Abstract
Purpose
We evaluated radiologic and clinical outcomes to compare the efficacy of anterior cervical discectomy and fusion (ACDF) and anterior corpectomy and fusion (ACCF) for multilevel cervical spondylotic myelopathy (CSM).
Methods
A total of 40 patients who underwent ACDF or ACCF for multilevel CSM were divided into two groups. Group A (n = 25) underwent ACDF and group B (n = 15) ACCF. Clinical outcomes (JOA and VAS scores), perioperative parameters (length of hospital stay, blood loss, operation time), radiological parameters (fusion rate, segmental height, cervical lordosis), and complications were compared.
Results
Both group A and group B demonstrated significant increases in JOA scores and significant decreases in VAS. Patients who underwent ACDF experienced significantly shorter hospital stays (p = 0.031), less blood loss (p = 0.001), and shorter operation times (p = 0.024). Both groups showed significant increases in postoperative cervical lordosis and achieved satisfactory fusion rates (88.0 and 93.3 %, respectively). There were no significant differences in the incidence of complications among the groups.
Conclusions
Both ACDF and ACCF provide satisfactory clinical outcomes and fusion rates for multilevel CSM. However, multilevel ACDF is associated with better radiologic parameters, shorter hospital stays, less blood loss, and shorter operative times.
Keywords: Cervical spondylotic myelopathy, Anterior cervical discectomy and fusion, Anterior cervical corpectomy and fusion
Introduction
Among several anterior and posterior techniques that have been suggested, controversy exists regarding the optimal approach for surgical treatment of cervical spondylotic myelopathy (CSM) [1–3]. Distinctions between anterior, posterior, and combined approaches for decompression are primarily based on the sagittal alignment of the spinal column, extent of disease, location of the compressive abnormality, presence of preoperative neck pain, and previous operations [2]. Among anterior approaches, anterior cervical corpectomy and fusion (ACCF) is associated with relatively good fusion rates [4–6]. Unfortunately, it is also associated with a higher incidence of complications, including vertebral artery [2, 7, 8], dural tears and CSF leakage [9]. Anterior cervical discectomy and fusion (ACDF) can decompress the anterior spinal cord and preserve the stability of the spinal column. It is also associated with a low prevalence of graft extrusion or migration. However, ACDF may not be the optimal surgical approach for CSM due to the risk of incomplete decompression, injury to the cord, limited visual exposure, and a high rate of pseudoarthrosis secondary to an increase in the number of fusion surfaces [1, 2, 7].
Based on these factors, the decision to treat multilevel CSM with ACDF rather than ACCF remains controversial. There are few reports comparing two-level ACDF to one-level ACCF [1, 7], and only one compares these approaches in multilevel constructs [3]. In the present study, we evaluate the efficacy of ACDF and ACCF in the treatment of multilevel CSM by comparing radiologic and perioperative parameters, clinical outcomes, and the incidence of complications.
Materials and methods
Materials
After obtaining Institutional Review Board approval, we reviewed the records of patients who underwent surgical treatment for multilevel CSM at a single institution by a single surgeon between 1994 and 2003 (K.J.S). Inclusion criteria were (1) patients who presented primarily with signs and symptoms of myelopathy, (2) patients with cord compression or signal change due to multilevel disc prolapses and/or osteophytes, and (3) patients with cord compression or signal change due to multilevel hypertrophied PLL or segmental type of ossification of the posterior longitudinal ligament (OPLL). Exclusion criteria were (1) patients with cervical myelopathy due to continuous or mixed OPLL, (2) patients with acute neurologic deterioration following trauma over a spondylotic canal, and (3) those with less than 5 years of follow-up. Based on these criteria, 40 patients were enrolled in this study. Patients were selected based on the timing of presentation and subsequently divided into two groups based on surgical methods. Group A (n = 25) included patients who underwent multilevel ACDF (Fig. 1a–i), [mean age 50.3 ± 7.5 years (min 42, max 73); 19 males and 6 females; 10 smokers, 18 three-level and 7 four-level discectomies (10 of C3–6, 8 of C4–7, 7 of C3–7), mean follow-up period of 87.3 ± 21.7 months (61–132)]. Group B (n = 15) was comprised of patients who underwent multilevel ACCF (Fig. 2a–i), [mean age 54.1 ± 9.8 years (min 45, max 70); 11 males and 4 females: 6 smokers, 10 two-level and 5 three-level corpectomies (4 of C3–6, 6 of C4–7, 5 of C3–7), mean follow-up period 94.3 ± 25.3 months (72–171)]. No significant intergroup differences were found in terms of age, the number of fusion levels, follow-up period, or gender (Table 1).
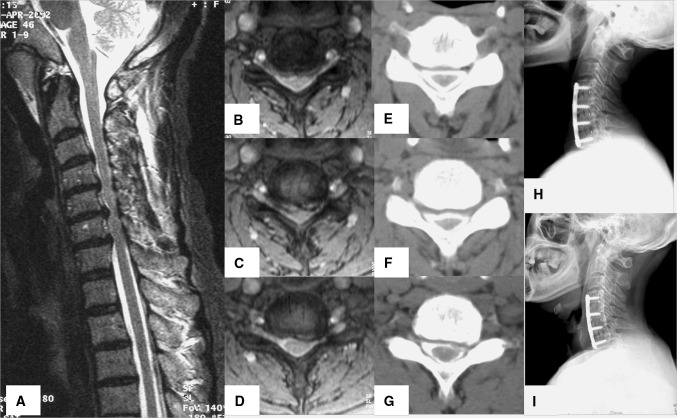
Fig. 1.
Sixty-six-year-old man who presented with cervical spondylotic myelopathy. T2-weighted sagittal MRI (a) showing cord compression and signal change due to multiple disc herniations and a thickened ligamentum flavum at C4–C7. Axial views (b, c, d) showing cord compression and deviation. Axial CT myelogram (e, f, g) again depicts cord compression and deviation along with bony spurs. Immediate postoperative lateral X-ray (h) showing C4–C5, C5–C6, C6–C7 discectomy and fusion with a cage filled with autogenous iliac cancellous graft. Six-year postoperative follow-up lateral X-ray (i) reveals complete bony fusion, maintenance of cervical lordotic curve, and preservation of adjacent disc spaces (C3–C4 and C7-T1)
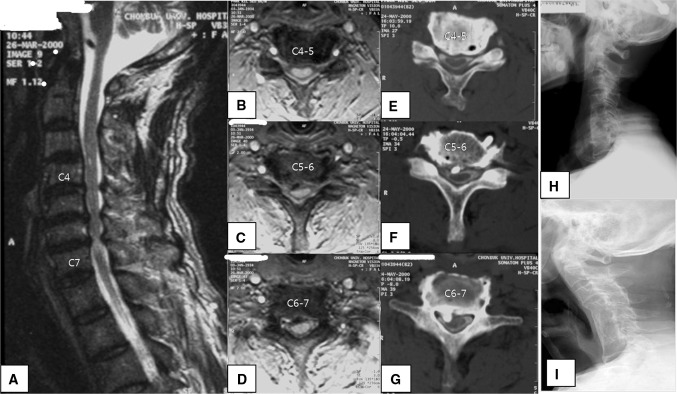
Fig. 2.
Sixty-six-year-old man who presented with cervical spondylotic myelopathy. T2-weighted sagittal MRI (a) showing cord compression and signal change due to multiple disc herniations, OPLL, and a thickened ligamentum flavum at C4–C7. Axial views (b, c, d) showing cord compression and deviation. Axial CT myelogram (e, f, g) again depicts cord compression and deviation along with OPLL. Immediate postoperative lateral X-ray (h) showing C5, C6 corpectomy, C4–C7 fusion with autogenous iliac tricortical graft. Eight-year postoperative follow-up lateral X-ray (i) revealing complete bony fusion, but the development of cervical kyphosis and the formation of ALD at C3–4 and C7-T1
Table 1.
Demographic data (mean ± SD)
| Group A (n = 25) | Group B (n = 15) | p value | |
|---|---|---|---|
| Age (years) | 50.3 ± 7.5 (42–73) | 54.1 ± 9.8 (45–70) | 0.897 |
| Gender (male:female) | 19:6 | 11:4 | 0.644 |
| Number of fusion levels | 18 three-level, 7 four-level | 10 two-level, 5 three-level | 0.734 |
| C3–6 fusion | 10 | 4 | |
| C4–7 fusion | 8 | 6 | |
| C3–7 fusion | 7 | 5 | |
| Follow-up (months) | 87.3 ± 21.7 (61–132) | 94.3 ± 25.3 (72–171) | 0.447 |
| Graft materials | Autogenous iliac bone: 10 Cage: 15 |
Autogenous iliac bone:12 Autogenous fibular bone:3 |
0.413 |
Anterior cervical discectomy and corpectomy were performed as described previously [8–10]. Of the 25 ACDF procedures, autogenous iliac bone graft with a CSLP plate (Cervical Spine Locking Plate, AO Synthes, Switzerland) was employed in 10, while a Solis cage (Stryker, Kalamazoo, MI, USA) filled with autogenous iliac bone chips and a CSLP plate was used in the remaining 15 procedures. A Philadelphia brace was used in all ACDF cases for 6 weeks followed by a soft collar brace for 4 weeks. Of the 15 corpectomy procedures, 12 were autogenous iliac bone grafts followed by the application of a Halo-vest while 3 were autogenous fibular bone grafts with Halo-vest application (Table 1). A complete corpectomy was performed, and the dorsal wall of the vertebral body and posterior longitudinal ligament was removed.
Methods
Clinical and radiologic follow-ups were performed immediately after operation, at 6 weeks, 3, 6, 9, 12, 18, and 24 months after surgery, and annually thereafter. The presence or absence of bone fusion, adjacent level disease (ALD), and radiological parameters were examined using anteroposterior (AP), lateral, and flexion/extension lateral plain radiographs.
Clinical outcomes (Japanese Orthopedic Association [JOA] scores, neck and arm pain Visual Analog Scale [VAS] scores, recovery rate), perioperative parameters (hospital stay, blood loss, operative time), radiologic parameters (fusion rate, segmental height, cervical lordosis), and the incidence of complications (ALD, revision surgery, hardware-related complications, hoarseness, pseudoarthrosis, dysphagia, donor site pain, graft-related complications, dural tears) were compared between groups. The recovery rate was calculated by the following equation: RR = (JOA score at follow-up−preoperative JOA score)/(17−preoperative JOA score) × 100 (%). All data were collected and reviewed by an independent observer.
Forty sets of AP and lateral radiographs of the cervical spine were combined to create a PowerPoint presentation from PACS system files. Patient records were then reviewed and the films were numbered. A single orthopedic surgeon who was blind to patient identity performed these analyses.
Radiographic fusion was defined as the absence of motion between spinous processes on flexion/extension radiographs and the absence of any radiolucent defect or halo around the iliac bone graft or cages, or the presence of a bridging bone anterior or posterior to a cage or iliac bone graft at the graft-endplate junction. To determine if fusion had occurred after corpectomy and fusion, the upper and lower ends of the construct were examined. To determine if fusion had occurred after discectomy and fusion, the fusion of the construct at each level adjacent to the strut was examined. Pseudoarthrosis was diagnosed if nonunion occurred at only one of the fusion sites. Cervical lordosis was defined as the angle formed between the lower endplate of C2 and the upper endplate of C7 by Cobb’s method on plain lateral radiographs with the patient in a neutral position. Segmental height was defined as the distance between the midlines of the involved cranial and caudal vertebral bodies. Adjacent level degeneration (ALD) was determined using modified Hilibrand criteria [12], which were divided into four stages (I, II, III, IV) depending on the presence of a decrease in disc height and posterior osteophyte formation. Stages II, III, and IV were considered indicative of ALD. Dysphagia was evaluated using the dysphagia score proposed by Bazaz et al. [13]. The occasional sensation of dysphagia with solid foods was considered moderate while frequent episodes were considered severe.
The Chi-square test was used to determine if differences and complications between groups before surgery were significant. Postoperative radiologic parameters, perioperative parameters, and clinical results were compared using independent t tests. All statistical analyses were performed using SPSS (version 12, SPSS, Chicago, IL, USA), and statistical significance was defined as p < 0.05.
Results
Clinical outcomes
The mean preoperative JOA score for group A was 11.1 ± 3.1. The JOA score improved significantly to 14.1 ± 2.3 points at 3 months postoperatively (independent t test, p = 0.027) and was maintained at 13.9 ± 2.2 points at the final follow-up (p = 0.039). The mean preoperative JOA score for group B was 11.4 ± 3.4. At 3 months postoperatively and at the final follow-up, the mean JOA scores in this group were 14.9 ± 2.7 (p = 0.021) and 13.6 ± 2.9 (p = 0.045), respectively. There were no significant differences in JOA scores between the groups at any point (Group A vs. B, 0.963 for preoperative, 0.757 for postoperative 3 months, and 0.891 for final follow-up) (Table 2).
Table 2.
Clinical outcomes
| Preop | Postop 3 months | Last F/U | ||
|---|---|---|---|---|
| JOA score | ||||
| Group A (n = 25) | 11.1 ± 3.1 | 14.1 ± 2.3 | 13.9 ± 2.2 | |
| Group B (n = 15) | 11.4 ± 3.4 | 14.9 ± 2.7 | 13.6 ± 2.9 | |
| p value | 0.963 | 0.757 | 0.891 | |
| VAS | ||||
| Group A (n = 25) | 6.84 ± 3.8 | 3.21 ± 2.7 | 3.76 ± 2.9 | |
| Group B (n = 15) | 5.97 ± 2.3 | 2.53 ± 2.3 | 2.96 ± 2.7 | |
| p value | 0.479 | 0.274 | 0.537 | |
| Recovery rate (%) | ||||
| Group A (n = 25) | 56.7 ± 30.6 | 52.3 ± 29.1 | ||
| Group B (n = 15) | 60.1 ± 23.3 | 59.7 ± 22.2 | ||
| p value | 0.854 | 0.347 | ||
Both groups showed significant improvement when comparing preoperative to 3-month postoperative VAS scores with 6.84 ± 3.8 (3–8) versus 3.21 ± 2.7 (1–7) in group A and 5.97 ± 2.3 (4–8) versus 2.53 ± 2.3 (0–4) in group B. However, the difference in improvement between groups was not statistically significant (Group A vs. B, 0.479 for preoperative, 0.274 for postoperative 3 months, and 0.537 for final follow-up).
For group A, the recovery rate was 56.7 ± 30.6 % 3 months postoperatively and 52.3 ± 29.1 % at final follow-up. For group B, the recovery rate was 60.1 ± 23.3 % 3 months postoperatively and 59.74 ± 22.2 % at the final follow-up. Recovery rates did not differ significantly between the groups.
Perioperative parameters
A summary of the perioperative parameters is presented in Table 3. The mean hospital stay was 10.74 ± 4.1 days (6–15 days) in group A and 18.43 ± 7.7 days (11–28 days) in group B (independent t test, p = 0.031). The mean blood loss was 301.71 ± 102.3 ml (230–450 ml) in group A and 574.57 ± 265.2 ml (310–1,040 ml) in group B (independent t test, p = 0.007). The mean operative time was 186.3 ± 58.3 min (140–230 min) in group A and 268.4 ± 65.2 min (201–367 min) in group B (independent t test, p = 0.024).
Table 3.
Perioperative parameters
| Group A (n = 25) | Group B (n = 15) | p value | |
|---|---|---|---|
| Hospital stay (days) | 10.74 ± 4.1 | 18.43 ± 7.7 | 0.031 |
| Blood loss (ml) | 621.33 ± 138.7 | 1011.28 ± 533.4 | 0.001 |
| OP time (min) | 186.3 ± 58.3 | 268.4 ± 65.2 | 0.024 |
Radiologic outcomes
A summary of the radiologic outcomes is presented in Table 4. The difference in segmental height immediately postoperatively and at the last follow-up was 2.1 ± 1.6 mm (1–5 mm) in group A and 4.7 ± 2.6 mm (3–7 mm) in group B (independent t test, p = 0.041). The average angle of cervical lordosis improved from 2.47 ± 5.56°(−3 to 9°) preoperatively to 10.21 ± 3.4° (7–14°) at 3 months postoperatively in group A and from 1.04 ± 11.07° (−10 to 13°) preoperatively to 6.07 ± 5.9° (1–13°) at 3 months postoperatively in group B (independent t test, p = 0.037). At final follow-up, the mean angle of cervical lordosis was maintained at 7.21 ± 4.1° (3–12°) in group A and at 3.93 ± 6.7° (−3 to 9°) in group B. The improvement in cervical lordosis was maintained to a significantly greater extent in group A than in group B (independent t test, p = 0.024). Solid fusion was achieved in 88.0 % (22/25) of subjects in group A and in 93.3 % (14/15) of subjects in group B at 6 months postoperatively and at final follow-up (Chi-square test, p = 0.537).
Table 4.
Radiological outcomes
| Preop | Postop 3 months | Last F/U | ||
|---|---|---|---|---|
| Difference of segmental height | ||||
| Group A (n = 25) | 2.1 ± 1.6 | |||
| Group B (n = 15) | 4.7 ± 2.6 | |||
| p value | 0.041 | |||
| Cervical lordosis | ||||
| Group A (n = 25) | 2.47 ± 5.56 (−3 to 9°) | 10.21 ± 3.4 (7 to 14°) | 7.21 ± 4.1 (3 to 12°) | |
| Group B (n = 15) | 1.04 ± 11.07 (−10 to 13°) | 6.07 ± 5.9 (1 to 13°) | 3.93 ± 6.7 (−3 to 9°) | |
| p value | 0.037 | 0.024 | ||
| Fusion rate (%) | ||||
| Group A (n = 25) | 88.0* | |||
| Group B (n = 15) | 93.3* | |||
| p value | 0.537 | |||
* Fusion rate at 6 months postoperatively
Complications
A summary of the incidence of complications is presented in Table 5. Adjacent level degeneration occurred in 64.0 % (16/25; 10 stage II, 3 stage III, 3 stage IV) of subjects in group A and in 53.3 % (8/15; 5 stage II, 1 stage III, 2 stage IV) of subjects in group B (p = 0.427). Two subjects (8.0 %) in group A required revision surgery. One underwent laminoplasty and anterior decompression with fusion for ALD while the other underwent combined antersior and posterior fusion for pseudoarthrosis. One subject (6.7 %) in group B required combined anterior and posterior fusion for pseudoarthrosis (p = 0.692). Hardware-related complications, including screw back-out or plate bending, occurred in two cases (8.0 %) in group A and in no cases in group B (p = 0.408). Pseudoarthrosis developed in 12.0 % (3/25) of subjects in group A and in 6.7 % (1/15) of subjects in group B (p = 0.537). Pseudoarthrosis developed in 20.0 % (2/10) of smokers in group A and 16.7 % (1/6) of smokers in group B, with no significant difference between groups. Two of the patients with pseudoarthrosis in group A were asymptomatic and did not require revision surgery. Three subjects (12.0 %) in group A and three subjects (20.0 %) in group B developed dysphagia that persisted for >6 weeks (p = 0.436). All cases resolved spontaneously within 6 months. Hoarseness developed in 8.0 % (2/25) of subjects in group A and in 13.3 % (2/15) of subjects in group B (p = 0.742). One subject (4.0 %) in group A and four subjects (26.7 %) in group B developed donor site pain that persisted for >6 weeks (p = 0.092). There were no cases of donor site infection in either group. There were no graft-related complications (migrations, fractures) in group A while there were two cases in group B (13.3 %, p = 0.158). There was one dural tear in group B (6.7 %, p = 0.688), which was repaired at the time of surgery without any additional issues. Although the complication rates were not significantly different between groups, there was a general trend of fewer complications in group B than in group A.
Table 5.
Complications
| Group A (n = 25) | Group B (n = 15) | p value | |
|---|---|---|---|
| ALD | 16/25 (64.0 %) (10 stage II, 3 stage III, 3 stage IV) | 8/15 (53.3 %) (5 stage II, 1 stage III, 2 stage IV) | 0.427 |
| Revision surgery | 2/25 (8.0 %) | 1/15 (6.7 %) | 0.692 |
| Hardware related | 2/25 (8.0 %) | 0/15 (0 %) | 0.408 |
| Pseudoarthrosis | 3/25 (12.0 %) | 1/15 (6.7 %) | 0.537 |
| Pseudoarthrosis in smokers | 2/10 (20.0 %) | 1/6 (16.7 %) | |
| Dysphagia | 3/25 (12.0 %) | 3/15 (20.0 %) | 0.436 |
| Hoarseness | 2/25 (8.0 %) | 2/15 (13.3 %) | 0.742 |
| Donor site pain | 1/25 (4.0 %) | 4/15 (26.7 %) | 0.092 |
| Graft related | 0/25 (0 %) | 2/15 (13.3 %) | 0.158 |
| Dural tear | 0/25 (0 %) | 1/15 (6.7 %) | 0.688 |
Discussion
The lower fusion rates previously reported for ACDF have been attributed to an increased number of grafts and interfaces that must consolidate with multilevel constructs and to increased stresses and resultant motion at the graft sites [5, 8]. In contrast, multilevel corpectomy utilizes only two graft-host interfaces (one single strut graft). Hilibrand et al. [5] achieved a 100 % fusion rate by performing corpectomies with fibula strut autografts in four-disc level fusions. Gore [4] achieved an 84 % union rate for three-disc level fusions, and Wada et al. [14] reported a 74 % union rate for two-disc level fusions utilizing corpectomies. Our series had a union rate of 93.3 % (14/15) in ACCF groups, which is higher than the ACDF group, although there was no significant difference.
The clinical outcomes of ACDF and ACCF are similar in many studies [1, 3, 15, 16]. In our series, both groups demonstrated a significant increase in JOA scores and a significant decrease in VAS scores that were maintained at the 5-year follow-up. Although the corpectomy group (ACCF) showed a higher fusion rate compared to the discectomy group (ACDF), the clinical outcomes were not significantly different from those patients who underwent discectomy with fusion. One possible explanation may be that the development of pseudoarthrosis does not necessarily affect clinical outcomes [17].
Both ACDF and ACCF can restore lordosis, but in multilevel ACDF, lordosis can be achieved and maintained easier than in ACCF. This is due to the multiple points of distraction and fixation in addition to the graft and interbody space shaping [3]. In the present study, cervical lordosis of fusion segments was significantly increased in both groups, but the increase was greater in the ACDF group than in the ACCF group.
A concern with multilevel anterior corpectomies is graft dislodgement, which has been reported in many articles [3, 4, 8, 11]. We noted a higher incidence of graft-related complications in the corpectomy group, but this difference was not statistically significant. This result may be due to sample size. A possible solution to this complication is the use of instrumentation [5, 19]. Anterior plating is effective when fusing less than three levels by maintaining appropriate cervical alignment and protecting the graft from dislodgement. This approach is not as effective when fusing three or more levels.
Lin Q et al. [3] have reported that their skip 1-level corpectomy, rather than 3-level corpectomy, achieved lower failure rates in the ACCF group (9.5 %) than previous reports have indicated [18, 20]. However, Sasso et al. [18] and Vaccaro et al. [20] reported an early failure rate of 50–71 % following anterior plating of three-level corpectomies, significantly higher than with two-level corpectomies. For this reason, we performed anterior corpectomy without a plate construct with subsequently longer immobilization using a Halo or 2-post external brace. The variability in surgical and external immobilization methods among groups is a limitation of our study.
The development of ALD predisposes patients to cervical kyphosis, late onset posterior neck pain, the need for revision surgery, and poor outcomes [12]. We found no significant difference between groups in the incidence of ALD or in the incidences of other complications, including the rate of pseudoarthrosis, hoarseness, dysphagia, donor site pain, and dural tears. However, the ACDF group showed significantly less blood loss, less segmental height loss, greater lordotic curve change, shorter operative time, and shorter hospital stay, suggesting that ACDF may be a safer alternative to ACCF for surgically managing multilevel CSM.
Our study is limited by its relatively small sample size, retrospective nature, and the heterogenous implants used for the two groups. Future prospective, randomized trials with larger sample sizes are warranted to further evaluate the optimal surgical approach for multilevel CSM.
Conclusions
There were no significant differences between ACDF and ACCF in fusion rate, clinical outcomes, or complications after surgical management of multilevel CSM. However, ACDF was associated with significantly less blood loss, less segmental height loss, greater lordotic curve change, shorter operative time, and shorter hospital stay. ACDF may therefore be a safer alternative to ACCF for the surgical management of multilevel CSM.
Acknowledgments
Conflict of interest
None.
Footnotes
This study was approved by the Institutional Review Board of Chonbuk National University Research Council (IRB-2009-81).
References
- 1.Oh MC, Zhang HY, Park JY, et al. Two-level anterior cervical discectomy versus one-level corpectomy in cervical spondylotic myelopathy. Spine. 2009;34:692–696. doi: 10.1097/BRS.0b013e318199690a. [DOI] [PubMed] [Google Scholar]
- 2.Rao RD, Gourab K, David KS. Operative treatment of cervical spondylotic myelopathy. J Bone Surg Am. 2006;88:1619–1640. doi: 10.2106/JBJS.F.00014. [DOI] [PubMed] [Google Scholar]
- 3.Lin Q, Zhou X, Wang X, et al. A comparison of anterior cervical discectomy and corpectomy in patients with multilevel cervical spondylotic myelopathy. Eur Spine J. 2012;21(3):474–481. doi: 10.1007/s00586-011-1961-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gore DR. The arthrodesis rate in multilevel anterior cervical fusions using autogenous fibula. Spine. 2001;26:1259–1263. doi: 10.1097/00007632-200106010-00016. [DOI] [PubMed] [Google Scholar]
- 5.Hilibrand AS, Fye MA, Emery SE, et al. Increased rate of arthrodesis with strut grafting after multilevel anterior cervical decompression. Spine. 2002;27:146–151. doi: 10.1097/00007632-200201150-00005. [DOI] [PubMed] [Google Scholar]
- 6.Hwang SL, Lee KS, Su YF, et al. Anterior corpectomy with iliac bone fusion or discectomy with interbody titanium cage fusion for multilevel cervical degenerated disc disease. J Spinal Disord. 2007;20:565–570. doi: 10.1097/BSD.0b013e318036b463. [DOI] [PubMed] [Google Scholar]
- 7.Iwasaki M, Kawaguchi Y, Kimura T, et al. Long-term results of expansive laminoplasty for ossification of the posterior longitudinal ligament of the cervical spine: more than 10 years follow up. J Neurosurg. 2002;96:180–189. doi: 10.3171/spi.2002.96.2.0180. [DOI] [PubMed] [Google Scholar]
- 8.Ellison TS, Hartman MB, Brigham CD (1997) Fusion rates for two-level ACDF+ plating versus subtotal corpectomy and fusion. Presented at the annual meeting of the North American Spine Society, New York, October 25
- 9.Cloward RB. The anterior approach for removal of ruptured cervical disks. J Neurosurg. 1958;6:496–511. doi: 10.3171/spi.2007.6.5.496. [DOI] [PubMed] [Google Scholar]
- 10.Matz PG, Pritchard PR, Hadley MN. Anterior cervical approach for the treatment of cervical myelopathy. Neurosurgery. 2007;60:S64–S70. doi: 10.1227/01.NEU.0000215399.67006.05. [DOI] [PubMed] [Google Scholar]
- 11.Orr RD, Zdeblick TA. Cervical spondylotic myelopathy. Approaches to surgical treatment. Clin Orthop Relat Res. 1999;359:58–66. doi: 10.1097/00003086-199902000-00007. [DOI] [PubMed] [Google Scholar]
- 12.Hilibrand AS, Carlson GD, Palumbo MA, et al. Radiculopathy and myelopathy at segments adjacent to the site of a previous anterior cervical arthrodesis. J Bone Joint Surg Am. 1999;81:519–528. doi: 10.2106/00004623-199904000-00009. [DOI] [PubMed] [Google Scholar]
- 13.Bazaz R, Lee MJ, Yoo JU. Incidence of dysphagia after anterior cervical spine surgery. Spine. 2002;27:2453–2458. doi: 10.1097/00007632-200211150-00007. [DOI] [PubMed] [Google Scholar]
- 14.Wada E, Suzuki S, Kanazawa A, et al. Subtotal corpectomy versus laminoplasty for multilevel cervical spondylotic myelopathy. A long-term follow up study over 10 years. Spine. 2001;26:1443–1447. doi: 10.1097/00007632-200107010-00011. [DOI] [PubMed] [Google Scholar]
- 15.Wang JC, McDonough PW, Endow KK, et al. A comparison of fusion rates between single-level cervical corpectomy and two-level discectomy and fusion. J Spinal Disord. 2001;14:222–225. doi: 10.1097/00002517-200106000-00006. [DOI] [PubMed] [Google Scholar]
- 16.Papadopoulos EC, Huang RC, Girardi FP, et al. Three-level anterior cervical discectomy and fusion with plate fixation: radiographic and clinical results. Spine. 2006;31:897–902. doi: 10.1097/01.brs.0000209348.17377.be. [DOI] [PubMed] [Google Scholar]
- 17.DePalma AF, Rothman RH, Lewinnek GE, et al. Anterior interbody fusion for severe cervical disc degeneration. Surg Gynecol Obstet. 1972;134:755–758. [PubMed] [Google Scholar]
- 18.Sasso RC, Ruggiero RA, Jr, Reilly TM, et al. Early reconstruction failures after multilevel cervical corpectomy. Spine. 2003;28:140–142. doi: 10.1097/00007632-200301150-00009. [DOI] [PubMed] [Google Scholar]
- 19.Ikenaga M, Shikata J, Tanaka C. Long-term results over 10 years of anterior corpectomy and fusion for multilevel cervical myelopathy. Spine. 2006;31:1568–1574. doi: 10.1097/01.brs.0000221985.37468.0f. [DOI] [PubMed] [Google Scholar]
- 20.Vaccaro AR, Falatyn SP, Scuderi GJ, et al. Early failure of long segment anterior cervical plate fixation. J Spinal Disord. 1998;11:410–415. [PubMed] [Google Scholar]