Abstract
Introduction
Thermoreversible hydrogels have potential in spine research as they provide easy injectability and mild gelling mechanism (by physical cross-link). The purpose of this study was to assess the potential of thermoreversible hyaluronan-based hydrogels (HA-pNIPAM) (pNIPAM Mn = 10, 20, 35 × 103 g mol−1) as nucleus pulposus cells (NPC) carrier.
Materials and methods
Cytocompatibility (WST-1 assay), viability (trypan blue), morphology (toluidine blue), sulphated glycosaminoglycan synthesis (DMMB assay) and gene expression profile (real-time PCR) of bovine NPC cultured in HA-pNIPAM were followed for 1 week and compared to alginate gel bead cultures. The injectability and cell survival in a whole disc organ culture model were assessed up to day 7.
Results
All HA, HA-pNIPAM and their degradation products were cytocompatible to NPC. HA-pNIPAM hydrogels with no volume change upon gelling maintained NPC viability and characteristic rounded morphology. Glycosaminoglycan synthesis was similar in HA-pNIPAM and alginate gels. Following NPC expansion, both gels induced re-differentiation toward the NPC phenotype. Significant differences between the two gels were found for COLI, COLII, HAS1, HAS2 and ADAMTS4 but not for MMPs and TIMPs. Higher expression of hyaluronan synthases (HAS1, HAS2) and lower expression of COLI and COLII mRNA were noted in cells cultured in HA-pNIPAM (pNIPAM = 20 × 103g mol−1). NPC suspension in HA-pNIPAM was injectable through a 22-G needle without loss of cell viability. Ex vivo, NPC viability was maintained in HA-pNIPAM for 1 week.
Conclusion
A HA-pNIPAM composition suitable for nucleus pulposus repair that provides an injectable carrier for NPC, maintains their phenotype and promotes extracellular matrix generation was identified.
Keywords: Thermoreversible hydrogel, Hyaluronic acid, Nucleus pulposus, Hyaluronan synthase
Introduction
Degeneration of the intervertebral disc (IVD) is recognized as a major cause of neck and low back pain. Current treatment options range from conservative strategies using medication and physical therapy to surgical procedures such as spinal fusion, disc arthroplasty and dynamic stabilization [1]. For early stages of degenerating discs, biological repair and regeneration techniques using anabolic factors, cells and biomaterials have been investigated in both in vitro and in vivo studies [2–6]. The aim of these interventions is to re-establish an extracellular matrix capable of fulfilling the mechanical requirements of absorbing and distributing loads while allowing for flexibility of the spinal column. For cell therapy, autologous disc cell implantation has been considered; potential limitations of this method are that nucleotomy is required and the cell activity of degenerating discs may be impaired. However, promising strategies to improve the activity of disc cells from patients such as co-culture with autologous mesenchymal stem cells have been demonstrated [7–9]. Direct implantation of mesenchymal stem cells—which are relatively easy to isolate from autologous bone marrow, adipose or other tissues—into degenerating discs has also shown regenerative effects [10–13]. The appropriate carrier material for injection of cells and/or anabolic factors into the disc space is under extensive investigation. Various types of biomaterials have been suggested to support regeneration of the IVD and, in particular, of the nucleus pulposus (NP) tissue [2]. The main functions of the carrier material are to retain cells and/or biological factors, to present a suitable three-dimensional environment, to partially and temporally replace lost extracellular matrix and to provide mechanical support. Given that the healthy NP consists of a highly hydrated extracellular matrix, hydrogels offer most promise as suitable materials for NP regenerative therapy. Hydrogels comprising natural components of the extracellular IVD matrix, such as collagen and hyaluronan, have been widely investigated and have already been tested for in vivo cell therapy in animal models [14–16]. It has to be noted that hyaluronan is the main macromolecular component of the IVD and has previously been used alone or in combination with other natural or synthetic materials for IVD and more specifically for nucleus pulposus cells (NPC) culture and tissue engineering [17–21]. However, to date the hyaluronan hydrogels proposed in the literature do not allow for a fine tuning of the macromolecular architecture. This is now possible thanks to recent developments in material synthesis, including reversible addition-fragmentation chain transfer polymerization (RAFT) and “click” chemistry. The combination of RAFT to generate specific molecular weight polymers with the efficient coupling mechanism of “click” chemistry allows highly controlled formation of hyaluronan hydrogels with distinct physical properties. An important property for the applicability of a hydrogel into the disc is its viscosity and hence its injectability. A thermoresponsive hydrogel that behaves as an injectable solution at room temperature and will form a solid gel upon temperature elevation by implantation into the IVD appears particularly attractive [22–24].
We have recently developed a set of cytocompatible hydrogels with thermoreversible properties by grafting poly(N-isopropylacrylamide) on a hyaluronan backbone (HA-pNIPAM) [25]. For NPC, the expression of the appropriate phenotype is a further fundamental requirement to assure the generation of an extracellular matrix that meets the specific mechanical demands of the tissue. The material is, therefore, expected to promote both re-differentiation of in vitro expanded NPC and activation of endogenous cells upon injection into the disc.
In this study, we tested the cytocompatibility of HA-pNIPAM hydrogels and their degradation products to NPC, as well as the viability and morphology of NPC cultured in the thermoreversible hydrogel beads in vitro. Furthermore, glycosaminoglycan production and gene profile of NPC encapsulated in HA-pNIPAM were compared to a standard three-dimensional alginate hydrogel culture. Finally, the injectability and cell survival of NPC embedded in the optimized thermoreversible hydrogel were assessed in a whole organ culture model. Overall, our results confirm the feasibility of this thermoresponsive hyaluronan gel formulation for IVD regenerative therapy.
Materials and methods
Synthesis and characterization of thermoreversible hyaluronan grafted poly(N-isopropylacrylamide) (HA-pNIPAM) hydrogels
Hydrosoluble hyaluronan propargylamide (HApa) was prepared from hyaluronan Streptococcus equi (HA) (Mn 1.7 × 106 g mol−1 measured using chemical assays) using an already established procedure [25]. The synthesis of azido terminated poly(N-isopropylacrylamide) (N3-pNIPAM) with Mn equal to 10, 20 and 35 × 103 g mol−1 (measured by MALDI–TOF) was performed by RAFT homopolymerization. After dissolution of HApa in distilled water at 0.5% w/v, poly(N-isopropylacrylamide) grafted hyaluronan (HA-pNIPAM) was obtained by adding N3-pNIPAM (corresponding to 25% of all propargylamine modified disaccharide subunits) together with CuSO4·5H20 and ascorbic acid sodium salt. The obtained HA-pNIPAM polymers with pNIPAM molecular weight of 10, 20 and 35 × 103 g mol−1 were named HA-pNIPAM10, HA-pNIPAM20, HA-pNIPAM35, respectively. After dialysis against 0.1 M NaCl and water, the solutions were frozen at −80°C and lyophilized to constant weight [25]. All chemicals used for the syntheses were purchased from Sigma-Aldrich.
Complex viscosity (η*) and storage modulus G′ of HA-pNIPAM solutions (with same HA content = 0.8% w/v) were analyzed using a CVOR-rheometer Bohlin instrument with Piezo rotary vibrator (PRV) option using a plate–plate geometry with silicon oil to avoid evaporation during measurements. The lower critical solution temperature (LCST) was measured using an Ultraviolet–Visible spectrophotometer Lambda 12 (Perkin-Elmer) with a Peltier temperature control system and is further referred as gel point. Water retention was assessed by weight measures of the amount of water lost or gained by the different HA-PNIPAM after 1 h in a phosphate buffered saline (PBS) (pH = 7.4) bath at 37°C. Further details on the experimental techniques used for the characterization of the hydrogels are available elsewhere [25].
Cytocompatibility of HA-pNIPAM and its degradation products to bovine NPC
Bovine NPC were isolated from the tails of young calves (4–6 months old) obtained from a local abattoir. Nucleus pulposus tissue was digested following an already established procedure [26]. Both the polymers and their degradation products were tested for cytocompatibility, as previously described [25]. Briefly, HA and HA-pNIPAM degradation products were prepared by enzymatic digestion with hyaluronidase (HAse) (type I–S, Sigma) (10, 50, 100, 500 or 1,000 U/mL), followed by heat inactivation. Polymers and degradation products were diluted in PBS (pH = 7.4) to prepare solutions with variable concentrations of hyaluronan (10, 20, 100 or 200 μg/mL) and variable pNIPAM molecular weights (10, 20 or 35 × 103 g mol−1). For pNIPAM solutions, the amount of pNIPAM was equivalent to the one in HA-pNIPAM solutions. Deactivated HAse (10–1,000 U/mL) was also added as a control.
NPC were seeded in 96-well plates at a density of 2,000 cells per 100 μL of culture medium, composed of Dulbecco’s modified Eagle medium (DMEM) containing 4.5 g/l glucose and supplied with 10% foetal calf serum (FCS) and 1% penicillin–streptomycin (P/S) (all products from Gibco). Cells were cultured at 37°C, 5% CO2, and 95% humidity. After 14 h, solutions of the polymers, the degradation products, or DMEM only (as positive control) were added (100 μL) and cells were cultured for additional 24 or 48 h. At each time point, viable cell number was determined by WST-1 assay (Roche Applied Science). Following medium removal, 100 μL of a 10% v/v solution of WST-1 reagent in DMEM supplemented with 0.5% FCS were added to each well. The absorbance at 450 nm was measured (at room temperature) with a multilabel plate reader (Victor 3, Perkin-Elmer) after 4 h of incubation at 37°C. Absorbance of the samples was normalized to the positive control (cells cultured in DMEM with 5% FCS).
NPC culture in HA-pNIPAM beads
NPC isolated as previously described and expanded for 1 week were resuspended (3 × 106 cells/mL) in 10% w/v HA-pNIPAM20 and HA-pNIPAM35 solutions. NCP seeded HA-pNIPAM beads were formed by dropping the NPC suspension though a 3-mL syringe (without needle) in PBS at 37°C. NPC-seeded alginate beads were formed using a 18 G needle according to a well-established procedure [27]. Gel beads were transferred to 12 well plates and cultured for 1 week in DMEM-10% FCS with medium change after 3 and 6 days of culture.
NPC viability and morphology in HA-pNIPAM beads
Cell viability was evaluated by live-dead (Invitrogen) and trypan blue exclusion assays at 1, 3 and 7 days of culture. Cells were collected by cooling the HA-pNIPAM beads at 4°C for 20 min. Alginate beads were dissolved using a sodium citrate solution [27] and resuspended in an equivalent volume of 0.9% w/v sodium chloride solution. For live-dead measures, a solution containing 5 μM of calcein AM and 1 μM of ethidium homodimer 1 (Molecular Probes) in DMEM was added in equal volume to the cell suspension. After 30 min of incubation, a 10 μL drop of cell suspension was viewed between two glass coverslips in a custom made holder with an inverted motorized optical microscope (Axiovert 200 M, Zeiss, Germany) equipped with a 5× objective (Zeiss), Axiocam HR camera (Zeiss) and filter sets #10 and #15 (Zeiss). The acquisition time was adjusted for each epifluorescence image. The red and green images were then combined using Axiovision 4.6.3 version software (Zeiss).
Samples for histology were embedded in cryo-embedding compound (OCT, Tissue-Tek) pre-warmed at 37°C and snap-frozen in liquid nitrogen. Cryosections (10 μm thick) were obtained using a microtome (HM 500 OMV, Microm, Germany). Sections were post-fixed with 4% buffered paraformaldehyde and stained with 0.5% toluidine blue. The sections were viewed in transmitted light under upright optical microscope (Axioplan 2, Zeiss, Germany) equipped with a 20× objective (Zeiss). The acquisition time was kept constant for all samples (100 ms).
DNA quantification and extracellular matrix synthesis of NPC in HA-pNIPAM beads
After 1 and 7 days of culture, HA-pNIPAM and alginate beads were collected and digested with a 0.5 mg/mL proteinase K solution (Roche Applied Science) at 56°C overnight. The sulphated glycosaminoglycans (GAG) and DNA content were quantified by 1,9-dimethylmethylene blue (DMMB) (Sigma-Aldrich) and PicoGreen (Invitrogen) assays, respectively. Nucleus pulposus tissue obtained from tails of 4–6 months old calves was analysed for DNA and GAG with the above-described methods and used as reference.
Gene expression of NPC cultured in HA-pNIPAM beads
Total RNA was extracted from homogenized constructs (TissueLyser, Qiagen) with TRI reagent (Molecular Research Center) and further purified using the NucleoSpin RNA isolation kit (Macherey–Nagel) according to the manufacturer’s instructions. Reverse transcription was performed using TaqMan Reverse Transcription kit (Applied Biosystems). Real-time polymerase chain reaction (RT-PCR) was performed with a SDS7500 instrument (Applied Biosystems) to assess the gene expression of NPC cultured in the hydrogel beads. Markers for extracellular matrix such as collagen I (COL1A1), collagen II (COL2A1), aggrecan (ACAN) were investigated together with the catabolic markers matrix metalloproteinase 3 and 13 (MMP3 and MMP13) and a disintegrin and metalloproteinase with thrombospondin motifs 4 and 5 (ADAMTS4 and ADAMTS5), and the anabolic markers tissue inhibitor of metalloproteinases-1 and 3 (TIMP1 and TIMP3) and hyaluronan synthase 1 and 2 (HAS1 and HAS2) (Table 1). Ribosomal 18s RNA was used an endogenous control [28]. The relative gene expression, normalized to the expression levels of subcultured NPC after expansion, was calculated using the ΔΔCt method.
Table 1.
Sequences of bovine primers and probes used for real-time PCR
| Gene | Accession number | Primer fw (5′–3′) | Primer rev (5′–3′) | Probe (5′FAM/3′TAMRA) |
|---|---|---|---|---|
| Procollagen 1A2 (COL1) | NM_174520 | TGC AGT AAC TTC GTG CCT AGC A | CGC GTG GTC CTC TAT CTC CA | CAT GCC AAT CCT TAC AAG AGG CAA CTG C |
| Procollagen 2A1 (COL2) | NM_001001135 | AAG AAA CAC ATC TGG TTT GGA GAA A | TGG GAG CCA GGT TGT CAT C | CAA CGG TGG CTT CCA CTT CAG CTA TGG |
| ACAN | NM_173981 | CCA ACG AAA CCT ATG ACG TGT ACT | GCA CTC GTT GGC TGC CTC | ATG TTG CAT AGA AGA CCT CGC CCT CCA T |
| HAS1 | XM_002695251 | GGC ACC CAC TGC ACC TTT | CGA GGT GTA CTT GGT GGC ATA G | CGT CAC CTC ACC AAC CGC ATG CT |
| HAS2 | NM_174079 | GGA TTA TGT ACA GGT TTG TGA TTC AGA | ACC TCC AAC CAT GGG ATC TTC | TCT CCA CAG ATG ATG CTG GGT CAA GC |
| TIMP1 | NM_174471 | GGA CCG CAG AAG TCA ATG AAA | GGG TGT AGA TGA ACC GGA TGT C | TAC CAG CGT TAT GAG ATC AAG ATG ACT AAG ATG |
| TIMP3 | NM_174473 | CCT TTG GCA CGA TGG TCT ACA | TTA AGG CCA CAG AGA CTT TCA GAA G | AAG CAG ATG AAG ATG TAC CGA GGA TTC A |
| MMP3 | XM_586521 | GGC TGC AAG GGA CAA GGA A | CAA ACT GTT TCG TAT CCT TTG CAA | CAC CAT GGA GCT TGT TCA GCA ATA TCT AGA AAA C |
| MMP13 | NM_174389 | CCA TCT ACA CCT ACA CTG GCA AAA G | GTC TGG CGT TTT GGG ATG TT | TCT CTC TAT GGT CCA GGA GAT GAA GAC CCC |
| ADAMTS4 | NM_181667 | CCC CAT GTG CAA CGT CAA G | AGT CTC CAC AAA TCT GCT CAG TGA | AGC CCC CGA AGG GCT AAG CGC |
| ADAMTS5 | NM_001166515 | GAT GGT CAC GGT AAC TGT TTG CT | GCC GGG ACA CAC CGA GTA C | AGG CCA GAC CTA CGA TGC CAG CC |
| 18s rRNA | Primer-probe 20× concentrate from Applied Biosystems (Cat.Nr. 4310893E) | |||
Injectability of NPC suspended in HA-pNIPAM
Cells were labelled with PKH26 red fluorescent dye (Sigma) and suspended in either a 10% w/v HA-pNIPAM solution in PBS or a 1.2% w/v alginate solution in 0.9% w/v NaCl at a density of 3 × 106 cells/mL. The cell suspension in HA-pNIPAM was slowly injected through a 22-G needle in the culture medium (DMEM-10% FCS) at 37°C. Alternately, alginate beads formed by dropping the cell suspension in a calcium chloride solution using a 22-G needle were washed in sodium chloride and then transferred to the culture medium. Cell viability was assessed by calcein AM staining at 24 h after injection (n = 3). Cells were observed with an inverted optical microscope according to the above-described procedure.
NPC seeded HA-pNIPAM culture in a whole organ culture model
Bovine IVD were dissected from the tail of young calves (4–6 months); cutting through the endplates was performed using a band saw. The endplates (~1 mm thickness) were rinsed with Ringer solution using a Pulsavac jet-lavage system (Zimmer). After washing the discs in a 10% penicillin–streptomycin solution, the discs were punched (Kai Medical, biopsy punch diameter = 3.5 mm, cutting length = 7 mm) on the lateral side. Freshly isolated bovine NPC were labelled with PKH26 red fluorescent dye (Sigma) according to the manufacturer’s instructions and suspended in 10% w/v HA-pNIPAM solution in PBS at a density of 3 × 106 cells/mL. Approximately, 50 μL of cell suspension was administered into the IVD defect (n = 3). The defect was then closed by press-fitting a 5 mm diameter annulus fibrosus plug (obtained from another IVD). The discs were then kept at 37°C for 1 h for gelation prior to transferring them to a DMEM-10% FCS solution. Medium was changed every second day. After 1 and 7 days of culture, the discs were cooled to 4°C. The liquefied cell suspension was collected out of the punched defect into an Eppendorf tube and an equal volume of a solution containing 5 μM of calcein AM and 1 μM of ethidium homodimer 1 in DMEM was added. Images were taken using an inverted motorized microscope according to the above-described procedure.
Statistical analysis
For cytocompatibility, NPC from three different animals were used and each experiment was performed in quadruplicates. One-way analysis of variance (ANOVA), followed by post hoc test (Tukey HSD) was performed with SPSS 18 software; p values <0.05 were considered to be statistically significant. For trypan blue, viability, DNA and GAG, NPC from two different animals were used and each experiment was performed in triplicates. After exclusion of the outliers, the normality of the data distribution was tested and Mann–Whitney test was performed with SPSS 18 software; only p values <0.05 (*) were considered to be statistically significant.
Results
Physical characteristics of HA-pNIPAM hydrogels
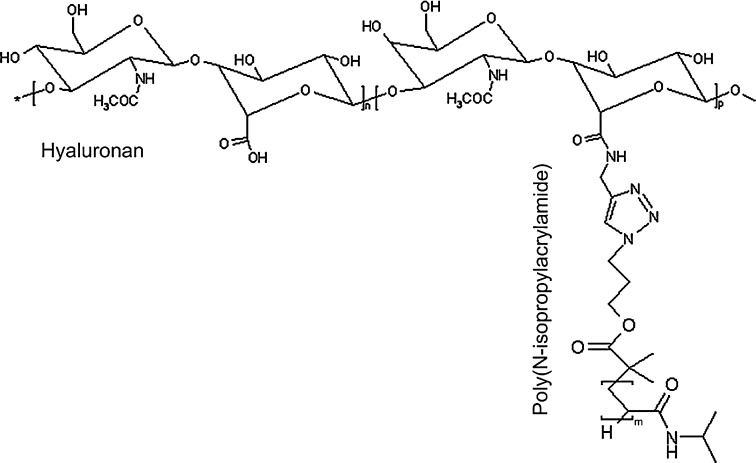
The molecular structure of the brush-like HA-pNIPAM macromolecule is reported in Scheme 1. The detailed synthesis procedure for the preparation of the HA-pNIPAM polymers with different pNIPAM molecular weights is described elsewhere [25]. As reported in Table 2, all polymer solutions (10% w/v in PBS) showed a low viscosity (η = 4.6–23.5 Pa s) at 25°C, with HA-pNIPAM20 composition being the most fluid one. Upon heating, a hydrogel is formed by the association of hydrophobic pNIPAM chains on the brushes. A gel point ~31°C was measured for compositions HA-pNIPAM20 and HA-pNIPAM35, while HA-pNIPAM10 did not display a clear gel point. The storage modulus (G′) and volume change showed a strong dependence on the pNIPAM molecular weight: HA with low pNIPAM Mn (gel HA-pNIPAM10) showed low G′ and swelling in PBS, while HA with high pNIPAM Mn (gel HA-pNIPAM35) showed high G′ and shrinkage in PBS. Gel HA-pNIPAM20 had a relatively low G′ (G′ = 140 Pa) and almost no volume change upon gelling.
Scheme 1.
Molecular structure of HA-pNIPAM
Table 2.
Complex viscosity (η*), gel point, storage modulus (G′) and volume change of hyaluronan grafted poly(N-isoproprylacrylamide) (HA-pNIPAM) compositions in PBS
| Hydrogel | η* (Pa s, 2 Hz, 25°C) | Gel point (°C) | G′ (kPa, 2 Hz, 37°C) | Volume change (%, after 1 h) |
|---|---|---|---|---|
| HA-pNIPAM10 | 23.5 | –a | 0.005 | +56 |
| HA-pNIPAM20 | 4.6 | 30.6 | 0.14 | −0.2 |
| HA-pNIPAM35 | 10.8 | 31.0 | 16 | −47 |
aHA-pNIPAM10 composition did not show a clear and sharp LSCT as measured by Ultraviolet–visible spectroscopy
Cytocompatibility to NPC
The cell survival in the presence of HA, HA-pNIPAM or degradation products was >90% for the whole range of pNIPAM molecular weights and HA concentrations tested. In Fig. 1, we report the cycompatibility measured after 48 h exposure to HA, HA-pNIPAM or degradation products with a HA concentration equal to 100 μg/mL, but similar trends were observed for the other HA concentrations (10, 20 and 200 μg/mL). Additionally, extensive HA backbone degradation induced NPC proliferation for both HA and HA-pNIPAM polymers. No significant differences in cell viability were found between 24 and 48 h of culture.
Fig. 1.
Influence of HA and HA-pNIPAM compositions (HA-pNIPAM10, HA-pNIPAM20 and HA-pNIPAM35) and their degradation products with constant HA concentration (100 μg/mL) and variable hyaluronidase (Hase) concentrations (0–1,000 U/mL) on nucleus pulposus cell number at 48 h as measured by WST-1 assay and normalized against positive control (cells with medium only): viability median, quartile, maximum and minimum; *p < 0.05 versus 0 U/mL Hase, **p < 0.05 versus 100 U/mL Hase, ***p < 0.05 versus 500 U/mL Hase, + p < 0.05 versus HA with equivalent concentration of HAse
NPC viability, morphology and extracellular matrix synthesis in HA-pNIPAM hydrogels
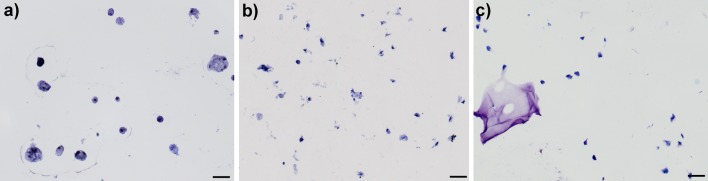
HA-pNIPAM20 and HA-pNIPAM35 compositions were chosen for the study of NPC behaviour in the hydrogels, while the HA-pNIPAM10 composition was not further investigated due to its high viscosity at room temperature and high swelling and low stability at 37°C. As shown in Figs. 2, 3, NPC viability after 1 day of culture was >80% in both hydrogel bead compositions. NPC viability in HA-pNIPAM20 hydrogels remained >80% also at days 3 and 7 of culture (comparable to alginate control). However, NPC viability in the HA-pNIPAM35 hydrogel decreased over time to 69 and 50% at 3 and 7 days of culture, respectively (Figs. 2, 3). Histological sections of the beads revealed that the characteristic rounded morphology of NPC was maintained in HA-pNIPAM20 hydrogel and alginate gel (Fig. 4). Conversely, NPC in HA-pNIPAM35 hydrogel lost their rounded shape and appeared stretched.
Fig. 2.
Representative images of calcein AM/ethidium homodimer 1 (live-dead) staining of nucleus pulposus cells cultured in HA-pNIPAM20 (a–c), HA-pNIPAM35 (d–f) and alginate (g–i) hydrogels for 1 (a, d, g), 3 (b, e, h) and 7 (c, f, i) days; green living cell, red dead cell. Scale bar 200 μm
Fig. 3.
Cell viability of nucleus pulposus cells assessed by trypan blue exclusion method after 1, 3 and 7 days of culture in HA-pNIPAM hydrogels (HA-pNIPAM20 and HA-pNIPAM35); #p < 0.05 versus HA-pNIPAM20, *p < 0.05 versus HA-pNIPAM35 day 1. Cell viability was maintained in HA-pNIPAM20 hydrogel, while it dropped significantly in HA-pNIPAM35 hydrogel
Fig. 4.
Representative images of toluidine blue staining of nucleus pulposus cells cultured for 7 days in a HA-pNIPAM20 hydrogel, b HA-pNIPAM35 hydrogel and c alginate gel. Scale bar 50 μm. The characteristic rounded morphology of nucleus pulposus cells was maintained in HA-pNIPAM20 and alginate hydrogels, while cells appeared stretched in HA-pNIPAM35 composition
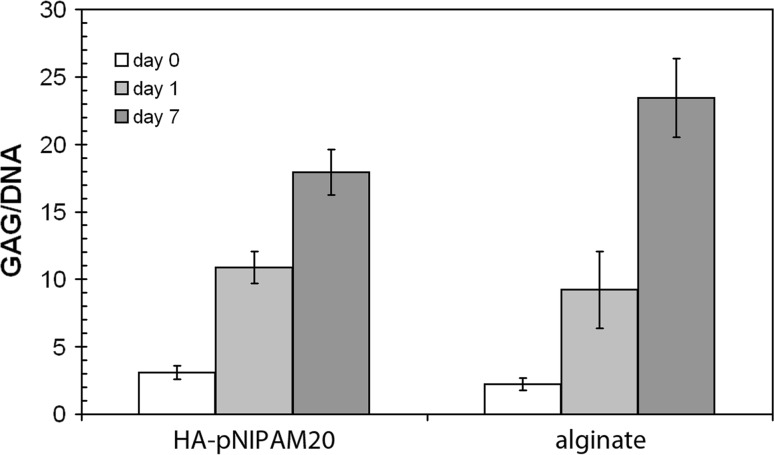
Since HA-pNIPAM20 hydrogel maintained a high cell viability and healthy NPC morphology after 1 week of culture, this composition was chosen for further studies of NPC behaviour in the thermoreversible HA-based hydrogels. The amount of DNA in HA-pNIPAM20 beads decreased slightly over the week of culture (0.45 ± 0.18, 0.46 ± 0.03 and 0.31 ± 0.04 μg/sample at day 0, 1 and 7 of culture, respectively), while it was constant in alginate beads (0.39 ± 0.03, 0.40 ± 0.01 and 0.43 ± 0.03 μg/sample at day 0, 1 and 7 of culture, respectively). After 1 week of culture, the GAG/DNA ratio was significantly higher for both HA-pNIPAM20 and alginate hydrogels (17.9 ± 1.7 and 23.5 ± 2.9, respectively) as compared to day 1 (10.9 ± 1.2 and 9.2 ± 2.9, respectively) (Fig. 5), while there was no significant difference between HA-pNIPAM20 and alginate gels. GAG and DNA were also measured in native nucleus pulposus tissue (from 20 bovine IVDs): DNA amounts of 0.05–0.1 μg/mg tissue and GAG contents of 50–100 μg/mg tissue were found, resulting in GAG/DNA ratios of 1,000–2,000.
Fig. 5.
Total sulphated glycosaminoglycan (GAG) content in the HA-pNIPAM20 hydrogel and alginate gel (constructs and media) relative to the amount of DNA; average and standard deviations are reported. An increase in GAG/DNA was found during the 7 days of culture without significant differences between the two gels
Gene expression of NPC cultured in HA-pNIPAM beads
Both gels induced re-differentiation toward the NP cell phenotype, as attested by the down-regulation of collagen I and up-regulation of aggrecan (Fig. 6). MMP3, MMP13 and HAS2 were up-regulated more than ten times in both hydrogels as compared to subcultured NPC. Significant differences between the two gels were found for collagen I, collagen II, HAS1, HAS2 and ADAMTS4 regulation.
Fig. 6.
Gene expression profile of nucleus pulposus cells (NPC) cultured for 1 week in HA-pNIPAM20 or alginate gel. Real-time PCR data were normalized to house-keeping gene (18s rRNA) and are presented relative to nucleus pulposus cells subcultured to P1; viability median, quartile, maximum and minimum, *p < 0.05 versus alginate. Compared to NPC in alginate gels, NPC in HA-pNIPAM20 hydrogel showed significant down-regulation of COL1 and COL2 and up-regulation of HAS1, HAS2 and ADAMTS4
Injectability of NPC suspended in HA-pNIPAM
Both the HA-pNIPAM20 and alginate hydrogels were injectable through a 22-G needle. Cell viability at 24 h after injection was 90 ± 2% for the HA-pNIPAM hydrogel and 71 ± 9% for the alginate gels (Fig. 7), confirming that the thermoreversible hydrogel can be used as an injectable cell carrier.
Fig. 7.
Representative images of a nucleus pulposus cavity created in bovine intervertebral disc with endplates by punching (punch diameter 3.5 mm, length 7 mm) and red PKH26 labelled nucleus pulposus cells after recovery and staining with calcein AM at b day 1 and c day 7 of culture in HA-pNIPAM20 hydrogel; yellow delivered living cell, green living cell from the surrounding tissue, red dead cell. Scale barsa 10 mm, b–c 200 μm. Cell viability was maintained for at least 1 week, although a decrease in cell density was observed
NPC seeded HA-pNIPAM culture in a whole organ culture model
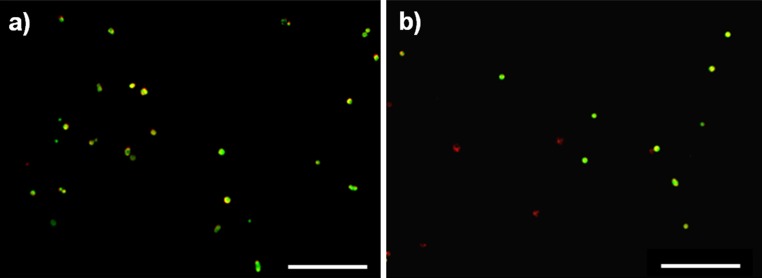
A well-defined NP cavity was created using a biopsy punch (Fig. 8a). NPC introduced in the defect using HA-pNIPAM20 hydrogel as cell carrier were >90% viable over 1 week of culture (Fig. 8b, c). The cell viability in the whole organ culture model was comparable to the one obtained for in vitro culture of HA-pNIPAM20 hydrogel beads.
Fig. 8.
Representative images of red PKH26 labelled nucleus pulposus cells (NPC) recovered and stained with calcein AM 24 h after injection through a 22-G needle of a NPC suspension in a HA-pNIPAM20 and b alginate gel; yellow living cell, red dead cell. Scale bar 200 μm. Cell viability after injection was higher in HA-pNIPAM20 hydrogel than in alginate gels
Discussion
We have recently developed a set of hyaluronan-based thermoreversible hydrogels, which are cytocompatible and cover a wide range of mechanical properties [25]. The aim of this study was to investigate the potential of HA-pNIPAM hydrogels for NPC encapsulation and select a hydrogel composition suitable for NPC injection in the IVD. A step-by-step approach was used: first, the injectability of a NPC suspension in HA-pNIPAM hydrogels was tested to investigate the potential of these hydrogels in a minimally invasive approach and then NPC behaviour in HA-pNIPAM hydrogels was studied with respect to the extracellular matrix production and gene expression regulation.
The rationale for the choice of hyaluronan as the backbone of the hydrogels is that it represents a chief component of the extracellular matrix of the nucleus pulposus tissue of the intervertebral disc and is known to play a key role in tissue development, cell proliferation and migration [29]. While unmodified hyaluronan solutions are rapidly degraded in vivo, chemically modified, physically or chemically cross-linked hyaluronan has shown a better stability. The grafting of the thermoresponsive polymer pNIPAM on the hyaluronan backbone has two main advantages: it decreases the viscosity of hyaluronan solutions at room temperature [25] and allows for a mild gelling mechanism (by physical cross-links and without any toxic by-products or pH shift) which is compatible with cell encapsulation. Moreover, pNIPAM forms a gel below body temperature (~32°C). pNIPAM has shown good biocompatibility [30] and although it is not biodegradable, low molecular weight pNIPAM can undergo renal clearance [31]. The physical properties of the gels (storage modulus, G′ at 37°C) have been reported in Table 2 and are comprised between 0.14 and 16 kPa. We did not perform a measurement of G′ of nucleus pulposus tissue. However, from the literature, human nucleus pulposus G′ is ~5 kPa [19]. Therefore, our gels have comparable storage modulus to human nucleus pulposus tissue.
In a previous study, we have shown that HA-pNIPAM and its degradation products are cytocompatible to the fibroblastic cell line h-TERT BJ1 [25]. In the present study, this was confirmed for NPC as well. Moreover, degraded HA-pNIPAM fragments induced NPC proliferation. A similar proliferative effect in the presence of HA fragment was found by Masters et al. [32] for valvular interstitial cells and in our previous study with h-TERT BJ1 fibroblasts [25].
An adequate cell carrier for nucleus pulposus should maintain cell viability and promote extracellular matrix synthesis. Although both HA-pNIPAM20 and HA-pNIPAM35 ensured a high viability after 1 day, HA-pNIPAM20 gel yielded a significantly higher viability after 1 week of culture. This is probably due to the differences in volume change between the two gels: while HA-pNIPAM20 hydrogel does not undergo a volume change upon gelling, HA-pNIPAM35 composition shrinks by ~30%, which might be too high to ensure good NPC survival. Volume changes also affected the cell morphology and rounded NPC were observed in HA-pNIPAM20 gel, while an unusual stretched NPC morphology was found in HA-pNIPAM35 gel.
Cell viability after 1 week of culture was ~90% in HA-pNIPAM20 hydrogels. Therefore, the slight DNA decrease is probably due to the loss of gel stability [33], rather than cell death. The sulphated glycosaminoglycan synthesized by NPC cultured in the HA-pNIPAM hydrogels is comparable to alginate gels after 1 week of culture. Similar GAG/DNA ratios were obtained for nucleus pulposus cells in collagen I-hyaluronan hydrogels [17] and articular chondrocytes culture in cross-linked HA-PEG by Michael addition [33] or photocrosslinked methacrylated HA [34]. While these GAG/DNA values are at least 50 times lower compared to our native NP tissue, the amount of GAG produced by the NPC within 1 week of culture in hydrogel beads in medium without specific growth factor supplementation comes up to our expectations. Furthermore, the steady increase in GAG/DNA is encouraging; nonetheless longer term cultures will be needed to confirm this trend.
At the gene expression level, NPC cultured in HA-pNIPAM20 hydrogel conserved the NP phenotype, as attested by collagen I down-regulation and aggrecan up-regulation. Moreover, the HA-pNIPAM20 hydrogel induced a significant up-regulation in HAS1 as compared to the alginate gel. When NPC were encapsulated in a 3D matrix, HAS2 was up-regulated as compared to monolayer cultured cells (HAS2/HAS1 was 9.8 and 32 for NPC cultured in HA-pNIPAM20 and alginate, respectively). The different HAS2/HAS1 ratio in the two gels might be related to different rate and size of the HA produced by the cells [35]. Furthermore, the higher expression of MMPs in HA-pNIPAM20 gel might be related to the presence of short hyaluronan fragments [36]. Finally, the up-regulation of HAS1 and TIMP1 genes is advantageous in terms of disc regeneration as both genes are regarded as anabolic markers for disc matrix synthesis. Alongside, a strong MMP3 and MMP13 up-regulation was observed in this study. Since we have used tails from young animals (3–6 months calves), it is possible that there is a stronger degradation and rapid matrix turn-over as compared to older animals [37]. At this stage, it is difficult to make a definitive statement as there is a dynamic balance between anabolic/catabolic genes and further investigations are needed to better understand the anabolic/catabolic balance of NPC cultured in these materials.
Injectability is a key feature for minimally invasive surgeries. High molecular weight hyaluronan solutions are characterized by a high viscosity and, therefore, a strong pressure needs to be applied for the injection which is not compatible with cell delivery. Alternately, a thermoreversible hydrogel consists of a low viscosity hyaluronan solution that can be injected and that will gelify in situ. As a proof of concept, HA-pNIPAM hydrogels with molecular weight pNIPAM of 20 × 103 g mol−1 were injected though a 22-G needle. This needle size represents a compromise between a needle as small as possible to reduce further damage of the IVD and a needle large enough to avoid a too high shear stress of the NPC in the needle (viscosity of the thermoreversible hydrogel at room temperature: 4.6–23.5 Pa s). 20-G needles have already been used to inject hMSCs and platelet-rich plasma in mini-pig IVDs [38], and 27-G needles have been used to inject MSCs and atelocollagen in rabbit IVDs [16]. A 22-G needle had been previously used for injection of mesenchymal stem cells embedded in hydrogel into cryopreserved bovine IVD [39]. Therefore, the 22-G needle size is within the usual range of needle sizes used for the injection of cell suspensions in viscous carriers. Additionally, bovine (and human) discs are bigger than rabbit or mini-pig discs, and, therefore, we can reasonably conclude that a 22-G needle may be used for injection purposes. The NPC survival in HA-pNIPAM20 at 24 h after injection was higher than in alginate gels, probably due to the mild gel formation mechanism of HA-pNIPAM. Indeed, while alginate gelling is based on ion cross-linking, the thermoreversible hydrogel gelling is due to the coil-to-globule transition of the pNIPAM segment, leading to hydrophobic associations of the pNIPAM chains. The chosen HA-pNIPAM composition (pNIPAM Mn = 20 × 103 g mol−1) undergoes no volume change upon gelling, meaning that the inter- and intra-polymer chain associations are balanced [25]. Therefore, the cells would undergo only a mild change of their surrounding upon gelling.
Whole organ culture models represent the most clinically relevant setup for the evaluation of regenerative therapies prior to in vivo studies [40]. Indeed, these ex vivo models add additional, tissue/organ-specific parameters to conventional in vitro cell culture studies. In the case of the intervertebral discs, such parameters include hypoxic environment, limited diffusion of nutrients, anabolic and catabolic agents, presence of chemokines and other factors. As a further step, bioreactors can be used to simulate the loading regimes to which IVDs are subjected in the body [40]. Therefore, in spite of certain limitations such as the absence of systemic reaction and difficulties in maintaining the organ viability for long terms, to date ex vivo cultures are the closest model to the in vivo situation [40]. In this study, the ability of HA-pNIPAM20 composition to form a gel in situ and maintain viable cells was studied over 1 week in an ex vivo model. The results confirm the in vitro findings with HA-pNIPAM20 composition forming a gel in a NP cavity and cells being viable for at least 1 week allowing for more extended evaluation using a whole organ IVD bioreactor system; however, long-term evaluations would be an essential step prior to in vivo testing.
Conclusion
In conclusion, the thermoreversible hydrogel provides an injectable carrier for NPC that maintains their phenotype and promotes extracellular matrix generation. The main limitations of this study are related to the short-term culture period. Longer-term NPC cultures will be needed to assess the expression of anabolic genes at the protein level, the GAG/DNA evolution as well as the stability of the thermoreversible hydrogel with time.
Acknowledgments
The authors would like to gratefully acknowledge Mr. Andrea Oswald for his help in the gene expression analyses and Mr. Markus Glarner for assistance with the hydrogel synthesis.
Conflict of interest
None.
References
- 1.Schizas C, Kulik G, Kosmopoulos V. Disc degeneration: current surgical options. Eur Cell Mater. 2010;20:306–315. doi: 10.22203/ecm.v020a25. [DOI] [PubMed] [Google Scholar]
- 2.Grad S, Alini M, Eglin D, Sakai D, Mochida J, Mahor S et al (2010) Cells and biomaterials for intervertebral disc regeneration. In: Athanasiou KA (ed) Synthesis lectures on tissue engineering, vol 2. Morgan & Claypool, San Rafael, pp 1–104
- 3.Masuda K. Biological repair of the degenerated intervertebral disc by the injection of growth factors. Eur Spine J. 2008;17(Suppl 4):441–451. doi: 10.1007/s00586-008-0749-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Masuda K, Lotz JC. New challenges for intervertebral disc treatment using regenerative medicine. Tissue Eng Part B Rev. 2010;16:147–158. doi: 10.1089/ten.teb.2009.0451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nishida K, Suzuki T, Kakutani K, Yurube T, Maeno K, Kurosaka M, et al. Gene therapy approach for disc degeneration and associated spinal disorders. Eur Spine J. 2008;17(Suppl 4):459–466. doi: 10.1007/s00586-008-0751-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Drury JL, Mooney DJ. Hydrogels for tissue engineering: scaffold design variables and applications. Biomaterials. 2003;24:4337–4351. doi: 10.1016/S0142-9612(03)00340-5. [DOI] [PubMed] [Google Scholar]
- 7.Sakai D. Future perspectives of cell-based therapy for intervertebral disc disease. Eur Spine J. 2008;17(Suppl 4):452–458. doi: 10.1007/s00586-008-0743-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Watanabe T, Sakai D, Yamamoto Y, Iwashina T, Serigano K, Tamura F, et al. Human nucleus pulposus cells significantly enhanced biological properties in a coculture system with direct cell-to-cell contact with autologous mesenchymal stem cells. J Orthop Res. 2010;28:623–630. doi: 10.1002/jor.21036. [DOI] [PubMed] [Google Scholar]
- 9.Vadala G, Studer RK, Sowa G, Spiezia F, Iucu C, Denaro V, et al. Coculture of bone marrow mesenchymal stem cells and nucleus pulposus cells modulate gene expression profile without cell fusion. Spine (Phila Pa 1976) 2008;33:870–876. doi: 10.1097/BRS.0b013e31816b4619. [DOI] [PubMed] [Google Scholar]
- 10.Hiyama A, Mochida J, Sakai D. Stem cell applications in intervertebral disc repair. Cell Mol Biol (Noisy-le-grand) 2008;54:24–32. [PubMed] [Google Scholar]
- 11.Hiyama A, Mochida J, Iwashina T, Omi H, Watanabe T, Serigano K, et al. Transplantation of mesenchymal stem cells in a canine disc degeneration model. J Orthop Res. 2008;26:589–600. doi: 10.1002/jor.20584. [DOI] [PubMed] [Google Scholar]
- 12.Miyamoto T, Muneta T, Tabuchi T, Matsumoto K, Saito H, Tsuji K, et al. Intradiscal transplantation of synovial mesenchymal stem cells prevents intervertebral disc degeneration through suppression of matrix metalloproteinase-related genes in nucleus pulposus cells in rabbits. Arthritis Res Ther. 2010;12:R206. doi: 10.1186/ar3182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yoshikawa T, Ueda Y, Miyazaki K, Koizumi M, Takakura Y. Disc regeneration therapy using marrow mesenchymal cell transplantation: a report of two case studies. Spine (Phila Pa 1976) 2010;35:E475–E480. doi: 10.1097/BRS.0b013e3181cd2cf4. [DOI] [PubMed] [Google Scholar]
- 14.Crevensten G, Walsh AJ, Ananthakrishnan D, Page P, Wahba GM, Lotz JC, et al. Intervertebral disc cell therapy for regeneration: mesenchymal stem cell implantation in rat intervertebral discs. Ann Biomed Eng. 2004;32:430–434. doi: 10.1023/B:ABME.0000017545.84833.7c. [DOI] [PubMed] [Google Scholar]
- 15.Sakai D, Mochida J, Yamamoto Y, Nomura T, Okuma M, Nishimura K, et al. Transplantation of mesenchymal stem cells embedded in Atelocollagen gel to the intervertebral disc: a potential therapeutic model for disc degeneration. Biomaterials. 2003;24:3531–3541. doi: 10.1016/S0142-9612(03)00222-9. [DOI] [PubMed] [Google Scholar]
- 16.Sakai D, Mochida J, Iwashina T, Hiyama A, Omi H, Imai M, et al. Regenerative effects of transplanting mesenchymal stem cells embedded in atelocollagen to the degenerated intervertebral disc. Biomaterials. 2006;27:335–345. doi: 10.1016/j.biomaterials.2005.06.038. [DOI] [PubMed] [Google Scholar]
- 17.Alini M, Li W, Markovic P, Aebi M, Spiro RC, Roughley PJ. The potential and limitations of a cell-seeded collagen/hyaluronan scaffold to engineer an intervertebral disc-like matrix. Spine (Phila Pa 1976) 2003;28:446–454. doi: 10.1097/01.BRS.0000048672.34459.31. [DOI] [PubMed] [Google Scholar]
- 18.Calderon L, Collin E, Velasco-Bayon D, Murphy M, O’Halloran D, Pandit A. Type II collagen–hyaluronan hydrogel–a step towards a scaffold for intervertebral disc tissue engineering. Eur Cell Mater. 2010;20:134–148. doi: 10.22203/ecm.v020a12. [DOI] [PubMed] [Google Scholar]
- 19.Cloyd JM, Malhotra NR, Weng L, Chen W, Mauck RL, Elliott DM. Material properties in unconfined compression of human nucleus pulposus, injectable hyaluronic acid-based hydrogels and tissue engineering scaffolds. Eur Spine J. 2007;16:1892–1898. doi: 10.1007/s00586-007-0443-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Halloran DO, Grad S, Stoddart M, Dockery P, Alini M, Pandit AS. An injectable cross-linked scaffold for nucleus pulposus regeneration. Biomaterials. 2008;29:438–447. doi: 10.1016/j.biomaterials.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 21.Su WY, Chen YC, Lin FH. Injectable oxidized hyaluronic acid/adipic acid dihydrazide hydrogel for nucleus pulposus regeneration. Acta Biomater. 2010;6:3044–3055. doi: 10.1016/j.actbio.2010.02.037. [DOI] [PubMed] [Google Scholar]
- 22.Chen JP, Cheng TH. Thermo-responsive chitosan-graft-poly(N-isopropylacrylamide) injectable hydrogel for cultivation of chondrocytes and meniscus cells. Macromol Biosci. 2006;6:1026–1039. doi: 10.1002/mabi.200600142. [DOI] [PubMed] [Google Scholar]
- 23.Roughley P, Hoemann C, DesRosiers E, Mwale F, Antoniou J, Alini M. The potential of chitosan-based gels containing intervertebral disc cells for nucleus pulposus supplementation. Biomaterials. 2006;27:388–396. doi: 10.1016/j.biomaterials.2005.06.037. [DOI] [PubMed] [Google Scholar]
- 24.Ohya S, Nakayama Y, Matsuda T. Thermoresponsive artificial extracellular matrix for tissue engineering: hyaluronic acid bioconjugated with poly(N-isopropylacrylamide) grafts. Biomacromolecules. 2001;2:856–863. doi: 10.1021/bm010040a. [DOI] [PubMed] [Google Scholar]
- 25.Mortisen D, Peroglio M, Alini M, Eglin D. Tailoring thermoreversible hyaluronan hydrogels by “click” chemistry and RAFT polymerization for cell and drug therapy. Biomacromolecules. 2010;11:1261–1272. doi: 10.1021/bm100046n. [DOI] [PubMed] [Google Scholar]
- 26.Zeiter S, der WM, Ito K. The fate of bovine bone marrow stromal cells in hydrogels: a comparison to nucleus pulposus cells and articular chondrocytes. J Tissue Eng Regen Med. 2009;3:310–320. doi: 10.1002/term.167. [DOI] [PubMed] [Google Scholar]
- 27.Gantenbein-Ritter B, Benneker LM, Alini M, Grad S. Differential response of human bone marrow stromal cells to either TGF-β(1) or rhGDF-5. Eur Spine J. 2011;20:962–971. doi: 10.1007/s00586-010-1619-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee CR, Grad S, MacLean JJ, Iatridis JC, Alini M. Effect of mechanical loading on mRNA levels of common endogenous controls in articular chondrocytes and intervertebral disk. Anal Biochem. 2005;341:372–375. doi: 10.1016/j.ab.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 29.Lapcik L, Jr, Lapcik L, Smedt S, Demeester J, Chabrecek P. Hyaluronan: preparation, structure, properties, and applications. Chem Rev. 1998;98:2663–2684. doi: 10.1021/cr941199z. [DOI] [PubMed] [Google Scholar]
- 30.Ibusuki S, Iwamoto Y, Matsuda T. System-engineered cartilage using poly(N-isopropylacrylamide)-grafted gelatin as in situ-formable scaffold: in vivo performance. Tissue Eng. 2003;9:1133–1142. doi: 10.1089/10763270360728044. [DOI] [PubMed] [Google Scholar]
- 31.Kohori F, Sakai K, Aoyagi T, Yokoyama M, Sakurai Y, Okano T. Preparation and characterization of thermally responsive block copolymer micelles comprising poly(N-isopropylacrylamide-b-dl-lactide) J Control Release. 1998;55:87–98. doi: 10.1016/S0168-3659(98)00023-6. [DOI] [PubMed] [Google Scholar]
- 32.Masters KS, Shah DN, Leinwand LA, Anseth KS. Crosslinked hyaluronan scaffolds as a biologically active carrier for valvular interstitial cells. Biomaterials. 2005;26:2517–2525. doi: 10.1016/j.biomaterials.2004.07.018. [DOI] [PubMed] [Google Scholar]
- 33.Jin R, Moreira Teixeira LS, Krouwels A, Dijkstra PJ, Blitterswijk CA, Karperien M, et al. Synthesis and characterization of hyaluronic acid-poly(ethylene glycol) hydrogels via Michael addition: an injectable biomaterial for cartilage repair. Acta Biomater. 2010;6:1968–1977. doi: 10.1016/j.actbio.2009.12.024. [DOI] [PubMed] [Google Scholar]
- 34.Chung C, Erickson IE, Mauck RL, Burdick JA. Differential behavior of auricular and articular chondrocytes in hyaluronic acid hydrogels. Tissue Eng Part A. 2008;14:1121–1131. doi: 10.1089/ten.tea.2007.0291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Recklies AD, White C, Melching L, Roughley PJ. Differential regulation and expression of hyaluronan synthases in human articular chondrocytes, synovial cells and osteosarcoma cells. Biochem J. 2001;354:17–24. doi: 10.1042/0264-6021:3540017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ohno S, Im HJ, Knudson CB, Knudson W. Hyaluronan oligosaccharide-induced activation of transcription factors in bovine articular chondrocytes. Arthritis Rheum. 2005;52:800–809. doi: 10.1002/art.20937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yoon ST. Molecular therapy of the intervertebral disc. Spine J. 2005;5:S280–S286. doi: 10.1016/j.spinee.2005.02.017. [DOI] [PubMed] [Google Scholar]
- 38.Chen WH, Liu HY, Lo WC, Wu SC, Chi CH, Chang HY, et al. Intervertebral disc regeneration in an ex vivo culture system using mesenchymal stem cells and platelet-rich plasma. Biomaterials. 2009;30:5523–5533. doi: 10.1016/j.biomaterials.2009.07.019. [DOI] [PubMed] [Google Scholar]
- 39.Chan SCW, Gantenbein-Ritter B, Leung VYL, Chan D, Cheung KMC, Ito K. Cryopreserved intervertebral disc with injected bone marrow-derived stromal cells: a feasibility study using organ culture. Spine J. 2010;10:486–496. doi: 10.1016/j.spinee.2009.12.019. [DOI] [PubMed] [Google Scholar]
- 40.Illien-Junger S, Gantenbein-Ritter B, Grad S, Lezuo P, Ferguson SJ, Alini M, et al. The combined effects of limited nutrition and high-frequency loading on intervertebral discs with endplates. Spine (Phila Pa 1976) 2010;35:1744–1752. doi: 10.1097/BRS.0b013e3181c48019. [DOI] [PubMed] [Google Scholar]