Abstract
Introduction
Notochordal cells and nucleus pulposus cells are co-existing in the intervertebral disc at various ratios among different mammalians. This fact rises the question about the interactions and the evolutionary relevance of this phenomenon. It has been described that these relatively large notochordal cells are mainly dominant in early lifetime of all vertebrates and then differences occur with ageing. Human, cattle, sheep, and goat lose the cells with age, whereas rodents and lagomorphs maintain these throughout their lifetime.
Materials and methods
Here, we addressed the importance of cell ratio using alginate bead 3-D co-culture of bovine nucleus pulposus cells (bNPC) and porcine notochordal cells (pNCs) for 14 days using culture inserts.
Result
We found a significant stimulation of bNPC in the presence of pNC in terms of cell activity and glycosaminoglycan production, but not for proliferation (DNA content). Relative gene expression was significantly stimulated for collagen type 2 and aggrecan.
Conclusion
The stimulating effect of NC was confirmed and the ideal ratio of NPC: NC was found to be ~50:50. This has direct implications for tissue-engineering approaches, which aim to repopulate discs with NP-like precursor cells.
Keywords: Co-culture, Notochord, Nucleus pulposus, Proteoglycan/DNA content, Relative gene expression
Introduction
Notochordal cells (NC) are remnant cells originating from the notochord present in all chordates in early embryogenesis and these cells are located in the center of the intervertebral disc [6, 17, 20, 37]. With ageing, these presumably progenitor-like cells disappear in some species and in other species they persist up to adulthood [5, 28]. In human, they disappear early in childhood [19]. Strikingly, these cells co-exist with nucleus pulposus cells (NPCs) at different ratios among different vertebrate species [28]. Rodents (rats and mice) and lagomorphs (e.g., rabbits) maintain a high number of NC cells throughout their lifetime, whereas in other animals such as bovine, goat, and sheep these cells disappear early in lifetime [19, 20].
Previous study on co-culture of non-chondrodystrophoid dog cells (e.g., Greyhound) with bovine NPCs seems to point toward regulatory mechanism and positive cell–cell interaction [1, 3, 22]. It has been speculated that these cells have precursor character and might belong to the exact same cell lineage as the disc cells since there were not too many differences reported between these two lineages [27, 32]. Other research groups are convinced that these cells are originating from another cell layer than the mesoderm, but are rather ectodermal origin. Here, we hypothesised that whether there is a ratio of NC relative to NPC cells, which is most favourable for both cell populations in terms of cell activity and extracellular matrix (ECM) production and whether these cells can influence each other by secretion of soluble factors as previous experiments have been demonstrated with co-cultures of a single cell–cell ratio [1, 3].
We hypothesize that cells of these two phenotypes are possibly influencing each other by soluble cytokines released into the media and that there is a mutualism between these cells. Thus, we systematically co-cultured porcine coccygeal NCs (in fact a NCs + NPC mix) and bovine coccygeal NPCs at different ratios, i.e., 0, 25, 50, 75 and 100%, respectively.
Materials and methods
Cell source and expansion
Porcine notochordal cells (pNCs) were isolated from the nucleus pulposus (NP) tissue of 4 to 5-month-old porcine tails obtained from the local abattoir. The high percentage of NCs in porcine NP tissue was confirmed by size and the haemocytometer using bright-field microscopy (~80%). Bovine nucleus pulposus cells (bNPCs) were harvested from the NP tissue of ~1-year-old bovine tails obtained from the local abattoir. Both cells were separated from native ECM by 0.19% pronase digestion (Roche, Basel, Switzerland) for 1 h and subsequent collagenase type 2 (Worthington, London, UK) digestion overnight (~14 h) and primary culture. The NCs from porcine NP tissue were expanded in monolayer up to Passage 2, which has been previously described as non-problematic concerning de-differentiation [3]. This expansion step of NCs was necessary since the cell yield of cell isolation was much lower (~1 × 106 cells) for porcine coccygeal disc cells relative to the bovine tails, and ~8 × 106 cells per cell type were used for each co-culture experiment.
3-D cell encapsulation and co-culture
The cells were encapsulated at a density of 4 × 106 cells/mL into 1.2% alginate by the application of a syringe/22G needle and by formation of ~30 μl droplets into a 102 mM CaCl2 salt solution [25]. Assuming porcine NP tissue to be 100% notochordal, the cells were kept in co-culture of pNC:bNPC ratios of 0, 25, 50, 75 and 100% in serum-free defined medium, containing 100 μg/mL penicillin/streptomycin, 50 μg/mL ascorbic acid, ITS + (Sigma, Buchs, Switzerland) and non-essential amino acids (Gibco + Sigma, Switzerland). All bead–bead co-cultures were conducted in duplicate in 12-well plates, using 0.4-μm pore size, high pore density, polyethylene terephthalate (PET) track-etched culture inserts (Becton, Dickinson and Company, Allschwil, Switzerland). The co-cultures were tracked on day 0, 7, and day 14. There were four co-culture pairings (thus, each N = 4 for the porcine and bovine animals).
Metabolic activity
Cell activity of the cells in alginate beads was measured using Alamar Blue® assay (Invitrogen, Bale, Switzerland) [2]. Two beads per condition were incubated in 500 μL of DMEM with 10% of FCS and Alamar Blue for 3.5 h in a 48-well plate. Relative fluorescence unit (RFU) was measured at an excitation wavelength of 547 nm and an emission wavelength of 582 nm using a microplate reader (Infinite 200, Tecan, Männedorf, Switzerland). RFU measured for each tissue was normalized with the amount of DNA.
Quantification of GAG and DNA content
Alginate beads from the Alamar Blue assay were digested with papain (Sigma–Aldrich, Bale, Switzerland) overnight at 60°C. The papain-digested samples were used for glycosaminoglycan (GAG) and DNA measurement. The GAG content was measured by the modified dimethylmethylene blue (DMMB) assay (pH 1.5) [7, 11]. The absorbance of the samples added to the DMMB buffer was read at 595 nm with a spectrophotometer. GAG concentrations were calculated from a standard curve obtained with chondroitin sulfate (Sigma–Aldrich). The amount of DNA in the sample was measured with bisbenzimidol fluorescent dye (Hoechst 33258, Sigma–Aldrich). Fluorescence was detected with Hoefer DyNAQuant (Amersham Bioscience, San Francisco, CA, US). A standard curve was generated with known concentrations of calf thymus DNA (Sigma–Aldrich, Buchs, Switzerland) and the amount of DNA of each sample was calculated from the standard curve.
Relative RT-PCR
Relative gene expression at major anabolic genes was monitored, i.e., ACAN, collagen type 1 and 2 (Col 1 and Col 2, respectively), and ribosomal 18S as a reference gene [23, 26, 34]. Due to DNA substitutions between the porcine and bovine codon sequences of one of the two primer regions, species-specific RT-PCR was designed (Table 1) for pNCs and bNPCs. Relative gene expression was estimated by the application of a threshold cycle (Ct) and calculation of ∆∆Ct and the statistics of the 2−∆∆C according to Livak and Schmittgen [24].
Table 1.
Primer sequences used for relative real-time RT-PCR
| Gene | Forward (5′–3′) | Reverse (5′–3′) |
|---|---|---|
| Bovine (Bos taurus) | ||
| Bt_r18S | ACG GAC AGG ATT GAC AGA TTG | CCA GAG TCT CGT TCG TTA TCG |
| Bt_ACAN | GGC ATC GTG TTC CAT TAC AG | ACT CGT CCT TGT CTC CAT AG |
| Bt_col1 A2 | GCC TCG CTC ACC AAC TTC | AGT AAC CAC TGC TCC ATT CTG |
| Bt_col2 A2* | CGG GTG AAC GTG GAG AGA CA | GTC CAG GGT TGC CAT TGG AG |
| Porcine (Sus scrofa domestica) | ||
| Ssd_r18S | TAG AAG GAA GAG GAA CCA T | TAA TGT CCA ACT CAC TGA AG |
| Ssd_ACAN | CAG TAA CTT CGT GCC TAG | GGT CCT CTA TCT CCA GTT |
| Ssd_col1 A2 | TAT CGG AAT TAA CCA GAC A | ACA GGA TTG ACA GAT TGA |
* Denotes primer match for both species
Statistical analyses
All data are given as relative to the pure cell population of the same culture day. Statistical significance was tested using one-way ANOVA and Bonferroni’s multiple comparison test using GraphPad Prism version 5.0d, GraphPad Software, San Diego, CA, USA, http://www.graphpad.com. Post-hoc power analysis was run with G*Power software [12] to determine the type II error of the statistical tests. The power analysis revealed that it was >0.80 for the gene expression tests and the Alamar Blue data using ANOVA, and it was ~70% for the DNA and GAG statistics.
Results
Cell proliferation, metabolism and GAG synthesis
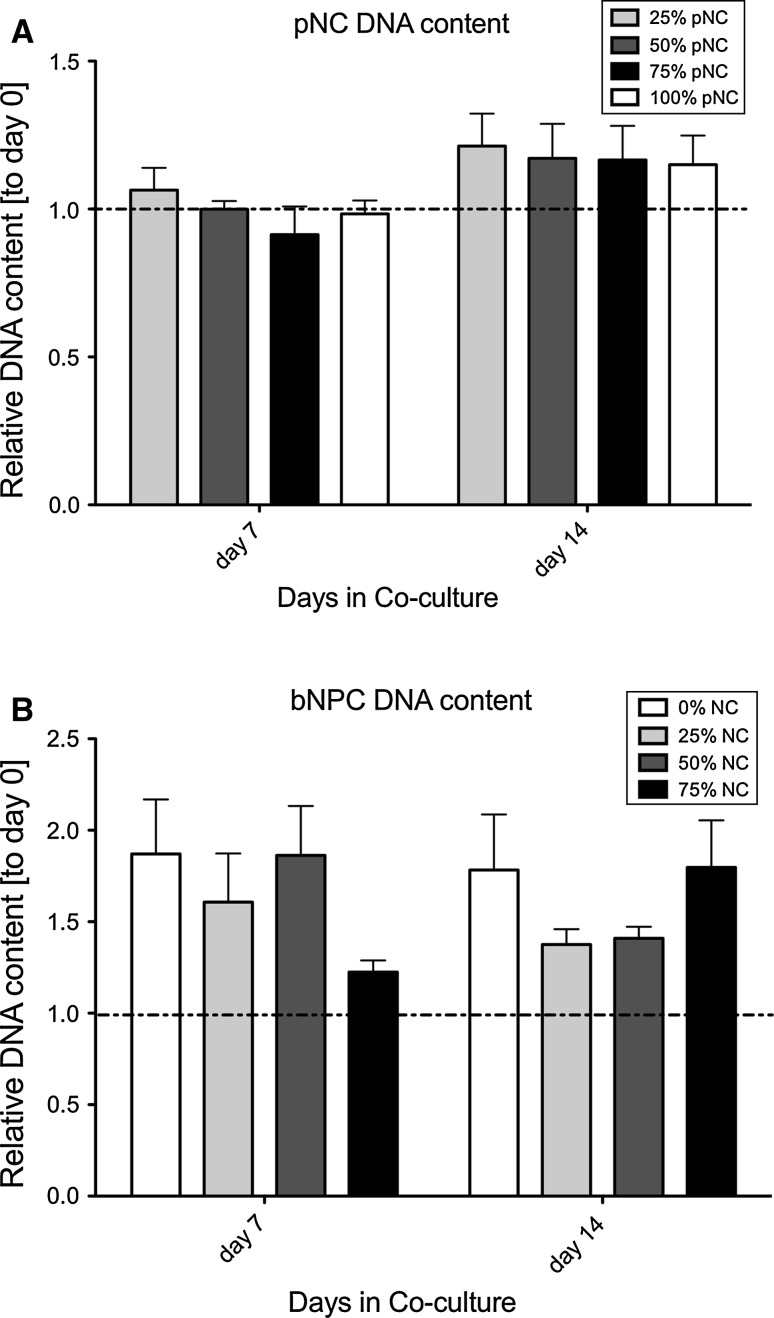
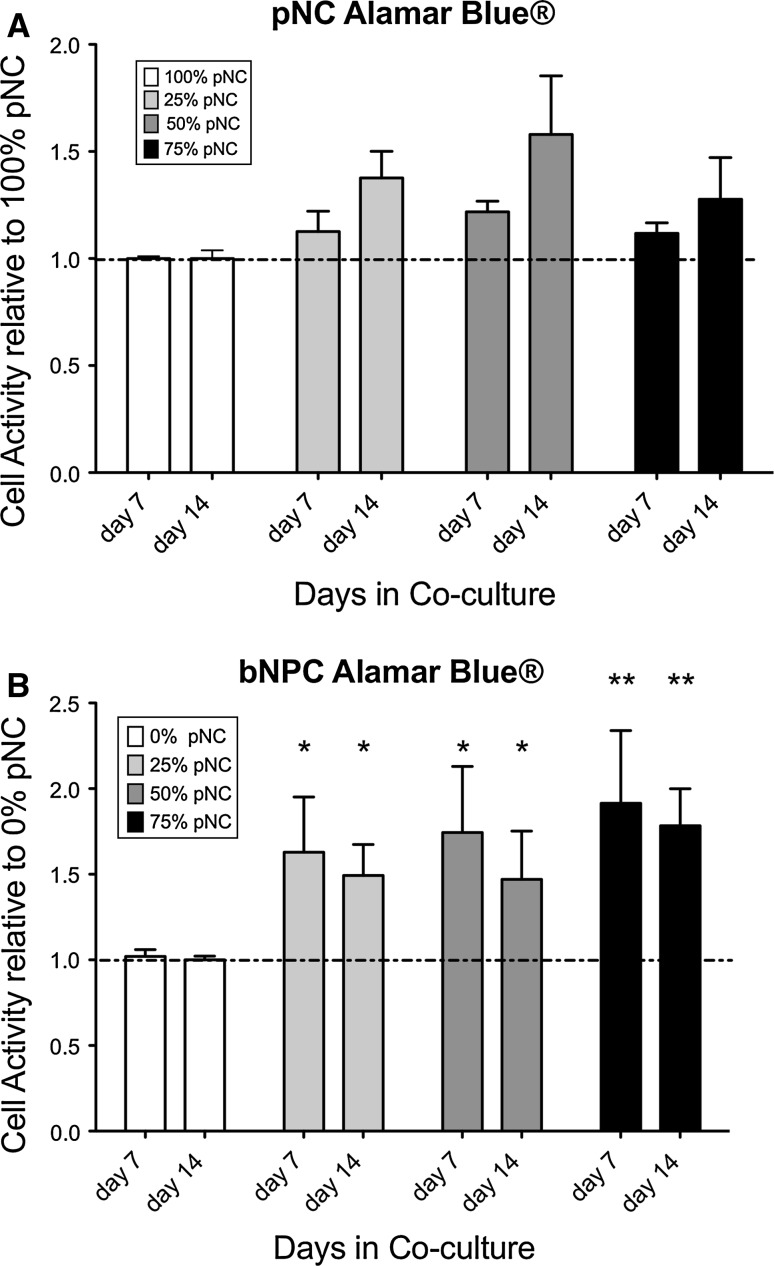
In both pNCs and bNPCs, the DNA content of beads was stable over culture time or even increased slightly relative to day 0 control after 14 days of co-culture (Fig. 1). There was no difference obvious among co-culture groups on the side of NC. There was a trend toward higher DNA content in the pure bNPC and the 25% bNPC and 75% pNC group; however, this was non-significant. As for the cell metabolism (Fig. 2a, b) the bNPCs were more strongly activated by the presence of pNCs (Fig. 2b) (p = 0.008) than vice versa (Fig. 2a), and this effect was the strongest in 75% of pNC co-culture and was also significant using Bonferroni testing for the 0 versus the 50% group and for the 0 versus the 75% group (Fig. 1a). We found a significant increase in GAG/DNA ratio for the 50% bNPC group after 14 days of co-culture (Fig. 3b), but no effect for pNC co-cultures (Fig. 3a).
Fig. 1.
DNA content of the alginate bead after 7 and 14 days co-culture for a porcine notochordal cells (pNC) and b for bovine nucleus pulposus (bNPC). N = 4 co-cultures repeats, plot of mean ± SEM
Fig. 2.
a, b Cell metabolism per DNA (cell) in porcine notochordal cells (pNCs) and bovine nucleus pulposus cells (bNPCs) cultured in 3-D alginate for 7 and 14 days, respectively. Plot of mean ± SEM. *p < 0.05, **p < 0.01
Fig. 3.
GAG/DNA ratio of a porcine notochordal cells (pNCs) and b bovine nucleus pulposus cells (bNPCs). N = 4 co-cultures repeats, mean ± SEM. *p < 0.05
Relative gene expression
For bNPCs, relative gene expression revealed up-regulation of ACAN by two to five times and slight up-regulation of Col 2 (Fig. 4b). It was most strongly up-regulated in the 50% pNC co-culture group. For pNCs, ACAN and collagen type 2 were found to be up-regulated by about 1,000 times, however, this effect was not significant for both mRNAs (Fig. 4a). This up-regulation by a factor of ~1,000 times for all three co-culture ratios containing bNPCs could be interpreted as a progenitor-like cell status of pNCs prior co-culture. On the other hand, Col 1 was found unchanged for the entire duration of the co-culture experiment, indicating maintenance of the original phenotype during the experiment.
Fig. 4.
Relative Gene Expression after 14 days in co-culture of bNPCs and pNCs in 3-D microspheres in alginate, respectively. N = 4 co-cultures repeats, mean ± SEM. *p < 0.05
Discussion
Cell identity of notochordal cells
The notochordal cells (NC) are entrapped during early embryogenesis (around day 20 in humans) and formation of the somites. This formation of the so-called prototissue which forms at the center of the embryo appears as a rod of tissue known as the notochord, which guides the embryonic development of the neural tube and the vertebral column, including the intervertebral disks [31]. The exact role of the entrapped notochordal cells after condensation is unclear, but they are believed to take part in the formation of the nucleus pulposus [4, 17, 37].
However, it seems obvious that these two cell populations differ in a number of characteristics such as cell size, nutrition [16], surface markers [13], and mechano-sensitivity [14]. Recently, the physiological requirements in terms of nutrition were compared between these two cell types and it was found that notochordal cells are more sensitive and consume more glucose than nucleus pulposus cells under the identical culture condition [16]. Furthermore, non-invasive femtosecond laser microscopy revealed clear size differences between these two cell types and seems to point toward two different cell lineages, if cell shape and size are considered [15]. Furthermore, the NC differs by the presence of large vacuoles, which can be separated by the size-scatter of FACS analysis [5]. These large vacuoles found in NC of the intervertebral disc has been attributed a possible functional role in osmoregulation [18].
There are also considerable differences in the nucleus pulposus cell shape among different animal species [19, 20]. Of interest are especially the two dog breeding lines, i.e., the chondrodystrophoid dogs, e.g., Dachs hound and Beagle and the non-chondrodystrophoid dogs, e.g., Mongrels, Greyhound, and German shepherd lines. There have been several morphological papers published, which describe the morphological differences between these cells [19, 20].
Transcriptomics, on the other hand, comparing the two cell populations revealed that there are only about two dozen genes really distinct between these two cell types [27, 30]. The search for specific markers to distinguish these two cell populations has just started [13]. Weiler et al. [38] found that cells in the human fetal and juvenile nucleus pulposus with the typical morphology of the notochord (physaliferous) express the markers cytokeratin (CK)-8, -18, -19 and galectin-3 [29]. Gilson et al. [13] found that pig NP cells, which are phenotypically similar to human infant nucleus pulposus cells, were all CK-8 positive. In human discs, the presence of notochordal cells has been associated with the occurrence of chordomas, which are malignant tumours that occur along the spine [36]. Brachyury (i.e., T gene) is a transcription factor associated with the notochord. It has been demonstrated that brachyury positive (T+) cells are associated with chordoma. Interestingly, human chordoma express high levels of aggrecan, collagen type 2, but lack expression of collagen X completely [36]. T+ cells are also related to cancer stem cells and express nanog and other stem cell-related markers and have been described as cells with higher “plasticity” [33].
It has also been shown that notochordal cells are perfectly adapted to low-oxygen environment. They can produce a better aligned ECM under hypoxic conditions [10]. Here, we cultured the cells under normoxic conditions. It is very likely that the current results would be even more pronounced under hypoxic conditions [10]. This should be tested in a further experiment.
Optimal cell ratio
We could demonstrate a significant increase of cell activity of the bNPCs in the presence of pNCs and activation of GAG/DNA ratio by a ratio of 1:1. The strongest activation of cell activity was found with a ratio of ~75% pNCs. Considering that the porcine nucleus pulposus contains not 100% notochordal cells, but to some extent, i.e., ~10–20% of pNPCs, the real ratio might have been shifted toward NPCs and thus was around 30:70 for pNC:bNPC. It is of interest that NPCs reacted positively to the addition of bovine NPCs since the “pure” population was so to say a natural “co-culture” of conspecific NCs and NPCs. The fact that cross-specific cell co-culture results in an activation of the notochordal cells could be either an artifact of the cross-species cytokines or a true effect pointing to a precursor state of notochordal cells, which can be triggered toward higher expression of aggrecan and collagen type 2 by the presence of additional NP cells. The fact that the DNA content in pNC was not significantly higher than in the bNPCs suggests that the expansion phase with serum in monolayer of the NCs did not bias the results of the GAG/DNA ratio. The fact that a 50% ratio of pNCs and bNPCs increased significantly GAG/DNA ratio of bNPCs exclusively is interesting with respect to the existence of pre-established regulator mechanisms, which can be triggered by reaching an optimum interaction between the two cell populations. Addition of bNPCs to the NPC population resulted in relative increase of cell metabolism in bNPCs (Fig. 2b). This activation is in line with the increase of GAG/DNA over time on the side of NPCs. GAG/DNA ratio on the side of NCs was elevated (around 50% higher than day 0) after 7 days and then were reduced to the level of the start of the co-culture. This is a contradiction to the gene expression results, which show an increase of aggrecan and collagen type 2.
Future experiments will test the influence of cell–cell contact by seeding pNCs and bNPCs into alginate beads enabling cell–cell interaction and also the importance of hypoxic conditions. Gene expression revealed a significant up-regulation of Col 1 and Col 2 by the pNCs in the presence of bNPCs, which confirms previous findings of Boyd [3] and Aguiar et al. [1] that NCs express certain stimulating cytokines, which seems evident and has been demonstrated using conditioning media [3, 9, 22]. Korecki et al. also detected that SOX-9 (transcription factor for chondrogenesis) was down-regulated if human mesenchymal stem cells (hMSCs) are exposed to notochordal conditioned medium (NCM) as compared to a chondrogenic medium containing TGF-β. NCM, however, also increased expression of collagen type 2, but not to the same extent as in the presence of TGF-β. They also concluded that NCM tended to increase laminin β1 mRNA expression, and also found significantly higher level of GAG production than for TGF-β-treated cells. In addition, NCM conditioning of MSCs tended to up-regulate collagen type II mRNA less strongly than with TGF-β from which the others concluded that NCM has the potential to thrive cells toward the “discogenic” phenotype rather than TGF-β alone. Interestingly, NCM stimulated the migration of IVD cartilage endplate chondrocytes in in vitro cell migration assays [21].
Conclusion
In conclusion, co-culture of porcine notochordal cells (pNC) together with bovine nucleus pulposus cells (bNPC) definitively stimulates both cells in a synergistic way. However, our data also show that bNPC are activated by the presence of pNC, which results in a higher GAG per cell production (significant in the case of a cell ratio of 1:1) and higher cell activity as measured by Alamar Blue assay. The NPC, on the other hand, neither activate the NC proliferation nor the GAG/DNA ratio, but instead the gene expression of collagen type 2 and aggrecan. We interpret this result as an indication that the notochordal cell phenotypes might have been at a progenitor state before the co-culture experiment. These data do not contradict the latest hypotheses about their origin being very related cell population to the chondrocyte-like disc cells [30]. The fact that the co-culture with cross-specific additional NPCs cells can trigger aggrecan and col 2 gene expression seems to point toward a progenitor-like status of notochordal cells. Moreover, these cells might be of key importance for the regeneration of the intervertebral disc using cell-based approaches [8, 13]. Although these notochord cells seem to be highly related to chondrocyte-like cells, the differential response in our co-culture experiments questions whether notochordal cells are irrelevant for the choice of animal models for disc regeneration [30, 35]. Although NC have stimulating effects on other IVD cells their implication for therapeutic usage for the human IVD can only be followed-up after clarification of their phenotypic status and potential to form chordoma. Much easier might be the clinical application of their yet unknown cytokines/substances which they secrete for potential medication.
Acknowledgments
This work was supported by the Swiss National Science Foundation (SNF #310030-127586/1) and the Department for Orthopaedic Surgery, Insel University Hospital, Bern.
Conflict of interest
None.
References
- 1.Aguiar DJ, Johnson SL, Oegema TR (1999) Notochordal cells interact with nucleus pulposus cells: regulation of proteoglycan synthesis. Exp Cell Res 246(1):129–137. doi:10.1006/excr.1998.4287 [DOI] [PubMed]
- 2.Ahmed SA, Gogal RM, Walsh JE. A new rapid and simple non-radioactive assay to monitor and determine the proliferation of lymphocytes: an alternative to [3H] thymidine incorporation assay. J Immunol Methods. 1994;170(2):211–224. doi: 10.1016/0022-1759(94)90396-4. [DOI] [PubMed] [Google Scholar]
- 3.Boyd LM, Chen J, Kraus VB, Setton LA. Conditioned medium differentially regulates matrix protein gene expression in cells of the intervertebral disc. Spine. 2004;29(20):2217–2222. doi: 10.1097/01.brs.0000142747.90488.1d. [DOI] [PubMed] [Google Scholar]
- 4.Butler WF. Comparative anatomy and development of the mammalian disc. In: Gosh P, editor. The biology of the intervertebral disc. Boca Raton: CRC Press; 1989. pp. 84–108. [Google Scholar]
- 5.Chen J, Yan W, Setton LA. Molecular phenotypes of notochordal cells purified from immature nucleus pulposus. Eur Spine J. 2006;15(Suppl 3):S303–S311. doi: 10.1007/s00586-006-0088-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Doskocil M, Valouch P, Pazderka V. On vertebral body growth. Funct Dev Morphol. 1993;3(3):149–155. [PubMed] [Google Scholar]
- 7.Enobakhare BO, Bader DL, Lee DA. Quantification of sulfated glycosaminoglycans in chondrocyte/alginate cultures, by use of 1,9-dimethylmethylene blue. Anal Biochem. 1996;243(1):189–191. doi: 10.1006/abio.1996.0502. [DOI] [PubMed] [Google Scholar]
- 8.Erwin WM. The enigma that is the nucleus pulposus cell: the search goes on. Arthritis Res Ther. 2010;12(3):118. doi: 10.1186/ar3001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Erwin WM, Ashman K, O’Donnel P, Inman RD. Nucleus pulposus notochord cells secrete connective tissue growth factor and up-regulate proteoglycan expression by intervertebral disc chondrocytes. Arthritis Rheum. 2006;54(12):3859–3867. doi: 10.1002/art.22258. [DOI] [PubMed] [Google Scholar]
- 10.Erwin WM, Las Heras F, Islam D, Fehlings MG, Inman RD. The regenerative capacity of the notochordal cell: tissue constructs generated in vitro under hypoxic conditions. J Neurosurg Spine. 2009;10(6):513–521. doi: 10.3171/2009.2.SPINE08578. [DOI] [PubMed] [Google Scholar]
- 11.Farndale RW, Buttle DJ, Barrett AJ. Improved quantitation and discrimination of sulphated glycosaminoglycans by use of dimethylmethylene blue 1. Biochim Biophys Acta. 1986;883(2):173–177. doi: 10.1016/0304-4165(86)90306-5. [DOI] [PubMed] [Google Scholar]
- 12.Faul F, Erdfelder E, Lang AG, Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39(2):175–191. doi: 10.3758/BF03193146. [DOI] [PubMed] [Google Scholar]
- 13.Gilson A, Dreger M, Urban JP. Differential expression levels of cytokeratin 8 in cells of the bovine nucleus pulposus complicates the search for specific intervertebral disc cell markers. Arthritis Res Ther. 2010;12(1):R24. doi: 10.1186/ar2931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guehring T, Nerlich A, Kroeber M, Richter W, Omlor GW. Sensitivity of notochordal disc cells to mechanical loading: an experimental animal study. Eur Spine J. 2010;19(1):113–121. doi: 10.1007/s00586-009-1217-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guehring T, Urban JP, Cui Z, Tirlapur UK. Noninvasive 3D vital imaging and characterization of notochordal cells of the intervertebral disc by femtosecond near-infrared two-photon laser scanning microscopy and spatial-volume rendering. Microsc Res Tech. 2008;71(4):298–304. doi: 10.1002/jemt.20557. [DOI] [PubMed] [Google Scholar]
- 16.Guehring T, Wilde G, Sumner M, Grünhagen T, Karney GB, Tirlapur UK, Urban JP. Notochordal intervertebral disc cells: sensitivity to nutrient deprivation. Arthritis Rheum. 2009;60(4):1026–1034. doi: 10.1002/art.24407. [DOI] [PubMed] [Google Scholar]
- 17.Horwitz T (1977) The human notochord: a study of its development and regression, variations, and pathologic derivative, chordoma. Horwitz: Indianapolis
- 18.Hunter CJ, Bianchi S, Cheng P, Muldrew K. Osmoregulatory function of large vacuoles found in notochordal cells of the intervertebral disc running title: an osmoregulatory vacuole. Mol Cell Biomech. 2007;4(4):227–237. [PMC free article] [PubMed] [Google Scholar]
- 19.Hunter CJ, Matyas JR, Duncan NA. The notochordal cell in the nucleus pulposus: a review in the context of tissue engineering. Tissue Eng. 2003;9(4):667–677. doi: 10.1089/107632703768247368. [DOI] [PubMed] [Google Scholar]
- 20.Hunter CJ, Matyas JR, Duncan NA. Cytomorphology of notochordal and chondrocytic cells from the nucleus pulposus: a species comparison. J Anat. 2004;205(5):357–362. doi: 10.1111/j.0021-8782.2004.00352.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim KW, Ha KY, Lee JS, Nam SW, Woo YK, Lim TH, An HS. Notochordal cells stimulate migration of cartilage end plate chondrocytes of the intervertebral disc in in vitro cell migration assays. Spine J. 2009;9(4):323–329. doi: 10.1016/j.spinee.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 22.Korecki CL, Taboas JM, Tuan RS, Iatridis JC. Notochordal cell conditioned medium stimulates mesenchymal stem cell differentiation toward a young nucleus pulposus phenotype. Stem Cell Res Ther. 2010;1(2):18. doi: 10.1186/scrt18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee CR, Grad S, Maclean JJ, Iatridis JC, Alini M. Effect of mechanical loading on mRNA levels of common endogenous controls in articular chondrocytes and intervertebral disk. Anal Biochem. 2005;341(2):372–375. doi: 10.1016/j.ab.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 24.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 25.Maldonado BA, Oegema TR. Initial characterization of the metabolism of intervertebral disc cells encapsulated in microspheres. J Orthop Res. 1992;10(5):677–690. doi: 10.1002/jor.1100100510. [DOI] [PubMed] [Google Scholar]
- 26.Marino JH, Cook P, Miller KS. Accurate and statistically verified quantification of relative mRNA abundances using SYBR Green I and real-time RT-PCR. J Immunol Methods. 2003;283(1–2):291–306. doi: 10.1016/S0022-1759(03)00103-0. [DOI] [PubMed] [Google Scholar]
- 27.Minogue BM, Richardson SM, Zeef LA, Freemont AJ, Hoyland JA. Transcriptional profiling of bovine intervertebral disc cells: implications for identification of normal and degenerate human intervertebral disc cell phenotypes. Arthritis Res Ther. 2010;12(1):R22. doi: 10.1186/ar2929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miyazaki T, Kobayashi S, Takeno K, Meir A, Urban J, Baba H. A phenotypic comparison of proteoglycan production of intervertebral disc cells isolated from rats, rabbits, and bovine tails; which animal model is most suitable to study tissue engineering and biological repair of human disc disorders? Tissue Eng Part A. 2009;15(12):3835–3846. doi: 10.1089/ten.tea.2009.0250. [DOI] [PubMed] [Google Scholar]
- 29.Oguz E, Tsai TT, Di Martino A, Guttapalli A, Albert TJ, Shapiro IM, Risbud MV. Galectin-3 expression in the intervertebral disc: a useful marker of the notochord phenotype? Spine. 2007;32(1):9–16. doi: 10.1097/01.brs.0000250302.74574.98. [DOI] [PubMed] [Google Scholar]
- 30.Risbud MV, Schaer TP, Shapiro IM. Toward an understanding of the role of notochordal cells in the adult intervertebral disc: from discord to accord. Dev Dyn. 2010;239:2141–2148. doi: 10.1002/dvdy.22350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rufai A, Benjamin M, Ralphs JR. The development of fibrocartilage in the rat intervertebral disc. Anat Embryol (Berl) 1995;192(1):53–62. doi: 10.1007/BF00186991. [DOI] [PubMed] [Google Scholar]
- 32.Sakai D, Nakai T, Mochida J, Alini M, Grad S. Differential phenotype of intervertebral disc cells: microarray and immunohistochemical analysis of canine nucleus pulposus and anulus fibrosus. Spine. 2009;34(14):1448–1456. doi: 10.1097/BRS.0b013e3181a55705. [DOI] [PubMed] [Google Scholar]
- 33.Sarkar D, Shields B, Davies ML, Müller J, Wakeman JA (2011) BRACHYURY confers cancer stem cell characteristics on colorectal cancer cells. Int J Cancer. doi:10.1002/ijc.26029 [DOI] [PubMed]
- 34.Schmittgen TD, Zakrajsek BA. Effect of experimental treatment on housekeeping gene expression: validation by real-time, quantitative RT-PCR. J Biochem Biophys Methods. 2000;46(1–2):69–81. doi: 10.1016/S0165-022X(00)00129-9. [DOI] [PubMed] [Google Scholar]
- 35.Shapiro IM, Risbud MV. Transcriptional profiling of the nucleus pulposus: say yes to notochord. Arthritis Res Ther. 2010;12(3):117. doi: 10.1186/ar3003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vujovic S, Henderson S, Presneau N, Odell E, Jacques TS, Tirabosco R, Boshoff C, Flanagan AM. Brachyury, a crucial regulator of notochordal development, is a novel biomarker for chordomas. J Pathol. 2006;209(2):157–165. doi: 10.1002/path.1969. [DOI] [PubMed] [Google Scholar]
- 37.Walmsley R (1953) The development and growth of the intervertebral disc. Edinburgh Med J 60:341–365 [PMC free article] [PubMed]
- 38.Weiler C, Nerlich AG, Schaaf R, Bachmeier BE, Wuertz K, Boos N (2010) Immunohistochemical identification of notochordal markers in cells in the aging human lumbar intervertebral disc. Eur Spine J. doi:10.1007/s00586-010-1392-z [DOI] [PMC free article] [PubMed]