the detection limit (15 pg/mL). The median P/S ratios were not different or were only marginally different among the patients with MPEs, PPEs, and CPEs.
5. Correlations between levels of pleural IL-33 and pleural ADA or IFN-γ levels
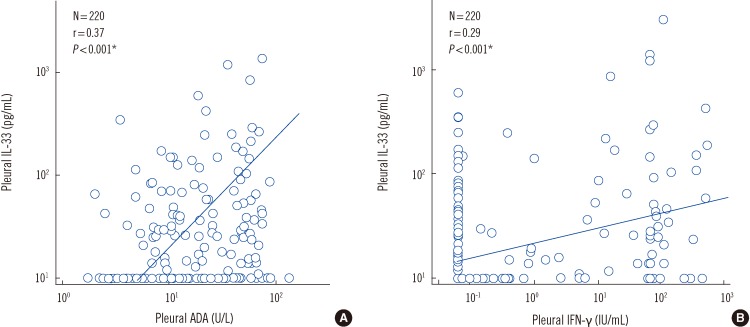
Correlations between levels of pleural IL-33 and pleural ADA or IFN-γ levels in patients with PEs were analyzed using Spearman rank correlations (Fig. 1). There were statistically significant positive correlations between IL-33 levels and ADA levels (r=0.37, P<0.001) and between IL-33 levels and IFN-γ levels (r=0.29, P<0.001) in the pleural fluid samples of the PE patients.
Fig. 1.
Correlations between pleural IL-33 and pleural ADA or IFN-γ levels. *P<0.001 by Spearman's rank correlation analysis.
Abbreviations: ADA, adenosine deaminase; IFN-γ, interferon gamma.
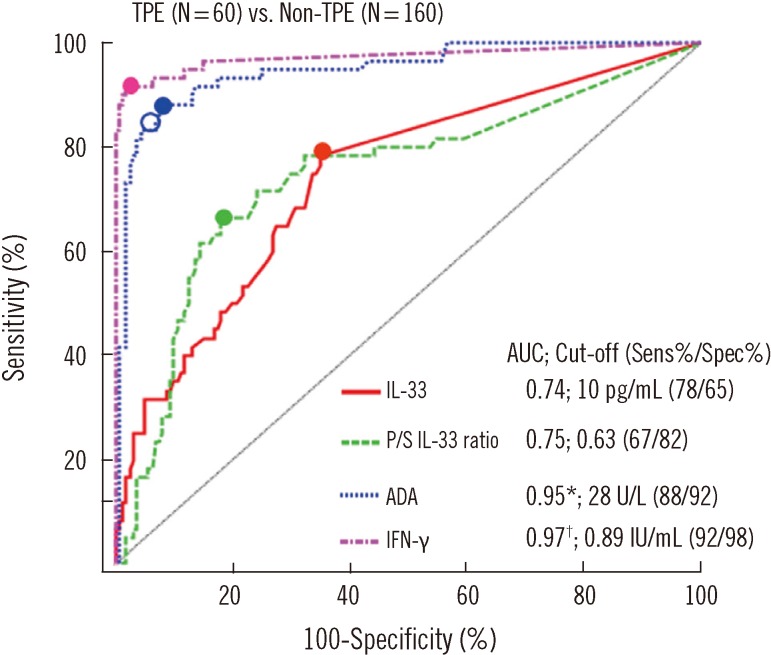
6. Diagnostic values of IL-33 and ST2 in PEs
In ROC curve analysis (Fig. 2), the diagnostic accuracies of pleural IL-33 (0.74) and P/S IL-33 ratio (0.75) for discrimination between patients with TPEs and patients with non-TPEs were fairly good. However, they were lower than that of pleural ADA (0.95, P<0.001) or IFN-γ (0.97, P<0.001). The diagnostic sensitivity and specificity of pleural IL-33 were lower than those of pleural ADA or IFN-γ. The area under the ROC curve (AUC) of pleural ST2 (0.61).
Fig. 2.
Comparison of diagnostic accuracies of pleural IL-33, pleural fluid-to-serum (P/S) IL-33 ratio, and other markers in pleural effusions for discriminating between TPEs and non-TPEs using ROC curves. Small closed circles indicate cut-offs that were determined by the highest Youden index. A small open circle on the ADA curve (blue dotted line) indicates the cut-off value (30 IU/mL) that presented in studies [30] that used the same manufacturer's reagent.
*P<0.05, IL-33 vs. ADA; †P<0.05, IL-33 vs. IFN-γ.
Abbreviations: TPE, tuberculous pleural effusion; AUC, area under the ROC curve; Sens, sensitivity; Spec, specificity; ADA, adenosine deaminase; IFN-γ, interferon gamma.
DISCUSSION
IL-33 promotes Th2 cytokine production and represses induction of IFN-γ in Th1-polarized cells [12]. On the other hand, it directly interacts with invariant NKT (iNKT) cells to induce IFN-γ production [13]. The accumulated evidence and conflicting hypotheses for IL-33 involvement in inflammatory responses and pathogenesis [12-15, 32, 33] raise questions regarding the IL-33 response in pleural disease. These questions are highlighted by the fact that TPEs have a more polarized Th1 reaction than MPEs [26, 34].
We found significantly higher levels of pleural IL-33 in TPEs than in all other types of PEs. Moreover, P/S IL-33 ratios were higher in patients with TPEs than in patients with other types of PEs. These results agree with evidence that the tuberculous pleurisy-involved cytokines IFN-γ and TNF are higher in the pleural fluid of patients with TPEs than in those with other types of PEs [18-20]. These data also agree with the fact that the concentration ratio (pleural fluid to circulatory blood) of tuberculous pleurisy-involved cytokine is higher in patients with TPE [20]. Further, our results demonstrated significant correlations between pleural IL-33 levels and the pleural levels of conventional TPE markers, IFN-γ and ADA [18, 19, 28-30].
Taken together, these findings indicate that IL-33 is more specifically associated with the pathophysiology of TPE than with other types of PE. Our data are consistent with the observations that IL-33 amplifies Th1 functions and induces a preferential increase in IFN-γ rather than Th2 cytokines production in vivo [13, 23]. Although we did not define the mechanism of IL-33 contribution to TPE pathogenesis, the significant relationship between IL-33 and tuberculous pleurisy observed in this study could be explained by the following hypotheses: First, IL-33 may render relatively specific immunomodulatory effects, in regard to induced cytokines and responding cells caused by tuberculosis rather than by other etiologies. Indeed, recognition of tuberculosis-infected macrophages by iNKT cells is followed by IFN-γ production [5]. In addition, IFN-γ is not only an upstream regulator of IL-33 [15-17] but also a downstream product of IL-33 signaling [13, 21-23]. Consequently, IFN-γ and IL-33 may form a coupled positive feedback loop in tuberculous pleurisy [13, 15-17, 22, 23]. IL-33 is more strongly induced in activated macrophages, but not in resting macrophages [12], and pleural granulomatous lesions, an organized collection of "activated" macrophages, are far more frequently seen in tuberculous pleurisy than in other types of pleurisy. In addition, variant cells in lung parenchyma and pleural cells may induce IL-33 via IFN-γ and TNF. Second, the "alarmin" effect [35] may be induced to alert the immune system of a local threat by destructed or necrotic cells during tuberculosis infection.
There was a significant elevation of IL-33 levels in TPE in current study, and reported cellular experiments presented pathophysiologic association between IL-33 and IFN-γ [13, 15-17, 22, 23]. Nevertheless, our data indicate that pleural IL-33 and the P/S IL-33 ratio were not efficient diagnostic markers for detection of TPE, even though our results for pleural IFN-γ and ADA were similar to those of previous studies [28-30].
Pleural ST2 levels in MPEs were significantly higher than in TPEs. Thus, our results confirmed previous observations of ST2 in MPE [26]. However, the poor diagnostic accuracy of pleural ST2 abrogates its utility as a marker for differential diagnosis of PE.
Our study has several limitations. First, we presented limited data for a single point cross-sectional test, did not analyze serial IL-33 levels and did not measure Th2 and Th1 cytokines other than IFN-γ in PEs. Therefore, we could neither confirm that a decrease of IL-33 preceded recovery from tuberculous pleurisy, nor define the heterogeneous effects of IL-33 during disease progression. A shift from a pro-Th 1 response to a pro-Th 2 response depends on changes in pleural micro-environmental conditions. For instance, in the early stages of tuberculous pleurisy, naïve macrophages may become activated macrophages (M1) by IL-33, and levels of which may correlate with Th1 cytokine levels in TPE for the increased Th1 cell-attracting M1 macrophages. In the advanced stages of tuberculous pleurisy, the M1 phenotype macrophages may subsequently change to M2 phenotype cells [36]. Thereafter, IL-33 may no longer form a positive feedback system with Th1 cytokines. In the end stage of immune activation that is close to the recovery phase of tuberculous pleurisy, the decrease of Th1 cytokines may not be followed by a decrease in IL-33. During this stage, IL-33 may be positively correlated with Th2 cytokines instead of Th1 cytokines. Second, our study did not examine the cellular levels of IL-33 in PEs, which is required for a more precise identification of the immune response and the cellular source of IL-33 in TPEs.
As a pilot study for IL-33 in pleural disease, we focused on pleural IL-33 levels in PEs and observed higher pleural IL-33 levels and P/S IL-33 ratios in tuberculous pleurisy and correlations between pleural IL-33 and conventional TPE markers. The results of our study reflect the involvement of IL-33 in the pathogenesis of tuberculous pleurisy. However, we strongly recommend the use of pleural ADA or IFN-γ, rather than pleural IL-33, for detection of TPE, because of the proven diagnostic power of these conventional markers.
Acknowledgement
This research was supported by Wonkwang University in 2011.
Footnotes
No potential conflicts of interest relevant to this article were reported.
References
- 1.Caramori G, Lasagna L, Casalini AG, Adcock IM, Casolari P, Contoli M, et al. Immune response to Mycobacterium tuberculosis infection in the parietal pleura of patients with tuberculous pleurisy. PLoS One. 2011;6:e22637. doi: 10.1371/journal.pone.0022637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baumann MH, Nolan R, Petrini M, Lee YC, Light RW, Schneider E. Pleural tuberculosis in the United States: incidence and drug resistance. Chest. 2007;131:1125–1132. doi: 10.1378/chest.06-2352. [DOI] [PubMed] [Google Scholar]
- 3.Sahn SA. State of the art. The pleura. Am Rev Respir Dis. 1988;138:184–234. doi: 10.1164/ajrccm/138.1.184. [DOI] [PubMed] [Google Scholar]
- 4.Sneller MC. Granuloma formation, implications for the pathogenesis of vasculitis. Cleve Clin J Med. 2002;69(Suppl 2):SII40–SII43. doi: 10.3949/ccjm.69.suppl_2.sii40. [DOI] [PubMed] [Google Scholar]
- 5.Sada-Ovalle I, Chiba A, Gonzales A, Brenner MB, Behar SM. Innate invariant NKT cells recognize Mycobacterium tuberculosis-infected macrophages, produce interferon-gamma, and kill intracellular bacteria. PLoS Pathog. 2008;4:e1000239. doi: 10.1371/journal.ppat.1000239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schierloh P, Yokobori N, Aleman M, Landoni V, Geffner L, Musella RM, et al. Mycobacterium tuberculosis-induced gamma interferon production by natural killer cells requires cross talk with antigen-presenting cells involving Toll-like receptors 2 and 4 and the mannose receptor in tuberculous pleurisy. Infect Immun. 2007;75:5325–5337. doi: 10.1128/IAI.00381-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Feng CG, Kaviratne M, Rothfuchs AG, Cheever A, Hieny S, Young HA, et al. NK cell-derived IFN-gamma differentially regulates innate resistance and neutrophil response in T cell-deficient hosts infected with Mycobacterium tuberculosis. J Immunol. 2006;177:7086–7093. doi: 10.4049/jimmunol.177.10.7086. [DOI] [PubMed] [Google Scholar]
- 8.Orme IM, Cooper AM. Cytokine/chemokine cascades in immunity to tuberculosis. Immunol Today. 1999;20:307–312. doi: 10.1016/s0167-5699(98)01438-8. [DOI] [PubMed] [Google Scholar]
- 9.Pasquinelli V, Townsend JC, Jurado JO, Alvarez IB, Quiroga MF, Barnes PF, et al. IFN-gamma production during active tuberculosis is regulated by mechanisms that involve IL-17, SLAM, and CREB. J Infect Dis. 2009;199:661–665. doi: 10.1086/596742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Asano M, Nakane A, Minagawa T. Endogenous gamma interferon is essential in granuloma formation induced by glycolipid-containing mycolic acid in mice. Infect Immun. 1993;61:2872–2878. doi: 10.1128/iai.61.7.2872-2878.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Crevel R, Ottenhoff TH, van der Meer JW. Innate immunity to Mycobacterium tuberculosis. Clin Microbiol Rev. 2002;15:294–309. doi: 10.1128/CMR.15.2.294-309.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schmitz J, Owyang A, Oldham E, Song Y, Murphy E, McClanahan TK, et al. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity. 2005;23:479–490. doi: 10.1016/j.immuni.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 13.Bourgeois E, Van LP, Samson M, Diem S, Barra A, Roga S, et al. The pro-Th2 cytokine IL-33 directly interacts with invariant NKT and NK cells to induce IFN-gamma production. Eur J Immunol. 2009;39:1046–1055. doi: 10.1002/eji.200838575. [DOI] [PubMed] [Google Scholar]
- 14.Smith DE. IL-33: a tissue derived cytokine pathway involved in allergic inflammation and asthma. Clin Exp Allergy. 2010;40:200–208. doi: 10.1111/j.1365-2222.2009.03384.x. [DOI] [PubMed] [Google Scholar]
- 15.Préfontaine D, Lajoie-Kadoch S, Foley S, Audusseau S, Olivenstein R, Halayko AJ, et al. Increased expression of IL-33 in severe asthma: evidence of expression by airway smooth muscle cells. J Immunol. 2009;183:5094–5103. doi: 10.4049/jimmunol.0802387. [DOI] [PubMed] [Google Scholar]
- 16.Masamune A, Watanabe T, Kikuta K, Satoh K, Kanno A, Shimosegawa T. Nuclear expression of interleukin-33 in pancreatic stellate cells. Am J Physiol Gastrointest Liver Physiol. 2010;299:G821–G832. doi: 10.1152/ajpgi.00178.2010. [DOI] [PubMed] [Google Scholar]
- 17.Wood IS, Wang B, Trayhurn P. IL-33, a recently identified interleukin-1 gene family member, is expressed in human adipocytes. Biochem Biophys Res Commun. 2009;384:105–109. doi: 10.1016/j.bbrc.2009.04.081. [DOI] [PubMed] [Google Scholar]
- 18.Wong CF, Yew WW, Leung SK, Chan CY, Hui M, Au-Yeang C, et al. Assay of pleural fluid interleukin-6, tumour necrosis factor-alpha and interferon-gamma in the diagnosis and outcome correlation of tuberculous effusion. Respir Med. 2003;97:1289–1295. doi: 10.1016/j.rmed.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 19.Orphanidou D, Gaga M, Rasidakis A, Dimakou K, Toumbis M, Latsi P, et al. Tumour necrosis factor, interleukin-1 and adenosine deaminase in tuberculous pleural effusion. Respir Med. 1996;90:95–98. doi: 10.1016/s0954-6111(96)90205-x. [DOI] [PubMed] [Google Scholar]
- 20.Barnes PF, Fong SJ, Brennan PJ, Twomey PE, Mazumder A, Modlin RL. Local production of tumor necrosis factor and IFN-gamma in tuberculous pleuritis. J Immunol. 1990;145:149–154. [PubMed] [Google Scholar]
- 21.Espinassous Q, Garcia-de-Paco E, Garcia-Verdugo I, Synguelakis M, von Aulock S, Sallenave JM, et al. IL-33 enhances lipopolysaccharide-induced inflammatory cytokine production from mouse macrophages by regulating lipopolysaccharide receptor complex. J Immunol. 2009;183:1446–1455. doi: 10.4049/jimmunol.0803067. [DOI] [PubMed] [Google Scholar]
- 22.Xu D, Jiang HR, Kewin P, Li Y, Mu R, Fraser AR, et al. IL-33 exacerbates antigen-induced arthritis by activating mast cells. Proc Natl Acad Sci U S A. 2008;105:10913–10918. doi: 10.1073/pnas.0801898105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smithgall MD, Comeau MR, Yoon BR, Kaufman D, Armitage R, Smith DE. IL-33 amplifies both Th1- and Th2-type responses through its activity on human basophils, allergen-reactive Th2 cells, iNKT and NK cells. Int Immunol. 2008;20:1019–1030. doi: 10.1093/intimm/dxn060. [DOI] [PubMed] [Google Scholar]
- 24.Cooper AM, Solache A, Khader SA. Interleukin-12 and tuberculosis: an old story revisited. Curr Opin Immunol. 2007;19:441–447. doi: 10.1016/j.coi.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Valdés L, San José E, Alvarez Dobaño JM, Golpe A, Valle JM, Penela P, et al. Diagnostic value of interleukin-12 p40 in tuberculous pleural effusions. Eur Respir J. 2009;33:816–820. doi: 10.1183/09031936.00085008. [DOI] [PubMed] [Google Scholar]
- 26.Oshikawa K, Yanagisawa K, Ohno S, Tominaga S, Sugiyama Y. Expression of ST2 in helper T lymphocytes of malignant pleural effusions. Am J Respir Crit Care Med. 2002;165:1005–1009. doi: 10.1164/ajrccm.165.7.2105109. [DOI] [PubMed] [Google Scholar]
- 27.Okamoto M, Hasegawa Y, Hara T, Hashimoto N, Imaizumi K, Shimokata K, et al. T-helper type 1/T-helper type 2 balance in malignant pleural effusions compared to tuberculous pleural effusions. Chest. 2005;128:4030–4035. doi: 10.1378/chest.128.6.4030. [DOI] [PubMed] [Google Scholar]
- 28.Aoe K, Hiraki A, Murakami T, Eda R, Maeda T, Sugi K, et al. Diagnostic significance of interferon-gamma in tuberculous pleural effusions. Chest. 2003;123:740–744. doi: 10.1378/chest.123.3.740. [DOI] [PubMed] [Google Scholar]
- 29.Greco S, Girardi E, Masciangelo R, Capoccetta GB, Saltini C. Adenosine deaminase and interferon gamma measurements for the diagnosis of tuberculous pleurisy: a meta-analysis. Int J Tuberc Lung Dis. 2003;7:777–786. [PubMed] [Google Scholar]
- 30.Boonyagars L, Kiertiburanakul S. Use of adenosine deaminase for the diagnosis of tuberculosis: a review. J Infect Dis Antimicrob Agents. 2010;27:111–118. [Google Scholar]
- 31.Light RW, Macgregor MI, Luchsinger PC, Ball WC., Jr Pleural effusions: the diagnostic separation of transudates and exudates. Ann Intern Med. 1972;77:507–513. doi: 10.7326/0003-4819-77-4-507. [DOI] [PubMed] [Google Scholar]
- 32.Oboki K, Ohno T, Kajiwara N, Saito H, Nakae S. IL-33 and IL-33 receptors in host defense and diseases. Allergol Int. 2010;59:143–160. doi: 10.2332/allergolint.10-RAI-0186. [DOI] [PubMed] [Google Scholar]
- 33.Miller AM. Role of IL-33 in inflammation and disease. J Inflamm (Lond) 2011;8:22. doi: 10.1186/1476-9255-8-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen YM, Yang WK, Ting CC, Tsai WY, Yang DM, Whang-Peng J, et al. Cross regulation by IL-10 and IL-2/IL-12 of the helper T cells and the cytolytic activity of lymphocytes from malignant effusions of lung cancer patients. Chest. 1997;112:960–966. doi: 10.1378/chest.112.4.960. [DOI] [PubMed] [Google Scholar]
- 35.Moussion C, Ortega N, Girard JP. The IL-1-like cytokine IL-33 is constitutively expressed in the nucleus of endothelial cells and epithelial cells in vivo: a novel 'alarmin'? PLoS One. 2008;3:e3331. doi: 10.1371/journal.pone.0003331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Joshi AD, Oak SR, Hartigan AJ, Finn WG, Kunkel SL, Duffy KE, et al. Interleukin-33 contributes to both M1 and M2 chemokine marker expression in human macrophages. BMC Immunol. 2010;11:52. doi: 10.1186/1471-2172-11-52. [DOI] [PMC free article] [PubMed] [Google Scholar]