Abstract
Norrin/Frizzled4 (Fz4) signaling activates the canonical Wnt pathway to control retinal vascular development. Using genetically engineered mice, we show that precocious Norrin production leads to premature retinal vascular invasion and delayed Norrin production leads to characteristic defects in intra-retinal vascular architecture. In genetic mosaics, wild type endothelial cells (ECs) instruct neighboring Fz4−/− ECs to produce an architecturally normal mosaic vasculature, a cell non-autonomous effect. However, over the ensuing weeks, Fz4−/− ECs are selectively eliminated from the mosaic vasculature, implying the existence of a quality control program that targets defective ECs. In the adult retina and cerebellum, gain or loss of Norrin/Fz4 signaling results in a cell-autonomous gain or loss, respectively, of blood retina barrier (BRB) and blood brain barrier (BBB) function, indicating an ongoing requirement for Frizzled signaling in barrier maintenance and substantial plasticity in mature CNS vascular structure.
The retinal vasculature has long been an object of scientific interest due to its central role in ophthalmologic diseases, including a variety of inherited disorders of retinal vascularization (Gariano and Gardner, 2005). Several of these inherited disorders – Norrie disease, osteoporosis-pseudoglioma syndrome, and familial exudative vitreoretinopathy – have recently been shown to arise from defects in the genes coding for the ligand (Norrin), receptor (Frizzled4; Fz4), co-receptor (Lrp5), and chaperone (TSPAN12) proteins of a single signaling pathway (abbreviated “Norrin/Fz4” signaling) that controls retinal vascular growth (reviewed in Ye et al., 2010). Norrin is produced by Muller glia (Ye et al., 2009), and it binds to Fz4 on endothelial cells (ECs), leading to activation of Lrp5 and the generation of a canonical Wnt signal. In mice carrying targeted mutations in the Norrin/Fz4 signaling complex, ECs in the developing vasculature fail to productively invade the early postnatal retina. The result is an adult retina with only a single layer of vessels on its vitreal face instead of the normal tri-layered arrangement.
Norrin is a highly divergent member of the transforming growth factor-beta superfamily, with no homology to the Wnt family of Frizzled ligands. Fz4 appears to be the only receptor for Norrin, based on the observations that (1) among the ten mammalian Frizzled receptors, only Fz4 exhibits high affinity Norrin binding (Smallwood et al., 2007), and (2), in mice, the embryonic lethality caused by ubiquitous Norrin over-expression is completely suppressed by eliminating Fz4 (Ye et al, 2009). Importantly, Fz4 can also bind Wnt ligands and induce canonical signaling in response to Wnt binding (Junge et al., 2009).
A central role for canonical Wnt signaling in CNS vascular development has independently emerged from studies of mice carrying targeted mutations in the genes coding for Wnt7a, Wnt7b, and betacatenin (Liebner et al., 2008; Stenman et al., 2008; Daneman et al., 2009). Without Wnt7a and Wnt7b production by the neuroepithelium or beta-catenin expression in ECs, developing ECs fail to productively invade the embryonic CNS and do not express proteins characteristic of the BBB. Major unanswered questions regarding Norrin and Wnt signaling in the vasculature relate to the roles of this pathway later in life, to the possibility that these roles may vary in different CNS regions, and to the identification of the downstream mediators of Norrin- and Wnt-dependent vascular phenotypes.
In the present study, we address a series of questions posed by the work described in the preceding paragraphs. Are there specific features of vascular architecture and artery/vein/capillary differentiation that are under the control of Norrin/Fz4 signaling? Are other vascular signaling systems affected by Norrin/Fz4 signaling? Can Norrin/Fz4 signaling be fine-tuned to yield different vascular architectures? What are the molecules associated with BBB/BRB differentiation that are controlled by Norrin/Fz4 signaling? How plastic is the BBB/BRB phenotype and, if there is plasticity, is it controlled by Norrin/Fz4 signaling? To address these questions we have used genetically engineered mice that permit temporal and tissue-specific control of Norrin/Fz4 signaling. The data reveal both cell-autonomous and cell-non-autonomous effects and they imply a central role for Norrin/Fz4 signaling in CNS vascular development and in the maintenance of the BBB/BRB state.
Results
Defects in vascular architecture and cell surface vascular receptors in NdpKO retinas
In the mouse, the retinal vasculature develops through a series of stages: first, radial growth at the vitreal face (P1-P9); second, growth of the deep vascular plexus in the outer plexiform layer (OPL) (P7-P12); and third growth of the intermediate vascular plexus in the inner plexiform layer (IPL) (P11-P17). In the absence of Norrin/Fz4 signaling, vascular growth across the vitreal surface of the retina is retarded and the intra-retinal capillary network does not form (Richter et al., 1998; reviewed in Ye et al., 2010). To further characterize the consequences of defective Norrin/Fz4 signaling, we quantified the proliferation of vascular cells at the vitreal surface of WT vs.NdpKO retinas at P5, P9, and P15 with a three hour EdU pulse label (Figure 1A; Ndp, the gene coding for Norrin is X-linked; Ndp−/Y males and Ndp−/− females will be referred to as NdpKO). At P5 and P9, EC proliferation lags in NdpKO compared to WT retinas, but at P15, NdpKO retinas exhibit an increase in EC proliferation, presumably in response to retinal hypoxia.
Figure 1. Defects in endothelial cell proliferation and artery-vein segregation in NdpKO retinas.
(A) Vascular cell proliferation at the vitreal surface measured with EdU labeling at P5, P9, and P15. Left, WT and NdpKO retina flat-mounts. Right, quantification of labeled vascular cells as a function of radial distance from the optic disc. Scale bar, 100 um.
(B) Left, flat-mount P10 retinas. Aberrant artery/vein crossings are seen in NdpKO retinas (arrows). Right, quantification of crossings for 15 WT and 16 NdpKO retinas at P10 (mean +/−S.D.). See also Figures S1 and 2. Scale bar, 500 um.
A second, and hitherto unrecognized, defect in retinas lacking Norrin/Fz4 signaling is the frequent crossing of arteries and veins (Figure 1B and S1), similar to that seen with loss of Neuropilin(Nrp)1 and neuron-specific hemizygous loss of VEGF-A (Haigh et al., 2003; Fantin et al., 2011). In WT retinas, major arteries and veins are segregated into alternating and radially arrayed territories, and they rarely cross. By contrast, at P10, NdpKO retinas show an average of 4–5 crossings of major arteries and veins, and many crossings among smaller vessels. Additionally, elastin, which normally coats arteries but not veins, is present on veins in NdpKO retinas (Figure S2A). These data suggest that Norrin/Fz4 signaling plays a role in signaling between arteries and veins or in specifying some aspects of artery vs. vein identity.
The retinal EC phenotypes in the absence of Norrin/Fz4 signaling could reflect, at least in part, a decreased response to retina-derived pro-angiogenic factors, such as VEGF, or an enhanced response to anti-angiogenic factors, such as class 3 Semaphorins, at least one member of which, Sema3E, regulates retinal vascular development (Fukushima et al. 2011; Kim et al., 2011). To test this idea, we localized transcripts coding for VEGF, VEGFR1, VEGFR2, and Nrp1 and Nrp2 (which function as class 3 Semaphorin receptors) in mature WT and NdpKO retinas by in situ hybridization (Figure 2A). Not surprisingly, in NdpKO retinas, VEGF transcripts are present at greatly increased abundance in the inner nuclear layer (INL), most likely in Muller glia (Figure 2A). We also localized VEGF-A, VEGF-B, and class 3 Semaphorin binding sites (Nrp1 and Nrp2) in situ by incubating alkaline phosphatase (AP) fusion proteins with fresh frozen WT and NdpKO retina sections (Figure 2B). Interestingly, in NdpKO retinas, VEGFR1, VEGFR2, Nrp1, and Nrp2 transcript levels are elevated in a spatially heterogenous manner within the vascular protrusions from the vitreal surface (arrows in Figure 2A). Binding sites for VEGF-A and VEGF-B but not Sema3F AP fusion proteins are present throughout the WT retinal vasculature, whereas binding sites for VEGF-A and Sema3F but not VEGFB AP fusion proteins are present in NdpKO retinal vasculature (Figure 2B and 2C).
Figure 2. Altered expression of receptors that control vascular development in NdpKO retinas.
(A) At ~P30, VEGF transcripts accumulate in the INL in NdpKO retinas, and VEGF-R1, VEGF-R2, Nrp1, and Nrp2 transcripts accumulate in aberrant EC clusters in the NdpKO retina (arrows). ONL, outer nuclear layer; INL, inner nuclear layer; GCL, ganglion cell layer. Scale bar (for A and B), 100 um.
(B) At ~P30, AP fusion protein ligands reveal VEGF-A and VEGF-B, but not Sema3F, binding sites throughout the WT retinal vasculature. In EC clusters in NdpKO retinas, binding sites are present for VEGF-A and Sema3F, but not for VEGF-B. Specificity is demonstrated for the AP-VEGF ligands by competition with 10 ug/ml VEGF-A.
(C) Summary of published ligand-receptor binding specificities (Geretti et al., 2008; Pellet-Many et al., 2008) and observed patterns of AP-ligand binding to retinal vasculature (panel B). VEGF-B(167) binds Nrp1 but VEGF-B(186) does not (Makinen et al., 1999). The AP-VEGF-B construct used here produces AP-VEGF-B(186) as the initial translation product; the extent of its proteolytic conversion to AP-VEGF-B(167) is unknown.
The AP-fusion protein binding experiments suggest that WT retinal vasculature displays VEGFR1 (which binds both VEGF-A and VEGF-B) and possibly VEGFR2 (which binds VEGF-A but not VEGF-B), but not detectable Nrp2 (which binds Sema3F with high affinity) or Nrp1 (which binds Sema3F with lower affinity) (Figure 2C; Geretti et al., 2008; Pellet-Many et al., 2008). By contrast, NdpKO retinal vasculature likely displays VEGFR2 but not VEGFR1 (since AP-VEGF-A binds but APVEGF-B does not), as well as Nrp1 and/or Nrp2 (since AP-Sema3F binds). We suggest that the VEGFR1 and VEGFR2 in situ hybridization signals in NdpKO retinal vasculature may derive from transcript variants that code for secreted extracellular VEGF-binding domains (e.g. soluble Flt-1 and its VEGFR2 homologue; Albuquerque et al., 2009), which would not be detected by AP-VEGF binding to fresh frozen sections. An increase in Nrp1 and/or Nrp2 in NdpKO retinal vasculature, inferred from the intensity of the in situ hybridization signal, would be predicted to confer enhanced responsiveness to both class 3 Semaphorins and VEGF. The failure of the NdpKO retinal vasculature to invade the retina - despite an apparent elevation in the abundance of tissue-derived VEGF and vascular VEGFR2, as inferred from the intensity of their in situ hybridization signals - implies a major role for angiogenesis inhibitors in determining the NdpKO phenotype.
BBB integrity and Norrin/Fz4 signaling
In earlier work, we described the presence of high concentrations of endogenous IgG in the cerebellum and retina in Fz4−/− mice, a likely consequence of defects in BBB and BRB integrity (Xu et al., 2004; Ye et al., 2009). To extend these observations to small molecule permeability and to more precisely localize the sites of BBB/BRB dysfunction, we stained tissue sections for covalent biotin adducts following intracardiac perfusion of Sulfo-NHS-biotin (MW=557 g/mole; Figure 3A). As expected, in both Fz4+/− and Fz4−/− mice extensive biotinylation was observed in the parenchyma of the liver and kidney, organs with fenestrated vasculature. Additionally, in the Fz4−/− CNS, BBB/BRB integrity is compromised in the cerebellum, olfactory bulb, spinal cord, and retina, but not in the cerebral cortex, striatum, or thalamus (Figure 3A, and data not shown).
Figure 3. Fz4 signaling in ECs is required for BBB and BRB integrity.
(A) Intracardiac perfusion with sulfo-NHS-biotin reveals BBB/BRB defects in cerebellum, olfactory bulb, spinal cord, and retina, but not cerebral cortex, in Fz4−/− mice. Biotin labeling of liver and kidney parenchyma serve as a positive control. Scale bar, 200 um.
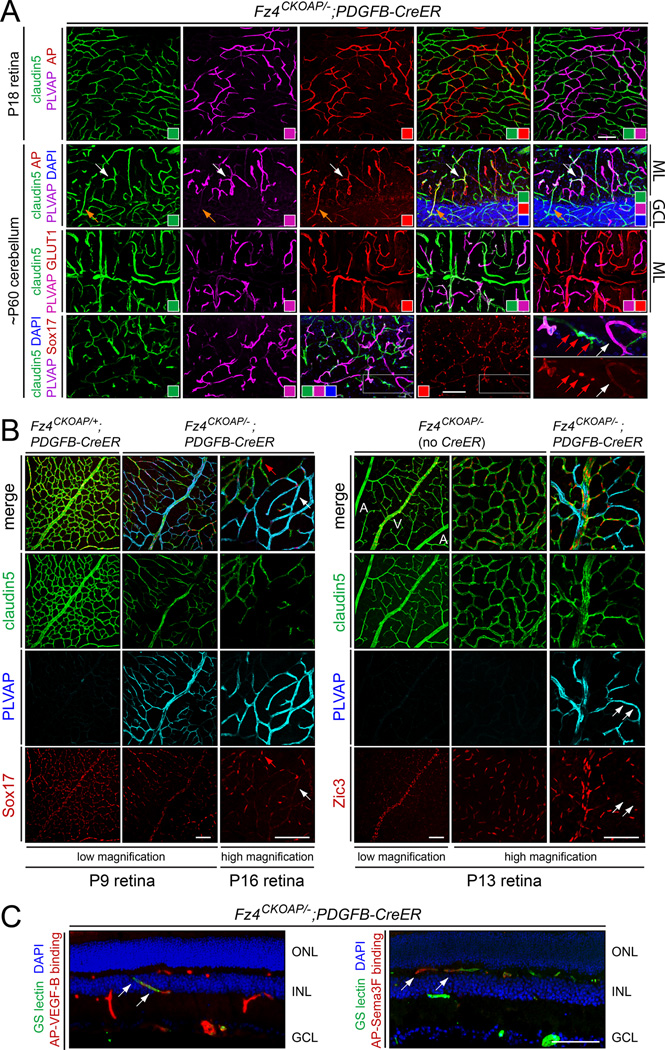
(B) Intracardiac perfusion of Fz4CKOAP/−;PDGFB-CreER mice with sulfo-NHS-biotin shows BBB defects and PLVAP induction in the molecular layer of the cerebellum and in the retina (shown in flat-mount) following early postnatal 4HT. GCL, granule cell layer; ML, molecular layer. Sulfo-NHS-biotin leakage at the edge of the cerebellum arises from the fenestrated vasculature of the choroid plexus. Scale bar, 100 um.
(C) Intra-peritoneal injection of Evans Blue one day before sacrifice reveals BBB defects in cerebellum and olfactory bulb in Fz4CKOAP/−;Tie2-Cre mice, but not in Fz4CKOAP/+;Tie2-Cre controls.
(D) In the adult Fz4CKOAP/−;Tie2-Cre cerebellum, all ECs are AP+ and many molecular layer and surface ECs are PLVAP+/claudin5−. In the Fz4CKOAP/+;Tie2-Cre cerebellum, all CNS ECs are PLVAP/claudin5+. See also Figure S3. Scale bar, 200 um.
To delete Fz4 specifically in ECs, we used a Fz4 conditional knockout allele in which the Fz4 coding region and 3’UTR are flanked by loxP sites and an AP reporter is located distal to the 3’ loxP site (Fz4CKOAP; Ye et al., 2009). When the Fz4CKOAP allele is placed over a conventional Fz4 null allele (Wang et al., 2001), Cre-mediated recombination produces cells that have simultaneously become Fz4−/− and express AP under the control of the Fz4 promoter. Cre-mediated deletion of Fz4 in ECs - using a Tie2-Cre transgene or a platelet-derived growth factor B (PDGFB)-CreER transgene with postnatal Tamoxifen or 4-hydroxytamoxifen (4HT) treatment - led to a loss of BBB/BRB integrity as assessed by Sulfo-NHS-biotin perfusion or following IP injection of Evans Blue (MW=961 g/mole) one day prior to sacrifice (Figure 3B and C). Interestingly, BBB disruption in the cerebellum is largely confined to the molecular layer (Figure 3B).
A survey of endothelial-specific markers in Fz4CKOAP/−;Tie2-Cre retinal, cerebellar, spinal cord, and olfactory bulb vasculature showed an increase in the abundance of plasmalemma vesicle associated protein (PLVAP), a structural component of endothelial fenestrae (Stan et al., 1999), and a reduction in the abundance of claudin5, a structural component of EC tight junctions (Figures 3B, 3D, S2B, and S4). These molecular changes in the retinal vasculature were observed as early as P5 (Figure S2B). The Fz4CKOAP/−;Tie2-Cre cerebellar vasculature shows minimal changes in the level of the glucose transporter GLUT1, a marker of brain, but not retina, ECs (Figure 3D). Although Cre-mediated recombination occurs in all ECs in Fz4CKOAP/−;Tie2-Cre mice, as judged by AP histochemistry (Ye et al., 2009) and AP immunostaining (Figure 3D), in the cerebellum, PLVAP+/claudin5− ECs are largely confined to vessels within and on the surface of the molecular layer (Figure 3D), consistent with the localized leakage of Sulfo-NHS-biotin (Figure 3B). In the olfactory bulb, PLVAP+/claudin5− ECs are localized to the glomerular and superficial nerve fiber layers, and in the cerebral cortex, PLVAP+/claudin5− ECs are confined to the cortical surface (Figure S3A). These data imply a hitherto unappreciated molecular heterogeneity among CNS ECs.
Previous work has shown that BBB integrity depends on the presence of pericytes (Armulik et al., 2010; Bell et al., 2010; Daneman et al., 2010), raising the possibility that loss of Norrin/Fz4 signaling in ECs might impair vascular permeability indirectly via an alteration in pericyte coverage or function or some other aspect of neurovascular organization. Arguing against this possibility, immunostaining for the pericyte markers PDGF receptor B in the Fz4CKOAP/−;Tie2-Cre cerebellum and NG2 in the mosaic Fz4CKOAP/−;PDGFB-CreER retina (described in detail in the next section) showed no diminution in pericyte coverage over Fz4−/− ECs (Figures S3C and S3D). Similarly, there were no alterations in the peri-vascular basement membrane around Fz4−/− ECs in the cerebellum as determined by immunostaining for perlecan (HSPG2) or laminin alpha 2 (Figures S3E and S3F), and there were no alterations in the distribution or abundance of aquaporin-4 in perivascular astrocyte end-feet (Figure S3B). Finally, we measured vascular permeability after treatment with imatinib (Gleevec), a tyrosine kinase inhibitor that blocks CNS vascular permeability in pericyte deficient mice by inhibiting EC trans-cytosis (Armulik et al., 2010). In these experiments we saw no evidence that imatinib decreased vascular permeability of Sulfo-NHS-biotin leakage (Figure S4). Taken together, these data argue that the CNS vascular permeability caused by defects in Norrin/Fz4 signaling is mechanistically distinct from that caused by loss of pericytes.
Although some or all of these molecular and functional changes might arise as a direct consequence of loss of Norrin/Fz4 signaling, the experiments described thus far cannot rule out the possibility that they might represent secondary responses to tissue stress. The possibility of such secondary effects is not implausible since VEGF signaling is known to increase vascular permeability and induce endothelial PLVAP expression (Roberts and Palade, 1995; Strickland et al., 2005). As described below, we have addressed the question of cell autonomy vs. non-autonomy by creating a genetically mosaic vasculature.
Genetics mosaics define cell autonomous vascular defects resulting from loss of Fz4 signaling
To create a Fz4−/−:Fz4+/− mosaic vasculature in which Fz4−/− cells are marked, we treated Fz4CKOAP/−;PDGFB-CreER mice with 4HT postnatally. In the text that follows, all experiments with Fz4CKOAP/−;PDGFB-CreER mice include control Fz4CKOAP/+;PDGFB-CreER littermates and involve 4HT administered between P4 and P12, most usually 0.1 mg 4HT administered between P4 and P8. For simplicity, the mice will simply be referred to by their genotypes, without noting the 4HT treatment history. In the experiments described below, we show mosaic vasculature in which Fz4−/− ECs are relatively abundant. Identical results were obtained when Fz4−/− ECs constitute only a few percent of the population.
Figure 4A shows genetically mosaic vasculature in the retina and cerebellum of Fz4CKOAP/−;PDGFB-CreER mice treated postnatally with 4HT. In the retinal vasculature, nearly all Fz4−/− ECs are AP+/PLVAP+/claudin5− and nearly all Fz4+/− ECs are AP-/PLVAP−/claudin5+. The principal exceptions are rare Fz4−/− ECs that are AP+/PLVAP+/claudin5+. In this experiment, 4HT was delivered at P4, a time prior to the vertical in-growth of retinal ECs that initiates the formation of the intraretinal capillary beds. Interestingly, the genetically mosaic retinal vasculature exhibits an essentially normal architecture despite the high fraction of Fz4−/− ECs. This observation supports the idea, initially suggested by Ye et al (2009), that neighboring WT ECs can largely correct the vascular migration defects of Fz4−/− ECs.
Figure 4. EC-specific genetic mosaics define cell-autonomous molecular defects.
(A) Mosaic vasculature in Fz4CKOAP/−;PDGFB-CreER mice treated with 4HT between P4 and P8. Anti-AP immunostaining (red; top two rows) marks Fz4−/− cells. In retina (top row), almost all AP+ ECs are PLVAP+/claudin5−. In cerebellum (lower three rows), most AP+ ECs in the molecular layer are PLVAP+/claudin5− and show reduced GLUT1. Second row, examples of minority AP+ EC phenotypes: PLVAP+/claudin5+ (white arrow) and PLVAP−/claudin5+ (yellow arrow). Sox17 is nuclear in Fz4+/− ECs (red arrows; enlarged regions, lower right panels), but is uniformly distributed at low level in many Fz4−/− ECs (white arrow). ML, molecular layer; GCL, granule cell layer. Colored squares show the colors in each panel. Scale bars, 100 um.
(B) In mosaic retinas, Sox17 in Fz4−/− ECs switches localization from nuclear (red arrow; left panels) to pan-cellular (white arrow), and Zic3 is lost (white arrows, right panels). PLVAP marks Fz4−/− cells. A, artery; V, vein. Scale bars, 100 um.
(C) In mosaic retinas, Fz4−/− ECs show a cell autonomous loss of AP-VEGF-B binding sites (green ECs in left panel) and acquisition of AP-Sema3F binding sites (red ECs in right panel). Scale bar, 100 um.
In Fz4CKOAP/−;PDGFB-CreER mice, the immunostaining pattern in the cerebellar vasculature shows a strong anti-correlation between claudin5 and PLVAP expression (Figure 4A and S5A). The principal exceptions are Fz4−/− ECs that are AP+/PLVAP+/claudin5+ or AP+/PLVAP−/claudin5+. Consistent with the pattern described above for the Fz4CKOAP/−;Tie2-Cre cerebellum (Figure 3D), the abundance of PLVAP+/claudin5− ECs is far lower in the granule cell layer than in the molecular layer. In contrast to molecular layer ECs, many Fz4−/−(i.e. AP+) ECs in the granule cell layer, remained PLVAP−/claudin5+.
The transcription factor Sox17 is required for normal vascular development in the mouse embryo and in zebrafish (Matsui et al., 2006; Sakamoto et al., 2007; Pendeville et al., 2008), and in an earlier study we observed reduced abundance of Sox17 transcripts in cultured ECs in the absence of Fz4 signaling (Ye et al., 2009). In mosaic Fz4CKOAP/−;PDGFB-CreER cerebellar and retinal vasculatures, Sox17 is localized to the nuclei of Fz4+/− ECs, but it is present at reduced abundance and in a pan-cellular distribution in Fz4−/− ECs (marked with PLVAP in Figure 4A, lower right, and Figure 4B, left panels).
Zic3, a second transcription factor that shows reduced transcript abundance in cultured ECs in the absence of Fz4 signaling, is undetectable in Fz4−/− retinal ECs (marked with PLVAP; Figure 4B, right panels). Interestingly, immunostaining of WT retinas showed that Zic3 is normally expressed in veins and capillaries but not arteries (Figure 4B), suggesting that Fz4−/− vein and capillary ECs may have adopted a more arterial fate. This interpretation is consistent with the idea that the retinal artery/vein crossing phenotype associated with a loss of Norrin/Fz4 signaling (Figures 1B, S1, and S2A) may reflect a failure to fully distinguish artery and vein identities.
We have also examined the cell autonomy of the two features of EC receptor composition that distinguish WT retinal vasculature from retinal vasculature that lacks Norrin/Fz4 signaling: loss of VEGF-B binding sites and acquisition of Sema3F binding sites (Figure 2B). As seen in Figure 4C, both attributes are cell autonomous properties of the Fz4−/− state.
Fz4 signaling and the plasticity of the endothelial barrier phenotype
In contrast to the retina, Norrin/Fz4 signaling in the brain plays little or no role in the development of vascular architecture (Xu et al., 2004; Luhman et al., 2008; Ye et al., 2009). However, the requirement for Fz4 signaling in promoting the PLVAP−/claudin5+ BBB+ state in cerebellum, spinal cord, and olfactory bulb indicates that Norrin and/or Wnts activate Fz4 in brain ECs, a plausible idea given that Fz4 is expressed in virtually all ECs and Ndp is expressed by glia throughout the CNS (Ye et al., 2009, 2011). To define the role of Norrin in this process, we examined BBB integrity and the expression of vascular markers in the NdpKO CNS. In the NdpKO cerebellum, ~5% of molecular layer ECs exhibit a conversion from a PLVAP−/claudin5+ state to a PLVAP+/claudin5− state (Figure 5A). These PLVAP+/claudin5− ECs also show reduced levels of occludin and GLUT1 and are associated with local disruptions of BBB integrity as determined by Sulfo-NHS-biotin leakage (Figure 5A, 5B, and S5B). As described above for the Fz4−/−:Fz4+/− mosaic vasculature, the conversion to a PLVAP+/claudin5−/BBBstate appears to occur far more readily in the molecular layer than in the granule cell layer (Figure 5A and B). The most striking aspect of this phenotype is its bimodality. In the NdpKO cerebellum, ECs are observed in either of two mutually exclusive states of gene expression, and Norrin/Fz4 signaling appears to determine the relative probability that an EC will exist in one or the other state.
Figure 5. Norrin/Fz4 signaling controls BBB plasticity, and in mosaic retinas Fz4−/− ECs are slowly eliminated.
(A) In the adult NdpKO cerebellum, a subset of ECs in the molecular layer are PLVAP+/claudin5−/occludin- (white arrows). In (A) and (B), colored squares show the colors in each panel. Scale bar, 100 um.
(B) Sites of BBB deficiency in the NdpKO cerebellum coincide with PLVAP+ ECs. See also Figures S4 and S5. ML, molecular layer; GCL, granule cell layer. Scale bar, 200 um.
(C) Adult conversion of ECs from Fz4+/− to Fz4−/− leads to induction of PLVAP and co-localized BBB breakdown. Diagram, sparse Cre-mediated conversion of ECs from Fz4+/− to Fz4−/−. Scale bar, 200 um.
(D) Restoration of Norrin expression in the adult NdpKO cerebellum restores the BBB. Six 9–12 week old NdpKO;Z/Norrin;GLAST-CreER mice, treated >3 weeks before sacrifice with 3×5 mg oral Tamoxifen, have a largely intact BBB (right), but six littermate control NdpKO mice (lacking either Z/Norrin or GLAST-CreER or both) sacrificed at the same age show patchy BBB breakdown (left). Untreated NdpKO;Z/Norrin;GLAST-CreER mice and NdpKO mice treated with Tamoxifen or with vehicle (sunflower seed oil) alone show cerebellar leakage indistinguishable from NdpKO mice.
(E) Progressive loss of Fz4−/− ECs in mosaic Fz4−/−:Fz4+/− retinal vasculature. Fz4CKOAP/−;PDGFB-CreER mice were exposed to 4HT at P4, one eye was enucleated at ~P18 and the other eye at ~P50, and retina immunostained for PLVAP and claudin5. Left, left and right retinas from the same mouse, harvested at P18 and P50, respectively. Center, the ratio of PLVAP+ to claudin5+ vasculature in the OPL declines over time. Data points derived from the two retinas of the same mouse are connected by a line. Right, density of adult (P53-P80) OPL vasculature in retinas from Fz4CKOAP/−;PDGFB-CreER and Fz4CKOAP/+;PDGFB-CreER littermates that had received 0.1 mg 4HT between P5 and P12 mean +/− S.D.). Scale bar, 500 um.
(F) Cell proliferation in OPL vasculature from nine pairs of Fz4CKOAP/−;PDGFB-CreER and Fz4CKOAP/+;PDGFB-CreER littermates following 0.1 mg 4HT treatment between P5 and P12 and EdU injections every 2–3 days from P21-P25 to P43-P56. Retinas were harvested between P53 and P80. Left, four panels from the retina with the highest level of EdU labeling showing EdU+ nuclei on/within vessels (yellow arrows), adjacent to vessels (red arrows), or far from vessels (white arrows). Center, number of EdU+ nuclei on, near, or far from Fz4−/− and Fz4+/− ECs in territories of 1 mm2; right, the same data, but individually normalized to the densities of Fz4−/− and Fz4+/− ECs within each territory. Scale bar, 100 um.
In all of the experiments described thus far, loss of Norrin or Fz4 began early in embryonic or postnatal life, thereby encompassing most or all of the periods when the retinal and cerebellar vasculatures are developing. Figure 5C shows that when Fz4 deletion is induced during adulthood, a conversion from a PLVAP− to a PLVAP+ molecular phenotype occurs, with a corresponding loss of local BBB/BRB integrity. Thus, ongoing Fz4 signaling in mature ECs is required to maintain the BBB/BRB state. This interpretation is further supported by recent experiments in which IP injection of a function-blocking anti-Fz4 mAb led to a compromised BRB (Paes et al, 2011).
We next asked whether the reciprocal phenotypic conversion is possible: that is, whether PLVAP+/claudin5−/BBB− ECs can convert to a PLVAP−/Claudin5+/BBB+ state. To address this question, we used a GLAST-CreER BAC transgene (P.M.S., A.R., and J.N., unpublished) that drives recombination in many CNS glia – including Muller glia in the retina and Bergmann glia in the cerebellum, the normal cellular sources of CNS Norrin (Ye et al., 2011) - to activate Norrin expression from a cytomegalovirus-actin (CAG) promoter LoxP-stop-LoxP Norrin knock-in allele at the Ubiquitin-B locus (referred to as Z/Norrin; Ye et al., 2009). As seen for six NdpKO;Z/Norrin;GLAST-CreER mice and six NdpKO control mice shown in Figure 5D, activating Norrin production in adulthood efficiently converted the patchy BBB− NdpKO vasculature to an almost completely BBB+ state. These data show that in fully mature ECs, the BBB+ state can be lost following a decrease in Fz4 signaling or acquired following an increase in Fz4 signaling.
The BBB rescue shown in Figure 5D most likely reflects a Norrin-dependent conversion of pre-existing cerebellar ECs from a PLVAP+/Claudin5−/BBB− to PLVAP−/Claudin5+/BBB+ state. An alternative possibility is that the PLVAP+/Claudin5− ECs were replaced by PLVAP−/Claudin5+ ECs. We have attempted to look for evidence of such replacement in 3–4 week old Tamoxifen-treated NdpKO;Z/Norrin;GLAST-CreER mice by EdU labeling. In this experiment, oral Tamoxifen delivery on experimental days 1, 3, and 5 was observed to largely restore BBB integrity by day 12. We therefore labeled with EdU on day 6, a time when the vasculature is presumably converting from a BBB− to a BBB+ state, and sacrificed the mice one day later. Examination of >1 mm3 of cerebellar tissue from each of two such mice showed no EdU incorporation into any vascular cells (data not shown), suggesting that replacement of PLVAP+/Claudin5−/BBB− ECs by newly postmitotic PLVAP−/Claudin5+/BBB+ ECs is an unlikely scenario.
ECs deficient in Norrin/Fz4 signaling are slowly lost
In the course of examining large numbers of mosaic retinas from Fz4CKOAP/−;PDGFB-CreER mice that had been treated with 4HT during early postnatal life, we noticed that the number of Fz4−/− ECs, as revealed by staining for PLVAP, appeared to decline with age. To quantify this observation, Fz4CKOAP/−;PDGFB-CreER mice were treated with 4HT at P4, one eye was removed at the time of retinal vascular maturation (P15–P20), and the other eye was removed more than one month later (P50–P70). Each retina was stained for claudin5, PLVAP, and AP, and the ratio of PLVAP+ to claudin5+ vasculature was quantified in flat-mount images of the entire retina at the level of the OPL (Figure 5E, left). In Fz4CKOAP/−;PDGFB-CreER retinas, the correlation between PLVAP+ and AP+ ECs is very nearly perfect (Figure 4A). Therefore, in the present experiment, we have used PLVAP rather than AP to quantify Fz4−/− ECs, since PLVAP can be visualized with a higher signal-to-noise ratio than can AP. As seen in Figure 5E center, the ratio of PLVAP+/claudin5− to PLVAP−/claudin5+ ECs declined with age in all six mice examined. Accompanying the selective loss of PLVAP+/claudin5− ECs is an overall lower vascular density in adult mosaic Fz4CKOAP/−;PDGFB-CreER retinas compared to their Fz4CKOAP/+;PDGFB-CreER littermates (Figure 5E right).
An analogous decline in vascular density occurs in the Fz4CKOAP/−;Tie2-Cre cerebellum, as seen by comparing Fz4CKOAP/−;Tie2-Cre and Fz4CKOAP/+;Tie2-Cre cerebellar sections in Figures S3C, S3E, and S3F. A much more modest loss of vascular density was noted previously in NdpKO cerebella (Luhmann et al., 2008). While these results are consistent with a cell autonomous mechanism for EC loss, the progressive cerebellar degeneration associated with loss of Fz4 signaling in the vasculature leaves open the possibility that non-autonomous effects from the cerebellar parenchyma could drive vascular pruning (Wang et al, 2001). By contrast, the genetic mosaic analysis of retinal vasculature (described in the preceding paragraph) is unaffected by this ambiguity as it involves a comparison of Fz4+/− and Fz4−/− ECs within the same tissue and in the absence of retinal degeneration.
To determine whether the progressive loss of Fz4−/− ECs in the mosaic retina is associated with EC proliferation, Fz4CKOAP/−;PDGFB-CreER and control Fz4CKOAP/+PDGFB-CreER littermates were injected with EdU every 2–3 days between ~P23 and ~P50 and the retinas examined 1–2 weeks later (Figure 5F). Within the OPL, Fz4CKOAP/−;PDGFB-CreER and Fz4CKOAP/+PDGFB-CreER retinas have on average 25+/−21 and 8+/−7 EdU+ nuclei per mm2, respectively, a significant difference (P=0.01).Interestingly, when EdU counts were normalized to the density of Fz4−/− and Fz4+/− ECs (identified by immunostaining), it was found that EdU+ nuclei were substantially enriched on or adjacent to Fz4−/− ECs (Figure 5F, right panels), and that this density of EdU+ nuclei was significantly higher than the densities associated with Fz4+/− ECs in both Fz4CKOAP/−;PDGFB-CreER and Fz4CKOAP/+PDGFB-CreER retinas. Figure 5F also shows that the density of EdU+ nuclei associated with Fz4+/− vasculature was modestly elevated in Fz4CKOAP/−;PDGFB-CreER compared to Fz4CKOAP/+PDGFB-CreER retinas. These data indicate that during the process of eliminating Fz4−/− ECs there is a low but detectable level of proliferation among vessel-associated cells, most likely ECs and pericytes. However, the overall conclusion is that newly generated ECs make at most a modest contribution to vascular remodeling in the adult Fz4CKOAP/−;Tie2-Cre retina, and that vascular remodeling consists largely of a selective removal of Fz4−/− ECs.
Modifying retinal vascular development by controlling the timing of Norrin production
To explore the vascular response to Norrin/Fz4 signaling at early times in development, we determined the consequences of precocious Norrin/Fz4 signaling using Z/Norrin;Foxg1-Cre mice (Figure 6). Foxg1-Cre activates target gene expression in the anterior retina by embryonic day (E)10 (Hébert and McConnell, 2000), an observation that we have confirmed by crossing Foxg1-Cre to Z/H2B-GFP, a knock-in that is identical to Z/Norrin except for the identity of the reporter (Figure 6C). By contrast, the endogenous Ndp gene is only expressed in the retina beginning late in gestation. Prenatal Ndp expression is confined to a region immediately adjacent to the optic disc; postnatally, Ndp is expressed in developing Muller glia throughout the retina (Ye et al., 2009, 2011). Figure 6 shows that prenatal activation of the Z/Norrin locus leads to precocious vascular invasion of the retina from the adjacent hyaloid vessels (i.e. vessels residing in the vitreous). Overall, 28/30 Foxg1-Cre;Z/Norrin eyes and 0/36 control eyes harvested between E17 and P0 exhibited vascular invasion into the retina (P=1.3 × 10−16; controls comprised littermates that lacked Foxg1-Cre and/or Z/Norrin). In Z/Norrin;Foxg1-Cre eyes, the invading vasculature was confined to the anterior retina, corresponding to the territory with Cre-mediated recombination.
Figure 6. Early production of Norrin induces precocious vascular invasion of the retina.
(A) Retina flat-mounts at E17 show extensive intravitreal (hyaloid) vasculature with intraretinal sprouts in the Z/Norrin;Foxg1-Cre retina (right) but not in the WT littermate retina (left). Scale bar, 100 um.
(B) At E17 there is vascular sprout (arrow) in the Z/Norrin;Foxg1-Cre retina (right) but not in the littermate control retina (left). The GS-lectin-stained lens is at center right.
(C) By P1, the Foxg1-Cre transgene has recombined the Z/H2B-GFP reporter in the anterior retina and lens. A, anterior; P, posterior. White arrow, boundary between recombined and non-recombined retinal zones.
(D) E17 Z/Norrin;Foxg1-Cre retina at higher magnification showing a vascular sprout (arrows) emerging from the hylaoid vasculature (top) and projecting into the retina. Scale bar, 100 um.
To explore the role of Norrin/Fz4 signaling at later stages, we used 4HT (delivered over 3–5 days by three IP injections of 200 ug each) to activate Norrin expression in Muller glia in NdpKO;Z/Norrin;GLAST-CreER mice beginning from P4 to P23 and then we assessed image-forming visual function in adulthood by the optokinetic response (OKR; Cahill and Nathans, 2008) and quantified retinal vascular architecture (Figure 7). The OKR depends on transmission of rod and cone signals to retinal ganglion cells via interneurons in the INL. In NdpKO and Fz4−/− mice, the OKR is absent, presumably because these interneurons, although present, are insufficiently supplied with oxygen and nutrients (Ye et al., 2009). In disorders characterized by incomplete intraretinal vascularization, the INL is the most vulnerable of the three retinal layers, as it cannot be efficiently supplied by either the choroidal circulation, which supplies the photoreceptors, or the vasculature at the vitreal surface, which supplies the ganglion cell layer. INL hypoxia can be readily demonstrated by visualizing the distribution of pimonidazole (Hypoxyprobe) adducts in the adult NdpKO retina (Figure S6). In the present experiments, a comparison of the OKR among 30 4HT-treated NdpKO;Z/Norrin;GLAST-CreER mice showed a bimodal distribution (Figure 7C, left), implying that the dependence of retinal interneuron function on vascular development exhibits a sharp threshold. 4HT injection on or before P8 leads to rescue of the OKR in ~50% of mice, but 4HT injection after P8 fails to rescue (Figure 7C, right).
Figure 7. Initiating Norrin production at different times during retinal vascular development leads to graded rescue of the NdpKO phenotype.
(A) Adult retina cross-sections show capillaries in the IPL and OPL in WT, a sparse IPL capillary network in NdpKO;Z/Norrin;GLAST-CreER mice treated with 3 × 0.2 mg 4HT over 3 days starting at P4 (mouse 4424) or P5 (mouse 4420), and a greatly reduced (4424) or absent (4420) OPL capillary network. In all cases, Norrin production restored claudin5. See also Figure S6. White arrowheads, normal locations of the three vascular layers. Scale bar, 100 um.
(B) Adult retina flat-mounts stained with GS-lectin, with the vertical position of the vasculature color-coded. NdpKO;Z/Norrin;GLAST-CreER mice treated with 3×0.2 mg 4HT over 3 days starting at P4 (mouse 4424) or P8 (mouse 4576) exhibit sparser IPL and OPL capillary networks. Scale bar, 100 um.
(C) Visual function among adult NdpKO;Z/Norrin;GLAST-CreER mice measured by OKR. Left, 90-second OKR traces in response to 30-seconds of rotating black and white vertical stripes sandwiched between 30-second rest periods. Mouse 4420 shows a normal OKR; mouse 4426 shows only spontaneous eye movements. Right, summary of OKR testing.
(D) Red boxes show regions of adult retina flat-mounts displayed in (C) and quantified in (E).
(E) Summary of 4HT injection times, adult retinal vascular coverage, and OKR in WT, NdpKO, and NdpKO;Z/Norrin;GLAST-CreER mice (numbered). Four retinal regions were imaged as shown in (D) and the fraction of the X-Y plane at the vitreal surface (blue), IPL (green), and OPL (red) occupied by blood vessels was determined.
Following OKR testing, the retinal vasculature from 19 NdpKO;Z/Norrin;GLAST-CreER mice was analyzed (Figure 7D). The vitreal surface, IPL, and OPL vascular beds were color coded, and the area occupied by the vasculature was quantified for each (Figure 7B and E). An example of a retina with an IPL vascular bed but no OPL vascular bed is seen in Figure 7A (mouse 4420). In some cases, graded differences in vascularization were observed across a single retina (e.g. mice 4424 and 4576 in Figures 7A and B). As seen in Figure 7E, several trends emerge from this data: (1) earlier 4HT treatment produces greater intra-retinal vascular coverage, (2) the IPL vasculature is rescued more efficiently than the OPL vasculature, and (3) lower vascular density within the retina (i.e. in the IPL and OPL) is associated with higher vascular density on the vitreal surface, an effect that is probably driven by retinal hypoxia. The second trend could reflect the greater distance from the vitreal face of the retina - from which the intraretinal vasculature originates - to the OPL compared to the IPL. However, we think it more likely reflects the earlier development of the OPL vasculature compared to the IPL vasculature. In particular, we imagine that there may be a distinct time windows during which the retina permits OPL or IPL invasion.
A less interesting explanation for the inability of later 4HT injections to rescue the NdpKO phenotype is an age-dependent decline in the efficiency of Cre-mediated recombination at the Z/Norrin locus. To test this possibility, we treated Z/H2B-GFP;GLAST-CreER mice with 4HT in the same manner as the NdpKO;Z/Norrin;GLAST-CreER mice and counted the number of GFP+ Muller glial nuclei per retina in adulthood. 4HT injection starting at P5, P8, and P10 produced on average 3085, 2060, and 1880 GFP+ nuclei per retina (n=2–3 retinas per time point). It seems unlikely that this modest difference in the efficiency of Cre-mediated recombination could account for more than a small fraction of the time-dependent decline in phenotypic rescue.
Taken together, the experiments in Figures 6 and 7 indicate that the retinal vasculature is responsive to Norrin/Fz4 signaling throughout its development. More generally, these experiments illustrate the profound effect that small changes in the timing of ligand-receptor signaling can have on the architecture of a vascular network.
Discussion
Together with earlier work, the experiments reported here show that Norrin/Fz4 signaling regulates multiple aspects of retinal vascular development including EC proliferation, migration, and invasion, and arterial and venous identity and topography. By creating mosaic Fz4−/−:Fz4+/− retinal and brain vasculatures, we show that Frizzled4 signaling in ECs controls a cell autonomous developmental program that is essential for BBB/BRB integrity and regulates the expression of transcription factors, cell surface receptors, and cell junction proteins. With gain or loss of Fz4 signaling in mature vasculature, this program can be activated or silenced, respectively. These observations imply that the BBB/BRB state remains plastic throughout life and that continuous Frizzled signaling is required for its maintenance.
In contrast to the cell autonomy of the BBB/BRB phenotype, the developing Fz4−/−:Fz4+/− mosaic retinal vasculature exhibits non-autonomous rescue of vascular network formation, indicating that angiogenesis is a social process in which information is distributed across the EC population. Finally, we observe that Fz4−/− ECs are selectively eliminated from the Fz4−/−:Fz4+/− mosaic retinal vasculature, implying the existence of a vascular quality control program.
The role of Frizzled signaling in vascular diversity and plasticity
Although Fz4 is expressed throughout the CNS and non-CNS vasculature, the Fz4−/− phenotype differs dramatically in different CNS regions. In the interior of the cerebral cortex, thalamus, and striatum, there is no apparent phenotype. In the spinal cord, nerve fiber and glomerular layers of the olfactory bulb, and molecular layer of the cerebellum, and at the surface of the cerebral cortex there is a loss of BBB integrity and a variable switch of ECs to a PLVAP+/claudin5− state with little or no effect on development of vascular architecture. By contrast, in the retina, there are multiple developmental defects – including a failure to form intra-retinal capillary beds and excessive artery/vein crossings - accompanied by a loss of BRB integrity and a complete switch to a PLVAP+/claudin5− state. We note that the primary nature of the retinal EC switch to a PLVAP+/claudin5− state was only revealed by studying Fz4−/−:Fz4+/− mosaics because the severe stunting of vascular development in Fz4−/− and NdpKO retinas leads to tissue hypoxia and compensatory neovascularization that preclude a clear distinction between primary and secondary phenotypic changes at the cellular level.
Ndp is expressed by glia throughout the CNS (Ye et al, 2011), but the NdpKO phenotype, like the Fz4−/− phenotype, varies in different CNS regions. In the cerebral cortex, thalamus, and striatum, there is no apparent phenotype. In the nerve fiber layer of the olfactory bulb and the molecular layer of the cerebellum there is a loss of BBB integrity but only a small minority of ECs switch to a PLVAP+/claudin5−/BBB− state. By contrast, in the retina the phenotype is uniform, severe, and indistinguishable from the Fz4−/− phenotype. Perhaps the most surprising aspect of the NdpKO and Fz4KO phenotypes in the cerebellum and olfactory bulb are their cellular heterogeneity: Norrin/Fz4 and Wnt/Fz4 signaling appears to act by biasing a cell-autonomous choice between distinct developmental states, with the result that adjacent and genetically identical ECs can exhibit strikingly different molecular phenotypes.
These findings - together with reports by others on the vascular consequences of gain or loss of Wnt7a, Wnt7b, and beta-catenin function, and of administration of anti-Fz4 mAb (Liebner et al., 2008; Stenman et al., 2008; Daneman et al., 2009; Paes et al., 2011) – are consistent with the following model (Figure S7): (1) all CNS ECs require canonical Wnt signaling to develop and maintain a BBB/BRB competent state; (2) in retinal ECs, this signaling pathway is activated exclusively by Norrin and Fz4; (3) in the nerve fiber and glomerular layers of the olfactory bulb, the molecular layer of the cerebellum, the most superficial layer of the cerebral cortex, and the spinal cord, Norrin and/or one or more Wnt ligands activate this pathway primarily through Fz4; (4) in other brain regions, additional Frizzled or non-Frizzled receptors and their Wnt ligands are fully redundant with Norrin and Fz4, and (5) when canonical Wnt signaling declines below a threshold level, the phenotypic response tends to be quantized rather than graded, and is manifested by an increase in the probability that ECs will switch from a PLVAP−/Claudin5+/BBB+ to a PLVAP+/Claudin5−/BBB− state.
Frizzled signaling and vascular architecture
The vascular defects reported in the Wnt7a−/−;Wnt7b−/− neural tube at E10-E12 (Stenman et al., 2008; Daneman et al., 2009) bear a striking resemblance to NdpKO and Fz4−/− defects in the retina: in both cases, surface vessels penetrate only a small distance into the target neural tissue. In the NdpKO retina, these abortive vascular protrusions persist throughout the life of the mouse and high levels of retina-derived VEGF are unable to stimulate further in-growth, although ECs on the retinal surface are induced to proliferate. These data suggest that if target-derived pro-angiogenic signaling drives vascular invasion into the CNS, then canonical Wnt and Norrin signaling act to gate that process.
The selective decrease in OPL capillaries in NdpKO;Z/Norrin;GLAST-CreER retinas that experienced late Norrin expression suggests an additional constraint on vascular growth: a time-dependent switch of retinal tissue from a permissive to a refractory state. In this case, the data suggest that the OPL is permissive for vascular invasion in early postnatal life and then converts to a more refractory state, whereas the IPL retains a permissive character at later times. If this model is correct, it will be interesting to identify the signaling molecules responsible for this inhibition. These or related molecules might also function to selectively exclude larger vessels from traversing regions of retina specialized for high acuity vision, such as the primate fovea.
Clinical implications of Frizzled signaling and BBB/BRB integrity
Loss of BBB/BRB integrity has been recognized in a wide variety of clinical contexts, including stroke, inflammatory and infectious diseases, diabetes, and traumatic brain injury (Ballabh et al., 2004). Strong evidence that pathologically increased vascular permeability plays a central role in a variety of ophthalmic disorders – including age-related macular degeneration and diabetic macular edema – comes from the rapid improvement in visual function produced by intra-ocular injection of anti-VEGF drugs (Truong et al., 2011). The rapid response arises from a decrease in vascular permeability and a concomitant decrease in retinal edema. Controlling edema is also central to the management of stroke and traumatic brain injury (Walcott et al., 2012), and the difference in permeability between tumor-associated and non-tumor vasculature is being exploited in neuro-imaging and neurooncology (Eichler et al., 2011). Finally, the accessibility of the CNS parenchyma to systemically administered drugs is determined largely by the BBB/BRB, and, therefore, variability in barrier function is of broad interest in clinical pharmacology (de Boer and Gaillard, 2007). The central role of Frizzled signaling in BBB/BRB development and maintenance implies that naturally occurring or induced variations in this signaling pathway will be of clinical interest.
Experimental Procedures
Mice
The following mouse alleles were used: Fz4− (Wang et al., 2001); Fz4CKOAP, Ndp−, Z/Norrin (Ye et al., 2009); PDGFB-CreER (Claxton et al., 2008), Tie2-Cre (Kisanuki et al., 2001), Foxg1-Cre (Hébert and McConnell, 2000); GLAST-CreER (a BAC transgenic line that has CreER inserted at the initiator methionine codon of the GLAST transporter gene); and Z/H2B-GFP (a LoxP-stop-LoxP knock-in at the Ubb locus).
Histochemistry and immunohistochemistry
Sources of antibodies and experimental procedures related to histochemistry, immunohistochemstry, in situ hybridization, and EdU and Hypoxyprobe labeling are described in Supplemental Information.
BBB and BRB integrity
For Sulfo-NHS-LC-Biotin perfusion, deeply anesthetized mice were perfused with 10–15 ml 0.5 mg/ml Sulfo-NHS-LC-Biotin (Thermo Scientific 21335) in PBS, followed by 1% PFA in PBS. Eyes were post-fixed in 1% PFA at 4°C for 4–6 hours prior to retina dissection. Wholemount retinas and 100 um vibratome sections of cerebella were incubated in Texas Red Streptavidin (Vector SA-5006) diluted in PBSTC + 10% normal goat or donkey serum, washed in PBSTC and mounted in Fluoromount G. For Evans Blue leakage, 100 ul of 2% Evans Blue (Sigma E-2129) was injected IP 24 hours prior to perfusion with 4% PFA in PBS. Brains were dissected and photographed intact.
Quantification of retinal vascular coverage and EdU+ nuclei
For the NdpKO;Z/Norrin;GLAST-CreER experiments, four Z-stacks covering 1.44 mm2 and offset approximately 0.3 mm from the optic disc were captured from each GS-lectin stained retina using a Zeiss LSM700 microscope. For each of the three retinal vascular beds, the fraction of the X-Y plane occupied by fluorescent GS-lectin was measured using ImageJ. For the analysis of Fz4−/− EC coverage in retinas from Fz4CKOAP/−;PDGFB-CreER mice, the ratio of PLVAP+ to claudin5+ vascular coverage in the OPL was quantified using ImageJ. For quantifying OPL vascular coverage and EdU+ nuclei, territories of 1 mm2 were analyzed. Parallel lines spaced at 100 um intervals were overlayed on the image and the number of crossings of Fz4+/− and Fz4−/− vessels were counted. EdU+ nuclei within each territory were categorized as residing on a vessel, adjacent to a vessel, or not near a vessel. Counts of EdU+ nuclei on or near vessels were normalized to the relative densities of the vessels of that genotype.
Alkaline phosphatase fusion protein binding
Human VEGF-A 165 or human VEGF-B 186 coding regions without their signal peptides were cloned 3’ of an AP coding region with a C-terminal triple-myc tag. The AP-Sema3F plasmid was a gift from Alex Kolodkin. Conditioned media containing the AP fusion proteins were collected from transiently transfected 293 cells and stored at 4°C. Fresh frozen retina sections were blocked in DMEM/F12 containing 10% FBS, with or without competitor VEGF peptide (Sigma V4512, 10 ug/ml), for 1 hour at 4°C and then incubated with conditioned medium, with or without competitor, for 1 hour at 4°C. The sections were then washed 6 times with cold DMEM/F12, fixed with 4% PFA for 15 min, and incubated at 65°C for 90 min. AP activity was detected with BCIP/NBT and blood vessels were visualized by staining with Alexa fluor 594 conjugated GS lectin.
Imatinib Treatment
Two-month old Fz4CKOAP/−;Tie2-Cre mice were injected intraperitoneally with Imatinib (LC Laboratories) at a dose of 150 mg/kg. Two hours later, the mice were perfused with 0.5 mg/ml Sulfo-NHS-biotin followed by 2% PFA.
Supplementary Material
Acknowledgements
We thank Hugh Cahill, Hao Chang, Alex Kolodkin, Se-Jin Lee, Max Tischfield, and Xin Ye for advice and/or helpful comments on the manuscript. Supported by the National Eye Institute (NIH), HHMI, the Johns Hopkins Brain Sciences Institute, the Foundation Fighting Blindness, and the Ellison Medical Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Albuquerque RJ, Hayashi T, Cho WG, Kleinman ME, Dridi S, Takeda A, Baffi JZ, Yamada K, Kaneko H, Green MG, et al. Alternatively spliced vascular endothelial growth factor receptor-2 is an essential endogenous inhibitor of lymphatic vessel growth. Nat. Med. 2009;15:1023–1030. doi: 10.1038/nm.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armulik A, Genové G, Mäe M, Nisancioglu MH, Wallgard E, Niaudet C, He L, Norlin J, Lindblom P, et al. Pericytes regulate the blood-brain barrier. Nature. 2010;468:557–561. doi: 10.1038/nature09522. [DOI] [PubMed] [Google Scholar]
- Ballabh P, Braun A, Nedergaard M. The blood-brain barrier: an overview: structure, regulation, and clinical implications. Neurobiol Dis. 2004;16:1–13. doi: 10.1016/j.nbd.2003.12.016. [DOI] [PubMed] [Google Scholar]
- Bell RD, Winkler EA, Sagare AP, Singh I, LaRue B, Deane R, Zlokovic BV. Pericytes control key neurovascular functions and neuronal phenotype in the adult brain and during brain aging. Neuron. 2010;68:409–427. doi: 10.1016/j.neuron.2010.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill H, Nathans J. The optokinetic reflex as a tool for quantitative analysis of nervous system function in mice: application to genetic and drug-induced variation. PLoS One. 2008;3:e2055. doi: 10.1371/journal.pone.0002055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claxton S, Kostourou V, Jadeja S, Chambon P, Hodivala-Dilke K, Fruttiger M. Efficient, inducible Cre-recombinase activation in vascular endothelium. Genesis. 2008;46:74–80. doi: 10.1002/dvg.20367. [DOI] [PubMed] [Google Scholar]
- Daneman R, Agalliu D, Zhou L, Kuhnert F, Kuo CJ, Barres BA. Wnt/beta-catenin signaling is required for CNS but not non-CNS, angiogenesis. Proc. Natl. Acad. Sci. U.S.A. 2009;106:641–646. doi: 10.1073/pnas.0805165106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daneman R, Zhou L, Kebede AA, Barres BA. Pericytes are required for blood-brain barrier integrity during embryogenesis. Nature. 2010;468:562–566. doi: 10.1038/nature09513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Boer AG, Gaillard PJ. Drug targeting to the brain. Annu. Rev. Pharmacol. Toxicol. 2007;47:323–355. doi: 10.1146/annurev.pharmtox.47.120505.105237. [DOI] [PubMed] [Google Scholar]
- Eichler AF, Chung E, Kodack DP, Loeffler JS, Fukumura D, Jain RK. The biology of brain metastases-translation to new therapies. Nat. Rev. Clin. Oncol. 2011;8:344–356. doi: 10.1038/nrclinonc.2011.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantin A, Schwarz Q, Davidson K, Normando EM, Denti L, Ruhrberg C. The cytoplasmic domain of neuropilin 1 is dispensable for angiogenesis, but promotes the spatial separation of retinal arteries and veins. Development. 2011;138:4185–4191. doi: 10.1242/dev.070037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukushima Y, Okada M, Kataoka H, Hirashima M, Yoshida Y, Mann F, Gomi F, Nishida K, Nishikawa S, Uemura A. Sema3E-PlexinD1 signaling selectively suppresses disoriented angiogenesis in ischemic retinopathy in mice. J. Clin. Invest. 2011;121:1974–1985. doi: 10.1172/JCI44900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gariano RF, Gardner TW. Retinal angiogenesis in development and disease. Nature. 2005;438:960–966. doi: 10.1038/nature04482. [DOI] [PubMed] [Google Scholar]
- Geretti E, Shimizu A, Klagsbrun M. Neuropilin structure governs VEGF and semaphorin binding and regulates angiogenesis. Angiogenesis. 2008;11:31–39. doi: 10.1007/s10456-008-9097-1. [DOI] [PubMed] [Google Scholar]
- Haigh JJ, Morelli PI, Gerhardt H, Haigh K, Tsien J, Damert A, Miquerol L, Muhlner U, Klein R, Ferrara N, et al. Cortical and retinal defects caused by dosage-dependent reductions in VEGF-A paracrine signaling. Dev. Biol. 2003;262:225–241. doi: 10.1016/s0012-1606(03)00356-7. [DOI] [PubMed] [Google Scholar]
- Hébert JM, McConnell SK. Targeting of cre to the Foxg1 (BF-1) locus mediates loxP recombination in the telencephalon and other developing head structures. Dev. Biol. 2000;222:296–306. doi: 10.1006/dbio.2000.9732. [DOI] [PubMed] [Google Scholar]
- Junge HJ, Yang S, Burton JB, Paes K, Shu X, French DM, Costa M, Rice DS, Ye W. TSPAN12 regulates retinal vascular development by promoting Norrin- but not Wnt-induced FZD4/beta-catenin signaling. Cell. 2009;139:299–311. doi: 10.1016/j.cell.2009.07.048. [DOI] [PubMed] [Google Scholar]
- Kim J, Oh WJ, Gaiano N, Yoshida Y, Gu C. Semaphorin 3E-Plexin-D1 signaling regulates VEGF function in developmental angiogenesis via a feedback mechanism. Genes Dev. 2011;25:1399–1411. doi: 10.1101/gad.2042011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kisanuki YY, Hammer RE, Miyazaki J, Williams SC, Richardson JA, Yanagisawa M. Tie2-Cre transgenic mice: a new model for endothelial cell-lineage analysis in vivo. Dev. Biol. 2001;230:230–242. doi: 10.1006/dbio.2000.0106. [DOI] [PubMed] [Google Scholar]
- Liebner S, Corada M, Bangsow T, Babbage J, Taddei A, Czupalla CJ, Reis M, Felici A, Wolburg H, Fruttiger M, et al. Wnt/beta-catenin signaling controls development of the blood-brain barrier. J. Cell Biol. 2008;183:409–417. doi: 10.1083/jcb.200806024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luhmann UF, Neidhardt J, Kloeckener-Gruissem B, Schäfer NF, Glaus E, Feil S, Berger W. Vascular changes in the cerebellum of Norrin /Ndph knockout mice correlate with high expression of Norrin and Frizzled-4. Eur. J. Neurosci. 2008;27:2619–2628. doi: 10.1111/j.1460-9568.2008.06237.x. [DOI] [PubMed] [Google Scholar]
- Makinen T, Olofsson B, Karpanen T, Hellman U, Soker S, Klagsbrun M, Eriksson U, Alitalo K. Differential binding of vascular endothelial growth factor B splice and proteolytic isoforms to neuropilin-1. J Biol. Chem. 1999;274:21217–21222. doi: 10.1074/jbc.274.30.21217. [DOI] [PubMed] [Google Scholar]
- Matsui T, Kanai-Azuma M, Hara K, Matoba S, Hiramatsu R, Kawakami H, Kurohmaru M, Koopman P, Kanai Y. Redundant roles of Sox17 and Sox18 in postnatal angiogenesis in mice. J. Cell Sci. 2006;119:3513–3526. doi: 10.1242/jcs.03081. [DOI] [PubMed] [Google Scholar]
- Paes KT, Wang E, Henze K, Vogel P, Read R, Suwanichkul A, Kirkpatrick LL, Potter D, Newhouse MM, Rice DS. Frizzled 4 is required for retinal angiogenesis and maintenance of the blood-retina barrier. Invest. Ophthalmol. Vis. Sci. 2011;52:6452–6461. doi: 10.1167/iovs.10-7146. [DOI] [PubMed] [Google Scholar]
- Pendeville H, Winandy M, Manfroid I, Nivelles O, Motte P, Pasque V, Peers B, Struman I, Martial JA, Voz ML. Zebrafish Sox7 and Sox18 function together to control arterial-venous identity. Dev. Biol. 2008;317:405–416. doi: 10.1016/j.ydbio.2008.01.028. [DOI] [PubMed] [Google Scholar]
- Pellet-Many C, Frankel P, Jia H, Zachary I. Neuropilins: structure, function and role in disease. Biochem. J. 2008;411:211–226. doi: 10.1042/BJ20071639. [DOI] [PubMed] [Google Scholar]
- Richter M, Gottanka J, May CA, Welge-Lüssen U, Berger W, Lütjen-Drecoll E. Retinal vasculature changes in Norrie disease mice. Invest. Ophthalmol. Vis. Sci. 1998;39:2450–2457. [PubMed] [Google Scholar]
- Roberts WG, Palade GE. Increased microvascular permeability and endothelial fenestration induced by vascular endothelial growth factor. J. Cell Sci. 1995;108:2369–2379. doi: 10.1242/jcs.108.6.2369. [DOI] [PubMed] [Google Scholar]
- Sakamoto Y, Hara K, Kanai-Azuma M, Matsui T, Miura Y, Tsunekawa N, Kurohmaru M, Saijoh Y, Koopman P, Kanai Y. Redundant roles of Sox17 and Sox18 in early cardiovascular development of mouse embryos. Biochem. Biophys. Res. Commun. 2007;360:539–544. doi: 10.1016/j.bbrc.2007.06.093. [DOI] [PubMed] [Google Scholar]
- Smallwood PM, Williams J, Xu Q, Leahy DJ, Nathans J. Mutational analysis of Norrin-Frizzled4 recognition. J. Biol. Chem. 2007;282:4057–4068. doi: 10.1074/jbc.M609618200. [DOI] [PubMed] [Google Scholar]
- Stan RV, Kubitza M, Palade GE. PV-1 is a component of the fenestral and stomatal diaphragms in fenestrated endothelia. Proc. Natl. Acad. Sci. U.S.A. 1999;96:13203–13207. doi: 10.1073/pnas.96.23.13203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenman JM, Rajagopal J, Carroll TJ, Ishibashi M, McMahon J, McMahon AP. Canonical Wnt signaling regulates organspecific assembly and differentiation of CNS vasculature. Science. 2008;322:1247–1250. doi: 10.1126/science.1164594. [DOI] [PubMed] [Google Scholar]
- Strickland LA, Jubb AM, Hongo JA, Zhong F, Burwick J, Fu L, Frantz GD, Koeppen H. Plasmalemmal vesicleassociated protein (PLVAP) is expressed by tumour endothelium and is upregulated by vascular endothelial growth factor-A (VEGF) J. Pathol. 2005;206:466–475. doi: 10.1002/path.1805. [DOI] [PubMed] [Google Scholar]
- Truong A, Wong TY, Khachigian LM. Emerging therapeutic approaches in the management of retinal angiogenesis and edema. J. Mol. Med. 2011;89:343–361. doi: 10.1007/s00109-010-0709-z. [DOI] [PubMed] [Google Scholar]
- Walcott BP, Kahle KT, Simard JM. Novel treatment targets for cerebral edema. Neurotherapeutics. 2012;9:65–72. doi: 10.1007/s13311-011-0087-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Huso D, Cahill H, Ryugo D, Nathans J. Progressive cerebellar, auditory, and esophageal dysfunction caused by targeted disruption of the frizzled-4 gene. J. Neurosci. 2001;21:4761–4771. doi: 10.1523/JNEUROSCI.21-13-04761.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Q, Wang Y, Dabdoub A, Smallwood PM, Williams J, Woods C, Kelley MW, Jiang L, Tasman W, Zhang K, Nathans J. Vascular development in the retina and inner ear: control by Norrin and Frizzled-4, a high-affinity ligand-receptor pair. Cell. 2004;116:883–895. doi: 10.1016/s0092-8674(04)00216-8. [DOI] [PubMed] [Google Scholar]
- Ye X, Wang Y, Cahill H, Yu M, Badea TC, Smallwood PM, Peachey NS, Nathans J. Norrin, frizzled-4, and Lrp5 signaling in endothelial cells controls a genetic program for retinal vascularization. Cell. 2009;139:285–298. doi: 10.1016/j.cell.2009.07.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye X, Smallwood P, Nathans J. Expression of the Norrie disease gene (Ndp) in developing and adult mouse eye, ear, and brain. Gene Expr. Patterns. 2011;11:151–155. doi: 10.1016/j.gep.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye X, Wang Y, Nathans J. The Norrin/Frizzled4 signaling pathway in retinal vascular development and disease. Trends Mol. Med. 2010;16:417–425. doi: 10.1016/j.molmed.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.