Abstract
Purpose
To evaluate the findings in a case of acute macular neuroretinopathy involving sudden development of scotomas accompanied by rapid focal increases in fundus autofluorescence.
Methods
The clinical presentation of the patient was documented by color fundus photographs, fundus autofluorescence, infrared imaging, and high-resolution spectral domain optical coherence tomography. The scotomas were assessed by Humphrey visual field 10-2 and MP-1 microperimetry.
Results
Visual field defects exhibited spatial correspondence with wedge-shaped lesions demonstrable in color fundus photographs and infrared imaging. It was notable that the lesions exhibited increased intensity on autofluorescence images obtained within 3 weeks of presentation. Optical coherence tomography revealed focal loss of photoreceptor inner segment/outer segment junctions in both eyes.
Conclusion
This case was distinguished by the relative rapidity with which the lesions became hyperautofluorescent in fundus autofluorescence images. Given that the bisretinoids that are the source of autofluorescence form in photoreceptor cells and are transferred to retinal pigment epithelium secondarily, the rapid increase in autofluorescence is unlikely to only reflect retinal pigment epithelium status and is more likely to be indicative of photoreceptor cell dysfunctioning and loss of structural integrity.
Keywords: acute macular neuroretinopathy, scotoma, fundus autofluorescence, RPE, retina, infrared imaging
Fundus autofluorescence is increasingly used in the diagnosis of retinal disorders of diverse origin. The spectral characteristics and spatial distribution of fundus autofluorescence are consistent with the source of this natural autofluorescence being the fluorescent pigments (lipofuscin) that accumulate with age inside retinal pigment epithelial cells.1 Here we describe findings from a patient who presented with bilateral scotomas of sudden onset. Our attention was drawn to the observation that the region of the fundus corresponding to each scotoma was marked by elevated fundus autofluorescence. We evaluated the fundus autofluorescence patterns in terms of the spatial correspondence with the lesions visualized by white light fundus imaging and infrared illumination. We considered this clinical presentation to be uniquely valuable to an examination of current interpretations of fundus autofluorescence imaging.
Case Report
A 52-year-old woman admitted to the hospital for pancreatitis complained of bilateral blurry vision for 5 days. Her medical history was significant for alcohol, tobacco, and cocaine abuse with a recent alcohol and cocaine binge leading to this hospitalization. She also had a history of asthma and chronic obstructive pulmonary disease for which she took bronchodilators and prednisone.
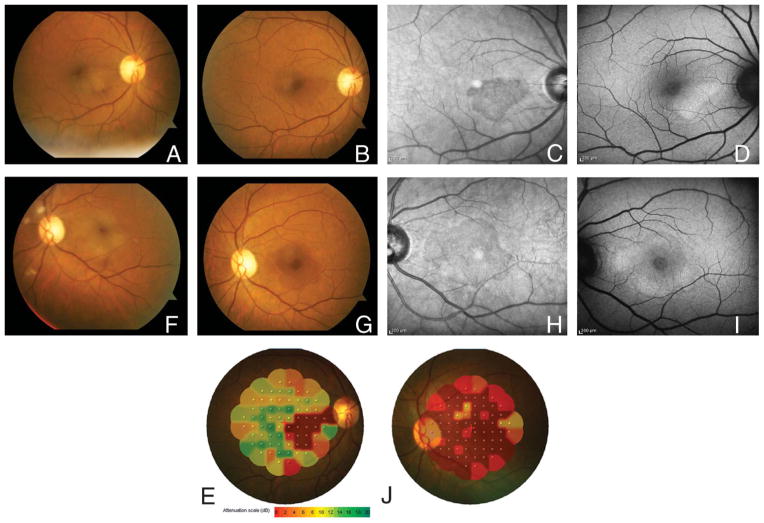
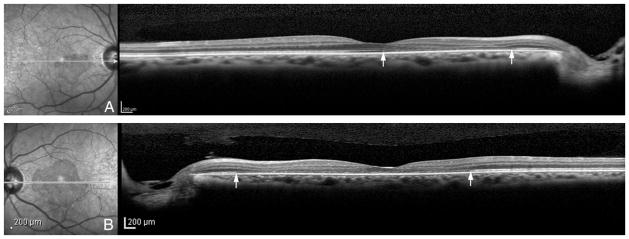
On examination, her best-corrected visual acuity was 20/100 in the right eye and counting-fingers in the left eye. A trace relative afferent pupillary defect was present in the left eye. Intraocular pressures and anterior segment examinations were normal in both eyes. On fundoscopic examination (Figure 1), wedge-shaped changes were noted in both maculas and cotton wool spots were present in the left eye. Humphrey visual field 10-2 revealed dense central scotomas in both eyes. Fluorescein angiography was essentially normal, with perhaps subtle parafoveal leakage. High-resolution spectral domain optical coherence tomography (OCT; Heidelberg Spectralis, Heidelberg Engineering, Heidelberg, Germany) at presentation disclosed loss of the photoreceptor inner segment/outer segment junction in both eyes (Figure 2). Particularly noticeable in the right eye was diminished separation of external limiting membrane (a faint reflective band above the inner segment/outer segment junction) and retinal pigment epithelium (RPE). There was no change in OCT during follow-up visit 2 weeks later. With infrared imaging (Figure 1, C and H), the wedge-shaped macular lesions were distinct. Fundus autofluorescence (Heidelberg Spectralis) (Figure 1, D and I) revealed regions of hyperautofluorescence corresponding to these lesions. MP-1 microperimetry (Nidek, Fremont, CA) (Figure 1, E and J) showed a close correlation between the lesions and the scotomas. Full-field electroretinography demonstrated normal scotopic and borderline photopic cone responses.
Fig. 1.
Color fundus photographs at presentation manifest multiple cotton wool spots and wedge-shaped macular lesions (A and F). Images acquired 2 weeks later (B and G) are marked by wedge-shaped darkened areas corresponding to hyporeflective regions in infrared images (C and H) and hyperautofluorescence in fundus autofluorescence images (D and I). Microperimetry testing in both eyes (E and J) shows defects that mirror findings described above.
Fig. 2.
High-resolution spectral domain optical coherence tomography (right) shows loss of the inner segment/outer segment junction in the right eye (A) and left eye (B), corresponding to wedge-shaped hyporeflective areas in infrared images (left) at presentation. Raster lines are presented in white.
Two weeks after presentation, her vision had improved to 20/70 in both eyes. At 1 month, her best-corrected visual acuity was 20/25 in the right eye and 20/30 in the left eye (with eccentric fixation). Repeat SD-OCT imaging showed persistent inner segment/outer segment junction defects despite improvement in visual acuity.
Discussion
Our patient presented with bilateral paracentral scotomas of sudden onset, cotton wool spots, and reddish brown macular lesions on white light fundus imaging that were pronounced with infrared illumination. All these features have been previously described in patients with acute macular neuroretinopathy (AMN),2–5 an uncommon clinical condition. A number of reports have also described outer retinal abnormalities in AMN,3,4,6 based on OCT findings. Our patient was a woman as is frequently the case in AMN. However, the history and severity of visual loss, including an afferent pupillary defect and eccentric fixation, are not typical.
This patient, as with an AMN case previously reported,5 was notable for the relative rapidity with which the localized area of hyperautofluorescence became visible by autofluorescence imaging. The fluorescent bisretinoid compounds in RPE that have the spectral characteristics consistent with fundus autofluorescence and that serve as the source of autofluorescence, form as a consequence of inadvertent reactions of vitamin A-aldehyde (all-trans-retinal) in photoreceptor outer segments.7 In a healthy retina, these bisretinoid compounds are kept to a minimum in photoreceptor cells by means of daily shedding of outer segment membrane followed by phagocytic transfer to RPE. This fluorescent material then accumulates with age in the lipofuscin of RPE.7 Contrary to what is sometimes assumed, synthesis of these fluorophores occurs before outer segment disk shedding and does not rely on phagocytosis for formation. The rate of formation is also not influenced by RPE catabolism. So how does one account for lesion-associated hyperautofluorescence that is acquired in a relatively short time—an autofluorescence that exceeds intensity levels built up elsewhere in the fundus over a lifetime?
As noted above, OCT imaging of our patient revealed disruption of the inner segment/outer segment junction, a sign of outer segment degeneration. As suggested previously about retinitis pigmentosa,7 areas of increased intensity on autofluorescence images are not necessarily a signal derived solely from RPE. Rather, one has to also consider the cellular source of RPE lipofuscin—the photoreceptor cell. We suggest that areas of increased fundus autofluorescence can be a sign of photoreceptor cell dysfunctioning and degeneration, wherein the lipofuscin synthetic pathway is accelerated because the photoreceptor cells are unable to efficiently detoxify the reactive all-trans-retinal that is generated each time a photon of light is absorbed by visual pigment. Evidence in support of this concept is provided by studies elucidating the mechanism of formation of these fluorescent compounds and work in animal models. Mishandling of all-trans-retinal by compromised photoreceptor cells would rapidly amplify bisretinoid formation in photoreceptor cells.7 We suggest that impaired bisretinoid-burdened photoreceptor cells and the debris resulting from degeneration can be an anomalous source of increased fundus autofluorescence signaling. Although not detectable in the present case, areas of hyperautofluorescence in AMN can also be contiguous with hypoautofluorescence.5 A decrease in autofluorescence such as this could be the result of autofluorescence bleaching and/or resolution of the degenerating debris. Recently developed approaches for quantifying autofluorescence signal8 will be valuable for better defining these areas of increased and decreased signal.
It is well known that autofluorescence imaging is informative of the RPE monolayer; under some conditions, including the relatively rapid increase in autofluorescence that was observed in our patient, we propose that this modality may also provide information on photoreceptor cell status. The interpretation of autofluorescence presented here does not eclipse a potential role for transient chorioretinal vasocontriction as an underlying cause of the macular lesion. Moreover, because the human fundus consists of layers of differing absorbing, reflecting, and fluorescing substances, the signal generating the infrared image is not necessarily the same as the signal generating fundus autofluorescence.
Acknowledgments
Supported by R24 EY019861, Foundation Fighting Blindness and a grant to the Department of Ophthalmology from Research to Prevent Blindness.
Footnotes
The authors have no conflicts of interest to disclose.
References
- 1.Delori FC, Dorey CK, Staurenghi G, et al. In vivo fluorescence of the ocular fundus exhibits retinal pigment epithelium lipofuscin characteristics. Invest Ophthalmol Vis Sci. 1995;36:718–729. [PubMed] [Google Scholar]
- 2.Corver HD, Ruys J, Kestelyn-Stevens AM, et al. Two cases of acute macular neuroretinopathy. Eye (Lond) 2007;21:1226–1229. doi: 10.1038/sj.eye.6702543. [DOI] [PubMed] [Google Scholar]
- 3.Hughes EH, Siow YC, Hunyor AP. Acute macular neuroretinopathy: anatomic localisation of the lesion with high-resolution OCT. Eye (Lond) 2009;23:2132–2134. doi: 10.1038/eye.2008.430. [DOI] [PubMed] [Google Scholar]
- 4.Vance SK, Spaide RF, Freund KB, et al. Outer retinal abnormalities in acute macular neuroretinopathy. Retina. 2011;31:441–445. doi: 10.1097/IAE.0b013e3181fe54fa. [DOI] [PubMed] [Google Scholar]
- 5.Yeh S, Hwang TS, Weleber RG, et al. Acute macular outer retinopathy (AMOR): a reappraisal of acute macular neuroretinopathy using multimodality diagnostic testing. Arch Ophthalmol. 2011;129:365–368. doi: 10.1001/archophthalmol.2011.22. [DOI] [PubMed] [Google Scholar]
- 6.Neuhann IM, Inhoffen W, Koerner S, et al. Visualization and follow-up of acute macular neuroretinopathy with the Spectralis HRA+OCT device. Graefes Arch Clin Exp Ophthalmol. 2010;248:1041–1044. doi: 10.1007/s00417-010-1324-y. [DOI] [PubMed] [Google Scholar]
- 7.Sparrow JR, Yoon K, Wu Y, Yamamoto K. Interpretations of fundus autofluorescence from studies of the bisretinoids of retina. Invest Ophthalmol Vis Sci. 2010;51:4351–4357. doi: 10.1167/iovs.10-5852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Delori FC, Greenberg JP, Woods RL, et al. Quantitative measurements of autofluorescence with the scanning laser ophthalmoscope. Invest Ophthalmol Vis Sci. 2011;52:9379–9390. doi: 10.1167/iovs.11-8319. [DOI] [PMC free article] [PubMed] [Google Scholar]