Abstract
Introduction
Spinal fusion as a treatment for degenerative disc disease is controversial. Prior authors have identified various MRI findings as being pain generators, which might help guide patient selection for lumbar fusion procedures. These findings have included disc desiccation, disc contour, high-intensity zone annular disruption, the presence of Modic endplate changes, and disc space collapse. The purpose of this study is to investigate which MRI findings in patients with degenerative disc disease predict clinical improvement with lumbar fusion.
Methods
A single-center surgical database of patients undergoing lumbar fusion was reviewed for patients whose indication for fusion surgery was primary disc pathology. We identified 51 patients (71 disc levels) who had completed 2-year prospectively collected outcomes questionnaires and had preoperative MRIs available for review. NRS (0–10) back and leg pain, Oswestry Disability Index (ODI) and SF-36 Physical Composite Summary scores were obtained preoperatively and at 1- and 2-year follow-up. MRIs were reviewed by three fellowship-trained spine surgeons who were asked to grade them for the following five characteristics: (a) disc desiccation, (b) disc contour, (c) presence of a high-intensity zone (HIZ) annular tear, (d) presence of Modic endplate changes and (e) disc height. Two-year outcome measures were compared to MRI findings to identify which findings correlated with improvement in outcome scores.
Results
Statistically significant improvements were noted in back pain, leg pain, SF-36 PCS and ODI in the group overall. Disc desiccation, disc contour, presence of an HIZ lesion, and the presence of Modic endplate changes did not correlate with 2-year outcomes. Disc height was correlated with 2-year change in outcome measures. Discs with preoperative height less than 5 mm demonstrated a 23.4 point ODI improvement compared to 9.2 points for discs >7 mm. Similarly, SF-36 PCS improved 9.5 points in discs <5 mm compared to 0.7 in discs greater than 7 mm. Discs between 5 and 7 mm demonstrated intermediate levels of improvement.
Conclusions
Several commonly utilized MRI criteria proposed as indications for lumbar fusion do not seem to correlate with 2-year improvement in clinical outcomes. Discs which are narrowed and collapsed, preoperatively, demonstrate better improvement at 2 years postoperatively as compared to discs which have maintained disc height. Significant disc space collapse may represent a subset of “degenerative disc disease” which responds more favorably to treatment with fusion.
Keywords: Lumbar fusion, Disc height, Clinical outcomes, ODI, SF36
Introduction
The surgical treatment of axial-predominant pain associated with lumbar disc degeneration has been controversial [13]. In contrast to conditions where neurogenic leg pain predominates, identifying the source of an individual patient’s axial low back pain is often difficult. Patients with clear segmental instability, such as spondylolisthesis, have been found to have predictable improvement with fusion [17, 19], but in the absence of clear instability, surgical indications remain a matter of considerable debate. With the rising rates of lumbar fusion surgery seen in the past decade, and the limited future health-care resources which will be allocated to spine care, a better understanding of which patients can reliably benefit from lumbar fusion surgery is a high public-health priority [12].
The question of which MRI findings are structural causes of low back pain and should be used in guiding treatment, particularly surgical treatment, is largely unanswered. Many radiographic abnormalities noted on MRI, which are often used as indications for lumbar fusion, are associated with normal aging of the lumbar spine and can be seen in asymptomatic individuals [3]. This has led to other diagnostic techniques such as discography in an attempt to better identify the pain source in these patients, but this technique also is controversial and has been shown to lead to marginal or no improvement in clinical outcomes [9, 24]. The search for noninvasive radiographic markers, which can predict improvement with fusion surgery has been challenging and the question of which radiographic findings are valid indications for fusion surgery remain a matter of debate.
The current study seeks to clarify the relationship between preoperative MRI findings and the results of lumbar fusion surgery. Previous studies have looked at certain radiographic features in isolation or have lacked validated health-related outcome measures. In the current study, we identified a group of patients undergoing lumbar fusion primarily for discogenic pain, who had prospectively collected clinical outcomes data. Preoperative MRI scans were then reviewed and we examined to determine which radiographic findings were correlated with successful results with surgery.
Methods
The current study is a retrospective study of a subset of a clinical database of prospectively collected health outcomes data. A single-center surgical database of patients undergoing lumbar fusion surgery at a tertiary spine care center was reviewed from 2001 to the present. At the time of enrollment, surgeons were required to select the primary indication for fusion in each patient based on previously defined criteria [17]. The database was screened for patients whose primary surgical indication was listed as “disc pathology”.
Of these patients, 51 patients (71 disc levels) were found to have preoperative MRIs available for review and had completed 2-year clinical outcomes data. All patients had axial or referred pain as their predominant symptom with or without associated radiculopathy. In all patients, they rated low back pain equal to or greater than leg pain. Patients with all other diagnoses other than primary disc pathology (spondylolisthesis, spinal stenosis, scoliosis, tumor, infection, etc.) were excluded. Patients who had a disc pathology as a secondary surgical indication in association with stenosis or spondylolisthesis as the surgeon’s primary indication for surgery were excluded. The study protocol was approved by the Institutional Review Board.
Standardized questionnaires were administered prior to surgery, and at 1 and 2 years post-surgery. Patients were asked to rate low back pain and leg pain independently on a 10-point numeric rating scale (back pain NRS and leg pain NRS), ranging from 0 (no pain) to 10 (worst possible pain) [22]. The questionnaires also included the Oswestry Disability Index (ODI) as a measure of low-back specific disability [15] and the Medical Outcomes Study Short Form-36 General Health Instrument (SF-36) as a measure of general health-related quality of life [32]. Outcome measure changes were evaluated based on mean changes from preoperative values to two-year post-operative values.
Preoperative MRI scans were graded independently by three fellowship-trained spine surgeons who were blinded to the clinical outcomes data. The MRIs were performed at a variety of centers, thus a standardized imaging protocol was not utilized in this study. All patients had a minimum of T1 and T2-weighted parasagittal and axial scans available for review. Each reviewer was informed as to which levels were fused at surgery and was asked to evaluate the levels for the criteria listed below. The definitions of all these MRI findings were operationalized and the definitions of each finding were listed on each individual grading sheet for the surgeon to review during the grading process. The reviewer was first asked to grade the disc contour using guidelines and definitions as outlined in the published recommendations from the combined task forces of the North American Spine Society, American Society of Spine Radiology, and the American Society of Neuroradiology [16]. A disc could be graded as normal, protrusion, or herniation (extrusion or sequestration).
Reviewers were then asked to grade the degree of disc degeneration with all associated findings. Disc desiccation was graded as present or absent. A high-intensity zone (HIZ) annular tear was noted to be present if an area of increased T2 signal, isointense to cerebrospinal fluid [2], was noted in the posterior or posterolateral annulus. Modic changes [26, 27] were graded as absent or Type I, II or III. Type I changes were defined as hypointensity on T1 weighted images and hyperintensity on T2-weighted images. Type II changes were defined as hyperintensity on T1-weighted images and isointensity or slight hyperintensity on T2-weighted images. Type III changes were defined as hypointensity on both T1 and T2-weighted images. For the purposes of statistical analysis, mixed changes were not allowed in the current study. Finally, the reviewer was asked to measure disc height on mid-sagittal images in the center of the endplate. An overall score for each finding was generated by combining the three readings. For categorical variables, the mode (majority reading) was utilized. For continuous variables (e.g. disc height), an average of the three readers’ findings was used.
Statistical analysis was by Statistical Package for the Social Sciences (SPSS v18.0) software (SPSS Inc., Chicago, IL). Mean differences in non-continuous variables (back and leg pain NRS) were compared using the Mann–Whitney test. Mean differences in continuous variables (ODI, SF-36 PCS) were compared using independent samples t test or one-way ANOVA. Tukey’s HSD procedure was used for post hoc comparisons between groups. Correlation coefficients between all variables were calculated using Spearman’s rho. In all cases, a two-tailed p value <0.05 were considered statistically significant.
Results
The baseline demographics of the study group are summarized in Table 1. Overall, there were 51 patients with 71 disc levels fused. The study group included 23 females and 28 males with an average age of 47 years (range 29–84 years); 10 patients (14%) were smokers and 6 patients (8%) were Workers’ Compensation patients; 33 patients (65%) had single-level fusions; 16 patients (31%) underwent two-level fusions, and 2 patients (4%) had three-level fusions. The majority of patients (60%) were treated with an instrumented posterolateral fusion, while the remainder had some type of interbody fusion (transforaminal interbody fusion, stand-alone anterior lumbar interbody fusion or anterior-posterior fusion). The overall change in outcome measures for the entire study group is shown in Table 2.
Table 1.
Patient demographics
| Variable | |
|---|---|
| Age, years | 47 (29–84) |
| M:F | 28:23 |
| Smoker | 10 (14%) |
| Worker’s comp | 6 (8%) |
| Number of levels | |
| 1 Level | 33 (65%) |
| 2 Level | 16 (31%) |
| 3 Level | 2 (4%) |
| Fusion type | |
| Posterolateral fusion | 43 (60%) |
| Transforaminal interbody fusion | 18 (25%) |
| Circumferential fusion | 8 (11%) |
| Anterior lumbar interbody fusion | 2 (3%) |
Table 2.
Overall clinical outcomes for entire study group
| Mean (standard deviation) clinical outcome scores | |||
|---|---|---|---|
| Baseline | Two-year post-operative | Change in score | |
| Back pain | 8.0 (2.0) | 4.9 (2.6) | −3.1 (2.7) |
| Leg pain | 7.4 (2.3) | 4.9 (2.9) | −2.7 (3.4) |
| ODI | 54.7 (11.7) | 37.1 (20.1) | −18.0 (18.3) |
| SF-36 PCS | 29.5 (6.8) | 36.0 (10.1) | 6.5 (9.5) |
ODI Oswestry Disability Index, SF-36 PCS Short Form-36 Physical Composite Summary Score
Overall correlations
Correlation coefficients (Spearman’s rho) were calculated between the preoperative MRI findings of the involved discs and the 2-year change in clinical outcome measures (back pain, leg pain, ODI and SF-36 PCS). These results are shown in Table 3. Of all the MRI findings, disc height was found to have a significant correlation with 2-year change in leg pain, ODI and SF-36 PCS (p < 0.05). Disc contour, desiccation, the presence of an HIZ lesion, and the presence of Modic endplate changes did not show statistically significant correlation with 2-year changes in outcome measures.
Table 3.
Correlation coefficients of MRI findings and 2-year change in clinical outcome measures
| Back pain | Leg pain | ODI | SF-36 PCS | |
|---|---|---|---|---|
| Desiccation | ||||
| r | −0.059 | −0.101 | 0.119 | 0.050 |
| p Value | 0.635 | 0.421 | 0.342 | 0.692 |
| HIZ | ||||
| r | −0.006 | 0.019 | 0.021 | −0.026 |
| p Value | 0.959 | 0.877 | 0.865 | 0.836 |
| Modic | ||||
| r | 0.053 | −0.233 | −0.039 | −0.118 |
| p Value | 0.672 | 0.059 | 0.758 | 0.349 |
| Disc | ||||
| r | −0.126 | −0.124 | −0.104 | −0.054 |
| p Value | 0.312 | 0.322 | 0.405 | 0.669 |
| Disc height | ||||
| r | −0.218 | −0.259 | −0.264 | −0.346 |
| p Value | 0.078 | 0.036 | 0.032 | 0.005 |
ODI Oswestry Disability Index, SF-36 PCS Short Form-36 Physical Composite Summary Score, HIZ High-Intensity Zone
Desiccation
Only 10 patients (14%) in the study group who underwent fusion had discs which did not appear desiccated, while 61 discs (85%) were desiccated. There was no difference in 2-year change of clinical outcome measures in patients whose discs were desiccated compared with those who were not (Table 4).
Table 4.
Mean change (SD) in outcome measures in patients with and without disc desiccation
| LBP | Leg pain | ODI | SF-36 PCS | |
|---|---|---|---|---|
| Not desiccated (n = 10) | −3.3 (2.4) | −3.2 (2.7) | −11.0 (10.6) | 6.2(8.9) |
| Desiccated (n = 61) | −3.1 (2.8) | −2.6 (6.3) | −19.3 (18.5) | 6.6 (9.7) |
| p Value | 0.79 | 0.59 | 0.06 | 0.89 |
Disc contour
Eleven discs (15%) were found to have normal contour, 52 (72%) were graded as protrusions and eight (11%) were graded as extrusions. Mean 2-year change in clinical outcomes is shown for these three groups in Table 5. There was no significant difference in improvement between discs with normal contour, protruding discs or discs with an extrusion.
Table 5.
Mean change (SD) in clinical outcome measures by disc contour
| LBP | Leg pain | ODI | SF-36 PCS | |
|---|---|---|---|---|
| Normal (n = 11) | −3.7 (3.4) | −3.1 (3.4) | −22.0 (21.3) | 6.4 (6.0) |
| Protrusion (n = 52) | −3.1 (2.7) | −3.5 (6.6) | −16.6 (18.0) | 6.9 (10.8) |
| Extrusion (n = 8) | −2.5 (1.8) | −1.3 (2.7) | −17.3 (14.4) | 4.6 (5.2) |
| p Value | 0.63 | 0.61 | 0.68 | 0.83 |
High-Intensity Zone
Eleven discs (15%) were found to have a high-intensity zone annular tear in the posterior or posterolateral annulus, while 60 discs (85%) did not demonstrate this lesion. Discs with and without this lesion did not have a significantly different 2-year change in clinical outcome measures (Table 6).
Table 6.
Mean change (SD) in clinical outcome measures based on presence of HIZ lesion
| LBP | Leg pain | ODI | SF-36 PCS | |
|---|---|---|---|---|
| No HIZ (n = 60) | −3.1 (2.6) | −2.6 (6.2) | −17.3 (17.8) | 6.8 (9.4) |
| HIZ (n = 11) | −3.4 (3.4) | −3.1 (4.3) | −19.1 (20.1) | 5.2 (10.4) |
| p Value | 0.71 | 0.65 | 0.84 | 0.617 |
Modic endplate changes
Forty discs (56%) were graded as having no Modic endplate changes, while 31 discs (43%) did demonstrate Modic changes. Of these, 10 discs (14%) had type 1 changes, 18 discs (25%) had type 2 changes, and 3 discs (4%) had type 3 changes. The presence of any Modic changes at all, did not demonstrate any effect on clinical outcome (Table 7). A subgroup analysis was then performed to see if certain types of Modic changes predicted 2-year improvement in outcome measures. This was not the case, as all Modic subtypes generated similar improvements when compared with each other or with discs which had no Modic changes at all (Table 8).
Table 7.
Mean change (SD) in clinical outcome measures by presence or absence of Modic endplate changes
| LBP | Leg pain | ODI | SF-36 PCS | |
|---|---|---|---|---|
| No Modic changes(n = 40) | −3.0 (2.8) | −3.9 (7.0) | −18.0 (18.7) | 7.1 (9.2) |
| Modic changes present (n = 31) | −3.3 (2.6) | −2.0 (3.3) | −17.0 (17.5) | 5.7 (10.1) |
| p Value | 0.63 | 0.194 | 0.83 | 0.56 |
Table 8.
Mean change (SD) in clinical outcome measures by Modic change subtype
| LBP | Leg Pain | ODI | SF-36 PCS | |
|---|---|---|---|---|
| None (n = 40) | −3.0 (2.8) | −3.9 (7.0) | −18.0 (18.7) | 7.1 (9.2) |
| Type 1 (n = 10) | −3.8 (2.8) | −3.8 (3.4) | −19.2 (17.9) | 7.0 (9.2) |
| Type 2 (n = 18) | −3.4 (2.6) | −1.2 (2.9) | −18.5 (18.2) | 6.3 (10.2) |
| Type 3 (n = 3) | −1.7 (1.2) | 0.0 (3.0) | −2.7 (5.0) | −1.7 (13.3) |
| p Value | 0.66 | 0.36 | 0.55 | 0.51 |
Disc height
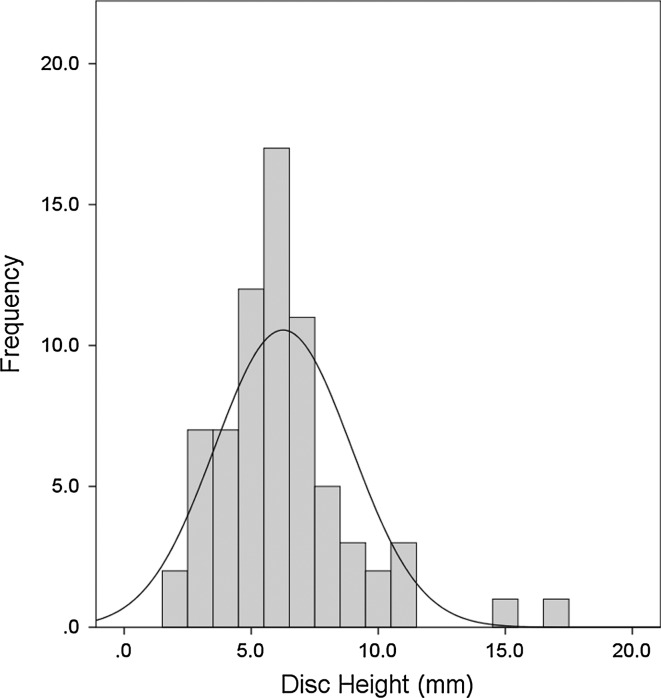
Figure 1 is a histogram of the distribution of the disc heights included in the study. The distribution approximates a normal curve (skewness = 1.5, kurtosis = 3.95) with a median disc height of 6.0 mm. For the purpose of further analysis, the discs were divided into three groups based on percentiles, with a disc height <5 mm representing the smallest third (33rd percentile), 5–7 mm representing the middle third, and >7 m (67th percentile) representing the largest third.
Fig. 1.
Histogram of distribution of disc heights for study sample
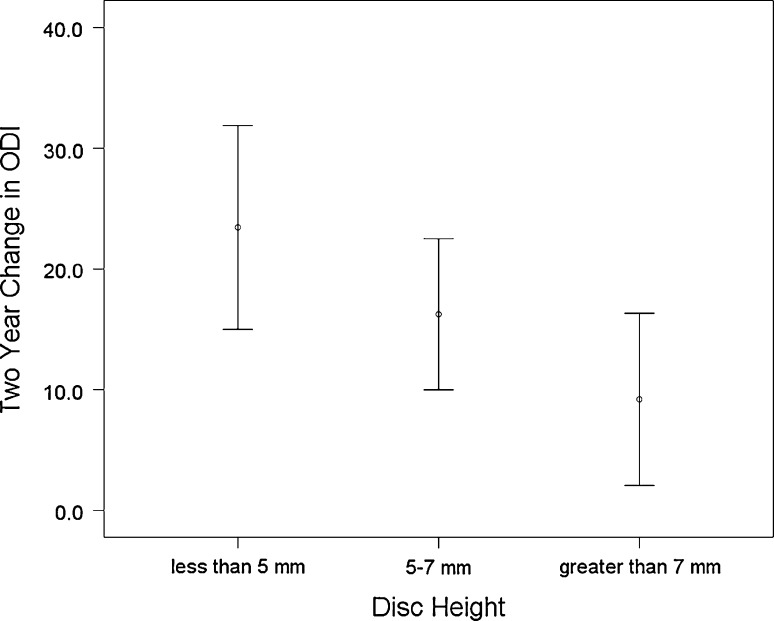
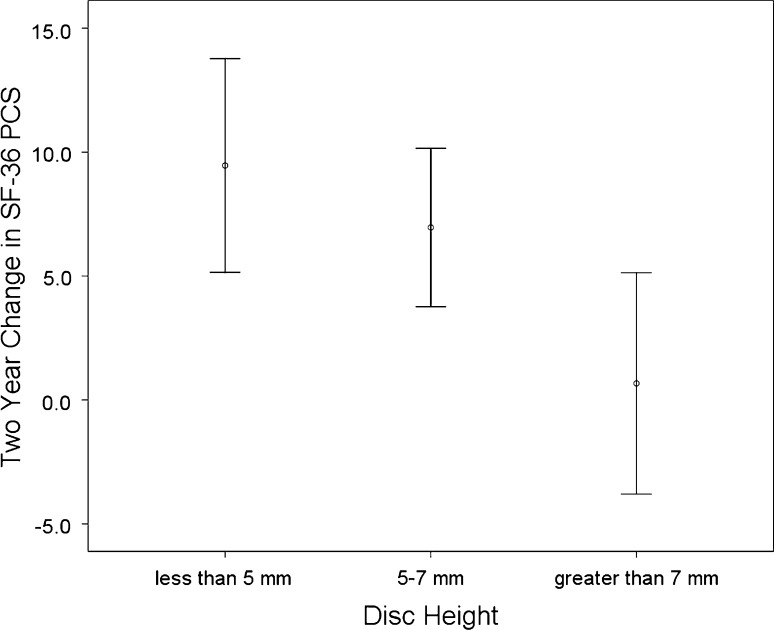
Figures 2 and 3 demonstrate that as disc height decreases, the mean 2-year improvement in clinical outcomes measures increases. Mean improvements in low back pain (F = 2.87, p = 0.06), leg pain (F = 4.30, p = 0.02), ODI (F = 3.89, p = 0.03) and SF-36 PCS (F = 4.75, p = 0.01) were all related to disc height. For instance, the mean improvement in ODI for discs <5 mm was 24.6 points compared with a 9.2 point improvement for discs >7 mm. Similarly, for SF-36 PCS, discs with height <5 mm had a mean improvement of 9.6 points compared with discs >7 mm, which had a mean improvement of 0.7 points. Discs with intermediate height of 5–7 mm demonstrated intermediate improvements in all four clinical outcome measures.
Fig. 2.
Two-year change in Oswestry Disability Index stratified by preoperative disc height
Fig. 3.
Two-year change in Short Form-36 Physical Composite Summary Score stratified by preoperative disc height
Discussion
The current study examined several MRI findings with respect to their capacity to predict improvement with lumbar fusion. We found that several MRI findings, which are commonly used to justify fusion procedures, were not able to predict improvement with fusion. These findings included disc desiccation, disc contour, high-intensity zone annular tears, and Modic vertebral endplate changes. Patients with these findings achieved improvement in health-related quality of life measures that were similar to study subjects who did not have these findings. Only narrowed disc space height was found to correlate with clinical improvement and appeared to do so in a dose-dependent manner.
Lumbar fusion has been a controversial treatment for lumbar degenerative disc disease largely because of the variability and unpredictability of clinical results. In fact, the term degenerative disc disease is problematic as it encompasses a wide spectrum of degenerative changes to the lumbar motion segment, many of which are a normal consequence of aging and can be seen in asymptomatic individuals. For fusion to be an effective treatment, correct identification of an individual patient’s “pain generator” would need to occur. Our study would suggest that many commonly used MRI findings are not reliable markers of who will improve after lumbar fusion. This finding certainly could explain much of the variability seen in clinical results of fusion in standard clinical practice. Our study does suggest that preoperative disc space narrowing, a relatively late-stage finding in the degenerative cascade, does seem to correlate with substantial clinical improvement. Targeting lumbar fusion to degenerative discs, which are collapsed and narrowed, may lead to an improvement in overall clinical results although certainly further investigation is needed.
Our data did not suggest that disc desiccation or disc contour correlated with clinical improvement following fusion. Disc desiccation is an early finding in lumbar disc degeneration and is nearly ubiquitous in patients over the age of 50, the majority of whom do not suffer from chronic back pain [28]. The fact that so many older people are pain-free despite having desiccated disc would argue against this being used as an indication for fusion. However, desiccation is used as an indication for fusion in younger patients, particularly if seen in combination with other findings such as annular tears or in the setting of positive discography [9]. Disc contour would not be expected to influence back pain improvement with fusion, as its main significance is with respect to neural compression and leg pain. Interestingly, patients in our study with disc protrusions did not experience greater relief of leg pain than patients with normal disc contour. However, the number of disc extrusions was too small to draw meaningful conclusion and such patients would typically undergo micro-discectomy alone in our practice.
When high-intensity zone annular tears, were first described by April and Bogduk in 1992 [2], many felt that it was a significant breakthrough in the diagnosis of low back pain syndromes. These authors found the lesion to be present in 28% of chronic low back pain patients contemplating fusion; 38 of 40 patients with this lesion had concordant pain with discography in this study, and the authors reported a positive predictive value of 90%. The hope was that this lesion represented a clearly diagnosable cause of low back pain, which could be reliably treated. However, further studies have called into question whether positive discography can be used as a surrogate measure for what is “surgically treatable” [6, 20, 24]. Other studies have shown the HIZ lesion to be present in 6–31% of asymptomatic subjects [5, 21, 30]. Carragee et al. [6] compared discography response in patients with an HIZ lesion with and without back pain. They found that the presence of an HIZ lesion did lead to a concordant response to discography in 70% of patients, whether they had a history of back pain or not. They concluded that the presence of an HIZ lesion did predict response to discography but was not pathognomonic for a structural low back pain illness. Our study similarly suggests that patients with an HIZ lesion did not have any greater improvement with fusion than patients without this lesion did.
Several studies now seem to support a positive association between Modic vertebral endplate signal changes and LBP [1, 23, 25, 27, 29, 31]. Type I changes in particular, which are characterized histologically by endplate fissuring, fibrovascular granulation tissue and edema in the marrow adjacent to the vertebral endplates, are thought to have a strong association with low back pain. One study found an association between Type I changes and segmental hypermobility [31] although this has been disputed [4]. Two recent European studies suggest that patients with type I Modic changes have a high rate of satisfactory outcome with lumbar fusion. Esposito et al. [14], in a review of 60 patients undergoing single-level lumbar fusion for degenerative disc disease, found superior outcomes for patients with type I changes compared to patients with other subtypes. Chataigner and co-authors [7] also noted superior outcomes with ALIF in patients with type I Modic changes but also noted that most of these patients also had disc space narrowing in their study. The current study could not identify a trend towards improved outcomes with Modic changes in general or type I changes specifically.
The association between disc narrowing and mechanical back pain has received increased attention in recent studies. DeShepper [11] and colleagues recently examined this association as part of the Rotterdam Study, a large prospective cohort study in the Netherlands, which has examined a number of disease states. In the 2,800 patients with lumbar radiographs, they found a positive association between disc narrowing with chronic low back pain and disability. Interestingly, they found this moderate association between nearly as strong as the relationship between joint space narrowing and pain in knee osteoarthritis [8] and stronger than the association of radiographic hand osteoarthritis and pain [10]. In results from the Swedish Lumbar Spine Study, Hägg [18] showed that disc narrowing and personality characterized by low neuroticism were the only factors, which predicted improvement with fusion for low back pain. Our study also demonstrated an association between disc narrowing and improvement with lumbar fusion. The cause for this is unknown. A possible explanation may be that a narrowed disc allows greater biomechanical stress to be borne by the adjacent endplates and facet joints, and that arthrodesis alleviates this pathologic stress transfer. Although the cause for this association is unclear, this association clearly was seen in our data across all four clinical outcome variables studied, and seemed to be dose dependent.
Our study certainly has limitations, which should temper the interpretation of our results. The small sample size and relatively low number of some MRI findings means that the study is underpowered to state definitively that there is no association between certain lesions (e.g. HIZ lesion, type I Modic change) and fusion outcomes. However, we did not see any trend towards an association with the sample size available. The sample size was also too small to analyze combinations of MRI characteristics such and disc collapse and Modic changes. Another important limitation is that the majority of fusions performed were posterolateral fusions. Many surgeons favor interbody fusions for discogenic pain. The sample size did not allow comparisons across different fusion types such as TLIF or anterior-posterior fusions. There may be the presence of selection bias in that patients who had disc pathology and did not have patient reported outcomes and/or MRI studies available were excluded. Radiologists were not included as readers in this study. The author’s needed to design the study to parallel what happens in clinical practice. That is, the MRI guides the surgeon in the decision-making process.
In conclusion, the current study demonstrates that of all commonly used MRI findings, which are used as an indication for lumbar fusion, disc space narrowing is the most strongly correlated with clinical improvement. The patient with a narrow, collapsed disc may represent a subset of the large, poorly characterized population of “degenerative disc disease” who may be effectively treated with lumbar fusion. Further prospective studies with large study samples will be needed to further clarify this association.
Conflict of interest
MD receives consulting fees from Medtronic. LYC receives research support from Norton Healthcare. CHC receives consulting fees from Medtronic and Alphatec and is on the Speaker's bureau for Synthes. SDG receives royalties from Medtronic and research support from Norton Healthcare. MD, LYC, CHC, KRB and SDG are employees of Norton Healthcare. JDZ has nothing to disclose.
References
- 1.Albert HB, Manniche C. Modic changes following lumbar disc herniation. Eur Spine J. 2007;16:977–982. doi: 10.1007/s00586-007-0336-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aprill C, Bogduk N. High-intensity zone: a diagnostic sign of painful lumbar disc on magnetic resonance imaging. Br J Radiol. 1992;65:361–369. doi: 10.1259/0007-1285-65-773-361. [DOI] [PubMed] [Google Scholar]
- 3.Boden SD, Davis DO, Dina TS, Patronas NJ, Wiesel SW. Abnormal magnetic resonance scans of the lumbar spine in asymptomatic subjects. A prospective investigation. J Bone Joint Surg Am. 1990;72:403–408. [PubMed] [Google Scholar]
- 4.Bram J, Zanetti M, Min K, Hodler J. MR abnormalities of the intervertebral discs and adjacent bone marrow as predictors of segmental instability of the lumbar spine. Acta Radiol. 1998;39:18–23. doi: 10.1080/02841859809172143. [DOI] [PubMed] [Google Scholar]
- 5.Buirski G, Silberstein M. The symptomatic lumbar disc in patients with low back pain: magnetic resonance imaging appearances in both a symptomatic and control population. Spine. 1993;18:1808–1811. doi: 10.1097/00007632-199310000-00016. [DOI] [PubMed] [Google Scholar]
- 6.Carragee EJ, Tanner CM, Khurana S, Hayward C, Welsh J, Date E, Truong T, Rossi M, Hagle C. The rates of false-positive lumbar discography in select patients without low back symptoms. Spine. 2010;25:1373–1380. doi: 10.1097/00007632-200006010-00009. [DOI] [PubMed] [Google Scholar]
- 7.Chataigner H, Onimus M, Polette A. Surgery for degenerative lumbar disc disease: should the black disc be grafted? Rev Chir Orthop Reparatrice Appar Mot. 1998;84:583–589. [PubMed] [Google Scholar]
- 8.Claessens AA, Schouten JS, Ouweland FA, Valkenburg HA. Do clinical findings associate with radiographic osteoarthritis of the knee? Ann Rheum Dis. 1990;49:771–774. doi: 10.1136/ard.49.10.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Colhoun E, McCall IW, Williams L, Cassar Pullicino VN, et al. Provocation discography as a guide in planning operations on the spine. J Bone Joint Surg Br. 1998;70:267–271. doi: 10.1302/0301-620X.70B2.2964449. [DOI] [PubMed] [Google Scholar]
- 10.Dahaghin S, Bierma-Zeinstra SM, Ginai AZ, Pols HA, Hazes JM, Koes BW. Prevalence and pattern of radiographic hand osteoarthritis and association with pain and disability (the Rotterdam study) Ann Rheum Dis. 2005;64:682–687. doi: 10.1136/ard.2004.023564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schepper EI, Damen J, Meurs JB, Ginai AZ, Popham M, Hofman A, Koes BW, Bierma-Zeinstra SM. The association between lumbar disc degeneration and low back pain. The influence of age, gender and individual radiographic features. Spine. 2010;35:531–536. doi: 10.1097/BRS.0b013e3181aa5b33. [DOI] [PubMed] [Google Scholar]
- 12.Deyo RA, Mirza SK, Martin BI, Kreuter W, Goodman DC, Jarvik JG. Trends, major medical complications, and charges associated with surgery for lumbar spinal stenosis in older adults. JAMA. 2010;303:1259–1265. doi: 10.1001/jama.2010.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deyo RA, Mirza SK, Turne JA, Martin BI. Overtreating chronic back pain: time to back off? J Am Board Fam Med. 2009;22:62–68. doi: 10.3122/jabfm.2009.01.080102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Esposito JL, Pinheiro-Franco S, Froelich D, Maitrot D. Predictive value of MRI vertebral endplate signal changes (Modic) on outcome of surgically treated degenerative disc disease: results of a cohort study including 60 patients. Neurochirurgie. 2006;52:315–322. doi: 10.1016/S0028-3770(06)71225-5. [DOI] [PubMed] [Google Scholar]
- 15.Fairbank JCT, Pynsent PB. The Oswestry Disability Index. Spine. 2000;25:2940–2953. doi: 10.1097/00007632-200011150-00017. [DOI] [PubMed] [Google Scholar]
- 16.Fardon DF, Milette PCN. Nomenclature and classification of lumbar disc pathology. Recommendations of the combined task forces of the North American Spine Society, American Society of Spine Radiology, and American Society of Neuroradiology. Spine. 2001;26:E93–E113. doi: 10.1097/00007632-200103010-00006. [DOI] [PubMed] [Google Scholar]
- 17.Glassman SD, Carreon LY, Djurasovic M, Dimar JR, Johnson JR, Puno RM, Campbell MJ. Lumbar fusion outcomes stratified by specific diagnostic indication. Spine J. 2009;9:13–21. doi: 10.1016/j.spinee.2008.08.011. [DOI] [PubMed] [Google Scholar]
- 18.Hägg O, Fritzell P, Ekselius L, Nordwall A, Swedish Lumbar Spine Study Group Predictors of outcome in fusion surgery for chronic low back pain. A report from the Swedish lumbar spine study. Eur Spine J. 2003;12:22–33. doi: 10.1007/s00586-002-0465-z. [DOI] [PubMed] [Google Scholar]
- 19.Herkowitz HN, Kurz LT. Degenerative lumbar spondylolisthesis with spinal stenosis. A prospective study comparing decompression with decompression and intertransverse process arthrodesis. J Bone Joint Surg [Am] 1991;73:802–808. [PubMed] [Google Scholar]
- 20.Holt EP., Jr The question of lumbar discography. J Bone Joint Surg Am. 1968;58A:720–726. doi: 10.2106/00004623-196850040-00007. [DOI] [PubMed] [Google Scholar]
- 21.Jensen MC, Brant-Zawadzki MN, Obuchowski N, Modic MT, Malkasian D, Ross JS. Magnetic resonance imaging of the lumbar spine in people without back pain. N Engl J Med. 1994;331:69–73. doi: 10.1056/NEJM199407143310201. [DOI] [PubMed] [Google Scholar]
- 22.Jensen MP, Turner JA, Romano JM. Correlates of improvement in multidisciplinary treatment of chronic pain. J Consult Clin Psychol. 1994;62:172–179. doi: 10.1037/0022-006X.62.1.172. [DOI] [PubMed] [Google Scholar]
- 23.Kuisma M, Karppinen J, Niinimäki J, Ojala R, Haapea M, Heliövaara M, Korpelainen R, Taimela S, Natri A, Tervonen O. Modic changes in endplates of lumbar vertebral bodies: prevalence and association with low back and sciatic pain among middle-aged male workers. Spine. 2007;32:1116–1122. doi: 10.1097/01.brs.0000261561.12944.ff. [DOI] [PubMed] [Google Scholar]
- 24.Madan S, Gundanna M, Harley JM, Boeree NR, Sampson M. Does provocative discography screening of discogenic back pain improve surgical outcome? J Spinal Disord Tech. 2002;15:245–251. doi: 10.1097/00024720-200206000-00014. [DOI] [PubMed] [Google Scholar]
- 25.Mitra D, Cassar-Pullicino VN, McCall IW. Longitudinal study of vertebral type 1 endplate changes on MR of the lumbar spine. Eur Radiol. 2004;14:1574–1581. doi: 10.1007/s00330-004-2314-4. [DOI] [PubMed] [Google Scholar]
- 26.Modic MT, Steinberg PM, Ross JS, Masaryk TJ, Carter JR. Degenerative disc disease: assessment of changes in vertebral body marrow with MR imaging. Radiology. 1988;166:193–199. doi: 10.1148/radiology.166.1.3336678. [DOI] [PubMed] [Google Scholar]
- 27.Modic MT. Modic type 1 and type 2 changes. J Neurosurg Spine. 2007;6:150–151. doi: 10.3171/spi.2007.6.2.150. [DOI] [PubMed] [Google Scholar]
- 28.Powell MC, Wilson M, Szypryt P, Symonds EM, Worthington BS. Prevalence of lumbar disc degeneration observed by magnetic resonance in symptomless women. Lancet. 1986;2:1366–1367. doi: 10.1016/S0140-6736(86)92008-8. [DOI] [PubMed] [Google Scholar]
- 29.Rahme R, Moussa R. The Modic vertebral endplate and marrow changes: Pathologic significance and relation to low back pain and segmental instability of the lumbar spine. Am J Neuroradiol. 2008;29:838–842. doi: 10.3174/ajnr.A0925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schellhas KP, Pollei SR, Gundry CR, Heithoff KB. Lumbar disc high-intensity zone: correlation of magnetic resonance imaging and discography. Spine. 1996;21:79–86. doi: 10.1097/00007632-199601010-00018. [DOI] [PubMed] [Google Scholar]
- 31.Toyone T, Takahashi K, Kitahara H, Yamagata M, Murakami M, Moriya H. Vertebral bone-marrow changes in degenerative lumbar disc disease: an MRI study of 74 patients with low back pain. J Bone Joint Surg Br. 1994;76:757–764. [PubMed] [Google Scholar]
- 32.Ware JE, Kosinski M, Keller SK. SF-36 physical and mental health summary scales: a user’s manual. Boston: The Health Institute; 1994. [Google Scholar]