Abstract
Purpose
The aim of this study is to establish standard MRI values for the cervical spinal canal, dural tube, and spinal cord, to evaluate age-related changes in healthy subjects, and to assess the prevalence of abnormal findings in asymptomatic subjects.
Methods
The sagittal diameter of the spinal canal and the sagittal diameter and cross-sectional area of the dural tube and spinal cord were measured on MRIs of 1,211 healthy volunteers. These included at least 100 men and 100 women in each decade of life between the third (20s) and eighth (70s). Abnormal findings such as spinal cord compression and signal changes in the spinal cord were recorded.
Results
The sagittal diameter of the spinal canal was 11.2 ± 1.4 mm [mean ± standard deviation (SD)]/11.1 ± 1.4 mm (male/female) at the mid-C5 vertebral level, and 9.5 ± 1.8/9.6 ± 1.6 mm at the C5/6 disc level. The cross-sectional area of the spinal cord was 78.1 ± 9.4/74.4 ± 9.4 mm2 at the mid-C5 level and 70.6 ± 11.7/68.9 ± 11.3 mm2 at the C5/6 disc level. Both the sagittal diameter and the axial area of the dural tube and spinal cord tended to decrease with increasing age. This tendency was more marked at the level of the intervertebral discs than at the level of the vertebral bodies, especially at the C5/6 intervertebral disc level. The spinal cord occupation rate in the dural tube at the C5 vertebral body level averaged 58.3 ± 7.0%. Spinal cord compression was observed in 64 cases (5.3%) and a T2 high-signal change was observed in 28 cases (2.3%).
Conclusions
Using MRI data of 1,211 asymptomatic subjects, the standard values for the cervical spinal canal, dural tube, and spinal cord for healthy members of each sex and each decade of life and the age-related changes in these parameters were established. The relatively high prevalence of abnormal MRI findings of the cervical spine in asymptomatic individuals emphasizes the dangers of predicating operative decisions on diagnostic tests without precisely correlating these findings with clinical signs and symptoms.
Keywords: Cervical spine, MRI, Standard values, Anatomy, Aging change
Introduction
The number of patients with cervical spondylotic myelopathy (CSM) is increasing in the aging population. Although CSM is the most common disease of the spinal cord that occurs during and after middle age, its pathophysiology remains unclear. In the pathophysiology of CSM, there are static factors such as preexisting developmental canal stenosis, bulging of the posterior margin of the intervertebral disc, and hypertrophy of the ligamentum flavum, as well as dynamic factors such as ligamentum flavum invagination (buckling), intervertebral disc protrusion into the spinal canal, and a pincer effect (anterior and posterior cord impingement) during neck extension.
The incidence of spinal stenosis is increasing with an increase in the number of elderly individuals in the population. Likewise, instances of spinal cord injuries without radiographic abnormality (SCIWORA), which are caused by minor trauma, have been increasing [5, 9]. SCIWORA is a syndrome describing spinal cord injury without evidence of fracture or dislocation of the spine on plain radiographs or computed tomography (CT) studies. The incidence, pathogenesis, and severity of SCIWORA vary with age groups because of anatomical and biomechanical differences in the spine. However, the symptoms of SCIWORA are irreversible once they occur. Therefore, an increase in SCIWORA incidence could induce large social losses.
Recently, it has become evident that magnetic resonance imaging (MRI) is the best clinical tool for evaluating traumatic spinal cord injury, and is therefore invaluable for the examination of patients with SCIWORA. MRI can reveal not only the degree of spinal canal stenosis but also the detailed intramedullary status of the spinal cord [11]. MRI is helpful in both the diagnosis and prognosis of SCIWORA because of its better contrast resolution, absence of bony artifacts, and multiplanar imaging capability [5]. Moreover, it provides adequate information about neural and extraneural injuries and can identify, for example, epidural hematomas and significant disc herniations that may require surgical intervention. We found two previous reports on normal cervical spinal canal parameters in the Japanese population, in which the bony spinal canal was evaluated on the basis of X-ray data [1, 8]. Furthermore, the only report we found on normal cervical spinal cord parameters was based on autopsy samples [3]. However, to the best of our knowledge, there have been only a few MRI-based reports describing the normal configuration of the cervical spinal canal, including the soft tissues, and the details of the relationships between the cervical spinal canal, dural tube, and spinal cord [2, 6, 7]. In the present study, we therefore sought to establish standard MRI values for these parameters in a large sample of healthy asymptomatic subjects.
However, developmental stenosis of the cervical spinal canal is currently defined on the basis of X-rays, and no MRI data are available for this diagnosis. X-rays evaluate only the bony cervical spinal canal, whereas MRI evaluates the dura and the spinal cord as well. Developmental stenosis of the cervical spinal canal can therefore be evaluated using MRI data, because it is considered that stenosis can be better assessed on the basis of the relationship between the spinal cord and the dura within the spinal canal.
The purpose of this research was to establish standard MRI values and those age-related changes for the cervical spinal canal, dural tube, and spinal cord, using MRI data of healthy members of each sex and in each decade of life, and elucidate the incidence of abnormal findings in normal asymptomatic subjects.
Materials and methods
Healthy Japanese volunteers were sought after the purpose of this study was officially announced. The exclusion criteria included a history of brain or spinal surgery, comorbid neurologic disease such as cerebral infarction and neuropathy, symptoms related to sensory or motor disorders (numbness, clumsiness, motor weakness, and gait disturbances) or having severe neck pain. Pregnant women and individuals who received workmen’s compensation or presented with symptoms after a motor vehicle accident were also excluded. After the Institutional Review Board approval was given, each patient signed a written consent form before examination.
There were approximately 100 volunteers representing each sex and decade, including individuals in the third to the eighth decade of life. A total of 1,230 volunteers were examined between February 2006 and February 2008. All the participants underwent both imaging studies and clinical examinations by two spinal surgeons (F.K. and Y.Y.). MRI data of 1,211 subjects were analyzed after excluding those with measurement difficulties resulting from problems such as motion or metal artifacts.
MRI scans were performed with a 1.5-Tesla superconductive magnet (Signa Horizon Excite HD version 12; GE Healthcare, UK). Scans were taken at slice thicknesses of 3 and 4 mm in the sagittal and axial planes, respectively. In sagittal scans, T1-weighted images [fast spin echo repetition time (TR), 450 ms; echo time (TE), 8.1 ms] and T2-weighted images (fast spin echo TR, 3,500 ms; TE, 102 ms) were obtained. Axial scans were performed using T2-weighted images (fast spin echo TR, 4,000 ms; TE, 102 ms). All images were transferred to the computer as DICOM data. Each parameter was measured by experienced radiation technologists using imaging software (Osiris4; Icestar Media Ltd, Essex, UK).
The sagittal diameter of the spinal canal, and the sagittal diameter and axial area of the dural tube and the spinal cord were measured using MRI data. All parameters were measured at each intervertebral disc level (C2/3–C7/T1) and vertebral body level (C3–C7). From these data, the spinal cord occupation rate in the dural tube was calculated (sagittal diameter of spinal cord/sagittal diameter of dural tube × 100).
Abnormal findings observed on MRI such as spinal cord compression, flattening, or high-signal changes in T2 sagittal images were individually recorded.
Results
The diameter of the spinal canal (mean ± SD) at the C5/6 intervertebral disc level for all ages as observed on sagittal images was 11.7 ± 1.6 mm in males and 11.6 ± 1.5 mm in females, while that at the C5 vertebral body level was 12.9 ± 1.4 mm in males and 12.5 ± 1.3 mm in females (Table 1).
Table 1.
Spinal canal diameter in sagittal images
| Level | Decades | |||||
|---|---|---|---|---|---|---|
| 20 | 30 | 40 | 50 | 60 | 70 | |
| Male | ||||||
| C2/3 | 14.4 ± 1.4 | 14.5 ± 1.4 | 14.0 ± 1.4 | 13.9 ± 1.3 | 13.8 ± 1.6 | 13.6 ± 1.6 |
| C3/4 | 13.3 ± 1.3 | 13.2 ± 1.5 | 12.6 ± 1.3 | 12.3 ± 1.4 | 12.4 ± 1.5 | 11.8 ± 1.5 |
| C4/5 | 13.0 ± 1.4 | 12.9 ± 1.5 | 12.4 ± 1.3 | 12.1 ± 1.4 | 11.9 ± 1.6 | 11.3 ± 1.6 |
| C5/6 | 12.7 ± 1.4 | 12.5 ± 1.5 | 11.8 ± 1.3 | 11.1 ± 1.5 | 11.3 ± 1.6 | 11.0 ± 1.7 |
| C6/7 | 12.9 ± 1.5 | 12.9 ± 1.4 | 12.5 ± 1.3 | 11.8 ± 1.7 | 12.0 ± 1.6 | 11.8 ± 1.7 |
| C7/T1 | 14.5 ± 1.4 | 14.8 ± 1.5 | 14.2 ± 1.4 | 13.9 ± 1.5 | 14.0 ± 1.7 | 13.6 ± 1.8 |
| C3 | 13.5 ± 1.2 | 13.4 ± 1.2 | 12.9 ± 1.3 | 12.8 ± 1.2 | 12.8 ± 1.3 | 12.6 ± 1.3 |
| C4 | 13.1 ± 1.3 | 13.1 ± 1.2 | 12.7 ± 1.2 | 12.5 ± 1.2 | 12.5 ± 1.3 | 12.2 ± 1.4 |
| C5 | 13.3 ± 1.4 | 13.3 ± 1.3 | 12.9 ± 1.1 | 12.5 ± 1.3 | 12.8 ± 1.5 | 12.4 ± 1.4 |
| C6 | 13.6 ± 1.3 | 13.7 ± 1.3 | 13.2 ± 1.3 | 12.8 ± 1.5 | 12.9 ± 1.4 | 12.6 ± 1.6 |
| C7 | 13.8 ± 1.3 | 14.0 ± 1.2 | 13.9 ± 1.1 | 13.6 ± 1.3 | 13.6 ± 1.3 | 13.6 ± 1.4 |
| Female | ||||||
| C2/3 | 14.3 ± 1.3 | 13.9 ± 1.3 | 13.9 ± 1.4 | 13.8 ± 1.4 | 13.1 ± 1.3 | 13.3 ± 1.5 |
| C3/4 | 12.9 ± 1.2 | 12.5 ± 1.3 | 12.5 ± 1.2 | 12.5 ± 1.3 | 11.9 ± 1.3 | 12.0 ± 1.6 |
| C4/5 | 12.6 ± 1.1 | 12.4 ± 1.4 | 12.3 ± 1.3 | 12.1 ± 1.4 | 11.5 ± 1.3 | 11.4 ± 1.6 |
| C5/6 | 12.6 ± 1.1 | 12.2 ± 1.3 | 11.6 ± 1.4 | 11.4 ± 1.3 | 10.9 ± 1.3 | 10.8 ± 1.6 |
| C6/7 | 12.8 ± 1.2 | 12.6 ± 1.3 | 12.3 ± 1.4 | 12.1 ± 1.5 | 11.4 ± 1.3 | 11.5 ± 1.4 |
| C7/T1 | 14.0 ± 1.3 | 14.1 ± 1.4 | 13.9 ± 1.4 | 13.7 ± 1.5 | 13.4 ± 1.4 | 13.5 ± 1.5 |
| C3 | 13.3 ± 1.1 | 13.1 ± 1.2 | 13.1 ± 1.2 | 13.0 ± 1.3 | 12.2 ± 1.2 | 12.3 ± 1.4 |
| C4 | 12.9 ± 1.1 | 12.8 ± 1.1 | 12.7 ± 1.2 | 12.5 ± 1.2 | 12.0 ± 1.2 | 12.1 ± 1.3 |
| C5 | 13.0 ± 1.2 | 12.9 ± 1.2 | 12.7 ± 1.2 | 12.6 ± 1.3 | 12.0 ± 1.2 | 12.0 ± 1.4 |
| C6 | 13.2 ± 1.1 | 13.1 ± 1.2 | 12.9 ± 1.4 | 12.8 ± 1.3 | 12.1 ± 1.4 | 12.2 ± 1.3 |
| C7 | 13.4 ± 1.2 | 13.5 ± 1.1 | 13.4 ± 1.1 | 13.3 ± 1.4 | 12.7 ± 1.2 | 13.0 ± 1.2 |
The values are mean ± SD (mm)
The dural tube diameter at the C5/6 intervertebral disc level for all ages as observed on sagittal images was 9.5 ± 1.8 mm in males and 9.6 ± 1.6 mm in females, while that at the C5 vertebral body level was 11.2 ± 1.4 mm in males and 11.1 ± 1.4 mm in females (Table 2). Dural tube area at the C5/6 intervertebral disc level for all ages as observed on axial images was 155.7 ± 32.1 mm2 in males and 149.6 ± 29.0 mm2 in females, while that at the C5 vertebral body level was 187.4 ± 32.6 mm2 in males and 177.0 ± 32.7 mm2 in females (Table 3).
Table 2.
Dural tube diameter in sagittal images
| Level | Decades | |||||
|---|---|---|---|---|---|---|
| 20 | 30 | 40 | 50 | 60 | 70 | |
| Male | ||||||
| C2/3 | 12.1 ± 1.2 | 12.1 ± 1.1 | 11.6 ± 1.3 | 11.6 ± 1.2 | 11.5 ± 1.3 | 11.1 ± 1.5 |
| C3/4 | 11.2 ± 1.2 | 11.2 ± 1.5 | 10.6 ± 1.3 | 10.3 ± 1.2 | 10.2 ± 1.7 | 9.5 ± 1.7 |
| C4/5 | 11.1 ± 1.3 | 10.9 ± 1.7 | 10.5 ± 1.4 | 9.9 ± 1.4 | 9.5 ± 1.8 | 8.8 ± 1.8 |
| C5/6 | 10.6 ± 1.4 | 10.4 ± 1.7 | 9.6 ± 1.4 | 8.7 ± 1.5 | 8.9 ± 1.6 | 8.6 ± 1.7 |
| C6/7 | 10.6 ± 1.4 | 10.4 ± 1.4 | 10.2 ± 1.3 | 9.4 ± 1.7 | 9.2 ± 1.5 | 9.2 ± 1.7 |
| C7/T1 | 11.9 ± 1.3 | 11.9 ± 1.3 | 11.8 ± 1.3 | 11.2 ± 1.6 | 11.2 ± 1.7 | 10.9 ± 1.6 |
| C3 | 11.8 ± 1.2 | 11.8 ± 1.1 | 11.3 ± 1.2 | 11.2 ± 1.2 | 11.2 ± 1.4 | 11.0 ± 1.3 |
| C4 | 11.7 ± 1.3 | 11.6 ± 1.3 | 11.3 ± 1.3 | 11.0 ± 1.2 | 11.0 ± 1.4 | 10.6 ± 1.4 |
| C5 | 11.8 ± 1.2 | 11.7 ± 1.4 | 11.4 ± 1.3 | 10.9 ± 1.3 | 11.0 ± 1.5 | 10.5 ± 1.4 |
| C6 | 11.7 ± 1.2 | 11.8 ± 1.3 | 11.4 ± 1.3 | 10.8 ± 1.5 | 10.7 ± 1.5 | 10.4 ± 1.6 |
| C7 | 12.2 ± 1.2 | 12.4 ± 1.2 | 12.4 ± 1.1 | 11.9 ± 1.5 | 12.0 ± 1.5 | 12.1 ± 1.4 |
| Female | ||||||
| C2/3 | 12.0 ± 1.1 | 11.8 ± 1.3 | 11.8 ± 1.2 | 11.5 ± 1.3 | 11.0 ± 1.2 | 11.2 ± 1.5 |
| C3/4 | 11.1 ± 1.1 | 11.0 ± 1.3 | 10.9 ± 1.2 | 10.6 ± 1.4 | 10.0 ± 1.4 | 9.9 ± 1.7 |
| C4/5 | 10.9 ± 1.1 | 10.8 ± 1.4 | 10.6 ± 1.3 | 10.3 ± 1.5 | 9.4 ± 1.4 | 9.3 ± 1.6 |
| C5/6 | 10.7 ± 1.2 | 10.5 ± 1.3 | 9.6 ± 1.4 | 9.4 ± 1.5 | 8.8 ± 1.4 | 8.5 ± 1.6 |
| C6/7 | 10.8 ± 1.2 | 10.5 ± 1.3 | 10.1 ± 1.3 | 9.6 ± 1.5 | 9.0 ± 1.4 | 9.1 ± 1.4 |
| C7/T1 | 11.7 ± 1.3 | 11.6 ± 1.3 | 11.5 ± 1.2 | 11.1 ± 1.4 | 10.5 ± 1.2 | 10.8 ± 1.4 |
| C3 | 11.8 ± 1.0 | 11.6 ± 1.1 | 11.6 ± 1.1 | 11.4 ± 1.3 | 10.8 ± 1.2 | 10.9 ± 1.4 |
| C4 | 11.5 ± 1.1 | 11.4 ± 1.3 | 11.4 ± 1.1 | 11.2 ± 1.3 | 10.6 ± 1.2 | 10.7 ± 1.3 |
| C5 | 11.7 ± 1.1 | 11.5 ± 1.3 | 11.4 ± 1.1 | 11.2 ± 1.4 | 10.4 ± 1.2 | 10.3 ± 1.4 |
| C6 | 11.8 ± 1.1 | 11.6 ± 1.3 | 11.2 ± 1.2 | 11.0 ± 1.3 | 10.3 ± 1.3 | 10.3 ± 1.4 |
| C7 | 12.1 ± 1.2 | 12.1 ± 1.1 | 12.0 ± 1.0 | 11.8 ± 1.3 | 11.3 ± 1.2 | 11.6 ± 1.2 |
The values are mean ± SD (mm)
Table 3.
Cross-sectional area of dural tube in axial images
| Level | Decades | |||||
|---|---|---|---|---|---|---|
| 20 | 30 | 40 | 50 | 60 | 70 | |
| Male | ||||||
| C2/3 | 218.8 ± 37.8 | 224.1 ± 39.1 | 211.4 ± 39.2 | 205.5 ± 37.3 | 207.6 ± 36.7 | 210.0 ± 39.1 |
| C3/4 | 176.6 ± 31.0 | 183.6 ± 31.0 | 170.7 ± 28.1 | 169.8 ± 29.3 | 168.3 ± 33.7 | 165.3 ± 34.9 |
| C4/5 | 175.0 ± 29.9 | 181.5 ± 34.0 | 169.8 ± 26.8 | 164.1 ± 30.0 | 159.3 ± 32.8 | 151.0 ± 36.0 |
| C5/6 | 168.1 ± 30.0 | 170.8 ± 31.1 | 157.3 ± 25.4 | 147.3 ± 30.2 | 147.4 ± 31.7 | 142.8 ± 32.7 |
| C6/7 | 165.1 ± 30.4 | 170.3 ± 31.1 | 158.1 ± 27.2 | 150.8 ± 33.2 | 146.6 ± 32.9 | 148.6 ± 33.1 |
| C7/T1 | 167.8 ± 27.7 | 175.2 ± 31.9 | 167.3 ± 30.6 | 160.9 ± 29.4 | 165.4 ± 35.9 | 159.6 ± 28.0 |
| C3 | 192.1 ± 32.4 | 196.6 ± 28.6 | 185.9 ± 29.6 | 181.4 ± 30.4 | 181.0 ± 29.7 | 186.5 ± 30.9 |
| C4 | 187.6 ± 31.6 | 194.2 ± 31.0 | 181.9 ± 29.6 | 182.7 ± 32.7 | 181.4 ± 32.1 | 180.0 ± 31.4 |
| C5 | 189.4 ± 33.2 | 196.4 ± 33.6 | 184.0 ± 28.1 | 185.0 ± 33.4 | 187.2 ± 32.0 | 181.9 ± 32.9 |
| C6 | 187.2 ± 33.0 | 194.0 ± 35.2 | 184.8 ± 29.5 | 181.0 ± 37.6 | 179.8 ± 35.8 | 179.9 ± 32.9 |
| C7 | 184.9 ± 31.4 | 195.3 ± 32.8 | 189.2 ± 27.5 | 186.5 ± 34.5 | 188.7 ± 34.8 | 193.0 ± 28.8 |
| Female | ||||||
| C2/3 | 206.6 ± 39.0 | 208.4 ± 39.9 | 204.1 ± 38.9 | 198.0 ± 40.2 | 195.2 ± 35.5 | 206.0 ± 37.9 |
| C3/4 | 173.7 ± 30.1 | 177.4 ± 32.4 | 170.0 ± 30.2 | 166.6 ± 33.3 | 161.1 ± 30.0 | 164.9 ± 33.2 |
| C4/5 | 168.1 ± 29.0 | 172.8 ± 33.7 | 165.0 ± 28.9 | 160.9 ± 28.6 | 152.4 ± 30.7 | 154.4 ± 34.2 |
| C5/6 | 161.8 ± 26.8 | 164.7 ± 30.4 | 150.2 ± 24.0 | 144.0 ± 25.3 | 139.9 ± 27.3 | 137.7 ± 28.6 |
| C6/7 | 156.6 ± 25.8 | 162.0 ± 28.4 | 153.0 ± 25.7 | 145.8 ± 28.7 | 139.9 ± 25.4 | 142.4 ± 27.9 |
| C7/T1 | 154.6 ± 27.3 | 161.3 ± 27.5 | 155.4 ± 27.5 | 153.2 ± 28.5 | 145.4 ± 22.8 | 157.7 ± 25.6 |
| C3 | 181.8 ± 31.1 | 188.0 ± 32.0 | 180.5 ± 29.5 | 176.1 ± 33.0 | 172.5 ± 26.4 | 180.0 ± 30.3 |
| C4 | 179.0 ± 32.1 | 184.3 ± 32.9 | 180.4 ± 30.3 | 174.5 ± 33.3 | 169.9 ± 28.5 | 173.3 ± 31.4 |
| C5 | 180.0 ± 33.8 | 188.0 ± 36.9 | 180.0 ± 31.5 | 176.7 ± 31.9 | 166.7 ± 27.2 | 171.1 ± 29.9 |
| C6 | 176.8 ± 30.0 | 184.5 ± 32.6 | 172.5 ± 27.9 | 171.7 ± 32.0 | 162.5 ± 28.2 | 165.0 ± 30.8 |
| C7 | 173.6 ± 31.5 | 181.7 ± 32.6 | 174.7 ± 30.2 | 173.3 ± 32.3 | 165.0 ± 26.7 | 178.0 ± 29.4 |
Values are mean ± SD (mm2)
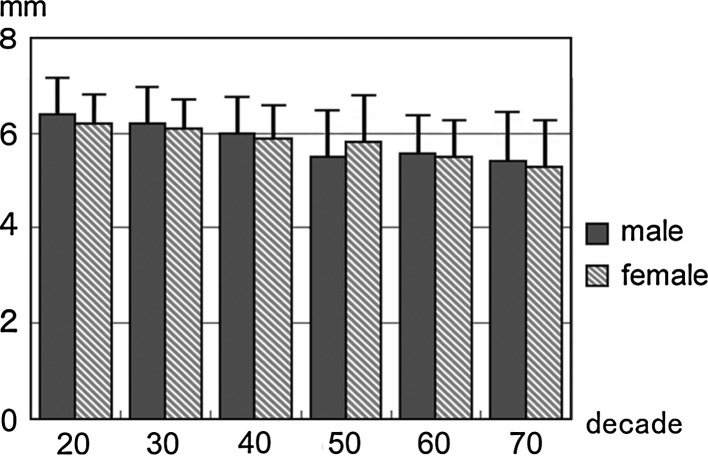
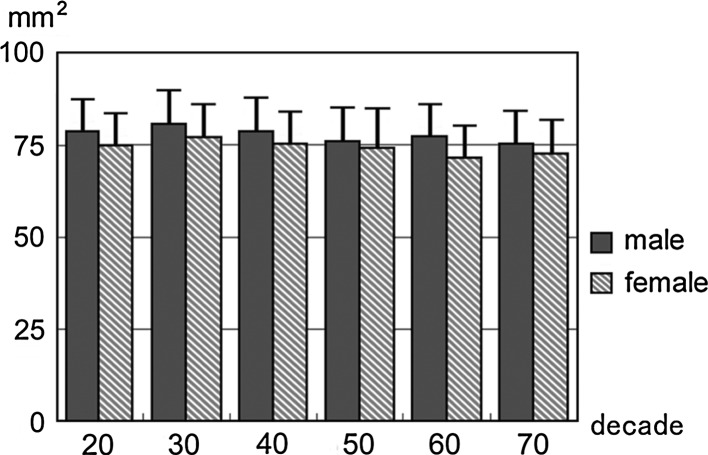
Spinal cord diameter at the C5/6 intervertebral disc level for all ages as observed on sagittal images was 5.9 ± 1.0 mm in males and 5.8 ± 0.9 mm in females, while that at the C5 vertebral body level was 6.5 ± 0.7 mm in males and 6.4 ± 0.7 mm in females (Table 4). Spinal cord area at the C5/6 intervertebral disc level for all ages as observed on axial images was 70.6 ± 11.7 mm2 in males and 68.9 ± 11.3 mm2 in females, while that at the C5 vertebral body level was 78.1 ± 9.4 mm2 in males and 74.4 ± 9.4 mm2 in females (Table 5).
Table 4.
Spinal cord diameter in sagittal images
| Level | Decades | |||||
|---|---|---|---|---|---|---|
| 20 | 30 | 40 | 50 | 60 | 70 | |
| Male | ||||||
| C2/3 | 6.9 ± 0.7 | 7.1 ± 0.7 | 7.0 ± 0.8 | 6.9 ± 0.7 | 7.0 ± 0.7 | 6.8 ± 0.8 |
| C3/4 | 6.9 ± 0.8 | 6.8 ± 0.8 | 6.5 ± 0.8 | 6.4 ± 0.8 | 6.3 ± 0.8 | 6.0 ± 1.1 |
| C4/5 | 6.7 ± 0.8 | 6.5 ± 0.9 | 6.2 ± 0.7 | 6.0 ± 0.8 | 5.9 ± 0.8 | 5.5 ± 1.1 |
| C5/6 | 6.4 ± 0.8 | 6.2 ± 0.8 | 6.0 ± 0.8 | 5.5 ± 1.0 | 5.6 ± 0.9 | 5.4 ± 1.1 |
| C6/7 | 5.9 ± 0.7 | 5.9 ± 0.6 | 5.8 ± 0.6 | 5.4 ± 0.9 | 5.5 ± 0.8 | 5.4 ± 0.8 |
| C7/T1 | 5.7 ± 0.6 | 5.8 ± 0.6 | 5.6 ± 0.6 | 5.4 ± 0.7 | 5.6 ± 0.6 | 5.4 ± 0.7 |
| C3 | 7.0 ± 0.7 | 7.0 ± 0.7 | 6.8 ± 0.8 | 6.7 ± 0.7 | 6.8 ± 0.7 | 6.8 ± 0.8 |
| C4 | 6.9 ± 0.8 | 6.9 ± 0.7 | 6.7 ± 0.7 | 6.6 ± 0.7 | 6.6 ± 0.6 | 6.4 ± 0.8 |
| C5 | 6.8 ± 0.8 | 6.7 ± 0.7 | 6.5 ± 0.7 | 6.4 ± 0.7 | 6.4 ± 0.6 | 6.3 ± 0.7 |
| C6 | 6.3 ± 0.7 | 6.4 ± 0.6 | 6.1 ± 0.7 | 5.9 ± 0.8 | 6.1 ± 0.6 | 6.0 ± 0.8 |
| C7 | 5.8 ± 0.7 | 5.8 ± 0.6 | 5.6 ± 0.6 | 5.6 ± 0.6 | 5.7 ± 0.6 | 5.7 ± 0.6 |
| Female | ||||||
| C2/3 | 6.7 ± 0.7 | 6.9 ± 0.7 | 6.9 ± 0.7 | 6.9 ± 0.8 | 6.7 ± 0.7 | 6.7 ± 0.8 |
| C3/4 | 6.6 ± 0.6 | 6.6 ± 0.7 | 6.4 ± 0.7 | 6.4 ± 0.9 | 6.1 ± 0.7 | 6.1 ± 0.9 |
| C4/5 | 6.4 ± 0.7 | 6.3 ± 0.8 | 6.2 ± 0.7 | 6.2 ± 0.9 | 5.8 ± 0.8 | 5.7 ± 0.9 |
| C5/6 | 6.2 ± 0.6 | 6.1 ± 0.6 | 5.9 ± 0.7 | 5.8 ± 1.0 | 5.5 ± 0.8 | 5.3 ± 1.0 |
| C6/7 | 5.9 ± 0.7 | 5.8 ± 0.7 | 5.7 ± 0.6 | 5.6 ± 0.8 | 5.4 ± 0.6 | 5.4 ± 0.8 |
| C7/T1 | 5.5 ± 0.7 | 5.5 ± 0.6 | 5.6 ± 0.5 | 5.4 ± 0.6 | 5.3 ± 0.6 | 5.4 ± 0.6 |
| C3 | 6.6 ± 0.6 | 6.8 ± 0.7 | 6.8 ± 0.6 | 6.8 ± 0.8 | 6.6 ± 0.7 | 6.6 ± 0.8 |
| C4 | 6.6 ± 0.7 | 6.6 ± 0.7 | 6.5 ± 0.6 | 6.6 ± 0.8 | 6.4 ± 0.7 | 6.3 ± 0.7 |
| C5 | 6.6 ± 0.5 | 6.5 ± 0.7 | 6.4 ± 0.6 | 6.4 ± 0.9 | 6.2 ± 0.6 | 6.1 ± 0.7 |
| C6 | 6.2 ± 0.6 | 6.1 ± 0.6 | 6.1 ± 0.5 | 6.1 ± 0.7 | 6.0 ± 0.6 | 5.8 ± 0.7 |
| C7 | 5.6 ± 0.6 | 5.7 ± 0.6 | 5.7 ± 0.5 | 5.6 ± 0.6 | 5.5 ± 0.6 | 5.6 ± 0.7 |
The values are mean ± SD (mm)
Table 5.
Cross-sectional area of spinal cord in axial images
| Level | Decades | |||||
|---|---|---|---|---|---|---|
| 20 | 30 | 40 | 50 | 60 | 70 | |
| Male | ||||||
| C2/3 | 77.9 ± 8.0 | 80.1 ± 7.7 | 79.5 ± 10.1 | 77.5 ± 8.7 | 77.4 ± 8.0 | 76.5 ± 9.1 |
| C3/4 | 78.0 ± 8.5 | 79.9 ± 8.7 | 78.1 ± 10.6 | 75.5 ± 9.9 | 76.3 ± 10.7 | 73.7 ± 10.1 |
| C4/5 | 79.3 ± 8.5 | 81.3 ± 10.6 | 78.5 ± 9.3 | 75.9 ± 11.3 | 74.9 ± 10.4 | 69.7 ± 15.0 |
| C5/6 | 74.6 ± 8.8 | 75.4 ± 10.3 | 72.3 ± 10.4 | 66.3 ± 12.9 | 68.5 ± 11.2 | 66.5 ± 12.4 |
| C6/7 | 64.9 ± 9.2 | 66.0 ± 8.8 | 63.8 ± 9.4 | 61.5 ± 10.9 | 62.7 ± 9.3 | 61.8 ± 11.3 |
| C7/T1 | 54.0 ± 6.6 | 55.6 ± 6.9 | 54.1 ± 7.7 | 51.1 ± 8.0 | 53.2 ± 6.4 | 53.1 ± 6.7 |
| C3 | 76.3 ± 6.8 | 78.4 ± 7.9 | 76.8 ± 9.2 | 74.8 ± 8.9 | 75.2 ± 8.1 | 75.0 ± 8.5 |
| C4 | 80.2 ± 8.8 | 82.3 ± 9.4 | 80.2 ± 9.2 | 78.5 ± 10.0 | 79.0 ± 10.0 | 78.2 ± 8.9 |
| C5 | 79.0 ± 8.6 | 80.9 ± 9.4 | 78.8 ± 9.6 | 76.1 ± 9.7 | 77.8 ± 8.5 | 76.0 ± 9.5 |
| C6 | 71.1 ± 7.7 | 74.3 ± 9.6 | 71.3 ± 8.0 | 69.8 ± 10.1 | 70.5 ± 8.0 | 69.8 ± 9.5 |
| C7 | 59.1 ± 7.4 | 60.4 ± 8.1 | 58.7 ± 9.1 | 57.3 ± 9.0 | 58.3 ± 7.3 | 58.4 ± 8.2 |
| Female | ||||||
| C2/3 | 72.4 ± 6.8 | 74.8 ± 8.6 | 73.9 ± 7.8 | 73.0 ± 8.1 | 71.4 ± 7.5 | 73.6 ± 8.2 |
| C3/4 | 74.0 ± 8.0 | 75.4 ± 8.9 | 75.0 ± 8.6 | 72.7 ± 9.7 | 70.1 ± 8.9 | 71.9 ± 9.5 |
| C4/5 | 75.4 ± 8.7 | 76.7 ± 9.4 | 76.1 ± 9.7 | 74.7 ± 11.3 | 70.6 ± 10.0 | 72.3 ± 11.8 |
| C5/6 | 72.4 ± 9.2 | 72.8 ± 10.3 | 70.6 ± 9.9 | 68.0 ± 11.8 | 64.6 ± 11.0 | 65.5 ± 12.7 |
| C6/7 | 62.7 ± 10.0 | 63.5 ± 10.1 | 63.0 ± 9.2 | 60.3 ± 10.2 | 58.2 ± 9.1 | 60.6 ± 10.4 |
| C7/T1 | 51.3 ± 7.4 | 52.3 ± 7.8 | 51.0 ± 5.8 | 50.9 ± 7.1 | 48.6 ± 6.1 | 52.3 ± 7.7 |
| C3 | 72.1 ± 7.0 | 74.3 ± 8.9 | 72.9 ± 7.6 | 71.7 ± 7.9 | 70.2 ± 7.3 | 71.9 ± 8.1 |
| C4 | 75.5 ± 8.2 | 78.7 ± 9.1 | 77.4 ± 8.2 | 76.0 ± 10.2 | 73.2 ± 8.1 | 75.4 ± 9.8 |
| C5 | 74.8 ± 8.7 | 77.3 ± 9.0 | 75.8 ± 8.5 | 74.7 ± 10.8 | 71.5 ± 8.3 | 72.8 ± 9.7 |
| C6 | 68.7 ± 8.5 | 69.9 ± 8.8 | 69.7 ± 8.9 | 68.3 ± 10.0 | 65.2 ± 8.0 | 68.2 ± 9.2 |
| C7 | 56.1 ± 8.5 | 56.6 ± 8.5 | 56.6 ± 7.9 | 56.2 ± 7.4 | 53.0 ± 7.1 | 57.3 ± 8.3 |
The values are mean ± SD (mm2)
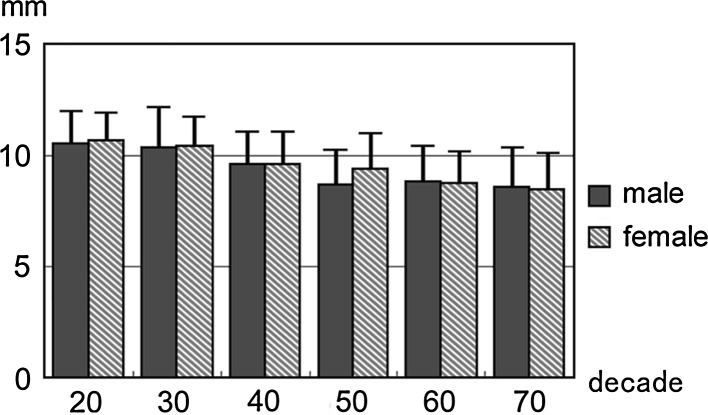
All the above-mentioned parameters tended to decrease with increasing age. This tendency was more marked at the intervertebral disc level than at the vertebral body level, especially around the C5/6 intervertebral disc level (Figs. 1, 2, 3, 4, 5, 6).
Fig. 1.
Dural tube diameter at the C5/6 in each sex and decade
Fig. 2.
Dural tube diameter at the C5 in each sex and decade
Fig. 3.
Spinal cord diameter at the C5/6 in each sex and decade
Fig. 4.
Spinal cord diameter at the C5 in each sex and decade
Fig. 5.
Cross-sectional area of spinal cord at the C5/6 in each sex and decade
Fig. 6.
Cross-sectional area of spinal cord at the C5 in each sex and decade
The average spinal cord occupation rate in the dural tube at the C5 vertebral body level was 58.3 ± 7.0% (Table 6). The border value (average + 2SD) was 72.3%. Therefore, we propose a spinal cord occupation rate of more than 75% in the dural tube as the criterion for the diagnosis of developmental stenosis of the cervical spinal canal.
Table 6.
Spinal cord occupation rate in dural tube (sagittal)
| Level | Decades | |||||
|---|---|---|---|---|---|---|
| 20 | 30 | 40 | 50 | 60 | 70 | |
| Male | ||||||
| C2/3 | 57.7 ± 6.4 | 59.0 ± 6.6 | 60.4 ± 6.4 | 59.9 ± 5.9 | 61.1 ± 6.9 | 62.1 ± 7.1 |
| C3/4 | 61.9 ± 6.5 | 61.3 ± 7.3 | 61.4 ± 6.5 | 61.8 ± 5.9 | 62.0 ± 7.0 | 62.9 ± 7.8 |
| C4/5 | 60.4 ± 7.1 | 59.9 ± 7.0 | 60.1 ± 6.1 | 61.1 ± 5.7 | 63.2 ± 7.6 | 62.5 ± 7.4 |
| C5/6 | 60.7 ± 7.2 | 60.9 ± 7.6 | 62.8 ± 7.5 | 62.9 ± 7.8 | 63.3 ± 7.7 | 63.4 ± 7.5 |
| C6/7 | 56.1 ± 7.3 | 57.6 ± 7.1 | 57.3 ± 7.0 | 58.0 ± 8.4 | 60.0 ± 7.4 | 60.1 ± 7.6 |
| C7/T1 | 48.6 ± 6.1 | 49.1 ± 7.0 | 48.0 ± 6.5 | 58.9 ± 7.3 | 50.5 ± 7.8 | 49.9 ± 8.1 |
| C3 | 59.5 ± 7.1 | 59.8 ± 6.6 | 60.0 ± 6.1 | 60.6 ± 6.0 | 61.4 ± 6.2 | 62.2 ± 7.0 |
| C4 | 59.7 ± 6.8 | 59.9 ± 6.6 | 59.5 ± 6.1 | 59.9 ± 6.6 | 60.3 ± 6.4 | 60.9 ± 6.7 |
| C5 | 58.0 ± 7.0 | 57.8 ± 7.1 | 58.0 ± 7.5 | 58.7 ± 6.1 | 58.8 ± 6.9 | 60.3 ± 7.3 |
| C6 | 54.6 ± 6.4 | 54.8 ± 7.5 | 54.1 ± 6.8 | 55.4 ± 7.4 | 58.1 ± 8.0 | 57.9 ± 7.8 |
| C7 | 47.3 ± 6.2 | 47.4 ± 6.3 | 45.7 ± 5.8 | 47.6 ± 7.1 | 48.1 ± 7.5 | 47.3 ± 6.8 |
| Female | ||||||
| C2/3 | 55.8 ± 6.9 | 58.5 ± 6.4 | 59.1 ± 6.3 | 59.8 ± 6.2 | 61.3 ± 6.3 | 60.8 ± 7.3 |
| C3/4 | 59.8 ± 6.0 | 60.6 ± 6.3 | 59.8 ± 6.4 | 61.2 ± 6.3 | 61.9 ± 6.4 | 61.8 ± 7.0 |
| C4/5 | 58.7 ± 6.8 | 58.5 ± 7.1 | 58.6 ± 6.0 | 60.5 ± 6.2 | 62.4 ± 7.2 | 61.9 ± 7.7 |
| C5/6 | 58.3 ± 6.6 | 58.5 ± 7.5 | 61.8 ± 6.9 | 61.7 ± 6.9 | 63.6 ± 6.2 | 63.0 ± 7.8 |
| C6/7 | 54.5 ± 7.1 | 55.7 ± 7.3 | 57.8 ± 7.4 | 58.8 ± 7.8 | 60.2 ± 6.8 | 60.1 ± 8.1 |
| C7/T1 | 47.7 ± 6.1 | 48.1 ± 6.2 | 49.1 ± 6.6 | 49.3 ± 7.0 | 50.9 ± 6.2 | 51.1 ± 7.8 |
| C3 | 56.8 ± 6.2 | 58.9 ± 6.3 | 59.1 ± 6.2 | 59.8 ± 6.7 | 61.4 ± 6.5 | 60.8 ± 7.4 |
| C4 | 57.8 ± 6.5 | 58.6 ± 6.4 | 57.7 ± 6.0 | 59.1 ± 6.8 | 60.1 ± 6.8 | 59.7 ± 7.2 |
| C5 | 56.4 ± 6.5 | 56.7 ± 6.7 | 56.6 ± 6.5 | 57.6 ± 6.2 | 60.5 ± 6.7 | 59.9 ± 7.5 |
| C6 | 52.3 ± 6.1 | 53.6 ± 7.1 | 55.1 ± 6.0 | 55.4 ± 6.9 | 58.6 ± 6.9 | 57.3 ± 8.2 |
| C7 | 46.7 ± 6.2 | 47.6 ± 5.3 | 48.1 ± 5.9 | 47.7 ± 6.2 | 49.6 ± 7.0 | 49.0 ± 7.6 |
The values are mean ± SD (%)
A variety of abnormal findings were recognized; these included compression of the spinal cord [n = 64 (5.3%)], flattening of the spinal cord [n = 38 (3.1%)], and high-signal changes in T2 sagittal images [n = 28 (2.3%)]. Other findings were as follows: synostosis (n = 14), Arnold–Chiari malformations (n = 7), ossification of the posterior longitudinal ligament of the spine (OPLL) (n = 5), C1/2 pseudo-tumor with subluxation (n = 1), and spinal tumor (n = 1).
Discussion
This research was conducted to establish standard MRI values for the cervical spinal canal, dural tube, and spinal cord, evaluate age-related changes in healthy subjects, and assess the incidence of abnormal findings in asymptomatic subjects. These parameters were established in each sex and each decade of life, and tended to decrease with increasing age. Age-related changes were more marked at the intervertebral disc level than at the vertebral body level, especially at the C5/6 level. The spinal cord occupation rate in the dural tube at the C5 vertebral body level averaged 58.3 ± 7.0%. Various abnormal findings were observed at a relatively high rate on MR images.
To date, only a few MRI-based reports on the normal configuration of the cervical spinal canal, including the soft tissue, have been documented in the literature [2, 7]. The cervical spinal canal and spinal cord measurements recorded in the present study were similar to those obtained using CT scanning after myelography (CTM) [4, 10]. The sagittal diameter of the spinal canal was approximately 12 mm and spinal cord diameter was approximately 6 mm in our MRI study, which were similar to the values obtained in the CTM study. However, the area of the axial spinal cord was approximately 52 mm2 in the CTM study, whereas it was approximately 75 mm2 in the present study. The values obtained by MRI may have been higher than those obtained by CTM because of factors such as spinal cord pulsation (motion artifact). In previous reports, the area of the axial spinal cord was approximately 90 mm2 using 0.5-Tesla MRI [2]. It is thought that these factors are more significant when using 0.5-Tesla MRI, which has a lower resolution and a longer inspection time than 1.5-Tesla MRI. The area of the axial spinal cord in autopsy specimens was found to be approximately 50 mm2, which is presumably reduced because of the fixation of specimens [3].
Among cervical spinal cord injuries, the frequency of cases without bony lesions varied from approximately 50% in subjects under 64 years of age to approximately 68% in subjects over 65 years of age [9]. It is predicted that the number of individuals with cervical stenosis will increase as the population ages. Furthermore, it is likely that spinal cord injuries without bony lesions will increase with the increase in prevalence of cervical stenosis.
The parameters evaluated in this study tended to decline with age. This tendency was greater at the intervertebral disc level than at the vertebral body level, especially at the C5/6 intervertebral disc level. Three explanations can be offered for this observation. First, degenerative changes in the cervical spine progress chiefly in the intervertebral discs, and such changes occur maximally at the C5/6 intervertebral disc level. Second, the spinal cord may atrophy as age increases. Third, both the cervical spine and spinal cord may possibly be relatively small in the elderly because they tend to have a smaller build.
The current definition of developmental stenosis of the cervical spinal canal is based on X-ray data, and no relevant MRI data on this subject have been published. We therefore proposed to define developmental stenosis of the cervical spinal canal on the basis of MRI data. Generally, a sagittal diameter of the spinal canal of less than 12–14 mm at the C5 vertebral body level as observed on X-ray is defined as developmental stenosis of the cervical spinal canal. However, this definition concerns only the container (the spinal canal) and ignores the contents (the spinal cord). Furthermore, as compared to a live specimen, there is a twofold difference in the axial area of the spinal cord in autopsy specimens, and a similar difference was demonstrated even in the present study. It is considered that true spinal canal stenosis is defined by the relationship between the spinal canal and the spinal cord. For example, it cannot be concluded that the spinal canal is actually in a stenosed condition when the spinal cord is thin and there is room in the canal, even if it is relatively narrow on X-ray. The relationship between the spinal cord and the dural tube (the contents and the container) were evaluated in the present study because these can be collectively evaluated on MRI. The dural tube was used to evaluate the size of the container on the basis of MRI, which can reveal the accurate size of the container including the soft tissue. We propose that developmental stenosis of the cervical spinal canal can be defined as an occupation rate of the dural tube of more than 75% (3/4) as observed on MRI.
A variety of abnormal findings such as spinal cord compression, signal changes in T2 images, Arnold–Chiari malformations, and OPLL were recognized; in addition, their prevalence was surprisingly high. These abnormal findings were all seen on cervical MRIs obtained from asymptomatic volunteers. These data emphasize on the dangers of predicating operative decisions on diagnostic tests without precisely correlating MRI findings with clinical signs and symptoms.
This study had a few limitations. First is the possibility of measurement errors. Measurement was performed only once because the number of specific measurements and the number of subjects were very large. However, the measurements were carried out by well-experienced radiation technologists with extensive knowledge of cervical anatomy. Second, all data in this study were derived only from Japanese volunteers, majority of whom belong to a single race. For this reason, it might be difficult to apply these findings to other races in a similar fashion. Japanese have most advanced aging society, and these data should help people of other races, who would prepare for an aged society, to understand age-related changes of cervical spine.
Conclusion
Standard MRI values for the cervical spinal canal, dural tube, and spinal cord were established for healthy members of each sex and each decade of life for 1,211 healthy subjects. The relatively high prevalence of abnormal MRI findings of the cervical spine of asymptomatic individuals emphasizes the dangers of predicating operative decisions on diagnostic tests without precisely correlating these findings with clinical signs and symptoms. The results of this research will prove useful in clinical and preventive medicine, especially because to the best of our knowledge, no previous report providing standard MRI values for the cervical spine and spinal cord are available.
Acknowledgments
This study was supported by institutional funds and by grant research funds, which are intended for promoting hospital functions, of the Japan Labor Health and Welfare Organization (Kawasaki, Japan). No benefits in any form have been or will be received from a commercial party related directly or indirectly to the subject of this manuscript.
Conflict of interest
None.
References
- 1.Higo M, Sako T, Suzuki Y, et al. Roentgenological study of the antero-posterior diameter in cervical developmental canal stenosis. Rinsho Seikei Geka. 1984;19(4):361–366. [Google Scholar]
- 2.Ishikawa M, Matsumoto M, Fujimura Y, et al. Changes of cervical spinal cord and cervical spinal canal with age in asymptomatic subjects. Spinal Cord. 2003;41(3):159–163. doi: 10.1038/sj.sc.3101375. [DOI] [PubMed] [Google Scholar]
- 3.Kameyama T, Hashizume Y, Ando T, et al. Morphometry of the normal cadaveric cervical spinal cord. Spine. 1994;19(18):2077–2081. doi: 10.1097/00007632-199409150-00013. [DOI] [PubMed] [Google Scholar]
- 4.Kimura I, Shingu H, Nasu Y, et al. Computed tomography of the spinal canal for the cervical spine and spinal cord injury. Rinsho Seikei Geka. 1983;18(5):541–551. [Google Scholar]
- 5.Machino M, Yukawa Y, Ito K, et al. Can magnetic resonance imaging reflect the prognosis in patients of cervical spinal cord injury without radiographic abnormality? Spine. 2011;36(24):E1568–E1572. doi: 10.1097/BRS.0b013e31821273c0. [DOI] [PubMed] [Google Scholar]
- 6.Okada E, Matsumoto M, Ichihara D, et al. Does the sagittal alignment of the cervical spine have an impact on disk degeneration? Minimum 10-year follow-up of asymptomatic volunteers. Eur Spine J. 2009;18(11):1644–1651. doi: 10.1007/s00586-009-1095-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Okada E, Matsumoto M, Fujiwara H, et al. Disc degeneration of cervical spine on MRI in patients with lumbar disc herniation: comparison study with asymptomatic volunteers. Eur Spine J. 2011;20(4):585–591. doi: 10.1007/s00586-010-1644-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sasaki T, Kadoya S, Iizuka H. Roentgenological study of the sagittal diameter of the cervical spinal canal in normal adult Japanese. Neurol Med Chir (Tokyo) 1998;38(2):8–83. doi: 10.2176/nmc.38.83. [DOI] [PubMed] [Google Scholar]
- 9.Shingu H, Ikata T, Katoh S, et al. Spinal cord injuries in Japan: a nationwide epidemiological survey in 1990. Paraplegia. 1994;32(1):3–8. doi: 10.1038/sc.1994.2. [DOI] [PubMed] [Google Scholar]
- 10.Thijssen HO, Keyser A, Horstink MW, et al. Morphology of the cervical spinal cord on computed myelography. Neuroradiology. 1979;18(2):57–62. doi: 10.1007/BF00344822. [DOI] [PubMed] [Google Scholar]
- 11.Yukawa Y, Kato F, Yoshihara H, et al. MR T2 image classification in cervical compression myelopathy. Predictor of surgical outcomes. Spine. 2007;32(15):1675–1678. doi: 10.1097/BRS.0b013e318074d62e. [DOI] [PubMed] [Google Scholar]