Abstract
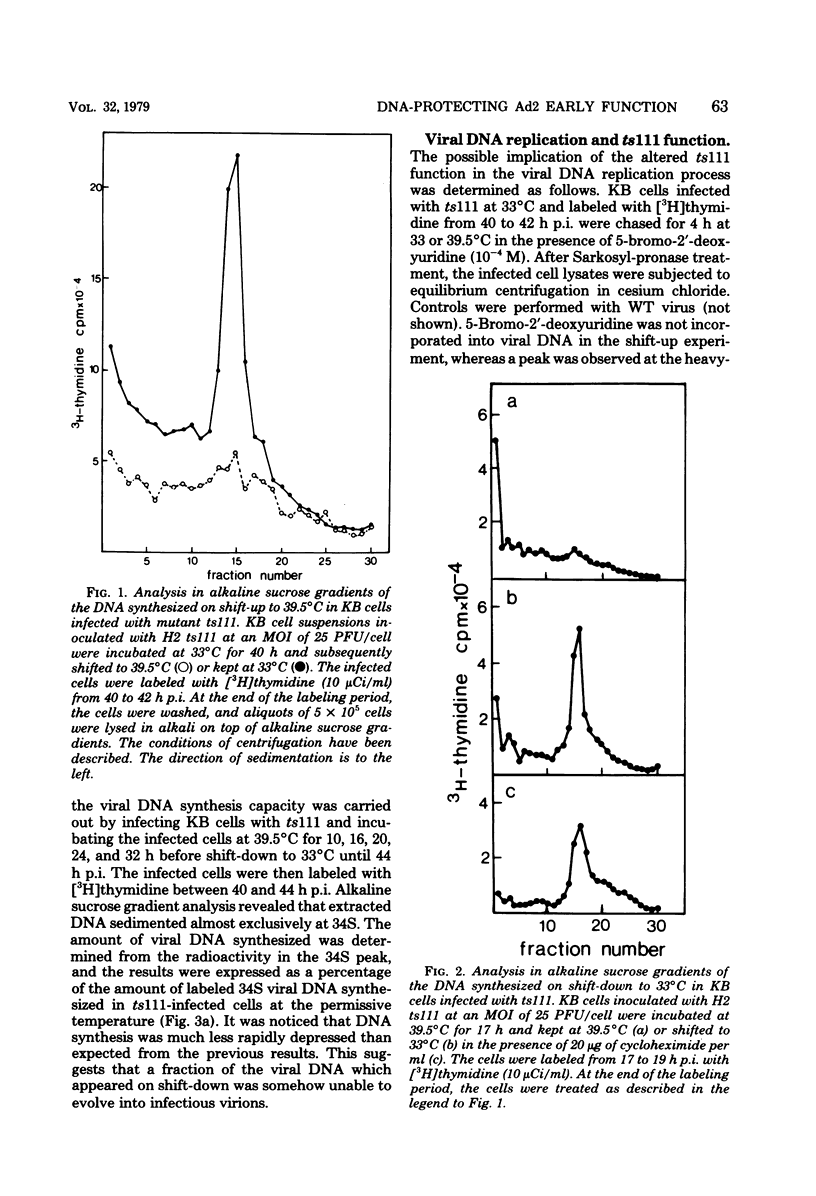
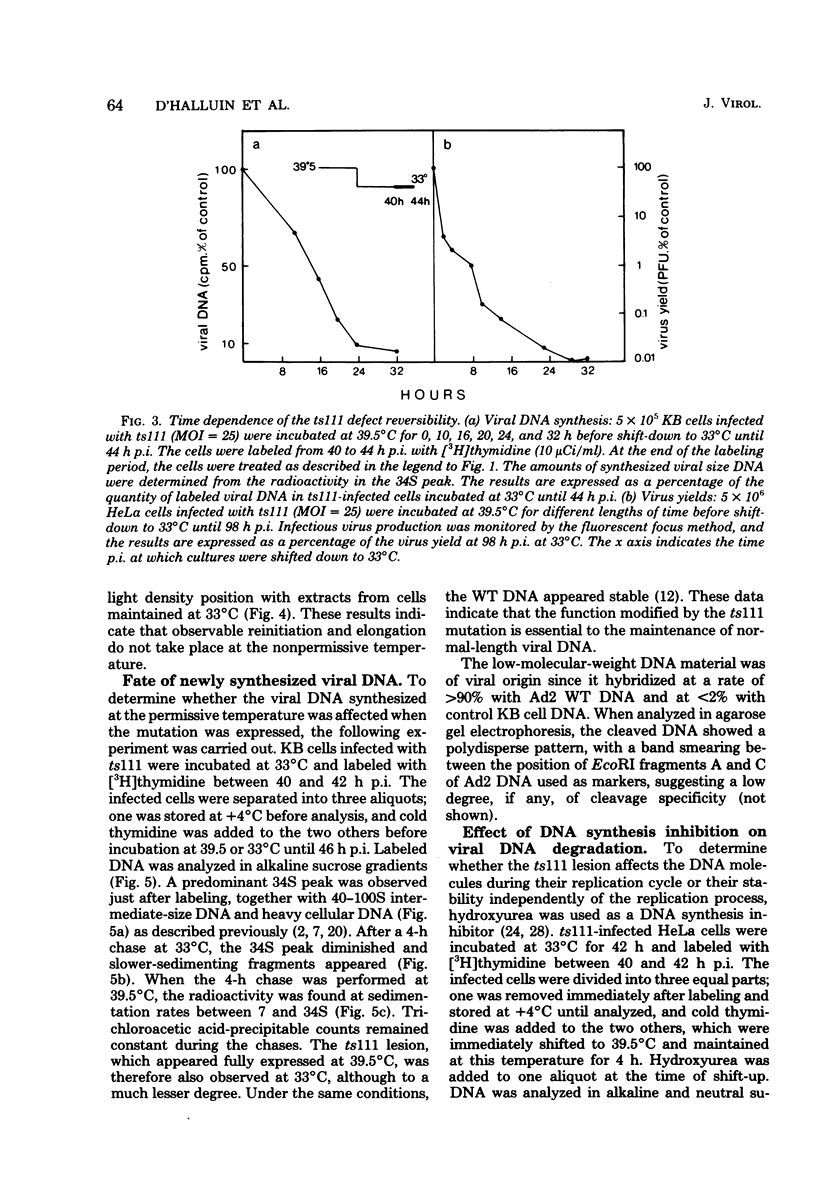
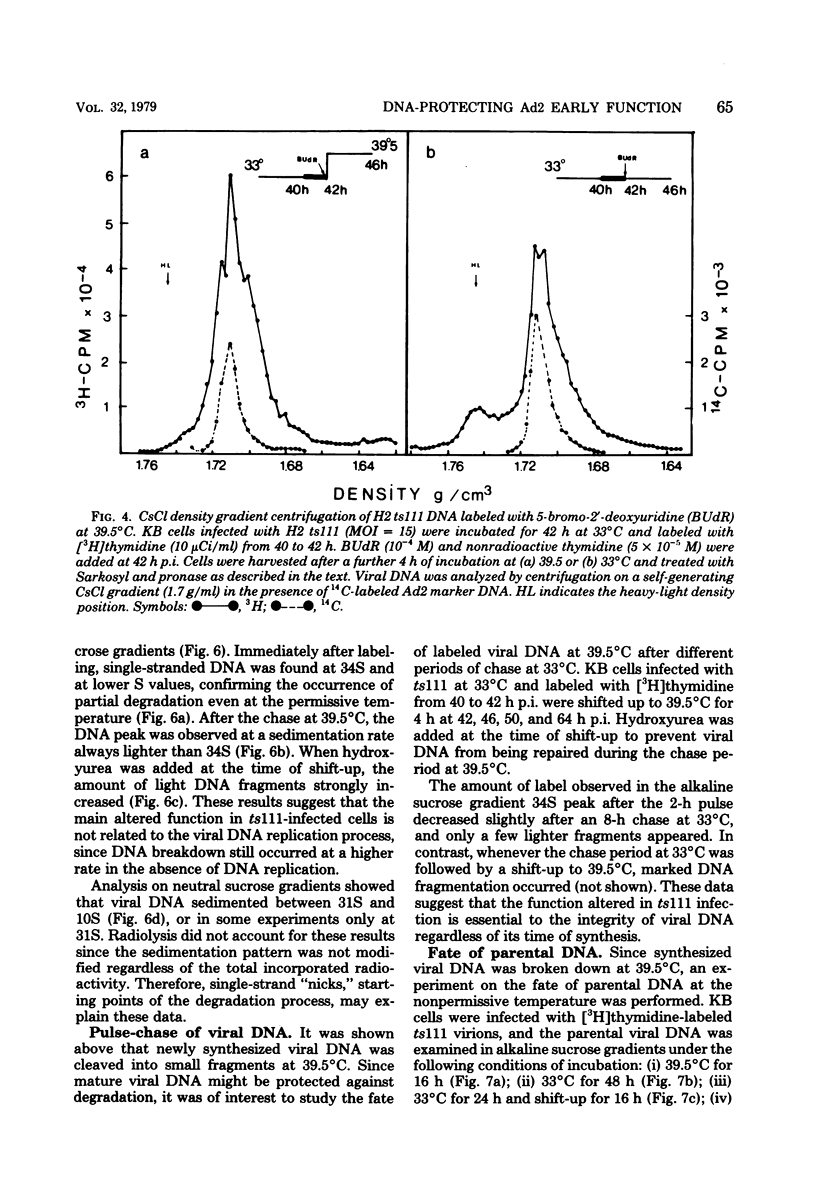
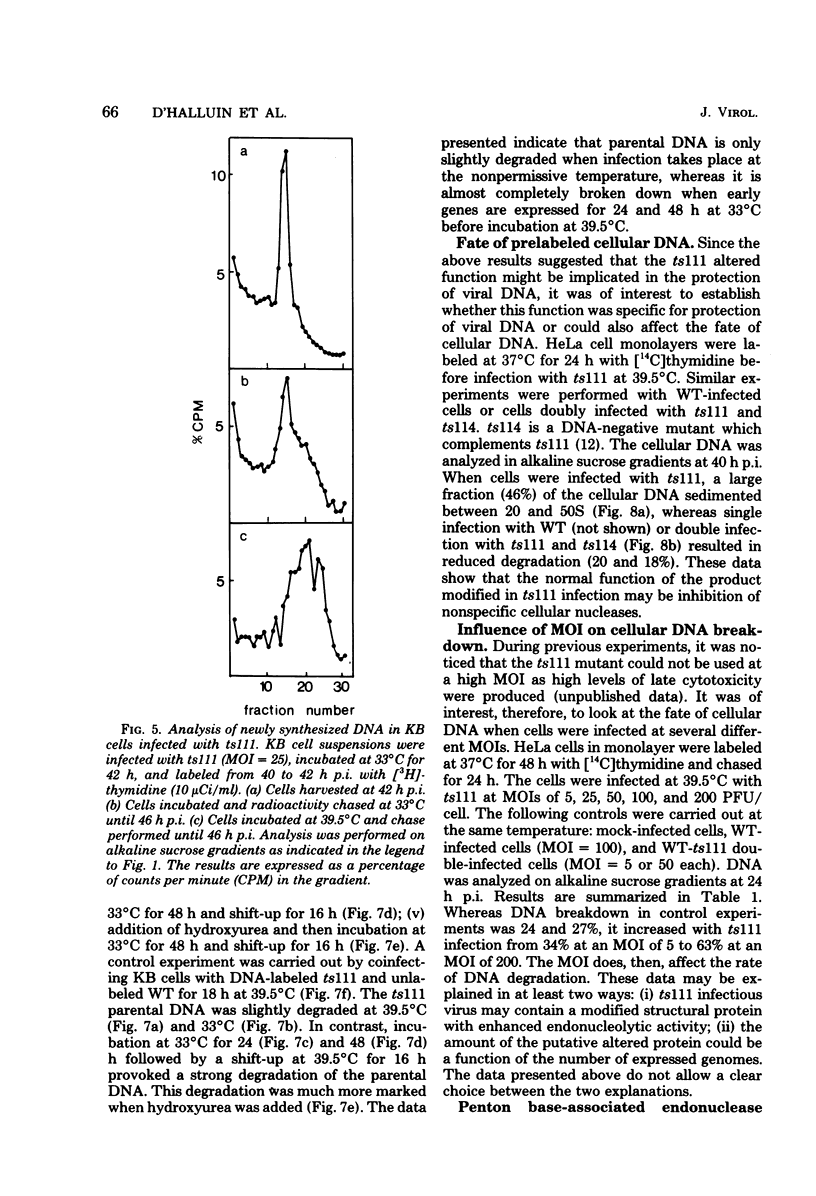
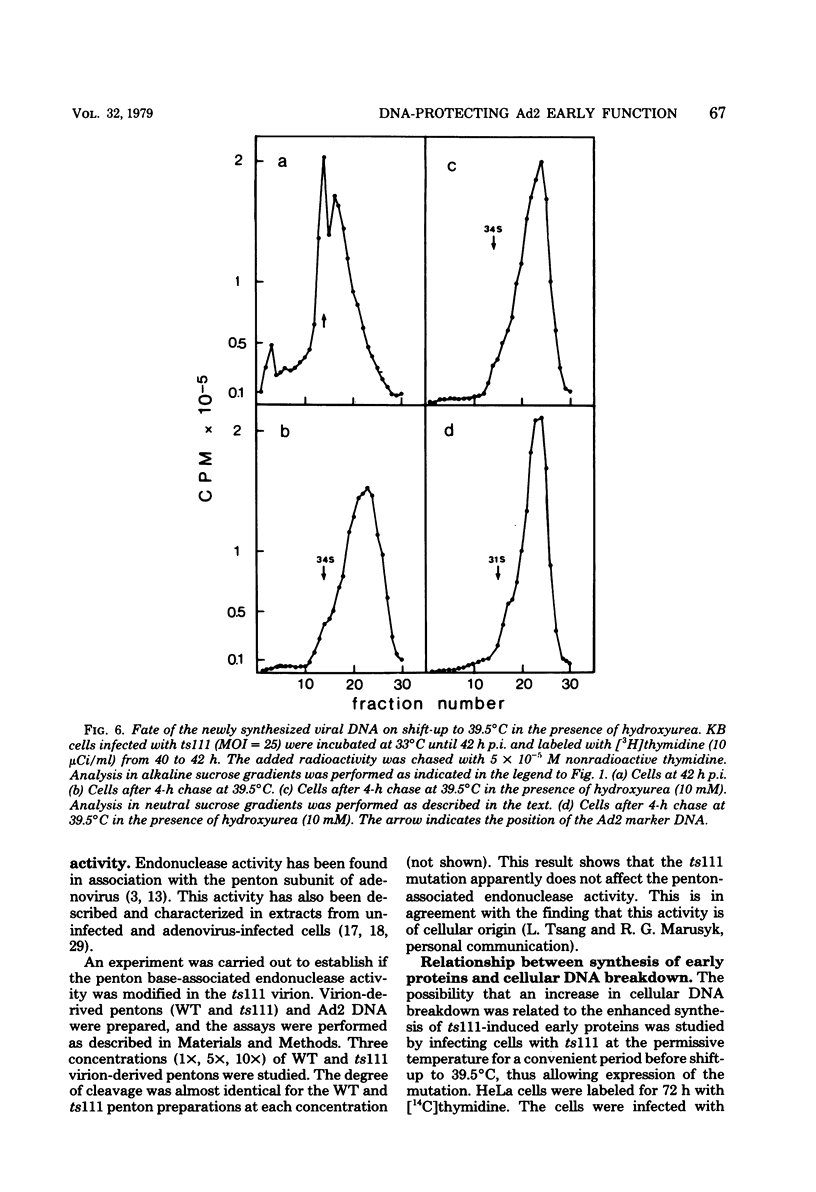
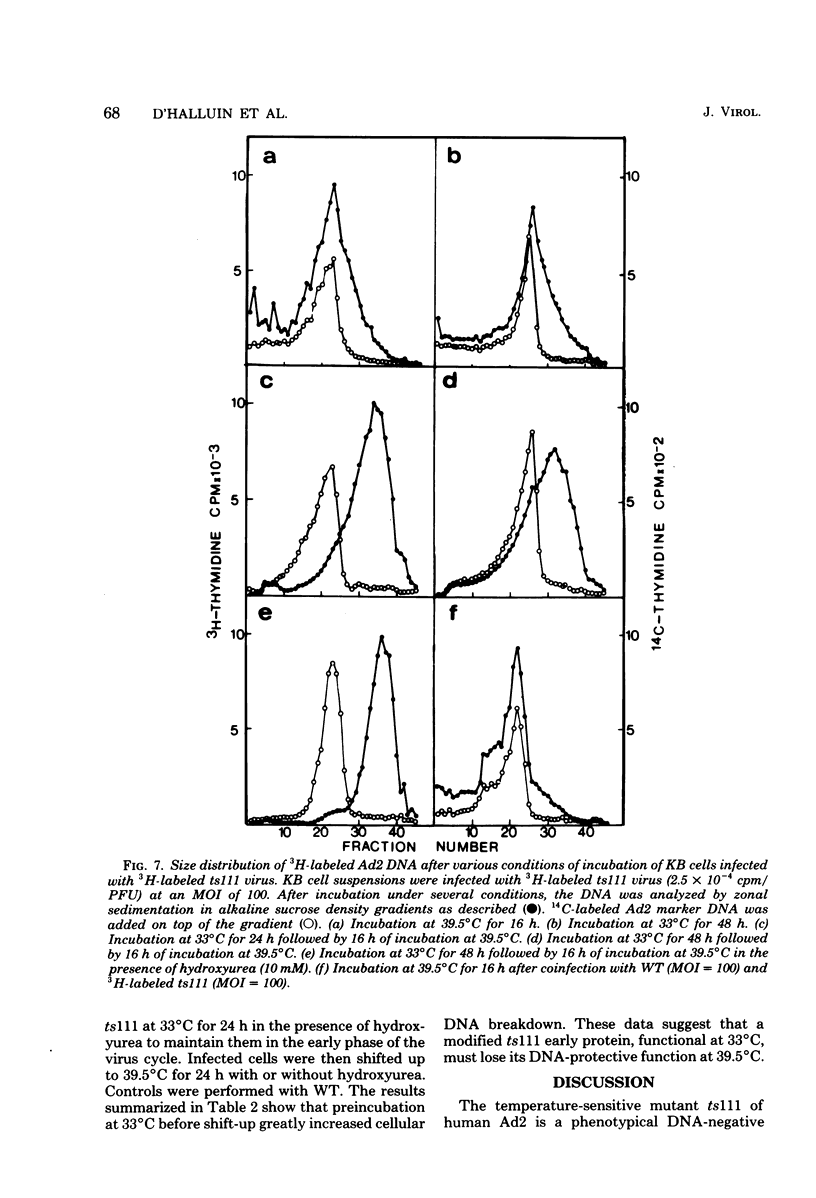
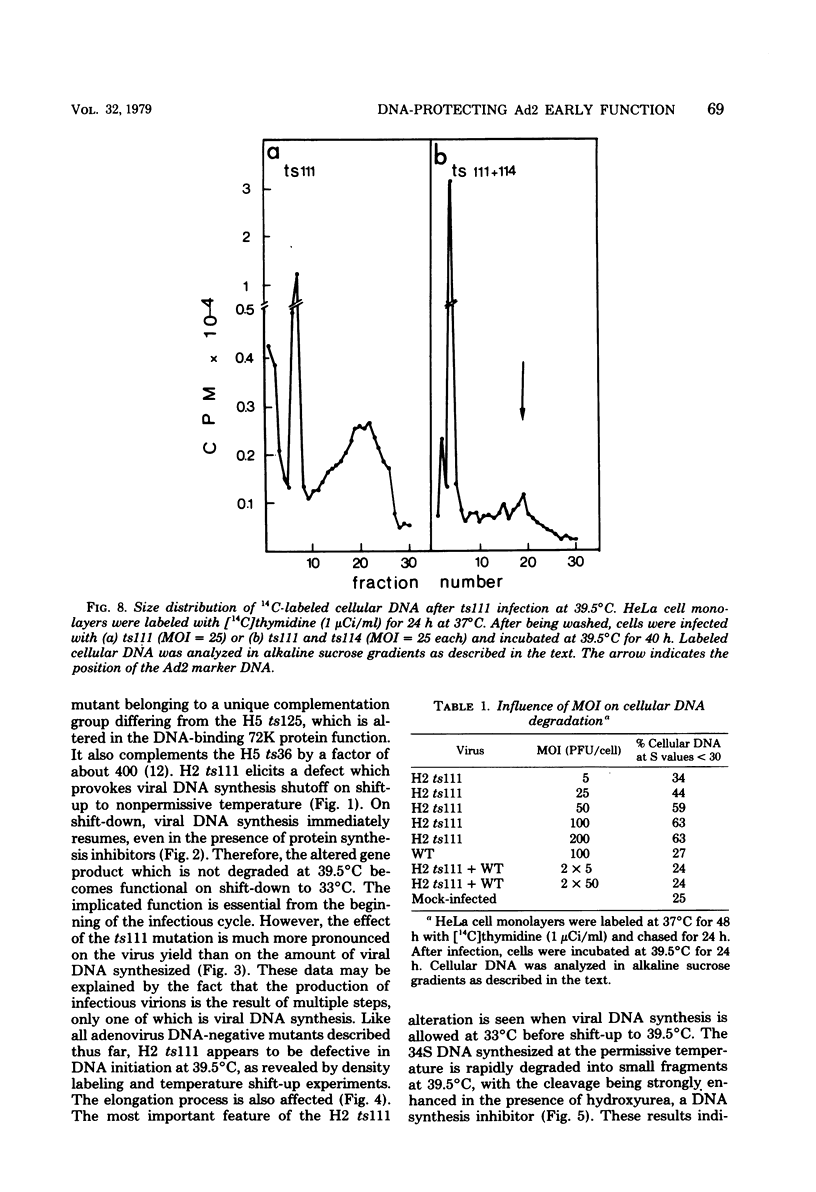
Studies were done to characterize a DNA-negative temperature-sensitive (ts) mutant of human adenovirus type 2, H2 ts111. The temperature-sensitive defect, which was reversible on shift-down in the absence of protein synthesis, was expressed as early as 2 h postinfection, and the results of density-labeling experiments are in agreement with at least a DNA replication initiation block. On shift-up, after allowing viral DNA synthesis at permissive temperatures, the newly synthesized viral DNA and the mature viral DNA were cleaved into fragments which sedimented as a broad peak with a mean coefficient of 10-12S. This cleavage was more marked in the presence of hydroxyurea as the DNA synthesis inhibitor. Parental DNA in infected cells was degraded to a much lesser extent regardless of the incubation temperature. In contrast, the parental DNA was strongly degraded when early gene expression was permitted at 33 degrees C before shift-up to 39.5 degrees C. Furthermore, cellular DNA was also degraded at 39.5 degrees C in ts111-infected cells, the rate of cleavage being related to the multiplicity of infection. This cleavage effect, which did not seem to be related to penton base-associated endonuclease activity, was also enhanced when early gene expression was allowed at 33 degrees C before shift-up. The ts111 defect, which was related to an initiation block and endonucleolytic cleavage of viral and cellular DNA, seemed to correspond to a single mutation. The implication of the ts111 gene product in protection of viral and cellular DNA by way of a DNase-inhibitory function is discussed.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Behme M. T., Lilley G. D., Ebisuzaki K. Postinfection control by bacteriophage T4 of Escherichia coli recBC nuclease activity. J Virol. 1976 Apr;18(1):20–25. doi: 10.1128/jvi.18.1.20-25.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burlingham B. T., Doerfler W., Pettersson U., Philipson L. Adenovirus endonuclease: association with the penton of adenovirus type 2. J Mol Biol. 1971 Aug 28;60(1):45–64. doi: 10.1016/0022-2836(71)90446-3. [DOI] [PubMed] [Google Scholar]
- Burlingham B. T., Doerfler W. Three size-classes of intracellular adenovirus deoxyribonucleic acid. J Virol. 1971 Jun;7(6):707–719. doi: 10.1128/jvi.7.6.707-719.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Halluin J. C., Milleville M., Boulanger P. A., Martin G. R. Temperature-sensitive mutant of adenovirus type 2 blocked in virion assembly: accumulation of light intermediate particles. J Virol. 1978 May;26(2):344–356. doi: 10.1128/jvi.26.2.344-356.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doerfler W. Nonproductive infection of baby hamster kidney cells (BHK21) with adenovirus type 12. Virology. 1969 Aug;38(4):587–606. doi: 10.1016/0042-6822(69)90179-2. [DOI] [PubMed] [Google Scholar]
- Ensinger M. J., Ginsberg H. S. Selection and preliminary characterization of temperature-sensitive mutants of type 5 adenovirus. J Virol. 1972 Sep;10(3):328–339. doi: 10.1128/jvi.10.3.328-339.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanning E., Schick J., Doerfler W. Studies on the occurrence of high molecular weight adenovirus type 2 DNA in productively infected cells. Virology. 1978 Jul 1;88(1):186–190. doi: 10.1016/0042-6822(78)90123-x. [DOI] [PubMed] [Google Scholar]
- Groneberg J., Brown D. T., Doerfler W. Uptake and fate of the DNA of adenovirus type 2 in KB cells. Virology. 1975 Mar;64(1):115–131. doi: 10.1016/0042-6822(75)90084-7. [DOI] [PubMed] [Google Scholar]
- Kathmann P., Schick J., Winnacker E. L., Doerfler W. Isolation and characterization of temperature-sensitive mutants of adenovirus type2. J Virol. 1976 Jul;19(1):43–53. doi: 10.1128/jvi.19.1.43-53.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledinko N. Temperature-sensitive mutants of adenovirus type 12 defective in viral DNA synthesis. J Virol. 1974 Sep;14(3):457–468. doi: 10.1128/jvi.14.3.457-468.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin G. R., Warocquier R., Cousin C., D'Halluin J. C., Boulanger P. A. Isolation and phenotypic characterization of human adenovirus type 2 temperature-sensitive mutants. J Gen Virol. 1978 Nov;41(2):303–314. doi: 10.1099/0022-1317-41-2-303. [DOI] [PubMed] [Google Scholar]
- Marusyk R. G., Morgan A. R., Wadell G. Association of endonuclease activity with serotypes belonging to the three subgroups of human adenoviruses. J Virol. 1975 Aug;16(2):456–458. doi: 10.1128/jvi.16.2.456-458.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nass K., Frenkel G. D. Adenovirus-induced inhibition of cellular DNase. J Virol. 1978 May;26(2):540–543. doi: 10.1128/jvi.26.2.540-543.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver D. B., Goldberg E. B. Protection of parental T4 DNA from a restriction exonuclease by the product of gene 2. J Mol Biol. 1977 Nov;116(4):877–881. doi: 10.1016/0022-2836(77)90276-5. [DOI] [PubMed] [Google Scholar]
- Philipson L., Lonberg-Holm K., Pettersson U. Virus-receptor interaction in an adenovirus system. J Virol. 1968 Oct;2(10):1064–1075. doi: 10.1128/jvi.2.10.1064-1075.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reif U. M., Winterhoff U., Lundholm U., Philipson L., Doerfler W. Purification of an endonuclease from adenovirus-infected KB cells. Eur J Biochem. 1977 Mar 1;73(2):313–325. doi: 10.1111/j.1432-1033.1977.tb11321.x. [DOI] [PubMed] [Google Scholar]
- Reif U., Winterhoff U., Doerfler W. Characterization of the pH 4.0 endonuclease from adenovirus-type-2-infected KB cells. Eur J Biochem. 1977 Mar 1;73(2):327–333. doi: 10.1111/j.1432-1033.1977.tb11322.x. [DOI] [PubMed] [Google Scholar]
- Sakaki Y. Inactivation of the ATP-dependent DNase of Escherichia coli after infection with double-stranded DNA phages. J Virol. 1974 Dec;14(6):1611–1612. doi: 10.1128/jvi.14.6.1611-1612.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schick J., Baczko K., Fanning E., Groneberg J., Burger H., Doerfler W. Intracellular forms of adenovirus DNA: integrated form of adenovirus DNA appears early in productive infection. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1043–1047. doi: 10.1073/pnas.73.4.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schick J., Doerfler W. High molecular weight virus DNA in KB cells infected with ts mutants of adenovirus type 2 under permissive and non-permissive conditions. J Gen Virol. 1979 Apr;43(1):217–222. doi: 10.1099/0022-1317-43-1-217. [DOI] [PubMed] [Google Scholar]
- Sergeant A., Tigges M. A., Raskas H. J. Nucleosome-like structural subunits of intranuclear parental adenovirus type 2 DNA. J Virol. 1979 Mar;29(3):888–898. doi: 10.1128/jvi.29.3.888-898.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiroki K., Shimojo H. Analysis of adenovirus 12 temperature-sensitive mutants defective in viral DNA replication. Virology. 1974 Oct;61(2):474–485. doi: 10.1016/0042-6822(74)90283-9. [DOI] [PubMed] [Google Scholar]
- Sussenbach J. S., van der Vliet P. C. Studies on the mechanism of replication of adenovirus DNA. I. The effect of hydroxyurea. Virology. 1973 Jul;54(1):299–303. doi: 10.1016/0042-6822(73)90142-6. [DOI] [PubMed] [Google Scholar]
- Suzuki E., Shimojo H. A temperature-sensitive mutant of adenovirus 31, defective in viral deoxyribonucleic acid replication. Virology. 1971 Feb;43(2):488–494. doi: 10.1016/0042-6822(71)90320-5. [DOI] [PubMed] [Google Scholar]
- Suzuki E., Shimojo H., Moritsugu Y. Isolation and a preliminary characterization of temperature-sensitive mutants of adenovirus 31. Virology. 1972 Aug;49(2):426–438. doi: 10.1016/0042-6822(72)90495-3. [DOI] [PubMed] [Google Scholar]
- Tanner D., Oishi M. The effect of bacteriophage T4 infection on an ATP-dependent deoxyribonuclease in Escherichia coli. Biochim Biophys Acta. 1971 Feb 11;228(3):767–769. doi: 10.1016/0005-2787(71)90747-7. [DOI] [PubMed] [Google Scholar]
- Timson J. Hydroxyurea. Mutat Res. 1975;32(2):115–132. doi: 10.1016/0165-1110(75)90002-0. [DOI] [PubMed] [Google Scholar]
- Tsuruo T., Arens M., Padmanabhan R., Green M. An endodeoxyribonuclease of human KB cells. Purification and properties of the enzyme. J Biol Chem. 1978 May 25;253(10):3400–3407. [PubMed] [Google Scholar]
- Wilkie N. M., Ustacelebi S., Williams J. F. Characterization of temperature-sensitive mutants of adenovirus type 5: nucleic acid synthesis. Virology. 1973 Feb;51(2):499–503. doi: 10.1016/0042-6822(73)90450-9. [DOI] [PubMed] [Google Scholar]



