Abstract
Introduction
Increased levels of proinflammatory cytokines seem to play a pivotal role in the development of back pain in a subpopulation of patients with degenerative intervertebral disc (IVD) disease. As current treatment options are mostly limited to surgical interventions or conservative treatment, anti-inflammatory substances might offer a novel, more target-orientated therapeutic approach. Triptolide (TPL), a natural substance found in the Chinese medicinal herb Tripterygium wilfordii Hook, has been demonstrated to possess anti-inflammatory effects in various cells, but no studies exist so far for the IVD. Therefore, the aim of this study was to determine the effects of TPL on human IVD cells by analyzing changes in gene expression and underlying molecular mechanisms.
Materials and methods
In order to investigate the anti-inflammatory, anabolic and anti-catabolic effect of TPL, dose-dependency experiments (n = 5) and time course experiments (n = 5) were performed on IL-1β prestimulated human IVD cells and changes in gene expression of IL-6/-8, TNF-α, PGE2S, MMP1/2/3/13, aggrecan and collagen-I/-II were analyzed by real-time RT-PCR. The molecular mechanisms underlying the effects observed upon TPL treatment were investigated by analyzing involvement of Toll-like receptors TLR2/4 (real-time RT-PCR, n = 5), NF-κB, MAP kinases p38, ERK and JNK (immunoblotting and immunocytochemistry, n = 4) as well as RNA polymerase II (immunoblotting, n = 3).
Results
Results showed that 50 nM TPL exhibited an anti-inflammatory, anti-catabolic and anabolic effect on the mRNA level for IL-6/-8, PGE2S, MMP1/2/3/13, aggrecan, collagen-II and TLR2/4, with most pronounced changes after 18 h for proinflammatory cytokines and MMPs or 30 h for TLRs and matrix proteins. However, we also observed an up-regulation of TNF-α at higher concentrations. The effects of TPL did not seem to be mediated via an inhibition of NF-κB or a decrease of RNA polymerase II levels, but TPL influenced activity of MAP kinases p38 and ERK (but not JNK) and expression of TLR2/4.
Conclusions
In conclusion, TPL may possess promising potential for the treatment of inflammation-related discogenic back pain in vitro, but its analgetic effect will need to be confirmed in an appropriate in vivo animal model.
Keywords: Intervertebral disc, Triptolide, Gene expression, Immunoblotting, Signaling pathway
Introduction
Tripterygium wilfordii Hook F, a vine native to several Asian countries, has been widely used in the traditional Chinese medicine. Triptolide (TPL), a diterpenoid triepoxide, was identified as its major active component. It has been demonstrated to possess strong immunosuppressive and anti-inflammatory effects [1, 2]. TPL has been used in treating inflammatory joint diseases [3, 4], but could potentially also be applied in other inflammation-related diseases. As TPL has been additionally shown to possess anti-proliferative and pro-apoptotic activity in various types of cancer cells, it is considered a possible new candidate in the group of new cancer therapeutics [5].
Current literature on the intervertebral disc (IVD) provides clear evidence for the relevance of inflammatory mediators in the development of back pain. Nucleus pulposus (NP) tissue has long been known to induce radiculopathic pain, due to chemical irritation of dorsal root ganglion nerves induced by proinflammatory cytokines present in this tissue [6–10]. Similarly, degenerative disc disease (leading to so-called discogenic back pain) seems to correlate with increased levels of proinflammatory cytokines such as interleukin 1β (IL-1β), interleukin 6 (IL-6), interleukin 8 (IL-8) and tumor necrosis factor α (TNF-α) [11–15]. Although basic knowledge on the increased expression of inflammatory mediators in certain cases of back pain (i.e. discogenic back pain, NP mediated back pain) exists, the molecular mechanisms underlying these processes are not yet elucidated. However, molecular biological research of the past decades indicates that the MAP kinase pathways as well as the transcription factor nuclear factor-κB (NF-κB) are the major mechanisms regulating inflammatory responses in vivo and in vitro. Additionally, TPL has been described to not only influence NF-κB [16–20] and MAP kinases [17, 18, 21], but also to reduce levels of RNA polymerase II (=enzyme that is essential in the process of transcription) in cancer cells [5], thus possibly explaining the broad spectrum of genes whose expression is influenced upon TPL treatment.
In order to suppress inflammation, corticosteroidal substances are frequently used, which are, however, known to have a significant risk for side effects. There is thus increased interest in alternative substances that possess a strong anti-inflammatory and anti-catabolic potential. In the present study, we examined the effects of TPL on the inflammatory and catabolic response of human IVD cells that were prestimulated with recombinant IL-1β to simulate an inflammatory situation. We also analyzed the molecular mechanisms mediating the inhibition of cytokine expression by investigating the role of the transcription factor nuclear factor kappa B (NF-κB), the Toll-like receptors TLR2 and TLR4, the mitogen-activated protein (MAP) kinases including p38, ERK (=extracellular signal-regulated kinase) and JNK (=c-Jun N-terminal kinase) as well as the involvement of RNA polymerase II.
Materials and methods
Materials
Materials used in this study are specified in Table 1.
Table 1.
Detailed information about materials used in this study
| Material | Supplier |
|---|---|
| Ampicillin | Gibco |
| BSA | Sigma-Aldrich |
| Bradford reagent | Bio-Rad |
| Collagenase NB4 | Serva |
| Dispase II | Roche |
| DMSO | Sigma |
| ERK/p-ERK antibodies | Cell Signaling |
| F-12/DMEM medium | Sigma-Aldrich |
| Fetal calf serum (FCS) | Tecomedical |
| HRP-mouse antibody | Amersham |
| HRP-rabbit antibody | Sigma |
| Hyperfilm ECL | Amersham |
| Hybond-P PVDF | Amersham |
| IL-1β recombinant | Peprotech |
| JNK/p-JNK antibodies | Cell Signaling |
| MTT | Sigma-Aldrich |
| p38/p-p38 antibodies | Cell Signaling |
| p65 antibody | Santa Cruz |
| PARP1 antibody | Santa Cruz |
| PCR Master Mix | Applied Biosystems |
| Penicillin | Gibco |
| PureLink RNA Kit | Invitrogen |
| Reverse transcription reagents | Applied Biosystems |
| RNA polymerase II antibody | Santa Cruz |
| Streptomycin | Gibco |
| SuperSignal West Dura | Socochim |
| Triptolide | Sigma |
| Trypsin | Invitrogen |
| Tubulin antibody | Cell Signaling |
Human intervertebral disc cell culture
Human IVD tissue was removed from patients undergoing spinal surgery for discectomy or interbody fusion for degenerative disc disease or disc herniation (for detailed information see Table 2) after obtaining informed consent in accordance with the local ethical guidelines.
Table 2.
Demographic data on surgical disc samples used in this study
| Number | Age | Sex | Pathology | Grade | Level |
|---|---|---|---|---|---|
| 1 | 60 | M | Disc herniation | 3 | L4/5 |
| 2 | 51 | F | Sympt. disc disease | 4 | L5/S1 |
| 3 | 42 | F | Disc herniation | 4 | L4/5 |
| 4 | 50 | M | Disc herniation | 4 | L4/5 |
| 5 | 47 | M | Disc herniation | 4 | L4/5 |
| 6 | 46 | F | Disc herniation | 3 | L5/S1 |
| 7 | 54 | M | Disc herniation | 4 | L4/5 |
| 8 | 27 | F | Disc herniation | 3 | L4/5 |
| 9 | 26 | F | Disc herniation | 3 | L4/5 |
| 10 | 43 | M | Disc herniation | 4 | L4/5 |
| 11 | 43 | F | Disc herniation | 4 | L4/5 |
| 12 | 50 | F | Disc herniation | 3 | L5/S1 |
| 13 | 49 | M | Disc herniation | 4 | L5/S1 |
| 14 | 26 | M | Disc herniation | 4 | L5/S1 |
| 15 | 45 | F | Disc herniation | 4 | L5/S1 |
| 16 | 40 | F | Disc herniation | 4 | L4/5 |
| 17 | 57 | M | Disc herniation | 4 | L4/5 |
| 18 | 44 | F | Disc herniation | 3 | L5/S1 |
| 19 | 60 | M | Disc herniation | 4 | L4/5 |
| 20 | 47 | M | Disc herniation | 4 | L4/5 |
| 21 | 36 | M | Disc herniation | 3 | C5/6 |
| 22 | 53 | M | Disc herniation | 4 | L4/5 |
| 23 | 48 | F | Disc herniation | 4 | L4/5 |
| 24 | 48 | F | Disc herniation | 3 | L4/5 |
| 25 | 42 | F | Disc herniation | 4 | L4/5 |
| 26 | 60 | M | Sympt. disc disease | 3 | L4/5 |
| 27 | 46 | F | Disc herniation | 4 | L5/S1 |
| 28 | 27 | F | Disc herniation | 4 | L4/5 |
| 29 | 42 | M | Disc herniation | 3 | L4/5 |
M male, F female, grade classification of intervertebral disc degeneration by Pfirrmann grade
IVD tissue was enzymatically digested (0.2% collagenase NB4, 0.3% dispase II) for 4–8 h and cells were thereafter cultured in DMEM/F12 supplemented with 10% FCS, penicillin (50 units/ml), streptomycin (50 μg/ml) and ampicillin (125 ng/ml) up to passage 2–3.
Viability measurement
Cellular viability after treatment with different concentrations of TPL (6, 18 and 30 h) was analyzed using the MTT assay as previously described, which has also been shown to be comparable to other viability/toxicity measurements (Picogreen assay, cell counting) [22]. Non-toxic concentrations were chosen for subsequent experiments.
Gene expression analysis
In order to investigate the effects of TPL on the expression of inflammatory mediators (IL-6, IL-8, TNF-α, PGE2S = prostaglandin E2 synthase), matrix degrading enzymes (MMP1, MMP2, MMP3, MMP13), Toll-like receptors (TLR2, TLR4) and anabolic genes (aggrecan collagen-I, collagen-II), dose-dependency (0.5, 5, 50 nM—all 18 h) and time course experiments (6, 18, 30 h—all 50 nM) were performed. Cells were serum starved for 2 h and then exposed to 5 ng/ml IL-1β for 2 h before treating cells with different concentration of TPL for 18 h. The most active concentration was chosen for further time course experiments. Untreated control cells as well as DMSO treated cells (=solvent) were included in each experiment. After treatment, cells were harvested by trypsin, RNA was isolated, reverse transcribed and gene expression was measured on the StepOne Plus PCR machine (Applied Biosystems) using real-time RT-PCR as previously described. Briefly, human specific probes and primers (see Table 3), TaqMan real-time RT-PCR Mix and cDNA were mixed, measured in duplicates and data was analyzed by using the comparative ct method (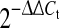 method, housekeeping gene = Tata Box binding protein = TBP). The assay was performed on samples from five independent experiments.
method, housekeeping gene = Tata Box binding protein = TBP). The assay was performed on samples from five independent experiments.
Table 3.
Primers/probes used for real-time RT-PCR (TaqMan® Gene Expression Assays, Applied Biosystems)
| Gene | Primer sequence number | Base pairs |
|---|---|---|
| Aggrecan | Hs00202971_m1 | 93 |
| Collagen-I | Hs00164004_m1 | 66 |
| Collagen-II | Hs00264051_m1 | 124 |
| Interleukin-6 (IL-6) | Hs00174131_m1 | 95 |
| Interleukin-8 (IL-8) | Hs00174103_m1 | 101 |
| Matrixmetalloproteinase-1 (MMP1) | Hs00233958_m1 | 133 |
| Matrixmetalloproteinase-2 (MMP2) | Hs00174131_m1 | 96 |
| Matrixmetalloproteinase-3 (MMP3) | Hs01548724_m1 | 98 |
| Matrixmetalloproteinase-13 (MMP13) | Hs00233992_m1 | 91 |
| Prostaglandin E2 Synthase (PGE2S) | Hs00228159_m1 | 66 |
| Tata Box binding protein (TBP) | Hs00427620_m1 | 91 |
| Toll-like receptor 2 (TLR2) | Hs00152932_m1 | 80 |
| Toll-like receptor 4 (TLR4) | Hs00152939_m1 | 89 |
| Tumor necrosis factor α (TNF-α) | Hs00174128_m1 | 80 |
Pathway analysis (NF-κB, MAP kinases)
In order to investigate potentially involved pathways, immunoblotting was performed for p65 (subunit of NF-κB) as well as for the phosphorylated (=activated) and unphosphorylated MAP kinases p38, ERK and JNK. Briefly, cells were stimulated with IL-1β or co-stimulated with IL-1β and 50 nM of TPL (the most potent concentration in gene expression experiments) for 15 min (MAP kinases) or 60 min (NF-κB). Unstimulated control cells were included as well.
For NF-κB immunoblotting, nuclear extracts were prepared according to standard protocols, protein content was measured by Bradford assay, nuclear extracts were fractioned by SDS-PAGE, proteins were transferred onto membranes and first incubated with a p65 antibody and then with the appropriate secondary HRP-labeled antibody before analyzing chemiluminescence. In order to confirm NF-κB immunoblotting results, nuclear translocation of p65 was additionally examined by immunocytochemistry in methanol-fixed cells using standard techniques.
For MAP kinase immunoblotting, whole cell extracts were prepared as previously described, protein content was measured by Bradford assay, whole cell extracts were fractioned by SDS-PAGE, proteins were transferred onto membranes and first incubated with antibodies recognizing phosphorylated or unphosphorylated p38, ERK (=p42/44) or JNK antibody and then with the appropriate secondary HRP-labeled antibody before analyzing chemiluminescence.
PARP1 and tubulin were used as loading controls for p65 and MAP kinase immunoblotting, respectively. Each assay was performed on samples from four independent experiments.
Analysis of RNA polymerase II protein expression level
The effect of TPL on RNA polymerase II expression was investigated by immunoblotting of whole cell extracts that were obtained from cells stimulated with either IL-1β alone or IL-1β prestimulated cells with 50 nM of TPL for 6, 18 or 30 h (see “Gene expression analysis”), using a specific antibody recognizing human RNA polymerase II. Tubulin was used as a loading control and the assay was performed on samples from three independent experiments.
Statistical analysis
Statistical analysis was performed by Mann–Whitney U Test (two-tailed) using the SPSS software. A significance level of P < 0.05 was considered statistically significant.
Results
Viability
TPL exhibited a cytotoxic effect at concentrations of 250 nM and higher already within 18 h (data not shown). For further experiments, non-toxic concentrations of 0.5, 5 and 50 nM were chosen, which did not exhibit any statistically significant cytotoxic effect within the time frame of the subsequent experiments (up to 30 h).
Gene expression
Confirming our data from previous studies, pre-treatment with IL-1β stimulated expression of IL-6, IL-8, TNF-α, MMP1, MMP2, MMP3, MMP13 as well as TLR2 and reduced expression of aggrecan, collagen-I and collagen-II (for detailed results, see Table 4), but did not influence expression of PGE2S and TLR4.
Table 4.
Effects of IL-1β stimulation on mRNA levels of candidate genes after 6, 18 and 30 h, indicated as fold change relative to no treatment (mean, SEM, P values)
| Gene | Time point (h) | Mean fold change | SEM | P value |
|---|---|---|---|---|
| Aggrecan | 6 | −1.4 | 0.3 | 0.001 |
| 18 | −1.8 | 0.5 | 0.001 | |
| 30 | −2.7 | 0.5 | <0.0001 | |
| Collagen-I | 6 | −1.0 | 0.2 | 0.001 |
| 18 | −1.3 | 0.5 | 0.015 | |
| 30 | −2.1 | 0.2 | <0.0001 | |
| Collagen-II | 6 | −2.1 | 0.2 | <0.0001 |
| 18 | −8.9 | 3.2 | <0.0001 | |
| 30 | −7.2 | 1.1 | <0.0001 | |
| IL-6 | 6 | 432.7 | 74.3 | <0.0001 |
| 18 | 6236.1 | 2601.3 | <0.0001 | |
| 30 | 7350.5 | 2557.3 | <0.0001 | |
| IL-8 | 6 | 558.4 | 175.2 | <0.0001 |
| 18 | 1102.5 | 291.3 | <0.0001 | |
| 30 | 1983.8 | 649.3 | <0.0001 | |
| MMP1 | 6 | 449.1 | 163.9 | <0.0001 |
| 18 | 1576.5 | 470.9 | <0.001 | |
| 30 | 2032.6 | 631.9 | <0.0001 | |
| MMP2 | 6 | 0.7 | 0.3 | 0.419 |
| 18 | 2.1 | 0.5 | <0.0001 | |
| 30 | 3.4 | 0.3 | <0.0001 | |
| MMP3 | 6 | 327.7 | 124.9 | <0.0001 |
| 18 | 1143.5 | 240.7 | <0.0001 | |
| 30 | 1768.8 | 636.0 | <0.0001 | |
| MMP13 | 6 | 79.1 | 20.6 | <0.0001 |
| 18 | 114.6 | 30.2 | <0.0001 | |
| 30 | 297.3 | 140.0 | <0.0001 | |
| TNF-α | 6 | 78.5 | 16.4 | <0.0001 |
| 18 | 41.1 | 14.4 | <0.0001 | |
| 30 | 46.0 | 11.3 | <0.0001 | |
| TLR2 | 6 | 16.5 | 1.9 | <0.0001 |
| 18 | 13.5 | 1.2 | <0.0001 | |
| 30 | 13.4 | 2.1 | <0.0001 |
Data was obtained by real-time RT-PCR (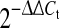 method) (n = 5). PGE2S and TLR4 were not significantly regulated by IL-1β stimulation (values not shown)
method) (n = 5). PGE2S and TLR4 were not significantly regulated by IL-1β stimulation (values not shown)
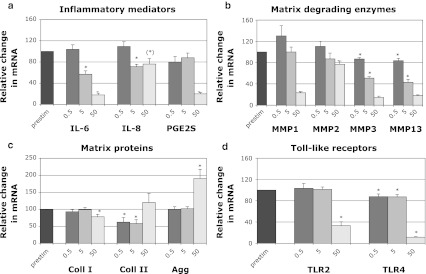
In the first set of experiments, IL-1β prestimulated cells were treated with different concentrations of TPL (0.5, 5, 50 nM) for 18 h. We observed a dose-dependent inhibition of the expression of inflammatory mediators (IL-6, IL-8, PGE2S), matrix degrading enzymes (MMP1, MMP2, MMP3, MMP13) and Toll-like receptors (TLR2, TLR4). For aggrecan, a 1.9-fold increase was observed with 50 nM after 18 h, while no changes occurred with the lower concentrations. Collagen-I and collagen-II were either not altered or slightly decreased after 18 h. For all results, see Fig. 1a–d. TNF-α expression was increased at concentrations of 5 nM (2.8-fold) and 50 nM (21.2-fold) (data not shown).
Fig. 1.
Effects of different concentrations of TPL (0.5, 5, 50 nM—18 h) on mRNA levels of candidate genes, indicated as fold change relative to IL-1β-prestimulation (set to 100%): a inflammatory mediators (IL-6, IL-8, PGE2S), b matrix degrading enzymes (MMP1, MMP2, MMP3, MMP13), c matrix proteins (aggrecan, collagen-I, collagen-II) and d Toll-like receptors (TLR2, TLR4). Data was obtained by real-time RT-PCR (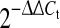 method) and is presented as Mean and SEM (n = 5). Asterisks indicate statistical significance (P < 0.05); an asterisk in brackets indicates barely significant (IL-8: P = 0.051)
method) and is presented as Mean and SEM (n = 5). Asterisks indicate statistical significance (P < 0.05); an asterisk in brackets indicates barely significant (IL-8: P = 0.051)
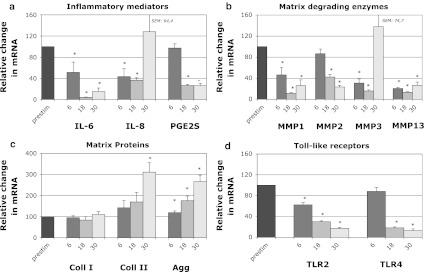
For the second set of experiments, IL-1β prestimulated cells were treated with 50 nM TPL for 6, 18 or 30 h (time course experiments). Results show that TPL exhibits its anti-inflammatory, anti-catabolic and anabolic effects already after 6 h with regard to IL-6, IL-8, MMP1, MMP3, MMP13, aggrecan and TLR2, but its effects were more pronounced after longer incubation periods (18 and 30 h), including an increase of collagen-II (Fig. 2a–d). The most distinct reduction in gene expression was observed at 18 h for IL-6 (100 → 4.1%), MMP1 (100 → 11.1%), MMP3 (100 → 15.7%), MMP13 (100 → 13.3%) and TLR4 (100 → 18.2%), but effects were also significant for IL-8 (100 → 36.5%), MMP2 (100 → 42.2%), PGE2S (100 → 26.4%) and TLR2 (100 → 30.1%). We observed a time-dependent up-regulation of aggrecan with a significantly increase after 18 h (1.8-fold), but the effect was more pronounced after 30 h (2.7-fold). Similarly, collagen-II expression was significantly induced after 30 h (3.1-fold), while collagen-I expression was not altered at any time point. While matrix proteins were most regulated after 30 h, effects declined for several genes at this time point already. Nevertheless, the inhibitory effect remained significant for IL-6, MMP1, MMP2, MMP13, PGE2S, TLR2 and TLR4 (Fig. 2a–d). In accordance with results of the dose-dependency experiments, TNF-α expression was up-regulated, especially after 30 h (6 h: 4.8-fold; 18 h: 101.0-fold; 30 h: 987.3-fold) (data not shown).
Fig. 2.
Effects of TPL (50 nM) on mRNA levels of candidate genes after different time points (6, 18, 30 h), indicated as fold change relative to IL-1β-prestimulation (set to 100%): a Inflammatory mediators (IL-6, IL-8, PGE2S), b matrix degrading enzymes (MMP1, MMP2, MMP3, MMP13), c matrix proteins (aggrecan, collagen-I, collagen-II) and d Toll-like receptors (TLR2, TLR4). Data was obtained by real-time RT-PCR (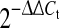 method) and is presented as Mean and SEM (n = 6). Asterisks indicate statistical significance (P < 0.05)
method) and is presented as Mean and SEM (n = 6). Asterisks indicate statistical significance (P < 0.05)
NF-κB and MAP kinase pathway
Immunoblotting for p65 indicates that IL-1β prestimulation caused nuclear translocation of p65, which is the first step of NF-κB activation. However, treating IL-1β prestimulated cells with 50 nM TPL was not able to prevent or reverse nuclear translocation of NF-κB. Figure 3a shows that the p65 band of TPL treated samples is not reduced compared to IL-1β stimulated samples, while untreated cells show a much smaller amount of target protein as detected by immunoblotting of nuclear extracts. Equal protein loading was confirmed by PARP1 detection. This pattern could be confirmed by immunocytochemistry as shown in Fig. 3b.
Fig. 3.
Effects of TPL (50 nM) on the induction/activity of NF-κB and MAP kinases, detected by immunoblotting (IB) and immunocytochemistry (IC). NF-κB induction was detected by a IB of nuclear extracts (n = 4, 60 min) and b IC (n = 4, 60 min). MAP kinase activity was detected by IB of whole cell extracts (n = 4, 15 min) for c p38, d ERK and e JNK. One representative sample is shown. For IB, PARP1 or tubulin was used as a loading control. For IC, Hoechst counterstaining of cell nuclei was performed
Immunoblotting for MAP kinases indicated that IL-1β prestimulation caused phosphorylation of p38, ERK and JNK, which is indicative of their activation. TPL treatment (50 nM) strongly reduced levels of phosphorylated p38 (Fig. 3c) and slightly reduced levels of phosporylated ERK (Fig. 3d), but not of JNK (Fig. 3e) compared to IL-1β stimulated samples. As expected, levels of unphosporylated p38, ERK and JNK were similar in all groups. Equal protein loading was confirmed by tubulin detection.
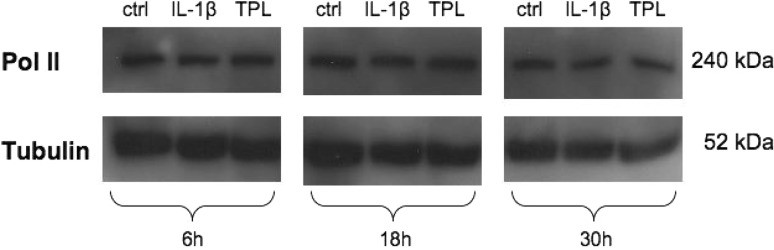
RNA polymerase II protein expression level
Immunoblotting for RNA polymerase II indicates that 50 nM TPL did not influence its expression levels at any investigated time point (6, 18, 30 h), compared to IL-1β stimulated samples or untreated samples (Fig. 4).
Fig. 4.
Effects of TPL (50 nM) on the expression levels of RNA polymerase II, detected by immunoblotting of whole cell extracts (n = 3) (6, 18, 30 h). One representative sample is shown. Tubulin was used as a loading control
Discussion
Tripterygium wilfordii Hook has its therapeutic origin in the traditional Chinese medicine (TCM), which—during the past years—has been recognized by researchers world-wide as an important and extensive source for revealing novel lead molecules for modern drug discovery. However, typical TCM preparations usually combine multiple herbs (or other natural substances) with the respective variability in composition (e.g. due to harvesting periods) and can thus not be regarded as standardized products. In order to create patentable and marketable products for conventional Western medicine, the active component has to be identified and its molecular mechanism of action should be elucidated. TPL has been identified as one of the major bioactive components of Tripterygium wilfordii Hook. The therapeutic potential of TPL or Tripterygium wilfordii Hook is currently investigated at the stage of clinical trials for the treatment of polycystic kidney disease, glomerulosclerosis, nephropathy, spondylitis and rheumatoid arthritis (http://clinicaltrials.gov). On a laboratory stage, the anti-inflammatory and anti-catabolic effects of TPL are currently being investigated in even more cell and tissue types, thus trying to broaden its therapeutic use. However, to the authors’ knowledge, no studies have been performed with regard to IVD diseases yet.
Results from this study on human IVD cells indicate that TPL can effectively reduce mRNA levels of major inflammatory mediators (IL-6, IL-8, PGES2) and matrix degrading enzymes (MMP1, MMP2, MMP3, MMP13), with highest effects after 18 h. However, mRNA expression of TNF-α was up-regulated upon TPL treatment. Additionally, 50 nM TPL significantly induced levels of relevant matrix proteins (aggrecan, collagen-II), especially after 30 h. Why lower concentrations of TPL seemed to inhibit collagen-II expression at earlier time points (18 h) is currently not clear. While matrix proteins responded strongly to TPL treatment after 30 h, effects were not as pronounced anymore at this time point for most inflammatory and catabolic genes, indicating that TPL may possess a limited bioactivity time frame. However, usage of a slow release system may overcome this restriction. Pathway analysis provides evidence that this effect may be (at least partially) mediated by the MAP kinases p38 and ERK (whose activity we saw to be influenced by TPL), while the transcription factor NF-κB, the MAP kinase JNK or the RNA polymerase II did not seem to be involved in signal transduction. Additionally, expression of Toll-like receptors TLR2 and TLR4 was reduced by TPL treatment.
The observed inhibition of mRNA for the major matrix degrading enzymes and the induction of matrix proteins in human IVD cells suggests that TPL can block tissue degradation and may thus potentially slow down or prevent further disc degeneration. A comparable anti-catabolic potential of TPL has been described in other cell types before, e.g. TPL was shown to inhibit expression of proMMPs 1 and 3 in human synovial fibroblasts [23] and expression of MMP3, MMP13 and ADAMTS4 in human OA chondrocytes [24].
In the present in vitro study, TPL was able to inhibit inflammatory responses in human IVD cells, which is similar to macrophages (inhibition of PGE2, IL-1α, IL-β and IL-6 expression) [23] and various kinds of fibroblasts (inhibition of PGE2, COX-2, IL-6 and IL-8 expression) [20, 25–27]. Although to the author’s knowledge only few studies exist to date that investigated the anti-inflammatory in vitro effect of TPL in chondrocytes [24], its effects were tested in a collagen-induced arthritis mouse model in vivo. Results indicate that TPL can reduce inflammatory responses and cartilage damage in the joint tissues by inhibiting expression of MMP3, MMP13, PGE2, COX-2, IL-1β, IL-6, TNF-α [16–18]. While we can confirm the inhibition of MMP3, MMP13 and IL-6 in human IVD cells in vitro, expression of TNF-α was induced in our system and will thus be subject to further scrutiny in next experiments. The increased level of TNF-α may not only be critical for any in vivo application, but may have also influenced our results if transferred to the protein level as it may be responsible for the sustained and unaltered NF-κB activation. Activation of NF-κB has been described to cause an anti-apoptotic effect, thus possibly masking any toxic effect of TPL [28]. However, in order to verify whether this is the case, experiments with NF-κB inhibition (e.g. using Ad5-I kappa B alpha Delta N; MG132 [28]) will need to be performed in the future. This will be important as in tumor cells, TPL could in contrast block TNF-α-induced activation of NF-κB, resulting in enhanced apoptosis induced by TNF-α [29].
Based on the obtained results, the next step will be to investigate the anti-inflammatory and analgetic behavior of TPL in vivo, using a well-established rodent model of radiculopathic pain [30]. As bioavailability and diffusion rates in vivo are unclear, the in vitro data obtained in this study can only provide a first basis to choose appropriate in vivo application modes.
Based on the promising gene expression results, we further sought to investigate the underlying molecular mechanism. Findings in the literature indicate that the NF-κB pathway is one of the molecular mechanisms underlying the cellular responses observed after TPL treatment [16–20]. However, the effects of TPL seem to be mediated by a complex interplay of various signaling mechanisms, including the MAP kinases p38 [17], ERK [21] or JNK [17, 18] as well as the transcription factor AP-1 [19]. We were able to show that in IVD cells, TPL may work in part by interfering with phosphorylation of the MAP kinases p38/ERK and by regulating expression of TLR2/4, but probably not by inhibiting NF-κB or JNK activity, although this is described for multiple other cell types [16–20], indicating that the involved signaling pathways are cell-specific. In addition, we investigated whether TPL is able to influence protein levels of RNA polymerase II as this has been described to be a major mechanism of action of TPL in cancer cells most recently [5]. However, in human disc cells, TPL did not reduce RNA polymerase II levels as shown by immunoblotting, indicating once more that TPL acts cell-specifically on certain mechanisms/pathways.
Clinical relevance
TPL seems to be a promising candidate to treat certain cases of discogenic or NP mediated back pain, in which increased levels of proinflammatory cytokines are responsible for pain sensation. The in vitro cell culture results clearly showed that TPL could effectively inhibit several proinflammatory cytokines and matrix degrading enzymes, which are thought to play a major role during symptomatic disc degeneration. Simultaneously, TPL induced expression of disc-specific matrix proteins. However, in vivo experiments will be needed to verify that TPL is an attractive, new therapeutic agent for degenerative disc disease by preventing further degradation of the tissue and exhibiting an analgetic effect due to inhibition of proinflammatory cytokines.
Acknowledgments
This study was made possible by grants from AOSpine (SRN 02/103 and AOSBRC-07-03). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of AOSpine. The authors gratefully acknowledge Andreas Plewnia for technical assistance.
Conflict of interest
None.
References
- 1.Chen BJ. Triptolide, a novel immunosuppressive and anti-inflammatory agent purified from a Chinese herb Tripterygium wilfordii Hook F. Leuk Lymphoma. 2001;42:253–265. doi: 10.3109/10428190109064582. [DOI] [PubMed] [Google Scholar]
- 2.Qiu D, Kao PN. Immunosuppressive and anti-inflammatory mechanisms of triptolide, the principal active diterpenoid from the Chinese medicinal herb Tripterygium wilfordii Hook. f. Drugs R D. 2003;4:1–18. doi: 10.2165/00126839-200304010-00001. [DOI] [PubMed] [Google Scholar]
- 3.Brinker AM, Ma J, Lipsky PE, Raskin I. Medicinal chemistry and pharmacology of genus Tripterygium (Celastraceae) Phytochemistry. 2007;68:732–766. doi: 10.1016/j.phytochem.2006.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yu DY. Clinical observation of 144 cases of rheumatoid arthritis treated with glycoside of Radix Tripterygium Wilfordii. J Tradit Chin Med. 1983;3:125–129. [PubMed] [Google Scholar]
- 5.Pan J (2010) RNA polymerase—an important molecular target of triptolide in cancer cells. Cancer Lett 292:149–152 [DOI] [PubMed]
- 6.Suzuki M, Inoue G, Gemba T, Watanabe T, Ito T, Koshi T, Yamauchi K, Yamashita M, Orita S, Eguchi Y, Ochiai N, Kishida S, Takaso M, Aoki Y, Takahashi K, Ohtori S. Nuclear factor-kappa B decoy suppresses nerve injury and improves mechanical allodynia and thermal hyperalgesia in a rat lumbar disc herniation model. Eur Spine J. 2009;18:1001–1007. doi: 10.1007/s00586-009-0940-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yamashita M, Ohtori S, Koshi T, Inoue G, Yamauchi K, Suzuki M, Takahashi K. Tumor necrosis factor-alpha in the nucleus pulposus mediates radicular pain, but not increase of inflammatory peptide, associated with nerve damage in mice. Spine (Phila Pa 1976) 2008;33:1836–1842. doi: 10.1097/BRS.0b013e31817bab2a. [DOI] [PubMed] [Google Scholar]
- 8.Kallakuri S, Takebayashi T, Ozaktay AC, Chen C, Yang S, Wooley PH, Cavanaugh JM. The effects of epidural application of allografted nucleus pulposus in rats on cytokine expression, limb withdrawal and nerve root discharge. Eur Spine J. 2005;14:956–964. doi: 10.1007/s00586-004-0773-6. [DOI] [PubMed] [Google Scholar]
- 9.Cuellar JM, Montesano PX, Carstens E. Role of TNF-alpha in sensitization of nociceptive dorsal horn neurons induced by application of nucleus pulposus to L5 dorsal root ganglion in rats. Pain. 2004;110:578–587. doi: 10.1016/j.pain.2004.03.029. [DOI] [PubMed] [Google Scholar]
- 10.Olmarker K, Blomquist J, Stromberg J, Nannmark U, Thomsen P, Rydevik B. Inflammatogenic properties of nucleus pulposus. Spine (Phila Pa 1976) 1995;20:665–669. doi: 10.1097/00007632-199503150-00006. [DOI] [PubMed] [Google Scholar]
- 11.Burke JG, Watson RW, McCormack D, Dowling FE, Walsh MG, Fitzpatrick JM. Intervertebral discs which cause low back pain secrete high levels of proinflammatory mediators. J Bone Joint Surg Br. 2002;84:196–201. doi: 10.1302/0301-620X.84B2.12511. [DOI] [PubMed] [Google Scholar]
- 12.Le Maitre CL, Freemont AJ, Hoyland JA. The role of interleukin-1 in the pathogenesis of human intervertebral disc degeneration. Arthritis Res Ther. 2005;7:R732–R745. doi: 10.1186/ar1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weiler C, Nerlich AG, Bachmeier BE, Boos N. Expression and distribution of tumor necrosis factor alpha in human lumbar intervertebral discs: a study in surgical specimen and autopsy controls. Spine (Phila Pa 1976) 2005;30:44–53. doi: 10.1097/01.brs.0000174529.07959.c0. [DOI] [PubMed] [Google Scholar]
- 14.Shamji MF, Setton LA, Jarvis W, So S, Chen J, Jing L, Bullock R, Isaacs RE, Brown C, Richardson WJ (2010) Proinflammatory cytokine expression profile in degenerated and herniated human intervertebral disc tissues. Arthritis Rheum 62:1974–1982 [DOI] [PMC free article] [PubMed]
- 15.Bachmeier BE, Nerlich AG, Weiler C, Paesold G, Jochum M, Boos N. Analysis of tissue distribution of TNF-alpha, TNF-alpha-receptors, and the activating TNF-alpha-converting enzyme suggests activation of the TNF-alpha system in the aging intervertebral disc. Ann N Y Acad Sci. 2007;1096:44–54. doi: 10.1196/annals.1397.069. [DOI] [PubMed] [Google Scholar]
- 16.Lin N, Liu C, Xiao C, Jia H, Imada K, Wu H, Ito A. Triptolide, a diterpenoid triepoxide, suppresses inflammation and cartilage destruction in collagen-induced arthritis mice. Biochem Pharmacol. 2007;73:136–146. doi: 10.1016/j.bcp.2006.08.027. [DOI] [PubMed] [Google Scholar]
- 17.Gong Y, Xue B, Jiao J, Jing L, Wang X. Triptolide inhibits COX-2 expression and PGE2 release by suppressing the activity of NF-kappa B and JNK in LPS-treated microglia. J Neurochem. 2008;107:779–788. doi: 10.1111/j.1471-4159.2008.05653.x. [DOI] [PubMed] [Google Scholar]
- 18.Kim YH, Lee SH, Lee JY, Choi SW, Park JW, Kwon TK. Triptolide inhibits murine-inducible nitric oxide synthase expression by down-regulating lipopolysaccharide-induced activity of nuclear factor-kappa B and c-Jun NH2-terminal kinase. Eur J Pharmacol. 2004;494:1–9. doi: 10.1016/j.ejphar.2004.04.040. [DOI] [PubMed] [Google Scholar]
- 19.Liou JT, Chen ZY, Ho LJ, Yang SP, Chang DM, Liang CC, Lai JH. Differential effects of triptolide and tetrandrine on activation of COX-2, NF-kappa B, and AP-1 and virus production in dengue virus-infected human lung cells. Eur J Pharmacol. 2008;589:288–298. doi: 10.1016/j.ejphar.2008.04.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lu Y, Fukuda K, Nakamura Y, Kimura K, Kumagai N, Nishida T. Inhibitory effect of triptolide on chemokine expression induced by proinflammatory cytokines in human corneal fibroblasts. Invest Ophthalmol Vis Sci. 2005;46:2346–2352. doi: 10.1167/iovs.05-0010. [DOI] [PubMed] [Google Scholar]
- 21.Lin J, Chen L, Lin Z, Zhao M. Inhibitory effect of triptolide on glioblastoma multiforme in vitro. J Int Med Res. 2007;35:490–496. doi: 10.1177/147323000703500408. [DOI] [PubMed] [Google Scholar]
- 22.Quero L, Klawitter M, Nerlich AG, Leonardi M, Boos N, Wuertz K (2010) Bupivacaine—the deadly friend of intervertebral disc cells? Spine J 11:46–53 [DOI] [PubMed]
- 23.Lin N, Sato T, Ito A. Triptolide, a novel diterpenoid triepoxide from Tripterygium wilfordii Hook. f. suppresses the production and gene expression of pro-matrix metalloproteinases 1 and 3 and augments those of tissue inhibitors of metalloproteinases 1 and 2 in human synovial fibroblasts. Arthritis Rheum. 2001;44:2193–2200. doi: 10.1002/1529-0131(200109)44:9<2193::AID-ART373>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 24.Liacini A, Sylvester J, Zafarullah M. Triptolide suppresses proinflammatory cytokine-induced matrix metalloproteinase and aggrecanase-1 gene expression in chondrocytes. Biochem Biophys Res Commun. 2005;327:320–327. doi: 10.1016/j.bbrc.2004.12.020. [DOI] [PubMed] [Google Scholar]
- 25.Tao QS, Ren JA, Li JS. Triptolide suppresses IL-1beta-induced chemokine and stromelysin-1 gene expression in human colonic subepithelial myofibroblasts. Acta Pharmacol Sin. 2007;28:81–88. doi: 10.1111/j.1745-7254.2007.00482.x. [DOI] [PubMed] [Google Scholar]
- 26.Lu Y, Liu Y, Fukuda K, Nakamura Y, Kumagai N, Nishida T. Inhibition by triptolide of chemokine, proinflammatory cytokine, and adhesion molecule expression induced by lipopolysaccharide in corneal fibroblasts. Invest Ophthalmol Vis Sci. 2006;47:3796–3800. doi: 10.1167/iovs.06-0319. [DOI] [PubMed] [Google Scholar]
- 27.Tao X, Schulze-Koops H, Ma L, Cai J, Mao Y, Lipsky PE. Effects of Tripterygium wilfordii hook F extracts on induction of cyclooxygenase 2 activity and prostaglandin E2 production. Arthritis Rheum. 1998;41:130–138. doi: 10.1002/1529-0131(199801)41:1<130::AID-ART16>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 28.Bergmann MW, Loser P, Dietz R, Harsdorf R. Effect of NF-kappa B Inhibition on TNF-alpha-induced apoptosis and downstream pathways in cardiomyocytes. J Mol Cell Cardiol. 2001;33:1223–1232. doi: 10.1006/jmcc.2001.1385. [DOI] [PubMed] [Google Scholar]
- 29.Liu Q (2010) Triptolide and its expanding multiple pharmacological functions. Int Immunopharmacol 11:377–383 [DOI] [PubMed]
- 30.Sasaki N, Kikuchi S, Konno S, Sekiguchi M, Watanabe K. Anti-TNF-alpha antibody reduces pain-behavioral changes induced by epidural application of nucleus pulposus in a rat model depending on the timing of administration. Spine (Phila Pa 1976) 2007;32:413–416. doi: 10.1097/01.brs.0000255097.18246.bc. [DOI] [PubMed] [Google Scholar]